SUMMARY
Background
Tuberculosis (TB) is a common diagnosis in HIV-infected patients on antiretroviral therapy (ART).
Objective
To describe TB-related practices in ART programmes in lower-income countries and identify risk factors for TB in the first year of ART.
Methods
Programme characteristics were assessed by standardized electronic questionnaire. Patient data from 2003-2008 were analyzed and incidence rate ratios (IRRs) calculated using Poisson regression models.
Results
Fifteen ART programmes in 12 countries in Africa, South America and Asia were included. Chest X-ray, sputum microscopy and culture were available free of charge in 13 (86.7%), 14 (93.3%) and eight (53.3%) programmes, respectively. Eight sites (53.3%) used directly observed therapy and five (33.3%) routinely administered isoniazid preventive therapy (IPT). A total of 19,413 patients aged ≥16 years contributed 13,227 person-years of follow-up; 1,081 new TB events were diagnosed. Risk factors included CD4 cell count (adjusted IRR comparing >350 cells/μL with <25 cells/μL 0.46, 95% CI 0.33-0.64, P<0.0001), gender (adjusted IRR comparing women with men 0.77, 0.68-0.88, P=0.0001) and use of IPT (IRR 0.24, 95% CI 0.19-0.31, p<0.0001).
Conclusions
Diagnostic capacity and practices vary widely across ART programmes. IPT prevented TB but was used in few programmes. More efforts are needed to reduce the burden of TB in HIV co-infected patients in lower income countries.
Keywords: tuberculosis, HIV, prevention, access, diagnostics, treatment practices, antiretroviral therapy, lower income countries, immunodeficiency, risk factor
INTRODUCTION
Tuberculosis (TB) remains a major public health problem worldwide. Human immunodeficiency virus (HIV) co-infection is a strong risk factor for TB, which increases the lifetime risk of progression from infection with Mycobacterium tuberculosis to active disease from 10% per lifetime to 5% to 15% per year.1 Importantly, HIV co-infection has contributed to the increase in TB incidence in recent decades and 10% to 15% of TB cases globally are now co-infected with HIV.2;3 Antiretroviral therapy (ART) has substantially improved the prognosis of HIV-infected patients in both industrialized and low-income countries.4 Access to ART in resource-limited settings, where 90% of people with HIV infection or AIDS live, has increased in recent years: the World Health Organization (WHO) estimates that about four million people were receiving ART in low- and middle-income countries by the end of 2008, a ten-fold increase during the past five years.5
In many resource-constrained settings TB is the most common AIDS-defining illness.6 TB is often present, but not necessarily diagnosed, at the start of ART.7 After starting ART an immune reconstitution syndrome may occur in patients with TB, and contribute to the high mortality in the first months of treatment observed in resource-limited settings.8 The incidence of TB can be substantially reduced by ART, both in adults6;9 and children,10;11 but additional interventions are needed to control TB in HIV-infected patients, including, for example, isoniazid preventive therapy (IPT) and screening of patients starting ART for TB: the concomitant start of ART and TB treatment can improve survival.12 Adding chest X-ray and mycobacterial sputum culture to screening for symptoms substantially increase the yield and accuracy of screening.13;14
We describe approaches to the prevention, diagnosis and treatment of TB in ART programmes in lower income settings and examined the determinants of incident TB in the first year of ART.
METHODS
We performed an electronic survey among sites participating in a network of ART treatment programmes in Africa, Asia and Latin America to assess programme level predictors of TB and obtained individual patient data to analyze the incidence and lethality of TB in the first year after starting ART.
The ART in Lower Income Countries collaboration
The ART in Lower Income Countries (ART-LINC) collaboration of the International epidemiological Databases to Evaluate AIDS (IeDEA) is a collaborative network of 24 HIV/AIDS treatment programmes in low- and middle-income countries in Africa, South America and Asia.15 The collaboration was set up in 2003 to define the prognosis of HIV-infected patients treated with ART in resource-limited settings, to compare experiences between different settings, delivery modes and types of monitoring, and to compare outcomes with those observed in industrialized nations. Patients are followed up every three to six months. The data collected at participating sites are cleaned, merged and analysed centrally according to agreed protocols.15 The present analysis includes data from January 1, 2003 to January 1, 2008, from 15 sites that prospectively recorded new TB episodes. The other nine sites did not systematically record new TB events in their databases.
