Abstract
Phosphoinositides are localized in various intracellular compartments and can regulate a number of intracellular functions, such as cytoskeletal dynamics and membrane trafficking. Phospholipase Ds (PLDs) are regulated enzymes that hydrolyse phosphatidylcholine (PtdCho) to generate the putative second messenger phosphatidic acid (PtdOH). In vitro, PLDs have an absolute requirement for higher phosphorylated inositides, such as phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2]. Whether this lipid is able to regulate the activity of PLD in vivo is contentious. To examine this hypothesis we studied the relationship between PLD and an enzyme critical for the intracellular synthesis of PtdIns(4,5)P2: phosphatidylinositol 4-phosphate 5-kinase α (Type Iα PIPkinase). We find that both PLD1 and PLD2 interact with the Type Iα PIPkinase and that PLD2 activity in vivo can be regulated solely by the expression of this lipid kinase. Moreover, PLD2 is able to recruit the Type Iα PIPkinase to its intracellular location. We show that the physiological requirement of PLD enzymes for PtdIns(4,5)P2 is critical and that PLD2 activity can be regulated solely by the levels of this key intracellular lipid.
Keywords: phosphatidic acid/phosphatidylinositol 4,5-bisphosphate/phosphatidylinositol 4-phosphate 5-kinase α/phospholipase D/porcine aortic endothelial cells
Introduction
Phospholipase D (PLD) catalyses the hydrolysis of phosphatidylcholine (PtdCho) to generate phosphatidic acid (PtdOH), which remains in the membrane, and choline, a water-soluble head group (Exton, 1998; Liscovitch et al., 2000). Thus far, two isoforms of PLD have been described, PLD1 and PLD2, both of which exist as two splice variants (Colley et al., 1997; Hammond et al., 1997; Park et al., 1997). The 124 kDa PLD1 appears localized to vesicles derived from the endosomal/lysosomal pathway (Ktistakis et al., 1995; Brown et al., 1998), but has also been detected at the plasma membrane (Brown et al., 1998); it has been suggested that it is involved in the regulation of membrane coating that occurs during vesicle formation (Austin and Shields, 1996; Ktistakis et al., 1996; Chen et al., 1997; Siddhanta et al., 1998) and in the regulation of secretion (Metz and Dunlop, 1990; Stutchfield and Cockcroft, 1993; Cockcroft, 1996; Bi et al., 1997; Morgan et al., 1997; Brown et al., 1998; Caumont et al., 1998). PLD1 activity is regulated by multiple inputs, including phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] (Liscovitch et al., 1991, 1994; Pertile et al., 1995; Schmidt et al., 1996b), protein kinase C (PKC) (Conricode et al., 1992; Eldar et al., 1993; Cockcroft, 1996a,b; Kiss, 1996; Ohguchi et al., 1996; Park et al., 1998; Kim et al., 2000), the Rho family proteins Cdc42, Rac (Hess et al., 1997; Plonk et al., 1998) and Rho (Malcolm et al., 1996; Hess et al., 1997; Karnam et al., 1997; Schmidt et al., 1997; Vinggaard et al., 1997), and Arf proteins (Brown et al., 1993; Cockcroft et al., 1994; Ktistakis et al., 1995; Kuribara et al., 1995; Whatmore et al., 1996) that act in an additive manner, leading to greater PLD1 activity than each alone (Hodgkin et al., 1999).
PLD2 is a 106 kDa protein that shares 50–55% homology with PLD1 but lacks the 116 amino acid loop region following the first HKD motif (Plonk et al., 1998). This isoform has been suggested to be localized to the plasma membrane (Plonk et al., 1998) and to an as yet undefined submembraneous vesicle compartment, which translocates to the membrane on stimulation with epidermal growth factor (EGF) (Honda et al., 1999). PLD2 is reported to have a much higher basal activity than PLD1 when expressed in Cos-7 cells or isolated from insect cells and it has been suggested that cellular proteins, such as fodrin and synucleins (Jenco et al., 1998), act to reduce the basal activity. Consequently, growth factor stimulation, rather than activating the enzyme catalytically, may repress the action of these endogenous inhibitors. Phorbol ester (phorbol 12-myristate 13-acetate) and members of the ARF family activate PLD2 2-fold only; however, deletion of the non-core N-terminal amino acids generates a protein with a much lower basal activity, which can be activated by ARF some 13-fold (Sung et al., 1999). Whether PLD2 can be activated in response to growth factors is still unclear.
Both mammalian PLD1 and PLD2 have an absolute requirement for the higher phosphorylated inositol lipids (Berstein et al., 1992; Colley et al., 1997; Hammond et al., 1997; Sciorra et al., 1999; Hodgkin et al., 2000). In keeping with this, we have recently demonstrated that PLD1 has a functional pleckstrin homology (PH) domain, specific for PtdIns(4,5)P2, that regulates both activity and localization (Hodgkin et al., 2000). Sequence analysis demonstrates considerable homology between the PH domains of PLD1 and PLD2, thus a similar specificity can be presumed.
Cellular PtdIns(4,5)P2 levels are, in part, governed by two families of enzymes, namely the Type I and Type II phosphatidylinositol 4-phosphate 5-kinases (PIPkinases) (Hinchliffe et al., 1998; Anderson et al., 1999). The Type II enzymes are composed of three isoforms that synthesize PtdIns(4,5)P2 by the 4-phosphorylation of PtdIns(5)P (Rameh et al., 1997). The role of these enzymes in the maintenance and synthesis of cellular PtdIns(4,5)P2 pools is not yet understood. Type I PIPkinases comprise at least three distinct isoforms that phosphorylate the 5-position of PtdIns(4)P (Ishihara et al., 1996; Loijens and Anderson, 1996; Shibasaki et al., 1997). However, in vitro, these enzymes will phosphorylate a number of inositol lipids such as PtdIns, PtdIns(3)P, PtdIns(4)P and PtdIns(3,4)P2 (Zhang et al., 1997; Tolias et al., 1998b). The Type I PIPkinases have been shown to associate with at least two low molecular weight G proteins of the Rho family (Chong et al., 1994; Ren et al., 1996; Tolias et al., 1998a) and recently to be activated by members of the ARF family (Honda et al., 1999; Jones et al., 2000). Previous data demonstrated that the Type I PIPkinases were activated by PtdOH (Jenkins et al., 1994), one product of PLD hydrolysis of PtdCho, and regulation of the Type I PIPkinase by ARF, in vitro, is dependent upon this acidic phospholipid (Honda et al., 1999). The Type I PIPkinases have been shown to be required for secretion in permeabilized cells, for Rho-mediated ezrin, radixin and moesin (ERM) phosphorylation (Matsui et al., 1999) and Rac-regulated capping/uncapping of actin in platelets (Tolias et al., 2000). Recent evidence has suggested that PIPkinases may also be involved in ARF-regulated vesicle coating and budding at the Golgi and lysosomal membranes (Arneson et al., 1999). A role in the internalization of activated receptors has also been suggested. Genetic studies in mice lacking a functional synaptojanin, a PtdIns(4,5)P2 5-phosphatase, have demonstrated increased PtdIns(4,5)P2 levels with an accumulation of clathrin-coated vesicles in the cytosol, further supporting a role for PtdIns(4,5)P2 in vesicular trafficking (Cremona et al., 1999).
