Abstract
Objective:
We evaluated quantitative EEG (QEEG) measures as predictive biomarkers for the development of dementia in Parkinson disease (PD). Preliminary work shows that QEEG measures correlate with current PD cognitive state. A reliable predictive QEEG biomarker for PD dementia (PD-D) incidence would be valuable for studying PD-D, including treatment trials aimed at preventing cognitive decline in PD.
Methods:
A cohort of subjects with PD in our brain donation program utilizes annual premortem longitudinal movement and cognitive evaluation. These subjects also undergo biennial EEG recording. EEG from subjects with PD without dementia with follow-up cognitive evaluation was analyzed for QEEG measures of background rhythm frequency and relative power in δ, θ, α, and β bands. The relationship between the time to onset of dementia and QEEG and other possible predictors was assessed by using Cox regression.
Results:
The hazard of developing dementia was 13 times higher for those with low background rhythm frequency (lower than the grand median of 8.5 Hz) than for those with high background rhythm frequency (p < 0.001). Hazard ratios (HRs) were also significant for > median θ bandpower (HR = 3.0; p = 0.004) compared to below, and for certain neuropsychological measures. The HRs for δ, α, and β bandpower as well as baseline demographic and clinical characteristics were not significant.
Conclusion:
The QEEG measures of background rhythm frequency and relative power in the θ band are potential predictive biomarkers for dementia incidence in PD. These QEEG biomarkers may be useful in complementing neuropsychological testing for studying PD-D incidence.
Dementia is common in Parkinson disease (PD).1,2 The addition of cognitive dysfunction to the motor disability of PD increases disability and mortality risk.3–5 Treatment with acetylcholinesterase inhibitors and NMDA antagonists has been shown to provide modest symptomatic benefit in PD dementia (PD-D).6–8 However, thus far there is no therapy known to prevent or delay the occurrence of dementia among patients with PD. It is likely that any neuroprotective therapy would have greatest efficacy if started at, or even prior to, the earliest symptoms of dementia. In the absence of a reliable biomarker for impending PD-D, large numbers of cognitively normal patients with PD would need to be treated with a putative neuroprotective agent in order to ensure that the expected incidence of dementia would be sufficient to demonstrate efficacy in a controlled trial.
The resting EEG is simple to acquire, requires minimal patient cooperation, and is not dependent on verbal or motor responses which may be affected in PD. Quantitative EEG (QEEG) measures may be ideal biomarkers to complement neuropsychological testing for studying cognitive decline among patients with PD. Our group and others have used QEEG methods to show correlation between dementia in subjects with PD and increased low-frequency and decreased high-frequency content of the resting EEG.9–15 Given these results, the present study investigates whether any of these measures might also serve as predictive biomarkers of PD-D incidence. We hypothesized that, among our longitudinally followed brain bank cohort, median cutoff QEEG values in subjects with PD without dementia are associated with PD-D incidence over time.
METHODS
Subjects.
Study subjects in the cohort were enrolled in the Banner Sun Health Research Institute Brain and Body Donation Program.16 The brain bank database was analyzed for subjects with PD evaluated over a 9-year period, from April 2000 (beginning of EEG examinations) to April 2009. Subjects underwent annual longitudinal movement disorder, behavioral neurology, and neuropsychological assessments as well as biennial EEG recordings. PD was diagnosed along the lines of the UK Parkinson's Disease Society brain bank clinical diagnostic criteria (bradykinesia plus at least one other cardinal feature of PD, no atypical features or secondary cause).17,18 The neuropsychological battery included Folstein Mini-Mental State Examination (MMSE), long-term memory score on the Auditory Verbal Learning Test (AVLT-LTM), Controlled Oral Word Association Test (COWAT), Stroop Interference, Trails B, Wechsler Adult Intelligence Scale (WAIS)–III Digit Span, and Judgment of Line Orientation test (JLO). After each series of evaluations, subjects were classified as having dementia (PD-D) or no dementia by consensus conference among a movement disorder neurologist (C.H.A.), behavioral neurologist (M.N.S.), and neuropsychologist per previously published criteria that use DSM-IV.19 Subjects with PD with no dementia with mild cognitive impairment (PD-MCI) were identified as per previously published criteria.19 Conference participants were blinded to EEG results. For this study, only subjects without PD with no dementia at the time of baseline EEG, and who had at least one subsequent cognitive evaluation, had EEG analyzed. Subjects using anticonvulsant or benzodiazepine medication at the time of the EEG examination were excluded from the study.