Survey of ART programmes
A cross-sectional survey of all 24 ART-LINC sites was performed in 2008. The questionnaire was written in English, translated to French and pilot tested in both languages. The web-based World Health Organization (WHO) Data Collector system was used to complete the questionnaire online or offline (https://extranet.who.int/datacol/home.asp). The questionnaire covered characteristicsof sites such as the level of care, the provision of services, criteria for ART eligibility, use of guidelines, costs of care to patients, laboratory monitoring; diagnosis and treatment of opportunistic infections and cancers, loss to follow up and ascertainment of deaths. Data on services to prevent HIV infection have been reported previously.16
Inclusion criteria and definitions
We included all ART-naïve patients aged16 years or older with a known date of starting ART and a documented baseline CD4 cell count. ART was defined as a minimum of three antiretroviral drugs and categorized into NNRTI-based regimen [two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI)], PI-based regimen [two NRTIs and one protease inhibitor (PI)] and other regimens. IPT was defined as preventive treatment of any duration with isoniazid, at or after initiation of ART. The endpoint was defined as any new pulmonary or extra-pulmonary TB event recorded after ART initiation. As in a previous analysis6 all TB events that were recorded at least six months after the last TB episode were included. Time was measured from the start of ART and ended at the earliest of: the date of a new TB event or death; the date of the last follow-up visit; or month 12 after starting ART. National TB incidence rates were obtained from the WHO Global Tuberculosis Report 2008.17
Statistical analysis
We examined the influence of site characteristics, including the access and costs to patients of chest X-ray, sputum microscopy and mycobacterial culture on TB incidence, using Poisson regression models with gamma-distributed random effects on sites with and without adjustment for baseline CD4 cell count (<25, 25-49, 50-99, 100-199, 200-350, >350 cells/μL), age (16-29, 30-39, 40-49, ≥50 years), gender, use of IPT, national TB incidence (0-100, 101-300, >300 cases per 100,000 population per year) and whether or not the local TB programme was an important point of entry into the ART programme. Similarly, we examined individual-level risk factors: CD4 cell count, age (categories see above), gender, type of ART regimen (NNRTI-based, PI-based, other regimen) and use of IPT. In sensitivity analyses we examined to what extent results of individual-level analyses were affected by the exclusion of the largest programme, the Academic Model Providing Access to Healthcare (AMPATH) in western Kenya,18 or by restricting analyses to African programmes, or programmes outside Africa. Results are reported as number of patients (%), medians with interquartile range (IQR) and crude and adjusted incidence rate ratios (IRR) with 95% confidence intervals (CI). All analyses were performed in Stata version 10.1 (Stata Corporation, College Station, TX, USA).
Ethical Approval
All study sites had local institutional review board or ethics committee approval to collect data and participate in ART-LINC. All data were stripped of patient identifying information prior to transfer to the analysis centre at the University of Bern, Switzerland.