In most, if not all of these cellular processes, a role for PLD has also been implicated. A feed-forward cycle has been postulated whereby the generation of PtdIns(4,5)P2 activates PLD, which leads to enhanced PtdOH formation able to activate further the Type I PIPkinase. This would lead to a rapid local increase in both PtdOH and PtdIns(4,5)P2, which has been suggested to be important in both the generation of membrane curvature, vesicle budding and the recruitment/activation of proteins involved in coating of vesicles (Liscovitch and Cantley, 1995). Although enticing, there is a paucity of evidence to corroborate this theory. In this report we demonstrate that both PLD1 and PLD2 interact with the murine Type Iα PIPkinase and that PLD2 is able to lead to the recruitment of Type Iα PIPkinase in porcine aortic endothelial (PAE) cells. Finally, expression of the Type Iα PIPkinase leads to the activation of PLD2 activity in vivo. These data suggest a molecular mechanism by which PtdIns(4,5)P2 can be generated in a localized environment, leading to activation of PLD2.
Results
Expression of a Type I PIPkinase in Cos-7 cells leads to the activation of an endogenous PLD
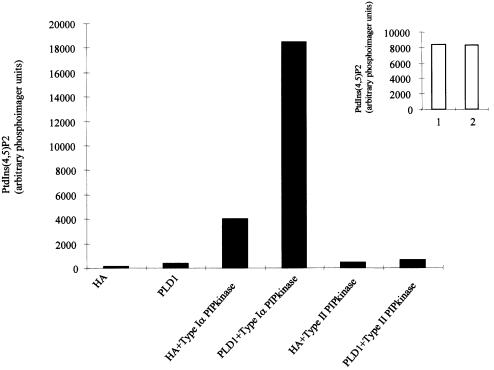
Cos-7 cells transfected with LacZ (cont) or murine Type Iα PIPkinase were treated as controls or stimulated with 12-O-tetradecanoylphorbol 13-acetate (TPA) or lysophosphatidic acid (LPA) and PLD activity was assessed. PLD activity is measured easily by the inclusion of a primary alcohol, such as butanol, which is used as a nucleophile, in place of water, leading to the production of phosphatidylbutanol (PtdBut). Unlike PtdOH, which can be synthesized in a cell by at least three separate mechanisms, the more metabolically stable PtdBut can only be formed by the action of PLD. Transfection of the Type Iα PIPkinase leads to an increase in endogenous PLD activity [from 856 ± 75 to 1432 ± 86 U (where U are arbitrary phosphoimager units); Figure 1]. As only 50% of the cells become transfected under these conditions, this suggests that the true stimulation would be at least 4-fold. This increase is equivalent to that seen in vivo using transfection with constitutively activated RhoA or with ARF-1, known activators of PLD1 (data not shown). TPA induced an increase of 1391 ± 81 U in non-transfected cells; however, in Type I PIPkinase-transfected cells TPA gave an increase of 2626 ± 207 U. Expression of Type I alone led to an increase of 575 ± 52 U, and added to the increase from the TPA treatment this would account for 1966 U of PLD activity. This suggests that Type Iα PIPkinase-induced PLD activity is unlikely to occur through increased PtdIns(4,5)P2 formation and subsequent hydrolysis leading to enhanced PKC activation. The fact that the activity in Type Iα PIPkinase-transfected cells stimulated with TPA is greater than the sum of the two activities alone may suggest that TPA may be able to activate the Type Iα PIPkinase.
Fig. 1. Cos-7 cells were transfected either with LacZ construct (1 µg) or with the Type Iα PIPkinase. These were labelled overnight with orthophosphate (10 µCi per dish) and stimulated as indicated for 30 min in the presence of 0.3% butan-1-ol. The reactions were quenched, and lipids were extracted and analysed as described in Materials and methods. The closed bars show the levels of 32P-labelled PtdIns(4,5)P2, while the open bars are the counts in PtdBut. The data are presented as the mean ± SD for triplicate samples (arbitary units from analysis using a phosphoimager) and are typical of two independent experiments.
Analysis of the phosphatidylinositol lipids after transfection of the Type I PIPkinase demonstrated a 2-fold increase in the level of PtdIns(4,5)P2 (Figure 1). These data are consistent with the hypothesis that transfection of the Type I PIPkinase enhances PtdIns(4,5)P2 synthesis, leading to the stimulation of an endogenous PLD activity.
Co-expression of Type Iα PIPkinase leads to the activation of PLD2
To investigate which isoform of PLD is activated by the Type Iα PIPkinase we studied the effect of its expression together with either PLD1 or PLD2 on PtdBut formation. As the extent of transfection differs between experiments, the counts obtained in the LacZ alone transfection have been subtracted. Thus, the data represent the PLD activity due to the transfection of the various constructs. Transfection of PLD1 into Cos-7 cells led to an increase in the basal activity that was stimulated potently by TPA, but we have been unable to demonstrate enhanced activation by co-transfection with the Type Iα PIPkinase (data not shown). This was also shown to be the case when the cells were stimulated with LPA. Although not conclusive, these data suggest that the endogenous PLD that is activated by expression of the Type Iα enzyme is not PLD1.
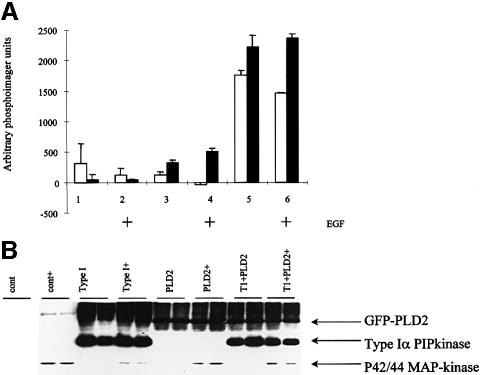
Transfection of PLD2 led to a small increase in both PtdOH (102 U) and PtdBut (327 U) in serum-starved cells. Co-transfection of PLD2 with the Type Iα PIPkinase led to a much larger increase in both PtdOH and PtdBut formation (1756 and 2216 U, respectively. Type I PIPkinase alone yielded an increase of 306 and 42 U in PtdOH and PtdBut formation, respectively (Figure 2A). No enhancements of these changes were seen after stimulation with EGF, although MAP-kinase was activated by this agonist (Figure 2B). The gel also shows that there was approximately equal green fluorescent protein (GFP)–PLD2 and Type I PIPkinase expression in the transfected cells (each lane represents a single transfection and is the protein recovered from the interface of the lipid extractions used to generate the PtdBut data) (Figure 2A). These data suggest that co-expression of the Type I PIPkinase is able to lead to activation of PLD2 in vivo.
Fig. 2. Cos-7 cells were transfected with Type I, PLD2 or a combination and were labelled overnight using orthophosphate. Cells were then left as controls or were stimulated with EGF (10 ng/ml) for 30 min in the presence of butan-1-ol (0.3%). Cells were quenched and the lipids and proteins were recovered and analysed by TLC (A) or western blotting (B) respectively. (A) Analysis of PtdOH (open bars) and PtdBut (closed bars). Lanes 1, 3 and 5 are controls, while lanes 2, 4 and 6 were treated with EGF. Lanes 1 and 2 were transfected with Type I PIPkinase (1 µg), lanes 3 and 4 were transfected with PLD2 (1 µg) and lanes 5 and 6 were transfected with Type I (1 µg) and PLD2 (1 µg). These data are expressed as the mean ± the range of values and are typical of two independent experiments. (B) Total protein was recovered from the interface of the lipid extraction used to generate the data for (A), redissolved in 8 M urea and separated using SDS–PAGE. Proteins were transferred to nitrocellulose and expression of the constructs determined using specific antibodies (anti-GFP for PLD and an antipeptide antibody for the Type Iα PIPkinase). To demonstrate that EGF was active (+), the blot was reprobed using a phospho-specific anti-p42/44 MAP-kinase antibody.