Standard protocol approvals, registrations, and patient consents.
The Sun Health and Mayo Clinic institutional review boards approved all procedures and written informed consent was obtained from study participants.
EEG recording.
Recordings during relaxed wakefulness were obtained from subjects seated in a recliner with eyes closed as previously described.14 Briefly, Ag/AgCl EEG electrodes were placed on the scalp using 20 standard 10–20 EEG electrode positions. Recordings were referenced to the Fz electrode, with a right mastoid electrode serving as ground. Electrode impedances were closely monitored and kept below 5 kΩ. Data were acquired using the Neuroscan Synamps2 system (Compumedics, Charlotte, NC) at a sampling rate of 1,000 Hz and a bandpass of 1–200 Hz. An EEG technician and neurophysiologist (J.N.C.) were present during the entire recording session to observe the behavioral state of the patient and to monitor on-line for signal quality. Patients were asked every minute if they were awake, and drowsiness was further excluded by monitoring for background changes and slow eye movements of drowsiness during the course of the recording. When muscle, eye movement/blink, or other artifacts were identified, the subject was coached until an artifact-free signal was obtained. The final recorded sample consisted of an awake artifact-free period of approximately 100–120 seconds.
EEG data processing.
The data from baseline EEG were processed off-line using Neuroscan EDIT software (Compumedics, Charlotte, NC). Consecutive, nonoverlapping, 4,096-point epochs were created from the continuous data, allowing for a frequency resolution of 0.244 Hz. Each epoch was manually inspected for artifacts, though rejection of artifacts was uncommon due to the monitoring of the online acquisition. The number of epochs accepted for further processing ranged from 25 to 30. In the rare cases when artifact was present in greater than 20% of the EEG traces, the subject was excluded from the study. The epochs were passed through a 10% cosine window, processed with a fast Fourier transform (FFT), and averaged to produce an averaged FFT power spectrum for each electrode. The background rhythm frequency (BRF) was defined as dominant peak in the power spectra of the posterior electrodes (P3, P4, Oz) determined by visual inspection of the FFT average.
Frequency bands were designated as follows: δ = 1.5–3.9 Hz; θ = 4–7.9 Hz; α = 8–12.9 Hz; β = 13–30 Hz. The global (over multiple electrodes) relative (%) EEG bandpower (defined here as “bandpower”) for each of the 4 frequency bands was calculated (using all electrodes except Fp1, Fp2, A1, and A2) as a percentage of total EEG power between 1.5 and 30 Hz from the FFT average.
Statistical analysis.
For subjects subsequently reclassified as PD-D, the date of onset of dementia was approximated by the midpoint between cognitive evaluations. The incidence of dementia for the entire sample was calculated using the Kaplan-Meier method. Mean and median values were calculated for all the variables. The median value was used as a “middle value” for each variable to determine a cutoff. Cox regression was used to calculate the incidence curves and hazard ratio (HR) using median cutoff categories (below or above the median cutoff value) of the multiple variables (e.g., QEEG measures). Analysis of the confounding effect of one variable on another was performed by using multivariable Cox regression modeling. A change in the HR of >20% in either direction by controlling for another variable in the modeling was designated as a confounding effect of that other variable.
RESULTS
There were a total of 138 subjects with PD, 21 of whom had dementia at baseline, leaving 117 subjects who met initial inclusion criteria. Seven subjects were excluded due to use of anticonvulsant or benzodiazepine medication at the time of the EEG examination, and 4 subjects were excluded based on the presence of artifacts in at least 20% of the EEG traces. Therefore, 106 subjects were included in the analysis. Twenty-four (23%) subjects had a diagnosis of PD-MCI at the baseline EEG examination.