RESULTS
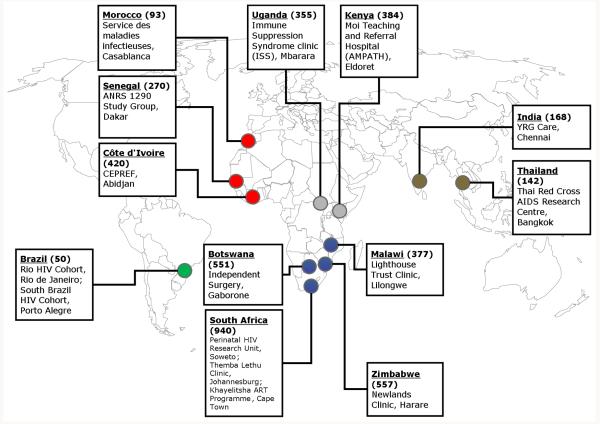
Figure 1 shows a map with the 15 programmes that prospectively recorded new TB events. There were three sites in West and North Africa, two in East and Central Africa, six in Southern Africa, two in Asia and two in South America. The map also gives the incidence of TB per 100,000 persons in 2006, which ranged from 50 per 100,000 in Brazil to 940 per 100,000 in South Africa.17 Eleven sites (73.3%) served urban or peri-urban populations, five sites (33.3%) were primary care centres and ten sites (67.7%) were funded by government. Patients were referred from TB programmes, antenatal clinics, voluntary counselling and testing centres or referred themselves to the ART clinic.
Figure 1. Geographical distribution of HIV treatment programmes participating in the ART-LINC of IeDEA collaboration.
Figures in brackets refer to TB incidence (any type of TB) per 100,000 population per year in 2006 (Source: World Health Organization17). Colours indicate IeDEA regions.
Table 1 shows the availability of chest X-ray, microbiological tests and treatment practices across sites. Chest X-ray and sputum microscopy were free of charge in 13 (86.7%) and 14 (93.3%) sites, respectively. Mycobacterial culture was free of charge in eight (53.3%) sites, at cost to patient in three (20.0%) and not available at four sites (26.7%). Treatment consisted of rifampicin-based regimens in all sites. Eight sites (53.4%) used directly observed therapy (DOT) either during the first two months or during the entire treatment period. ITP was given routinely by five sites (33.3%) and in selected cases in another three (20.0%). The eight sites that prescribed IPT prophylaxis excluded TB by screening for symptoms (cough, fever, night sweat). Other criteria included weight loss and the general condition of the patient. Seven sites additionally used sputum smears, six performed chest X-ray and five used mycobacterial culture to rule out TB before starting IPT.
Table 1.
Tuberculosis related examinations and practices in 15 antiretroviral treatment programmes in Africa, Asia and Latin America.
| Characteristic | N | (%) |
|---|---|---|
| Chest X-ray | ||
| Free of charge | 13 | (86.7) |
| At cost to patient | 2 | (13.3) |
|
| ||
| Sputum microscopy | ||
| Free of charge | 14 | (93.3) |
| At cost to patient | 1 | (6.7) |
|
| ||
| Mycobacterial culture | ||
| Free of charge | 8 | (53.3) |
| At cost to patient | 3 | (20.0) |
| Not available | 4 | (26.7) |
|
| ||
| Mycobacterial culture on site | 6 | (40.0) |
|
| ||
| Tuberculin skin test | ||
| Free of charge | 6 | (40.0) |
| Not available | 9 | (60.0) |
|
| ||
| Screening by interferon-based test | ||
| Selected cases | 1 | (6.7) |
| Never / not available | 14 | (93.3) |
|
| ||
| TB DOT | ||
| During the 1st 2 months | 4 | (26.7) |
| During entire treatment period | 4 | (26.7) |
| Not used | 7 | (46.6) |
|
| ||
| TB treatment location | ||
| In HIV clinic | 4 | (26.7) |
| In specialized TB clinic | 11 | (73.3) |
|
| ||
| Isoniazid preventive therapy | ||
| Routinely used | 5 | (33.3) |
| Used when clinically indicated | 3 | (20.0) |
| Not used | 7 | (46.7) |
|
| ||
| TB case definitions used* | ||
| WHO definitive case definition | 11 | (73.3) |
| WHO clinical case definition | 12 | (80.0) |
TB, tuberculosis; WHO, World Health Organization
Categories not exclusive
A total of 31,294 patients were enrolled in the 15 programmes and 19,413 patients (62.0%) met criteria for inclusion in analyses. Figure 2 illustrates the selection of eligible patients and the reasons for exclusions. Among included patients 7654 (39.4%) were from Southern Africa, 5799 (29.9%) from East and Central Africa, 3049 (15.7%) from West and North Africa, 1946 (10.0%) from Asia, and 965 patients (5.0%) from Latin America. The median year of starting ART was 2005 (IQR 2004-2005), median age was 35 years (IQR 30-42 years) and 12161 patients (62.6%) were female. The median CD4 cell count was 115 cells/μL (IQR 46-191 cells/μL); 5087 patients (26.2%) started ART with fewer than 50 CD4 cells/μL. A total of 8137 patients (41.9%) were known to be in advanced clinical stage (WHO stage III/IV), but clinical stage was missing in 7348 patients (37.9%). Most patients were on a NNRTI-based regimen (16861 patients, 86.9%). Compared to included patients, adult patients who were excluded from analyses (Figure 2) had higher CD4 cell counts (median 145 cells/μL), were older (median 36 years), more likely to be male (4636/11098, 41.8%), more likely to be on a PI-based regimen (694/11098, 6.3%) and less likely to be on IPT (326/11098, 2.9%); P<0.0001 for all differences.