Murine Type Iα PIPkinase interacts with PLD
To assess whether PLD2 and Type Iα PIPkinase interact, we co-transfected Cos-7 cells with haemagglutinin (HA)-tagged PLD2 and EE-tagged Type Iα PIPkinase. Immunoprecipitation was carried out using antibodies directed against the Glu-Glu (EE) tag (Type I PIPkinase), followed by western blot analysis using the anti-HA antibody (PLD2). Analysis of the whole cell lysates showed that HA-PLD2 was expressed equally (Figure 3A, lanes 5 and 7), but was only immunoprecipitated by the EE antibody when co-expressed with the EE-Type Iα PIPkinase (Figure 3A, lane 3). These data demonstrate that PLD2 is able to interact with the Type Iα PIPkinase.
Fig. 3. (A) Cos-7 cells were transfected as indicated, lysed and immunoprecipitated using the EE antibody directed against the tagged Type Iα PIPkinase. The immunoprecipitates and total lysates were separated by SDS–PAGE and immunoprobed using an anti-HA antibody directed against the tagged PLD2. PLD2 was expressed to equal levels either in the presence or absence of the Type Iα PIPkinase. PLD2 was immunoprecipitated only when both PLD2 and Type Iα PIPkinase were co-transfected (lane 3). (B) Deletions were generated in the Type I PIPkinase as indicated (δx–y defines the deleted bases in the cDNA, where 1 refers to the start ATG) and cloned into a pGEX-4T expression vector. Proteins were induced, purified and used to affinity purify PLD2 from transfected Cos-7 cell lysates. The proteins were separated by SDS–PAGE, transferred to nitrocellulose and probed using an anti-HA antibody against the tag for PLD2. Only deletion δ1–912 did not bind PLD2. Interestingly, there is no overlap between δ325–1626 and δ1–420, suggesting that either the PLD2 binding site resides across this stretch or that there are two distinct binding sites. The experiment shown was carried out independently on two occasions.
To define regions of PIPkinase that interact with PLD2, deletion mutants of the Type Iα PIPkinase (Figure 3B) were constructed and expressed as recombinant glutathione S-transferase (GST)-tagged proteins in Escherichia coli. These were purified using glutathione beads and then used to affinity purify PLD2 that had been expressed in Cos-7 cells (Figure 3B, blot). As controls, either GST alone or GST–Type IIα PIPkinase was used. The full-length Type Iα PIPkinase was able to affinity purify PLD2. Deletion of the first 120 amino acids (δ1–420) did not prevent affinity purification of PLD2. However, deletion of the first 306 amino acids (δ1–912) completely abolished PLD2 binding. Deletions in the other direction demonstrated that even removal of the C-terminal 425 amino acids (δ325–1626), leaving only the N-terminal 108 amino acids, still allowed binding of PLD2. It should be noted that there is no overlap between δ325–1626 and δ1–420, suggesting that either these two regions form distinct PLD2 binding sites or that they are both part of a single binding site. No affinity purification of PLD2 was achieved with either GST alone or GST–Type II PIPkinase.
Although we were unable to show that Type Iα PIPkinase could regulate PLD1 activity in vivo, we were able to demonstrate that these two proteins interact (Figure 4). After co-transfection of the two proteins, wild-type PLD1 was immunoprecipitated and the Type Iα PIPkinase activity associated with this enzyme was assessed. Co-transfection of the two cDNAs led to an enhancement of the PIPkinase activity immunoprecipitated with PLD1 (there was an increase in the PIPkinase activity in Type I-transfected cells alone, which may represent immunoprecipitation of endogenous PLD1) (Figure 4). Immunoprecipitation of the Type I activity, using an anti-myc antibody, demonstrated that its expression, either in the presence or the absence of PLD1, was equivalent (Figure 4, inset). No PIPkinase activity was associated with PLD1 immunoprecipitates when it was co-expressed with the Type IIα PIPkinase. These data demonstrate that both isoforms of PLD are able to interact with the Type Iα PIPkinase, but not with the Type II enzyme. However, PtdIns(4,5)P2 generated by this enzyme can only activate PLD2 in vivo.
Fig. 4. PLD1 interacts with the Type Iα PIPkinase. Cos-7 cells were transfected with the HA protein (control) or with PLD1, myc-tagged Type Iα, TypeII PIPkinase, or in combinations as shown. The cells were lysed and immunoprecipitated using an anti-PLD1 polyclonal antibody. The immunoprecipitations were then used in a PIPkinase assay. As can be seen, co-transfection leads to enhanced immunoprecipitation of Type I activity. The inset shows the total Type I PIPkinase activity after immunoprecipitation using a myc antibody for co-expression with the HA protein (lane 1) or with PLD1 (lane 2).
PLD2 recruits the Type Iα PIPkinase when co-expressed in PAE cells
To assess whether Type Iα PIPkinase and PLD2 interact in vivo, we co-expressed these proteins in confluent PAE cells by microinjection of the cDNAs and stained for the various proteins 3 h later. This experiment was carried out using both GFP-tagged PLD2 and myc-tagged Type I PIPkinase or GFP-tagged Type Iα PIPkinase and HA-tagged PLD2. In both cases the same result was observed. PLD2 was localized in a submembraneous vesicular compartment (Figure 5A, 2), which did not co-localize with markers for either the endoplasmic reticulum or the Golgi (data not shown). Expression of the Type I PIPkinase alone showed a complex staining pattern associated with the plasma membrane, cytosol and in intracellular structures that resemble microtubuli (Figure 5A, 1). Co-expression of PLD2 and Type Iα PIPkinase resulted in a dramatic relocalization of the Type Iα PIPkinase to the submembraneous PLD2-positive patches (Figure 5B). This co-localization was also seen when viewing the cells through a Z section (Figure 5C). As the Type Iα PIPkinase is able to associate with a number of different proteins (Rac1, PLD1 and PLD2, PKD and ARFs) and to test the requirement for PIPkinase activity for this recruitment, we also tested the localization of the GFP–δ325–1626 deletion mutant, which contains only the first 108 amino acids but is still able to bind to PLD2 (Figure 3C). When injected alone, the GFP–δ325–1626 was completely cytosolic (Figure 5A, 3); however, co-injection with PLD2 also led to a complete co-localization at the sub-membraneous patches (Figure 5D). Thus, PLD2 is able to recruit the Type Iα PIPkinase to its own intracellular location.
Fig. 5. Confluent PAE cells were microinjected with various constructs, left for 3 h, and then fixed and stained. In (A, 1–3), the constructs were injected alone. In (B, 1–3), they were injected with Type Iα PIPkinase together with GFP–PLD2. (B, 1) shows the GFP–PLD2 staining, (B, 2) is the Type Iα PIPkinase expression visualized using a polyclonal antibody and (B, 3) is the merge. (C, 1–3) Cells microinjected with both Type Iα PIPkinase and PLD2, but viewed through a Z plane. (D, 1–3) Co-injection of the GFP–δ325–1626 together with HA-tagged PLD2. The lasers were set up such that no bleed through from one channel to the other was detected.