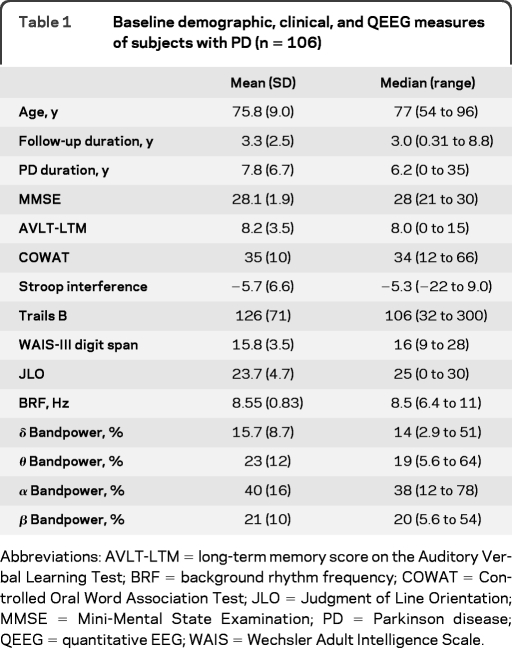
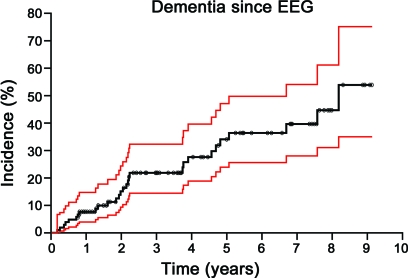
Mean and median values for the baseline characteristics of the 106 subjects with PD are given in table 1. Age ranged from 54 to 96 years with a mean of 76 years. The duration of follow-up after the EEG examination ranged from 0.31 to 8.8 years with a mean of 3.3 years. Forty-seven subjects (44%) were women. Figure 1 shows the Kaplan-Meier curve of all 106 subjects with PD for dementia incidence with 95% confidence limit bands. The incidence of dementia within 5 years of the baseline EEG examination for the aggregate group was 34%.
Table 1.
Baseline demographic, clinical, and QEEG measures of subjects with PD (n = 106)
Abbreviations: AVLT-LTM = long-term memory score on the Auditory Verbal Learning Test; BRF = background rhythm frequency; COWAT = Controlled Oral Word Association Test; JLO = Judgment of Line Orientation; MMSE = Mini-Mental State Examination; PD = Parkinson disease; QEEG = quantitative EEG; WAIS = Wechsler Adult Intelligence Scale.
Figure 1. Incidence of dementia since EEG among 106 subjects with Parkinson disease using Kaplan-Meier method (black).
Red lines indicate the 95% confidence band. Circles indicate the duration of follow-up for subjects who did not develop dementia (censored observations).
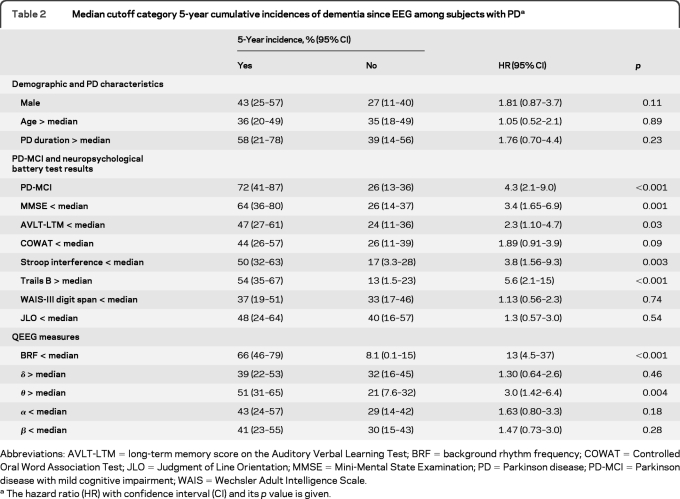
Table 2 shows the 5-year cumulative incidence of dementia using median cutoff categories for the demographic variables, PD duration, MMSE score, and QEEG measures. The HR values and their significance are also given.
Table 2.
Median cutoff category 5-year cumulative incidences of dementia since EEG among subjects with PDa
Abbreviations: AVLT-LTM = long-term memory score on the Auditory Verbal Learning Test; BRF = background rhythm frequency; COWAT = Controlled Oral Word Association Test; JLO = Judgment of Line Orientation; MMSE = Mini-Mental State Examination; PD = Parkinson disease; PD-MCI = Parkinson disease with mild cognitive impairment; WAIS = Wechsler Adult Intelligence Scale.