Figure 2. Selection of study population.
In the first year of ART, 1081 new TB events were diagnosed during 13227 person-years of follow-up, for an overall incidence rate of 8.2 per 100 person-years (95% CI 7.7-8.7 per 100 person-years). During the same period 924 patients died (mortality rate 7.0 per 100 person-years; 95% CI 6.5-7.5 per 100 person-years) and 1726 patients were lost to follow-up (rate 13.0 per 100 person-years; 95% CI 12.4-13.7 per 100 person-years). Patients lost to follow-up were similar to patients remaining in care with regard to age (P=0.93) but were more likely to be male (788/1726, 45.7% versus 6464/17687, 36.5%, P<0.0001) and had lower CD4 cell counts (94 cells/μL versus 117 cells/μL, P<0.0001). Among patients diagnosed with TB, 59 patients died in the first year after the diagnosis, during 280.2 person-years of follow-up: the overall mortality rate was thus 21.1 per 100 person-years (95% CI 16.3-27.2 per 100 person-years).
Table 2 shows crude and adjusted IRR according to the availability of diagnostic tests. Incidence rates were higher in sites where chest X-ray, sputum microscopy ormycobacterial culture were available free of charge compared to sites where patients were charged for these examinations, or sites where culture was not available. These associations became stronger in multivariable analyses, but failed to reach conventional levels of statistical significance.
Table 2.
Crude and adjusted incidence rate ratios of tuberculosis according to the availability of examinations in 15 antiretroviral treatment programmes in Africa, Asia and Latin America.
| Tuberculosis diagnostics |
No. of sites (%) |
Crude IRR (95% CI) |
P value | Adjusted IRR (95% CI)* |
P value |
|---|---|---|---|---|---|
| Chest X-ray | 0.72 | 0.29 | |||
| Routinely available | 13 (86.7) | 1.0 | 1.0 | ||
| Charges applicable † | 2 (13.3) | 0.80 (0.23-2.80) | 0.53 (0.16-1.73) | ||
| Not available | 0 | - | - | ||
|
| |||||
| Sputum microscopy | 0.46 | 0.41 | |||
| Routinely available | 14 (93.3) | 1.0 | 1.0 | ||
| Charges applicable † | 1 (6.7) | 0.53 (0.10-2.87) | 0.49 (0.87-2.73) | ||
| Not available | 0 | - | - | ||
|
| |||||
| Mycobacterial culture | 0.88 | 0.30 | |||
| Routinely available | 8 (53.3) | 1.0 | 1.0 | ||
| Charges applicable † | 3 (20.0) | 1.20 (0.39-3.67) | 1.22 (0.27-5.53) | ||
| Not available | 4 (26.7) | 0.86 (0.31-2.38) | 0.45 (0.16-1.27) | ||
IRR, incidence rate ratio.