Local generation of PtdIns(4,5)P2 is required for TPA-stimulated PtdBut formation
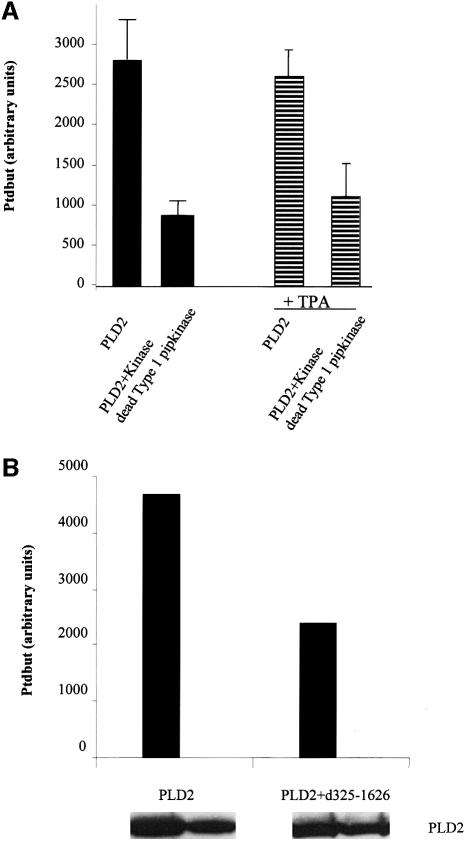
To determine whether the Type I PIPkinase activity was required for the activation of PLD2, we constructed a kinase-dead (KD) version of the Type I PIPkinase by substituting a single amino acid in the ATP-binding loop as described previously (Ishihara et al., 1998). This mutant was expressed in Cos-7 cells, immunoprecipitated and assayed for PIPkinase activity. The wild-type enzyme yielded 109 146 U, while the KD mutant produced 1435. This mutant was transfected into Cos-7 cells, in the presence or the absence of PLD2, and the activity of the expressed PLD2 was assessed. Overexpression of the KD mutant significantly reduced the basal activity of PLD2 (Figure 6A). The reason for the incomplete inhibition probably resides in the fact that both PLD2 and Type Iα KD were co-transfected. Thus, the expression of the KD may not be high enough to completely abrogate the heterologously expressed PLD2 activity (anti-GFP antibodies showed that GFP–PLD2 with GFP–wild-type Type Iα PIPkinase were expressed at equimolar levels).
Fig. 6. (A) Cos-7 cells were transfected with PLD2 alone or in the presence of the KD Type I PIPkinase. The cells were labelled overnight and incubated in the presence of butan-1-ol for 30 min. The lipids were extracted and the formation of PtdBut was analysed (filled bars). In a separate experiment, PLD2 was expressed alone or together with the KD Type Iα and then stimulated with TPA (striped bars). The data are expressed as the means from three separate transfections ± SD. The difference is significant with P <0.05. (B) Cos-7 cells were transfected with PLD2 alone or in the presence of GFP–δ325–1626 and labelled as above, but were treated with TPA in the presence of butan-1-ol for 30 min. The data are represented as the means of two separate transfections. The panel underneath shows the expression levels of PLD2 in the experiment and was carried out after recovery of the protein from the interface after extraction of the lipids (see Materials and methods).
Previous data showed that PtdIns(4,5)P2 was also required for TPA-stimulated PLD activity in vivo. We therefore investigated whether the kinase activity was also required. PLD2 was co-expressed together with the δ325–1626 construct, which we showed was able to bind PLD-2, but is inactive with respect to kinase activity. Expression of this mutant results in a 50% decrease in the TPA-induced PLD2 activity (Figure 6B). Western analysis showed that there was no significant change in the expression of PLD2 in these transfections. Identical data were obtained using the KD mutant Type Iα instead of the δ325–1626 mutant (Figure 6A, striped bars). These data suggest that interaction with Type Iα PIPkinase is required for the generation of PtdIns(4,5)P2, which is required for the activation of PLD2 by TPA.
Type Iα PIPkinase and PLD interact in vivo
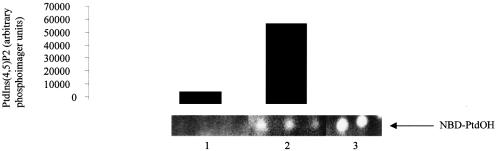
Although the previous data are consistent with the hypothesis that PLD family members and the Type Iα PIPkinase interact, these experiments were carried out with overexpressed proteins. To assess the in vivo significance, we employed anti-peptide antibodies specific for the Type I PIPkinase and assessed whether they were able to immunoprecipitate PLD activity assayed in vitro. PAE cells were grown to confluency, lysed using a mild detergent buffer and the proteins were immunoprecipitated using specific antibodies to the Type I or Type II PIPkinase as a control. After extensive washing, the beads were assayed for PIPkinase activity by phosphorylation of PtdInsP to PtdIns(4,5)P2 in the presence of N-(N-[6-[7-nitrobenz-2-'2-oxa-1,3-diazol-4-yl]amino]caproyl) (NBD)– PtdCho as a substrate for PLD. The reactions were carried out for 20 min and the products were separated by thin layer chromatography (TLC). PIPkinase activity was assessed by phosphoimager analysis of the 32P incorporated into PtdIns(4,5)P2, while PLD activity was assessed by the generation of NBD–PtdOH. As a control, HA-tagged PLD2 was expressed in Cos-7 cells and immunoprecipitated using the anti-HA antibody (Figure 7, lane 3). No PLD activity was found when either protein G– Sepharose or protein G–Sepharose coupled to anti-Type II PIPkinase antibodies was used (Figure 7). In contrast, anti-Type I PIPkinase antibodies immunoprecipitated PLD activity. As the assays were carried out in the presence of 10 µM PtdIns(4,5)P2, the increased PLD activity is not a reflection of the amount of conversion of PtdIns(4)P to PtdIns(4,5)P2. These data suggest that, in vivo, PLD can associate with Type I PIPkinase isoforms in PAE cells.
Fig. 7. PAE cells were grown to confluence, lysed and immunoprecipitated using either an anti-Type II (lane 1) or anti-Type I antibody (lane 2). The beads were then assayed for PIPkinase activity in the presence of NBD-labelled PtdCho. The reaction was carried out for 20 min and the lipids were extracted, separated by TLC, and NBD–PtdOH was viewed using an eagle Eye video camera. Radioactivity incorporated into PtdIns(4,5)P2 was quantitated using a phosphoimager. Lane 3 shows the immunoprecipitation of HA-tagged PLD2 expressed in Cos-7 cells. The production of NBD–PtdOH is shown, which co-migrated with NBD–PtdOH generated by the phosphorylation in vitro of NBD–1,2-diacylglycerol.