The hazard ratio (HR) with confidence interval (CI) and its p value is given.
Demographic and PD characteristics.
Age, sex, and PD duration median cutoff categories did not yield significant HR values.
PD-MCI and neuropsychological battery test results.
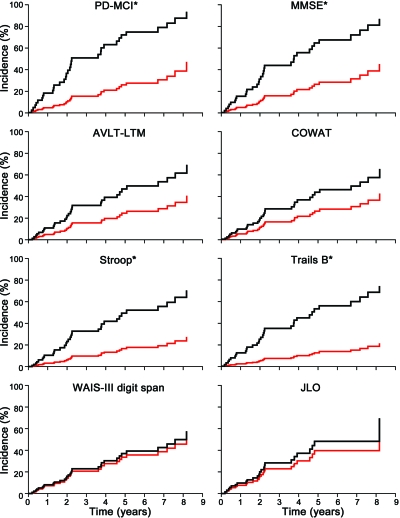
Figure 2 shows the cumulative incidence curves of the median cutoff categories for PD-MCI and the neuropsychological tests. The presence of PD-MCI showed a significant HR of 4.3. Trails B, Stroop interference, MMSE, and AVLT-LTM all had significant HRs, whereas COWAT, JLO, and WAIS-III digit span did not. The high to low rank order of HRs for significant neuropsychological test variables is Trails B > Stroop interference > MMSE > AVLT-LTM.
Figure 2. Cumulative incidence curves for median cutoff categories for Parkinson disease (PD)–mild cognitive impairment (MCI) and neuropsychological tests.
PD-MCI: yes black, no red; Mini-Mental State Examination (MMSE): < median black, > median red; long-term memory score on the Auditory Verbal Learning Test (AVLT-LTM): < median black, > median red; Controlled Oral Word Association Test (COWAT): < median black, > median red; Stroop-interference: < median black, > median red; Trails B: > median black, < median red; Wechsler Adult Intelligence Scale (WAIS)–III Digit Span: < median black, > median red; Judgment of Line Orientation (JLO): < median black, > median red. Asterisks indicate statistical significance as defined by p < 0.05.
QEEG measures.
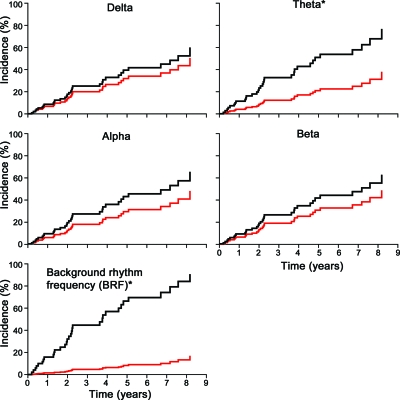
Figure 3 shows the cumulative incidence curves of the median cutoff categories for the QEEG measures. For the QEEG measures, the BRF HR of < median BRF (median cutoff value of 8.5 Hz) compared to > median BRF was significant at 13, and the HR was significant at 3.0 for > median θ bandpower compared to < median θ bandpower. The incidence of dementia within 5 years was 66% for those with lower than median BRF vs 8.1% for higher than median BRF. The incidence of dementia within 5 years was 51% for those with > median θ bandpower vs 21% for those with < median θ bandpower. Bandpower for δ, α, and β bands did not show significant HRs using the median cutoff categories.
Figure 3. Cumulative incidence curves for median cutoff categories for each of the quantitative EEG measures.
BRF: < median black, > median red; α bandpower: < median black, > median red; β bandpower: < median black, > median red; δ bandpower: > median black, < median red; θ bandpower: > median black, < median red. Asterisks indicate statistical significance as defined by p < 0.05.