95% CI, 95% confidence interval
Adjusted for baseline CD4 cell count, age, gender, isoniazid preventive therapy, main point of entry into ART programme (referral from TB programme or other), and national TB incidence.
Charges to patients
Table 3 shows crude and adjusted IRR for TB in the first year of ART. TB was associated with lower CD4 cell counts (P<0.0001 from the adjusted model) and male gender (P=0.0001). The association with gender was similar across age groups (P=0.60 from test of interaction). A total of 1534 patients (7.9%) received IPT. Patients receiving and not receiving IPT had the same median age (35 years, P=0.60) and similar median CD4 cell counts at the start of ART (113 cells/μL compared to 115 cells/μL, P=0.97), but patients receiving IPT were more likely to be female than patients not receiving IPT (72.7% compared to 61.8%, P<0.0001). The use of IPT was protective, with similar IRRs from crude and adjusted analyses. The adjusted IRR was 0.24 (95% CI 0.19-0.31, P<0.0001). There was little evidence that the effect of IPT was modified by the degree of immunodeficiency (P=0.65 from test of interaction). Finally, sensitivity analyses showed that estimates were robust to the exclusion of the largest programme, or the restriction of the analysis to African or non-African programmes.
Table 3.
Crude and adjusted incidence rate ratios for tuberculosis in the first year of antiretroviral therapy. Data from 15 antiretroviral treatment programmes in Africa, Asia and Latin America.
| Characteristic | No. of patients (%) |
No. of patients with TB |
Crude IRR (95% CI) |
P value | Adjusted IRR (95% CI) |
P value | |
|---|---|---|---|---|---|---|---|
| CD4 cell count (cells/μL) | <0.0001 | <0.0001 | |||||
| <25 | 3037 | (15.6) | 208 | 1 | 1 | ||
| 25–49 | 2050 | (10.6) | 147 | 0.97 (0.78-1.20) | 0.97 (0.79-1.20) | ||
| 50–99 | 3527 | (18.2) | 208 | 0.74 (0.62-0.91) | 0.76 (0.62-0.92) | ||
| 100–199 | 6469 | (33.3) | 340 | 0.62 (0.53-0.75) | 0.63 (0.53-0.75) | ||
| 200–350 | 3247 | (16.7) | 135 | 0.55 (0.44-0.68) | 0.55 (0.44-0.69) | ||
| >350 | 1083 | (5.6) | 43 | 0.41 (0.30-0.57) | 0.46 (0.33-0.64) | ||
|
| |||||||
| Age (years) | 0.002 | 0.14 | |||||
| 16-29 | 4295 | (22.1) | 191 | 1 | 1 | ||
| 30-39 | 8836 | (45.5) | 517 | 1.33 (1.12-1.57) | 1.21 (1.02-1.43) | ||
| 40-49 | 4536 | (23.4) | 280 | 1.40 (1.16-1.68) | 1.19 (0.99-1.44) | ||
| ≥50 | 1746 | (9.0) | 93 | 1.25 (0.97-1.60) | 1.09 (0.84-1.40) | ||
|
| |||||||
| Gender | <0.0001 | 0.0001 | |||||
| Male | 7252 | (37.4) | 498 | 1 | 1 | ||
| Female | 12161 | (62.6) | 583 | 0.71 (0.62-0.80) | 0.77 (0.68-0.88) | ||
|
| |||||||
|
First-line antiretroviral
regimen |
0.055 | 0.047 | |||||
| NNRTI-based | 16861 | (86.9) | 1000 | 1 | 1 | ||
| PI-based | 1145 | (5.9) | 28 | 0.67 (0.45-1.02) | 0.67 (0.44-1.02) | ||
| Other | 1407 | (7.2) | 53 | 1.27 (0.92-1.76) | 1.29 (0.93-1.78) | ||
|
| |||||||
|
Isoniazid preventive
therapy |
<0.0001 | <0.0001 | |||||
| No | 17879 | (92.1) | 992 | 1 | 1 | ||
| Yes | 1534 | (7.9) | 89 | 0.25 (0.19-0.31) | 0.24 (0.19-0.31) | ||
IRR, incidence rate ratio
95% CI, 95% confidence interval
TB, tuberculosis
NNRTI, non-nucleoside reverse transcriptase inhibitor
PI, protease inhibitor
P values from Wald tests. Models were adjusted for all variables listed in the table and the national TB incidence.