Discussion
A number of studies have demonstrated that PLD activity requires the presence of PtdIns(4,5)P2. It has been suggested that the inositide interacts with a site within domain IV (Sciorra et al., 1999), but contradicting evidence has now pointed to the effect being mediated through a PtdIns(4,5)P2 selective PH domain (Hodgkin et al., 2000). The PH domain binding site appears to have two functions, as point mutations abolish PLD activity, whilst deletion of the domain prevents membrane association. The demonstration that Rho family proteins are able to regulate PIPkinase activity (Chong et al., 1994; Ren et al., 1996; Tolias et al., 1998a) together with the use of a number of bacterial toxins, such as the Clostridium difficile toxin B (which inactivates Rho family proteins) and Clostridium sordelli toxin (which inhibits both Ras and Rho family proteins), has been used to implicate the importance in vivo of phosphoinositides in the regulation of PLD activity (Schmidt et al., 1996a,b, 1997). These data are, however, limited as the small molecular weight G proteins, which are targets for these toxins, are also potential activators of PLD1. In toxin B-pretreated HEK 293 cells, the reduced GTPγs-mediated PLD activity could be fully restored by the re-addition of PtdIns(4,5)P2 (Schmidt et al., 1996b). These data, although implying a specific role for PtdIns(4,5)P2 in the regulation of PLD activity, do not demonstrate that PLD activity can be regulated in vivo by the levels of this lipid. In this study we demonstrate that the endogenous PLD in Cos-7 cells can be stimulated solely by the overexpression of a Type I PIPkinase. Furthermore, we suggest that this occurs through the interaction between the two proteins. Recruitment of the Type Iα PIPkinase to the intracellular compartment where PLD2 resides would lead to enhanced synthesis of polyphosphoinositides. Although both PLD1 and PLD2 have an absolute requirement for PtdIns(4,5)P2 and are able to interact with the Type I PIPkinase, we were only able to show activation of PLD2. As PLD1 activity is regulated by multiple, independent inputs: two separate GTPases, a Rho family member and an ARF family member and PKC, in a phosphorylation-independent manner, it may be that PtdIns(4,5)P2 levels alone are not enough to regulate this enzyme. PLD2, in contrast, is not regulated by the same multiple inputs.
The mechanism behind the regulation of PLD family members by phosphoinositides is not clear, as it has been demonstrated that PLD2 activity may be negatively regulated in cells by proteins such as fodrin and synucleins (Jenco et al., 1998) and by actinin and amphiphysins (Lee et al., 2000; Park et al., 2000). It is possible that the role of polyphosphoinositides is to alleviate the inhibition by these proteins. The recent demonstration that ARF family members are able to regulate Type Iα PIPkinase activity (Honda et al., 1999; Jones et al., 2000), together with the data from this study, suggest that ARF-mediated regulation of PLD activity may occur through enhanced local PtdIns(4,5)P2 synthesis. Alternatively, phosphoinositides may regulate ARF activation. The regulation of ARF by phosphoinositides is complex. PtdIns(4,5)P2 has been shown to mediate the activation of an ARF GAP and its interaction with ARF (Randazzo, 1997); this would lead to an increase in the GDP loading. However, PtdIns(4,5)P2 has also been suggested to positively regulate the activity of an ARF–guanine nucleotide exchange factor (ARNO), leading to an increase in the levels of GTP bound ARF (Paris et al., 1997). Further complexity arises through the ability of PtdIns(4,5)P2 to stimulate guanine nucleotide exchange on ARF (Terui et al., 1994). At present, there are no methods to look specifically for activated ARF in vivo and the importance in vivo of ARF family members in regulating PLD2 remains controversial.
A number of studies have previously suggested a link between PtdIns(4,5)P2, PtdOH and vesicle formation (Liscovitch and Cantley, 1995; Pertile et al., 1995; Arneson et al., 1999). The generation of PtdIns(4,5)P2 by PIPkinase, and the subsequent activation of PLD2 to generate PtdOH, positively regulates PIPkinase, leading to high local concentrations of these lipids in the membrane, which may be important in the induction of membrane curvature. It may also be important in the recruitment of adapter proteins involved in the generation of a coat required to make the vesicle. Whether this feedback occurs is not known. Previous data from Moritz et al. (1992) showed that treatment of membranes with bacterial PLD, which leads to increased PtdOH formation, also led to the activation of an endogenous PIPkinase.
The generation of PtdOH at membranes may be a general mechanism for regulating PIPkinase at these intracellular domains. Recently, it has been demonstrated that endophillin-1, which is able to stimulate synaptic vesicle budding, acts as a lysophosphatidic acid acyl-transferase generating PtdOH. The authors suggest that this may be required to activate a PIPkinase able to generate PtdIns(4,5)P2 and thus leading to the recruitment/activation of dynamin (Schmidt et al., 1999). A role for PtdIns(4,5)P2 in clathrin-coated vesicle formation has been genetically established in mice that are homozygous for a deletion of synaptojanin, a PtdIns(4,5)P2 5-phosphatase (Cremona et al., 1999).
The data presented in this paper are consistent with the hypothesis that PLD2 is able to lead to the recruitment of the Type Iα PIPkinase that leads to enhanced PtdIns(4,5)P2 production, which is able to regulate PLD2 activity. This study provides the first evidence in vivo for activation of PLD by PtdIns(4,5)P2 and places PLD2 as a downstream effector of the Type Iα PIPkinase. This may have important implications as a number of studies have suggested that both PIPkinase and PLD activities are up-regulated in the development of cancer.
Materials and methods
General reagents
Unless otherwise stated all reagents were of analytical grade. PtdCho, phosphatidylserine (PtdSer), PtdIns, PtdInsP and PtdInsP2 were purified from bovine brain. NBD–PtdCho was purchased from Molecular Probes. All radiochemicals were purchased from Amersham International. Anti-HA tag antibody was generated from clone 12CA5, while the myc epitope was from clone 9e10. All secondary antibodies were purchased from Dako Products.
Plasmids
A cDNA encoding the murine Type Iα PIPkinase was generated from a murine brain cDNA library using PCR and cloned into either Pjex 4T (Pharmacia) for expression in bacteria, or pCDNA3 for expression in cos cells. Deletions were generated using PCR and cloned into the above vectors and into pEGFP-C2 for GFP fusion protein expression. PLD1 and HA-PLD2 were subcloned into pCDNA3. GFP–PLD2 was cloned into PEGFP-C2
Cell culture
Cos-7 cells were routinely maintained at ∼30% confluency and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 8% fetal calf serum (FCS) (v/v). The cells were split 1 day before transfection to 30%, and transfected the following day using DEAE–dextran. Briefly, cells were washed twice with phosphate-buffered saline (PBS) and incubated with plasmid DNA [2 µg per 6 cm dish in 560 µl of PBS + 30 µl of DEAE–dextran (10 mg/ml)] for 30 min. Four millilitres of DMEM–8% FCS containing 80 µM chloroquine were added and left for a further 2.5 h. This medium was replaced with 2 ml of DMEM–8% FCS–10% DMSO, left for 2 min and replaced with fresh DMEM–8% FCS. Transfection was carried out for a further 24 h, after which the cells were labelled and treated as below. PAE cells were maintained in DMEM–8% FCS and routinely passaged to 30% confluency. Microinjection of plasmids (0.1 µg/ml) into the nucleus of PAE cells was carried out using an automated Eppendorf injector. The cells were allowed to recover for 3 h and then were fixed and processed as described below.