The multivariable modeling analysis showed that the relationship between the hazard of dementia and BRF was not confounded by other factors. In this modeling, adjustment for sex, age, or PD duration reduced the HR for BRF by less than 4%. Adjustment for PD-MCI or MMSE reduced the HR for BRF by less than 15%, and no other neuropsychological test (except Trails B) by more than 9%. The adjustment for Trails B did reduce the BRF HR to 9.3, which is still a high HR. Adjustment for the δ, θ, α, or β bandpower reduced the HR for BRF by less than 8%. For the relationship between the hazard of dementia and θ bandpower, age category, sex, and the other QEEG bandpower measures were not confounding. However, the 3.0 HR for θ bandpower was reduced by PD duration to 2.1 and by MMSE to 2.4. In addition, the multivariable modeling revealed that controlling for BRF reduced the HR for θ band to only 1.16.
DISCUSSION
We found that certain QEEG measures are potential predictive biomarkers for PD-D incidence. Those with a lower than median BRF had a significantly higher incidence of dementia (HR = 13) over time than those with higher than median BRF, while those with high θ bandpower have a smaller but still significant increased incidence of PD-D (HR = 3.0) than those with lower than median θ bandpower. PD duration at baseline, sex, and age median cutoff categories did not elicit significant HRs for risk of dementia. Moreover, in the multivariable modeling, adjustment for PD duration, sex, and age did not reduce the HR for BRF by more than 8%. The presence of PD-MCI and the median cutoff scores for Stroop interference, AVLT-LTM, and MMSE all had significant HRs. Trails B score had the next highest HR after BRF, but accounting for Trails B still produced a high BRF HR of 9.3. The BRF hazard was not reduced in the multivariable modeling by more than 15% by either PD-MCI or any of these neuropsychological test results. This suggests that the BRF predictive biomarker for dementia is not simply acting as a surrogate for any of PD duration, age, PD-MCI, or the neuropsychological tests with respect to dementia risk. Moreover, the multivariable modeling suggests that the BRF is not simply acting as a biomarker of current cognitive impairment without dementia criteria being fulfilled. θ bandpower HR was considerably less than for BRF and was confounded by both PD duration and MMSE. This may suggest that θ bandpower is perhaps partially reflecting current cognitive impairment without dementia criteria being fulfilled. However, the θ bandpower HR was still above 2 after the multivariable analysis. It is important to note that besides BRF and θ bandpower, the other QEEG measures analyzed did not yield significant HRs. In our previously published cross-sectional EEG study, all these measures showed group differences between PD-D and PD no dementia groups.14 Our findings suggest that QEEG biomarkers have differential ability to act as various biomarker types, i.e., for concurrent dementia (surrogate biomarker) vs prediction of future dementia (predictive biomarker).
The predictive value of abnormal QEEG measures at the level of the individual patient needs more study, although it is conceivable that such measures may eventually assume a role in prognostication or in guiding decisions regarding early therapy. QEEG measures may also be useful as a biomarker tool in the study of PD-related cognitive decline, particularly for studying therapeutic intervention in PD-D. The promise of biomarkers is based partly on their potential to be more objective and precise than assessments of symptoms and examination findings of PD cognitive decline. Today, neuropsychological testing is considered to be the gold standard for assessing PD cognitive decline. However, neuropsychological testing can be affected by cooperation, effort, and degree of parkinsonism in verbal and motor responses. EEG acquisition requires only relaxed wakefulness and requires no effort or verbal and motor responses. In our QEEG cross-sectional study, we found that Unified Parkinson's Disease Rating Scale scores, Hoehn & Yahr staging, and levodopa equivalents did not account for the QEEG changes in PD cognitive decline.14 Moreover, EEG detects neurophysiologic activity related to cortical neurons that participate in cognitive functions.20–24 All these combined characteristics, along with low cost and good test-retest reliability, suggest that QEEG may effectively complement neuropsychological testing in therapeutic and other studies concerning PD cognitive decline.25,26
Among this population of longitudinally followed individuals fulfilling clinical diagnostic criteria for PD, the incidence of dementia was 34% of subjects without dementia by 5 years. The difference in dementia incidence at 5 years between high and low median cutoff values was 58% for BRF and 30% for θ bandpower measures. Thus, these measures could be used to risk-stratify potential study candidates prior to entry into trials of treatments intended to prevent or delay dementia in PD. For example, if subjects were screened for BRF <8.5 Hz, the 5-year incidence of dementia would increase from 34% to 66%. Thus, the sample size needed to detect a 25% reduction in the incidence of dementia would be 300 patients instead of 900 patients (α = 0.05, β = 0.2). Moreover, studies could assess the ability of a treatment to prevent the QEEG changes associated with transition to PD-D as a secondary outcome measure. This would afford an opportunity to see if the intervention could improve or prevent the worsening of cortical neuron physiology as assessed by QEEG measures.