DISCUSSION
This survey of TB-related preventive, diagnostic and treatment practices in 15 ART programmes in Africa, Asia and Latin America showed that approaches differed widely across programmes. For example, mycobacterial culture was available free of charge in about half of programmes and only few programmes routinely administered IPT. The analysis of risk factors for TB in the first year of ART showed, based on data from almost 20,000 individuals, that more advanced immunodeficiency and male gender were associated with a higher risk of TB. The use of IPT was associated with a substantially lower risk of TB.
IPT was routinely administered in some small sites, used when clinically indicated in others and not used in over half of the ART programmes: overall, only 8% of patients received IPT. In this observational study IPT appeared to reduce the risk of TB to a greater extent than expected from the randomised trials,19 possibly because of confounding by indication if patients at lower risk of TB were more likely to receive IPT. Of note, the beneficial effect of IPT appeared to be independent of the degree of immunodeficiency. IPT implementation is still a long way behind the target of the Global Plan to Stop TB, reaching less than 0.1% of people estimated to be HIV-positive globally.17 Aït-Khaled and colleagues20 recently called for the scale-up of IPT under the banner of the “Three I’s” (Infection control to prevent nosocomial transmission of TB in health care settings, Intensified TB case finding and IPT), stressing that IPT must be implemented in a safe and structured way, to prevent the development of multidrug-resistant TB. The experience in Botswana showed that IPT can be safe and well-tolerated in HIV-positive patients.21
Getahun and colleagues from WHO recently reported that in countries with a high prevalence of HIV more women than men are diagnosed with TB.22 Evidence from the pre-HIV era suggests that young and middle-aged women are more likely to progress from infection to disease than men.23 In the present study, we found that male gender was associated with a higher risk of TB, and that this association was independent of age. This might reflect differences between women and men in access to and utilisation of ART, which substantially reduces the risk of TB.6;9-11 Interestingly, in a previous analysis of the ART-LINC programmes we found that the proportion of female ART recipients was similar to or higher than the UNAIDS estimates of the proportion of HIV-infected adults who are women in the respective countries.24 Furthermore, we found no gender difference in the risk of TB in patients starting ART in industrialized countries.6
The lack of a uniform TB case definition is an important limitation of our study. We relied on the TB events recorded by the sites, which included for example presumptive cases in some programmes but not others. Our results comparing incidence rates of TB across sites with different diagnostic capabilities must therefore be interpreted with caution. The definition of IPT was also somewhat imprecise, and included IPT of any duration at or after the start of ART. The lack of a standardised definition is, however, less likely to have affected the analyses of individual patient level factors, including use of IPT: these analyses were controlled for between-programme variations. The appropriate duration and drug combinations of chemoprophylaxis against TB is the topic of ongoing research.25;26 For example, a recent trial from South India concluded that a 6-month regimen of ethambutol and isoniazid was as effective as three years of isoniazid alone.25