In vivo PLD assay
Cells were transfected as above, left for 24 h and labelled overnight with [32P]orthophosphate (10 µCi) in phosphate-free DMEM. The cells were washed twice with RPMI-salts buffer and incubated in this buffer containing butan-1-ol (0.3% v/v) for 30 min. This medium was aspirated, and 0.45 ml of 2.4 M HCl added. The cells were maintained and scraped on ice, removed to a clean Eppendorf tube and the dishes washed with 0.5 ml of methanol, which was pooled with the HCl. Half a millilitre of chloroform containing 5 µg of Folsch lipid extract was added, together with 0.25 ml of water. The two phases were mixed vigorously, centrifuged (2 min at 21 000 g in an Eppendorf bench centrifuge) and the lower phase was removed carefully, so as not to disturb the protein interface (see below), and was washed once with theoretical upper phase (chloroform:methanol:1 M HCl 15:245:235, 0.7 ml), with the lower phase being removed to a clean Eppendorf tube. The first upper phase was then back extracted with 0.2 ml of chloroform, which after mixing and centrifugation was removed to the tube containing the theoretical upper phase wash. This was mixed and centrifuged and the lower phase removed to the tube containing the first lower phase. The samples were dried and kept at −20°C. Samples were analysed for PtdBut formation and for PtdIns/PtdInsP/PtdInsP2 labelling (see the following section). To assess the expression levels of various constructs, the protein layer at the initial interface was recovered after lipid removal by the addition of 0.5 ml of methanol to the upper phase. The Eppendorf tube was centrifuged (21 000 g for 10 min), and the protein pellets were washed once with 70% acetone. These were allowed to dry and were resuspended overnight in 50 mM Tris pH 8.0, 8 M urea. SDS–PAGE sample buffer was added, the samples were boiled, resolved by SDS–PAGE and transferred to nitrocellulose. The blots were probed with various antibodies (at dilutions indicated in the figure legends) and visualization was carried out using ECL according to the manufacturer’s instructions (Amersham International).
Co-immunoprecipitations
Cells were transfected as described and left for 48 h, after which they were lysed [1 ml lysis buffer (50 mM Tris pH 8.0, 50 mM KCl, 10 mM EDTA, 1% NP-40)], scraped, and nuclei and cellular debris removed by centrifugation (14 000 r.p.m., 4°C Eppendorf centrifuge). Immunoprecipitations were carried out using monoclonal antibodies against epitope tags 12CA5 (anti-HA tag), 1E10 (anti-myc tag) and an antibody derived against the EE tag. Immunoprecipitation of the endogenous Type Iα PIPkinase from PAE cells was carried out using an anti-peptide antibody. The immunoprecipitates were collected using protein G–Sepharose, and washed five times with IP wash buffer (50 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA 0.1% Tween-20), then once with PIPkinase buffer. Immunoprecipitations were either used for western blotting or were used to assess the PIPkinase and/or PLD activities as described below.
Lipid vesicles were prepared using 1 nmol of PtdInsP isolated from pig brain together with 3 nmol of PtdOH. Reactions were carried out at 30°C for 5 min in PIPkinase buffer (50 mM Tris pH 7.4, 10 mM MgCl2, 1 mM EGTA, 70 mM KCl) containing cold ATP (20 µM) and 1 µCi of [32P]ATP in a final volume of 100 µl. Reactions were quenched with 0.5 ml of chloroform:methanol [1:1 (v:v)] and the phases were split by the addition of 125 µl of 2.4 M HCl. The lower phases were removed to a new tube, dried and separated by TLC either using an acidic (chloroform:methanol:acetone:glacial acetic acid:water 240:78:72:70:42) or an alkaline solvent [chloroform:methanol:ammonia (28%):water 45:35:2:8]. Incorporation into PtdIns(4,5)P2 was quantitated using a phosphoimager.
To assess PLD and PIPkinase activity in the same reaction the following lipid vesicles were made: 3 nmol PtdOH, 1 nmol PtdInsP, 1 nmol PtdIns(4,5)P2, 10 nmol PtdSer and 2 nmol NBD-labelled PtdCho. Reactions were carried out for 20 min, after which they were extracted as above and reaction products separated using the acidic solvent system. Incorporation into PtdIns(4,5)P2 was carried out as above, while the PLD activity was assessed by the production of NBD–PtdOH.
Type Iα PIPkinase deletions
Deletions in the 3′ direction were carried out using a constant 5′ primer tagged with the myc epitope and a unique restriction site, while deletions in the other direction were with a constant 3′ primer tagged with the myc epitope. PCR was carried out using Pfu and the reaction products were restricted and cloned into either pCDNA3 for eukaryotic expression, or in a GST vector for bacterial expression and purification. In some cases, the products were cloned into a GFP vector. Site-directed mutagenesis was carried out using the Quick Change kit from Stratagene. All constructs were verified by sequencing.
GST–PIPkinase affinity purification of PLD2
Lysates were prepared from Cos-7 cells expressing HA-PLD2 and were incubated with GST fusion proteins purified from bacterial lysates by incubation with glutathione–Sepharose. Incubations were carried out for 2 h, after which the affinity beads were washed three times with immunoprecipitation buffer (see above). The beads were then placed in SDS loading buffer, separated by SDS–PAGE, transferred to nitrocellulose and probed using antibodies against the HA tag. GST alone, GST–Type IIα PIPkinase or glutathione beads were used as controls for this experiment. No binding to any of these was found.
Immunolocalizations in PAE cells
Constructs were microinjected into the nucleus of PAE cells, allowed to recover for 3 h and then fixed with formaldehyde (3.6% in PBS). The cells were permeabilized with Triton X-100 (0.1% in PBS) and blocked with PBS–bovine serum albumin (1%). Coverslips were incubated with primary antibody [PIPkinase (1:50), anti-HA (1:100)] for 1 h, washed in PBS three times and incubated with the corresponding secondary antibody conjugated to either fluorescein isothiocyanate or Texas red. The coverslips were washed with PBS, after which they were mounted using vectashield. Fluorescence was viewed using a Leica confocal microscope.
Acknowledgments
Acknowledgements
We would like to acknowledge the radioisotope department at the NKI for their constant help and advice, N.Ktistakis (The Babraham Insitute) for the PLD1 plasmid and antisera and members of H3 for advice and encouragement. N.D. is an AVL fellow. C.D’S. is supported by the Dutch Cancer Society grant number NKB 99-2055. Work in M.J.O.'s laboratory is supported by the Wellcome Trust.