We do not know why the BRF and θ bandpower showed significant HRs while δ, α, and β bandpower measures did not. The different EEG frequency bands have had various normal functions and pathophysiology correlates proposed. The background “α rhythm” is a normal EEG activity detected from dendrites of cortical neurons that is generated by subcortical and corticocortical circuits.20 Thus, early subcortical or cortical PD pathology may contribute to the pathologic downward spectral shift of the BRF frequency. The downward spectral peak shift of the BRF seen in our results was significant, but perhaps it did not result in significantly lower overall α bandpower because most of the BRF peak still stayed within the α band. α background rhythm has been implicated to correlate with the “default network,” which is cortical neuronal network activity that occurs during the cognitive resting state.27 There is evidence that high levels of amyloid deposition may create an aberrant default network in asymptomatic subjects, thereby representing a presymptomatic state for AD.28 Accordingly, Lewy body pathology in the frontal lobe or other regions that project to posterior cortical regions may disrupt the default network in PD, giving rise to abnormal background α frequency. This concept needs further investigation. The θ bandpower increase could have been significant because even a small amount of BRF peak “overflow” into the θ range, which is normally small, resulted in a significant increase in the usually low θ bandpower. Alternatively, the θ bandpower increase may arise from an independent pathologic generator. Increased θ power has been observed in attention deficit disorder, and patients with PD without dementia are known to demonstrate attention deficits.19,29,30 Increased δ activity represents severe disruption of cortical activity, and its presence much more closely correlates with coincident cognitive decline rather than predicts it.14 β rhythms are thought to represent primarily neocortical activity, but their wide frequency range of 13–30 Hz may make β bandpower an insensitive early predictor of PD-D.21–23 Pathologic and biochemical studies of subjects with PD who have had QEEG assessed will eventually provide insight about the substrates of early predictive QEEG changes for PD-D.
QEEG parameters can be useful in defining the risk of incident dementia in PD. Both a lower than median BRF and, to a lesser extent, a higher than median EEG power in the θ band are associated with a higher risk of dementia in this population. These parameters are potential biomarkers for cognitive decline in PD and may prove useful in clinical trials. QEEG, as an assessment of the physiologic activity of cortical neurons, may provide the most feasible window into the cortical dysfunction associated with the cognitive decline of patients with PD. Measurement of additional subjects with longer duration of follow-up will enable further refinement of prediction models.
- AVLT-LTM
- long-term memory score on the Auditory Verbal Learning Test
- BRF
- background rhythm frequency
- COWAT
- Controlled Oral Word Association Test
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FFT
- fast Fourier transform
- HR
- hazard ratio
- JLO
- Judgment of Line Orientation
- MMSE
- Mini-Mental State Examination
- PD
- Parkinson disease
- PD-D
- Parkinson disease with dementia
- PD-MCI
- Parkinson disease with mild cognitive impairment
- QEEG
- quantitative EEG
- WAIS
- Wechsler Adult Intelligence Scale
Editorial, page 94
AUTHOR CONTRIBUTIONS
Dr. Klassen: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. J.G. Hentz: analysis or interpretation of data, statistical analysis. Dr. Shill: drafting/revising the manuscript, acquisition of data. Dr. Driver-Dunckley: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Evidente: analysis or interpretation of data. Dr. Sabbagh: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Adler: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, obtaining funding. Dr. Caviness: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, obtaining funding.