The lack of detailed data on morbidity, with information on clinical stage missing in a substantial proportion of patients, is another limitation. We adjusted analyses for the degree of immunodeficiency, but not for co-morbidities or clinical stage. Our results, including those from the individual patient data analyses, may therefore have been affected by confounding or residual confounding. Also, we acknowledge that the sites included in the present study will not be representative of ART programmes in their region or country: they are mainly urban and were recruited to the collaboration because they capture data in electronic databases. Many patients had to be excluded from analyses because of missing information, particularly missing CD4 cell counts, and some patients were lost to follow-up in the first year of ART. Loss to follow-up was associated with lower CD4 cell counts and may introduce bias in estimates of TB incidence and mortality. Simulation studies have, however, shown that this bias will be modest if fewer than 10% of patients are lost to follow-up.27;28 Our study in a geographically very diverse set of ART programmes clearly identified important issues related to diagnostic practices and the implementation of IPT, interventions that are essential to reduce early morbidity and mortality in patients on ART in resource-limited settings.20;29
The most effective screening strategy for TB among HIV-infected patients is a matter of ongoing research. A study in Harare, Zimbabwe, showed that even smear-positive TB may be missed in HIV-positive patients by screening for symptoms. Sensitivity and specificity of symptom screening and sputum culture were similar in HIV-positive and HIV-negative patients, but the positive predictive value was higher and the negative predictive value lower in HIV-positive patients.30 A recent study from Asia proposed a clinical algorithm for HIV-infected patient to rule out TB, based on three symptoms.14 Adding chest X-ray to screening for symptoms increased the sensitivity of screening but requires experienced radiography readers.14 The same study also showed that sputum culture is required for diagnosis in most cases with symptoms. Microbiological sputum examination of all individuals without any prior selection can yield an additional four TB cases per 100 individuals screened.31 A study in a South African township demonstrated that active case finding targeting HIV-infected people is successful in finding previously undiagnosed TB cases.32 The trend seen in our study towards higher TB incidence according to availability of diagnostic examinations and costs to patients may indicate the importance of diagnostic procedures in ART programmes.
Although culture is more sensitive, sputum smear examination by light microscopy is often the only diagnostic test available in lower income countries with high TB burden. But the proportion of smear-negative pulmonary TB may range up to 60% in HIV-positive TB patients.14;22 Accurate TB diagnosis in HIV-infected patients should ideally rely not only on sputum smear microscopy: mycobacterial culture plays an important role in TB diagnosis and can be part of a diagnostic algorithm.33
In conclusion, not all ART programmes routinely offer access to TB diagnostics before and during ART, and about a third routinely used IPT. Improving diagnostic capacity, intensifying case finding and implementing IPT more widely must be of high priority in ART programmes in resource-limited settings, to reduce the high early mortality observed in these settings. Further studies are needed to monitor trends in screening practices and to define optimal TB screening and prevention strategies in ART programmes.
ACKNOWLEDGEMENTS
We are indebted to the patients and clinic staff for their contributions to these research efforts. We acknowledge all patients whose data were used in this study. We also would like to thank all who contributed to recording and entering data as well as preparing and sending it to the ART-LINC of IeDEA collaboration. We thank Jack Whitescarver and Paolo Miotti (NIH/OAR), Carolyn Williams (NIH/NIAID), Michel Kazatchkine, Jean-François Delfraissy, Brigitte Bazin and Séverine Blesson (ANRS) for encouraging and supporting this collaborative work. The ART-LINC Collaboration was funded by the United States National Institute of Health (Office of AIDS Research) and the French Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS).
Footnotes
COMPETING INTERESTS
None declared.