References
- Anderson R.A., Boronenkov,I.V., Doughman,S.D., Kunz,J. and Loijens,J.C. (1999) Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J. Biol. Chem., 274, 9907–9910. [DOI] [PubMed] [Google Scholar]
- Arneson L.S., Kunz,J., Anderson,R.A. and Traub,L.M. (1999) Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J. Biol. Chem., 274, 17794–17805. [DOI] [PubMed] [Google Scholar]
- Austin C.D. and Shields,D. (1996) Formation of nascent secretory vesicles from the trans-Golgi network of endocrine cells is inhibited by tyrosine kinase and phosphatase inhibitors. J. Cell Biol., 135, 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein G., Blank,J.L., Smrcka,A.V., Higashijima,T., Sternweis,P.C., Exton,J.H. and Ross,E.M. (1992) Reconstitution of agonist-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11, and phospholipase C-β 1. J. Biol. Chem., 267, 8081–8088. [PubMed] [Google Scholar]
- Bi K., Roth,M.G. and Ktistakis,N.T. (1997) Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol., 7, 301–307. [DOI] [PubMed] [Google Scholar]
- Brown F.D., Thompson,N., Saqib,K.M., Clark,J.M., Powner,D., Thompson,N.T., Solari,R. and Wakelam,M.J. (1998) Phospholipase D1 localizes to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol., 8, 835–838. [DOI] [PubMed] [Google Scholar]
- Brown H.A., Gutowski,S., Moomaw,C.R., Slaughter,C. and Sternweis,P.C. (1993) ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell, 75, 1137–1144. [DOI] [PubMed] [Google Scholar]
- Caumont A.S., Galas,M.C., Vitale,N., Aunis,D. and Bader,M.F. (1998) Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem., 273, 1373–1379. [DOI] [PubMed] [Google Scholar]
- Chen Y.G., Siddhanta,A., Austin,C.D., Hammond,S.M., Sung,T.C., Frohman,M.A., Morris,A.J. and Shields,D. (1997) Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J. Cell Biol., 138, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan,A., Bokoch,G.M. and Schwartz,M.A. (1994) The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell, 79, 507–513. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. (1996a) ARF-regulated phospholipase D: a potential role in membrane traffic. Chem. Phys. Lipids, 80, 59–80. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. (1996b) Phospholipase D: regulation by GTPases and protein kinase C and physiological relevance. Prog. Lipid Res., 35, 345–370. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. et al. (1994) Phospholipase D: a downstream effector of ARF in granulocytes. Science, 263, 523–526. [DOI] [PubMed] [Google Scholar]
- Colley W.C., Sung,T.C., Roll,R., Jenco,J., Hammond,S.M., Altshuller,Y., Bar-Sagi,D., Morris,A.J. and Frohman,M.A. (1997) Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol., 7, 191–201. [DOI] [PubMed] [Google Scholar]
- Conricode K.M., Brewer,K.A. and Exton,J.H. (1992) Activation of phospholipase D by protein kinase C. Evidence for a phosphorylation-independent mechanism. J. Biol. Chem., 267, 7199–7202. [PubMed] [Google Scholar]
- Cremona O. et al. (1999) Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell, 99, 179–188. [DOI] [PubMed] [Google Scholar]
- Eldar H., Ben-Av,P., Schmidt,U.S., Livneh,E. and Liscovitch,M. (1993) Up-regulation of phospholipase D activity induced by overexpression of protein kinase C-α. Studies in intact Swiss/3T3 cells and in detergent-solubilized membranes in vitro. J. Biol. Chem., 268, 12560–12564. [PubMed] [Google Scholar]
- Exton J.H. (1998) Phospholipase D. Biochim. Biophys. Acta, 1436, 105–115. [DOI] [PubMed] [Google Scholar]
- Hammond S.M. et al. (1997) Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-α. J. Biol. Chem., 272, 3860–3868. [DOI] [PubMed] [Google Scholar]
- Hess J.A., Ross,A.H., Qiu,R.G., Symons,M. and Exton,J.H. (1997) Role of Rho family proteins in phospholipase D activation by growth factors. J. Biol. Chem., 272, 1615–1620. [DOI] [PubMed] [Google Scholar]
- Hinchliffe K.A., Ciruela,A. and Irvine,R.F. (1998) PIPkins1, their substrates and their products: new functions for old enzymes. Biochim. Biophys. Acta, 1436, 87–104. [DOI] [PubMed] [Google Scholar]
- Hodgkin M.N., Clark,J.M., Rose,S., Saqib,K. and Wakelam,M.J. (1999) Characterization of the regulation of phospholipase D activity in the detergent-insoluble fraction of HL60 cells by protein kinase C and small G-proteins. Biochem. J., 339, 87–93. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin M.N., Masson,M.R., Powner,D., Saqib,K.M., Ponting,C.P. and Wakelam,M.J. (2000) Phospholipase D regulation and localisation is dependent upon a phosphatidylinositol 4,5-biphosphate-specific PH domain. Curr. Biol., 10, 43–46. [DOI] [PubMed] [Google Scholar]
- Honda A. et al. (1999) Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell, 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Shibasaki,Y., Kizuki,N., Katagiri,H., Yazaki,Y., Asano,T. and Oka,Y. (1996) Cloning of cDNAs encoding two isoforms of 68-kDa Type I phosphatidylinositol-4-phosphate 5-kinase. J. Biol. Chem., 271, 23611–23614. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Shibasaki,Y., Kizuki,N., Wada,T., Yazaki,Y., Asano,T. and Oka,Y. (1998) Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J. Biol. Chem., 273, 8741–8748. [DOI] [PubMed] [Google Scholar]
- Jenco J.M., Rawlingson,A., Daniels,B. and Morris,A.J. (1998) Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by α- and β-synucleins. Biochemistry, 37, 4901–4909. [DOI] [PubMed] [Google Scholar]
- Jenkins G.H., Fisette,P.L. and Anderson,R.A. (1994) Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem., 269, 11547–11554. [PubMed] [Google Scholar]
- Jones D.H., Morris,J.B., Morgan,C.P., Kondo,H., Irvine,R.F. and Cockcroft,S. (2000) Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem., 275, 13962–13966. [DOI] [PubMed] [Google Scholar]
- Karnam P., Standaert,M.L., Galloway,L. and Farese,R.V. (1997) Activation and translocation of Rho (and ADP ribosylation factor) by insulin in rat adipocytes. Apparent involvement of phosphatidylinositol 3-kinase. J. Biol. Chem., 272, 6136–6140. [DOI] [PubMed] [Google Scholar]
- Kim Y., Han,J.M., Han,B.R., Lee,K.A., Kim,J.H., Lee,B.D., Jang,I.H., Suh,P.G. and Ryu,S.H. (2000) Phospholipase D1 is phosphorylated and activated by protein kinase C in caveolin-enriched microdomains within the plasma membrane. J. Biol. Chem., 275, 13621–13627. [DOI] [PubMed] [Google Scholar]
- Kiss Z. (1996) Regulation of phospholipase D by protein kinase C. Chem. Phys. Lipids, 80, 81–102. [DOI] [PubMed] [Google Scholar]
- Ktistakis N.T., Brown,H.A., Sternweis,P.C. and Roth,M.G. (1995) Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc. Natl Acad. Sci. USA, 92, 4952–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N.T., Brown,H.A., Waters,M.G., Sternweis,P.C. and Roth,M.G. (1996) Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J. Cell Biol., 134, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H., Tago,K., Yokozeki,T., Sasaki,T., Takai,Y., Morii,N., Narumiya,S., Katada,T. and Kanaho,Y. (1995) Synergistic activation of rat brain phospholipase D by ADP-ribosylation factor and rhoA p21, and its inhibition by Clostridium botulinum C3 exoenzyme. J. Biol. Chem., 270, 25667–25671. [DOI] [PubMed] [Google Scholar]
- Lee C., Kim,S.R., Chung,J.K., Frohman,M.A., Kilimann,M.W. and Rhee,S.G. (2000) Inhibition of phospholipase D by amphiphysins. J. Biol. Chem., 275, 18751–18758. [DOI] [PubMed] [Google Scholar]