DISCLOSURE
Dr. Klassen reports no disclosures. J.G. Hentz receives research support from Allergan, Inc., Astellas Pharma Inc., Dynatherm Medical, Inc., Millennium Pharmaceuticals, Inc., U.S. Department of Defense, Arizona Biomedical Research Commission, the NIH (NCI, Small Business Technology Transfer Program), and the Michael J Fox Foundation. Dr. Shill serves on a scientific advisory board for Teva Pharmaceutical Industries Ltd. and Ipsen; serves as a consultant for Medtronic, Inc.; and receives research support from Avid Radiopharmaceuticals, Inc., Chelsea Therapeutics, Teva Pharmaceutical Industries Ltd., Schering-Plough Corp., Boehringer Ingelheim, Kalaco Scientific, Inc., the NIH, Arizona Biomedical Research Commission, and International Essential Tremor Foundation, and the Michael J Fox Foundation for Parkinson Research. Dr. Driver-Dunckley has received research support from Merck Serono and GlaxoSmithKline. Dr. Evidente serves/has served on scientific advisory boards for Teva Pharmaceutical Industries Ltd., Merz Pharmaceuticals, LLC, Allergan, Inc., and Ipsen; serves as a consultant for Allergan, Inc., Teva Pharmaceutical Industries Ltd., Merz Pharmaceuticals, LLC; has received speaker honoraria from Teva Pharmaceutical Industries Ltd.; and receives research support from Allergan, Inc., Allon Therapeutics, Inc., UCB, Arizona Biomedical Research Commission, Arizona Department of Health Services, the NIH/NIA, and Michael J. Fox Foundation for Parkinson's Research (Prescott Family Initiative). Dr. Sabbagh serves/has served on scientific advisory boards for Ipsen, AmeriSciences, GlaxoSmithKline, Janssen/Pfizer Inc, Eisai Inc., Medtronic, Inc., Merck Serono, Merz Pharmaceuticals, LLC, Eli Lilly and Company, Takeda Pharmaceutical Company Limited, and Allon Therapeutics, Inc.; serves on the editorial boards of Clinical Neurology News, Journal of Alzheimer's Disease, and BMC Neurology; receives publishing royalties for The Alzheimer's Answer: Reduce Your Risk and Keep Your Brain Healthy (John Wiley & Sons, 2008); serves as a consultant for Elan Corporation; receives research support from Elan Corporation, Wyeth, Medivation, Inc., Avid Radiopharmaceuticals, Inc., Baxter International Inc., Eli Lilly and Company, GE Healthcare, Eisai Inc., Bayer Schering Pharma, Janssen/Pfizer Inc, Bristol-Myers Squibb, the NIH/NIA, the Alzheimer's Disease Cooperative Study, and ADNI; and receives royalties from AmeriSciences on the commercial product Cognivite. Dr. Adler has served on scientific advisory boards for Bachmann Strauss Dystonia and Parkinson Foundation; receives publishing royalties for Parkinson's Disease and Movement Disorders (Humana Press, 2000); has served as a consultant to Allergan, Inc., Biogen Idec, Eli Lilly and Company, GlaxoSmithKline, Ipsen, Medtronic, Inc., Merck Serono, and Merz Pharmaceuticals, LLC; and receives research support from Arizona Biomedical Research Commission, U.S. Department of Defense, NIH/NINDS, Michael J. Fox Foundation for Parkinson's Disease Research, and National Parkinson's Foundation. Dr. Caviness serves on the editorial board of Parkinsonism and Related Disorders; serves as a consultant for Teva Pharmaceutical Industries Ltd.; and receives research support from the Huntington's Disease Study Group.