REFERENCES
- (1).Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, et al. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N Engl J Med. 1992;327(24):1697–1703. doi: 10.1056/NEJM199212103272401. [DOI] [PubMed] [Google Scholar]
- (2).Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- (3).Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367(9514):938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- (4).Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- (5).World Health Organization . Scaling up priority HIV/AIDS interventions in the health sector. 2009 Progress Report. World Health Organization; Geneva: [Accessed 23 September 2010]. 2009. Towards universal access. Available from http://www.who.int/hiv/pub/2009progressreport/en/index.html. [Google Scholar]
- (6).Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, Sprinz E, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45(11):1518–1521. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177(7):680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- (10).Braitstein P, Nyandiko W, Vreeman R, Wools-Kaloustian K, Sang E, Musick B, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28(7):626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- (11).Edmonds A, Lusiama J, Napravnik S, Kitetele F, Van RA, Behets F. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38(6):1612–1621. doi: 10.1093/ije/dyp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51(7):823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. New Engl J Med. 2010;362(8):707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- (15).Dabis F, Balestre E, Braitstein P, Miotti P, Brinkhof WGM, Schneider M, et al. Antiretroviral Therapy in Lower Income Countries (ART-LINC): International collaboration of treatment cohorts. Int J Epidemiol. 2005;34:979–986. doi: 10.1093/ije/dyi164. [DOI] [PubMed] [Google Scholar]
- (16).Spaar A, Graber C, Dabis F, Coutsoudis A, Bachmann L, McIntyre J, et al. Prioritising prevention strategies for patients in antiretroviral treatment programmes in resource-limited settings. AIDS Care. 2010;22(6):775–783. doi: 10.1080/09540120903349102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).World Health Organization . WHO report 2008. World Health Organization; Geneva: [Accessed 23 September 2010]. 2008. Global tuberculosis control 2008: surveillance, planning, financing. Available from http://www.who.int/tb/publications/global_report/2008/en/ [Google Scholar]
- (18).Einterz RM, Kimaiyo S, Mengech HN, Khwa-Otsyula BO, Esamai F, Quigley F, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82(8):812–818. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- (19).Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD000171.pub2. CD000171. [DOI] [PubMed] [Google Scholar]
- (20).Aït-Khaled N, Alarcon E, Bissell K, Boillot F, Caminero JA, Chiang CY, et al. Isoniazid preventive therapy for people living with HIV: public health challenges and implementation issues. Int J Tuberc Lung Dis. 2009;13(8):927–935. [PubMed] [Google Scholar]
- (21).Mosimaneotsile B, Mathoma A, Chengeta B, Nyirenda S, Agizew TB, Tedla Z, et al. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: a Botswana Experience, 2004-2006. J Acquir Immune Defic Syndr. 2010;54(1):71–77. doi: 10.1097/QAI.0b013e3181c3cbf0. [DOI] [PubMed] [Google Scholar]
- (22).Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- (23).Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis. 1998;2(2):96–104. [PubMed] [Google Scholar]
- (24).Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 2008;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- (25).Swaminathan S, Menon P, Perumal V, Santhanakrishnan RK, Ramachandran R, Chinnaiah P, et al. Efficacy of a 6-month vs a 36-month Regimen for Prevention of TB in HIV-infected Persons in India: A Randomized Clinical Trial; 17th Conference on Retroviruses and Opportunistic Infections San Francisco, USA; Feb 16-19, 2010; 2010. Abstract 103. [Google Scholar]
- (26).Samandari T, Mosimaneotsile B, Agizew T, Nyirenda S, Tedla Z, Sibanda T, et al. Randomized, Placebo-controlled Trial of 6 vs 36 Months Isoniazid TB Preventive Therapy for HIV-infected Adults in Botswana; 17th Conference on Retroviruses and Opportunistic Infections San Francisco, USA; Feb 16-19, 2010; 2010. Abstract 104LB. [Google Scholar]
- (27).Egger M, Spycher B, Sidle J, Weigel R, Genge EH, Fox MP, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 8(1):e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Fenner L, Brinkhof MW, Keiser O, Weigel R, Cornell M, Moultrie H, et al. Early Mortality and Loss to Follow-up in HIV-Infected Children Starting Antiretroviral Therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010;54(5):524–532. doi: 10.1097/QAI.0b013e3181e0c4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Lawn SD, Harries AD, Wood R. Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5(1):18–26. doi: 10.1097/COH.0b013e328333850f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Corbett EL, Zezai A, Cheung YB, Bandason T, Dauya E, Munyati SS, et al. Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ. 2010;88:13–21. doi: 10.2471/BLT.08.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kranzer K, Houben R, Glynn JR, Bekker LG, Wood R, Lawn SD. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175(1):87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).World Health Organization . Recommendations for HIV-prevalent and resource-constrained settings. World Health Organization; Geneva: [Accessed 23 September 2010]. 2006. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents. Available from http://www.who.int/entity/tb/publications/2006/tbhiv_recommendations.pdf. [Google Scholar]