- Liscovitch M. and Cantley,L.C. (1995) Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell, 81, 659–662. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Chalifa,V., Danin,M. and Eli,Y. (1991) Inhibition of neural phospholipase D activity by aminoglycoside antibiotics. Biochem. J., 279, 319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Chalifa,V., Pertile,P., Chen,C.S. and Cantley,L.C. (1994) Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J. Biol. Chem., 269, 21403–21406. [PubMed] [Google Scholar]
- Liscovitch M., Czarny,M., Fiucci,G. and Tang,X. (2000) Phospholipase D: molecular and cell biology of a novel gene family. Biochem. J., 345, 401–415. [PMC free article] [PubMed] [Google Scholar]
- Loijens J.C. and Anderson,R.A. (1996) Type I phosphatidylinositol- 4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J. Biol. Chem., 271, 32937–32943. [DOI] [PubMed] [Google Scholar]
- Malcolm K.C., Elliott,C.M. and Exton,J.H. (1996) Evidence for Rho-mediated agonist stimulation of phospholipase D in rat1 fibroblasts. Effects of Clostridium botulinum C3 exoenzyme. J. Biol. Chem., 271, 13135–13139. [DOI] [PubMed] [Google Scholar]
- Matsui T., Yonemura,S. and Tsukita,S. (1999) Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol., 9, 1259–1262. [DOI] [PubMed] [Google Scholar]
- Metz S.A. and Dunlop,M. (1990) Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem. J., 270, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C.P., Sengelov,H., Whatmore,J., Borregaard,N. and Cockcroft,S. (1997) ADP-ribosylation-factor-regulated phospholipase D activity localizes to secretory vesicles and mobilizes to the plasma membrane following N-formylmethionyl-leucyl-phenylalanine stimulation of human neutrophils. Biochem. J., 325, 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A., De Graan,P.N., Gispen,W.H. and Wirtz,K.W. (1992) Phosphatidic acid is a specific activator of phosphatidylinositol- 4-phosphate kinase. J. Biol. Chem., 267, 7207–7210. [PubMed] [Google Scholar]
- Ohguchi K., Banno,Y., Nakashima,S. and Nozawa,Y. (1996) Regulation of membrane-bound phospholipase D by protein kinase C in HL60 cells. Synergistic action of small GTP-binding protein RhoA. J. Biol. Chem., 271, 4366–4372. [DOI] [PubMed] [Google Scholar]
- Paris S., Beraud-Dufour,S., Robineau,S., Bigay,J., Antonny,B., Chabre,M. and Chardin,P. (1997) Role of protein–phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor Arno. J. Biol. Chem., 272, 22221–22226. [DOI] [PubMed] [Google Scholar]
- Park J.B., Kim,J.H., Kim,Y., Ha,S.H., Yoo,J.S., Du,G., Frohman,M.A., Suh,P.G. and Ryu,S.H. (2000) Cardiac phospholipase D2 localizes to sarcolemmal membranes and is inhibited by α actinin in an ARF-reversible manner. J. Biol. Chem., 275, 21295–21301. [DOI] [PubMed] [Google Scholar]
- Park S.K., Provost,J.J., Bae,C.D., Ho,W.T. and Exton,J.H. (1997) Cloning and characterization of phospholipase D from rat brain. J. Biol. Chem., 272, 29263–29271. [DOI] [PubMed] [Google Scholar]
- Park S.K., Min,D.S. and Exton,J.H. (1998) Definition of the protein kinase C interaction site of phospholipase D. Biochem. Biophys. Res. Commun., 244, 364–367. [DOI] [PubMed] [Google Scholar]
- Pertile P., Liscovitch,M., Chalifa,V. and Cantley,L.C. (1995) Phosphatidylinositol 4,5-bisphosphate synthesis is required for activation of phospholipase D in U937 cells. J. Biol. Chem., 270, 5130–5135. [DOI] [PubMed] [Google Scholar]
- Plonk S.G., Park,S.K. and Exton,J.H. (1998) The α-subunit of the heterotrimeric G protein G13 activates a phospholipase D isozyme by a pathway requiring Rho family GTPases. J. Biol. Chem., 273, 4823–4826. [DOI] [PubMed] [Google Scholar]
- Rameh L.E., Tolias,K.F., Duckworth,B.C. and Cantley,L.C. (1997) A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature, 390, 192–196. [DOI] [PubMed] [Google Scholar]
- Randazzo P.A. (1997) Functional interaction of ADP-ribosylation factor 1 with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem., 272, 7688–7692. [PubMed] [Google Scholar]
- Ren X.D., Bokoch,G.M., Traynor-Kaplan,A., Jenkins,G.H., Anderson,R.A. and Schwartz,M.A. (1996) Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol. Biol. Cell, 7, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Wolde,M., Thiele,C., Fest,W., Kratzin,H., Podtelejnikov,A.V., Witke,W., Huttner,W.B. and Soling,H.D. (1999) Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature, 401, 133–141. [DOI] [PubMed] [Google Scholar]
- Schmidt M. et al. (1996a) A role for Rho in receptor- and G protein-stimulated phospholipase C. Reduction in phosphatidylinositol 4,5-bisphosphate by Clostridium difficile toxin B. Naunyn Schmiedeberg’s Arch. Pharmacol., 354, 87–94. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Rumenapp,U., Nehls,C., Ott,S., Keller,J., von Eichel-Streiber,C. and Jakobs,K.H. (1996b) Restoration of Clostridium difficile toxin-B-inhibited phospholipase D by phosphatidylinositol 4,5-bisphosphate. Eur. J. Biochem., 240, 707–712. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Rumenapp,U., Keller,J., Lohmann,B. and Jakobs,K.H. (1997) Regulation of phospholipase C and D activities by small molecular weight G proteins and muscarinic receptors. Life Sci., 60, 1093–1100. [DOI] [PubMed] [Google Scholar]
- Sciorra V.A., Rudge,S.A., Prestwich,G.D., Frohman,M.A., Engebrecht,J. and Morris,A.J. (1999) Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J., 18, 5911–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y., Ishihara,H., Kizuki,N., Asano,T., Oka,Y. and Yazaki,Y. (1997) Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J. Biol. Chem., 272, 7578–7581. [DOI] [PubMed] [Google Scholar]
- Siddhanta A. and Shields,D. (1998) Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J. Biol. Chem., 273, 17995–17998. [DOI] [PubMed] [Google Scholar]
- Stutchfield J. and Cockcroft,S. (1993) Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem. J., 293, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung T.C., Altshuller,Y.M., Morris,A.J. and Frohman,M.A. (1999) Molecular analysis of mammalian phospholipase D2. J. Biol. Chem., 274, 494–502. [DOI] [PubMed] [Google Scholar]
- Terui T., Kahn,R.A. and Randazzo,P.A. (1994) Effects of acid phospholipids on nucleotide exchange properties of ADP-ribosylation factor 1. Evidence for specific interaction with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem., 269, 28130–28135. [PubMed] [Google Scholar]
- Tolias K.F., Couvillon,A.D., Cantley,L.C. and Carpenter,C.L. (1998a) Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol. Cell. Biol., 18, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias K.F., Rameh,L.E., Ishihara,H., Shibasaki,Y., Chen,J., Prestwich,G.D., Cantley,L.C. and Carpenter,C.L. (1998b) Type I phosphatidylinositol-4-phosphate 5-kinases synthesize the novel lipids phosphatidylinositol 3,5-bisphosphate and phosphatidylinositol 5-phosphate. J. Biol. Chem., 273, 18040–18046. [DOI] [PubMed] [Google Scholar]
- Tolias K.F., Hartwig,J.H., Ishihara,H., Shibasaki,Y., Cantley,L.C. and Carpenter,C.L. (2000) Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol., 10, 153–156. [DOI] [PubMed] [Google Scholar]
- Vinggaard A.M., Provost,J.J., Exton,J.H. and Hansen,H.S. (1997) Arf and RhoA regulate both the cytosolic and the membrane-bound phospholipase D from human placenta. Cell Signal., 9, 189–196. [DOI] [PubMed] [Google Scholar]
- Whatmore J., Morgan,C.P., Cunningham,E., Collison,K.S., Willison,K.R. and Cockcroft,S. (1996) ADP-ribosylation factor 1-regulated phospholipase D activity is localized at the plasma membrane and intracellular organelles in HL60 cells. Biochem. J., 320, 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. et al. (1997) Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J. Biol. Chem., 272, 17756–17761. [DOI] [PubMed] [Google Scholar]