REFERENCES
- 1. Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 2010;289:18–22 [DOI] [PubMed] [Google Scholar]
- 2. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844 [DOI] [PubMed] [Google Scholar]
- 3. Reid WG, Hely MA, Morris JG, et al. A longitudinal of Parkinson's disease: clinical and neuropsychological correlates of dementia. J Clin Neurosci 1996;3:327–333 [DOI] [PubMed] [Google Scholar]
- 4. Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993;43:2227–2229 [DOI] [PubMed] [Google Scholar]
- 5. Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology 2002;59:1708–1713 [DOI] [PubMed] [Google Scholar]
- 6. Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004;351:2509–2518 [DOI] [PubMed] [Google Scholar]
- 7. Poewe W, Wolters E, Emre M, et al. Long-term benefits of rivastigmine in dementia associated with Parkinson's disease: an active treatment extension study. Mov Disord 2006;21:456–461 [DOI] [PubMed] [Google Scholar]
- 8. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009;8:613–618 [DOI] [PubMed] [Google Scholar]
- 9. Sirakov AA, Mezan IS. EEG findings in parkinsonism. Electroencephalogr Clin Neurophysiol 1963;15:321–322 [DOI] [PubMed] [Google Scholar]
- 10. Neufeld MY, Inzelberg R, Korczyn AD. EEG in demented and non-demented parkinsonian patients. Acta Neurol Scand 1988;78:1–5 [DOI] [PubMed] [Google Scholar]
- 11. Serizawa K, Kamei S, Morita A, et al. Comparison of quantitative EEGs between Parkinson disease and age-adjusted normal controls. J Clin Neurophysiol 2008;25:361–366 [DOI] [PubMed] [Google Scholar]
- 12. Soikkeli R, Partanen J, Soininen H, Paakkonen A, Riekkinen P., Sr Slowing of EEG in Parkinson's disease. Electroencephalogr Clin Neurophysiol 1991;79:159–165 [DOI] [PubMed] [Google Scholar]
- 13. Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD. EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia 1994;5:23–28 [DOI] [PubMed] [Google Scholar]
- 14. Caviness JN, Hentz JG, Evidente VG, et al. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson's disease. Parkinsonism Relat Disord 2007;13:348–354 [DOI] [PubMed] [Google Scholar]
- 15. Fonseca LC, Tedrus GM, Letro GH, Bossoni AS. Dementia, mild cognitive impairment and quantitative EEG in patients with Parkinson's disease. Clin EEG Neurosci 2009;40:168–172 [DOI] [PubMed] [Google Scholar]
- 16. Adler CH, Hentz JG, Joyce JN, Beach T, Caviness JN. Motor impairment in normal aging, clinically possible Parkinson's disease, and clinically probable Parkinson's disease: longitudinal evaluation of a cohort of prospective brain donors. Parkinsonism Relat Disord 2002;9:103–110 [DOI] [PubMed] [Google Scholar]
- 17. Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146 [DOI] [PubMed] [Google Scholar]
- 18. Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study: 1992. Neurology 2001;57:S34–S38 [PubMed] [Google Scholar]
- 19. Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord 2007;22:1272–1277 [DOI] [PubMed] [Google Scholar]
- 20. Steriade M, Gloor P, Llinas RR, et al. Basic mechanisms of cerebral rhythmical activities. Electroencephalogr Clin Neurophysiol 1990;76:481–508 [DOI] [PubMed] [Google Scholar]
- 21. Kozelka JW, Pedley TA. Beta and mu rhythms. J Clin Neurophysiol 1990;7:191–207 [DOI] [PubMed] [Google Scholar]
- 22. Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science 1985;228:750–752 [DOI] [PubMed] [Google Scholar]
- 23. Nakamura S, Sadato N, Oohashi T, Nishina E, Fuwamoto Y, Yonekura Y. Analysis of music-brain interaction with simultaneous measurement of regional cerebral blood flow and electroencephalogram beta rhythm in human subjects. Neurosci Lett 1999;275:222–226 [DOI] [PubMed] [Google Scholar]
- 24. Spiegel A, Tonner PH, Renna M. Best practice & research. Clin Anaesthesiol 2006;20:57–67 [DOI] [PubMed] [Google Scholar]
- 25. Salinsky MC, Oken BS, Morehead L. Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol 1991;79:382–392 [DOI] [PubMed] [Google Scholar]
- 26. Gasser T, Bacher P, Steinberg H. Test-retest reliability of spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol 1985;60:312–319 [DOI] [PubMed] [Google Scholar]
- 27. Sperling RA, LaViolette PS, O'Keefe KO, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009;63:178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laufs H. Endogenous brain oscillations and related networks detected by surface EEG-combined fMRI. Hum Brain Mapp 2008;29:762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Becker K, Holtmann M. Role of electroencephalography in attention-deficit hyperactivity disorder. Exp Rev Neurother 2006;6:731–739 [DOI] [PubMed] [Google Scholar]
- 30. Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I: qualitative and quantitative electroencephalography. Clin Neurophysiol 2003;114:171–183 [DOI] [PubMed] [Google Scholar]