Abstract
Extracellular cAMP stimulates the rapid tyrosine phosphorylation and nuclear translocation of the Dictyostelium STAT protein Dd-STATa. Here we show that it also induces serine phosphorylation by GskA, a homologue of glycogen synthase kinase-3 (GSK-3). Tyrosine phosphorylation occurs within 10 s of stimulation, whereas serine phosphorylation takes 5 min, matching the kinetics observed for the cAMP regulation of GskA. Phosphorylation by GskA enhances nuclear export of Dd-STATa. The phosphorylated region, however, is not itself a nuclear export signal and we identify a region elsewhere in the protein that mediates nuclear export. These results suggest a biphasic regulation of Dd-STATa, in which extracellular cAMP initially directs nuclear import and then, via GskA, promotes its subsequent export. It also raises the possibility of an analogous regulation of STAT nuclear export in higher eukaryotes.
Keywords: Dictyostelium discoideum/GSK-3/nuclear export/serine phosphorylation/STAT transcription factors
Introduction
The STAT proteins were discovered as transducers of the cellular response to cytokines (reviewed by Ihle and Kerr, 1995; Schindler and Darnell, 1995). They contain a DNA-binding domain, an SH2 domain, a tyrosine phosphorylation site and most contain a transcriptional activation domain at their C-terminus. When a cytokine binds to its receptor, an associated Janus kinase (JAK) is activated and phosphorylates STAT on a tyrosine residue. This leads to dimerization through binding of the phosphotyrosine on one STAT molecule to the SH2 domain of the other. STAT signalling pathways are also found in other organisms. The Drosophila genes hopscotch and marelle encode JAK and STAT homologues, respectively (Binari and Perrimon, 1994). They are required for blood cell development and control segmentation, imaginal disc cell proliferation and polarity in the developing eye (Harrison et al., 1995; Strutt and Strutt, 1999). A STAT protein homologue has been cloned from the non-metazoan organism Dictyostelium (Kawata et al., 1997). This protein, Dd-STATa, is homologous to metazoan STAT proteins, possessing an SH2 domain, a tyrosine phosphorylation site and a DNA-binding domain. Dd-STATa does not, however, contain a transcriptional activation domain at its C-terminus and it is known to act as a repressor of transcription (Mohanty et al., 1999).
All growing and developing Dictyostelium cells appear to contain a small amount of Dd-STATa in their nuclei, the only apparent exception being mature spore cells (Araki et al., 1998). However, during development some cells come to exhibit a strong nuclear enrichment. This is most apparent between 6 and 10 h development, when Dictyostelium cells undergo the transition from unicellular amoebae to a multicellular aggregate (the mound). Immediately after formation of the mound >95% of cells show strong nuclear staining with anti-Dd-STATa antibodies. Consistent with these observations, tyrosine phosphorylation of Dd-STATa increases over the same time scale and is rapidly stimulated by extracellular cAMP: the central regulatory signal for aggregation.
After 12 h development a tip appears on top of the mound and this coordinates later development. The tip extends upwards and the mound reshapes into a tall finger-like column of cells, which under some environmental conditions can topple over to form a freely motile slug. There is a dramatic fall in the number of cells showing nuclear enrichment of Dd-STATa during tip formation, so that in the slug only ∼15% have strong Dd-STATa nuclear localization and these are present in the slug tip. The ultimate product of Dictyostelium development is the fruiting body, which consists of a spore head, a stalk and a basal disc. Fruiting body formation (culmination) is blocked in mutants that lack Dd-STATa protein (Mohanty et al., 1999). Dd-STATa null mutants also exhibit abnormal expression of the ecmB gene. In the wild type, strong expression of ecmB is seen at culmination in cells as they enter the stalk tube (Williams et al., 1989). In a Dd-STATa mutant, ecmB is prematurely and ectopically expressed in pre-stalk cells before they enter the stalk tube (Mohanty et al., 1999).
The Dictyostelium homologue of glycogen synthase kinase-3 (GSK-3), GskA, is also required for the correct pattern of gene expression during development. GSK-3 is a highly conserved protein kinase found in all eukaryotic groups and regulates a wide range of cellular processes, ranging from intermediate metabolism and gene expression to cytoskeletal integrity (Plyte et al., 1992). Genetic studies suggest that GSK-3 family members play a crucial role during development. The Drosophila homologue of GSK-3, Shaggy (encoded by the gene sgg), is required for establishing segment polarity, cell specification and correct patterning of the zygotic neuroectoderm (Ruel et al., 1993; Siegfried et al., 1994; Cadigan and Nusse, 1997). GskA is required to establish the correct pattern of cell types in the Dictyostelium mound (Harwood et al., 1995) and its activity increases at this stage of development. GskA activity is stimulated by extracellular cAMP, through a process that is mediated by the cAMP receptor carC and the tyrosine kinase ZAK-1 (Kim et al., 1999; Plyte et al., 1999).
Among the targets of mammalian GSK-3 are a number of transcription factors and GSK-3 phosphorylation has different effects on each of them. Phosphorylation of c-jun interferes with its binding to DNA and hence represses c-jun-directed transcription (Boyle et al., 1991). Conversely, GSK-3 phosphorylation of CREB activates gene transcription (Fiol et al., 1994). GSK-3 phosphorylation of the lymphoid cell transcription factor NF-ATc causes enhanced nuclear exit (Beals et al., 1997). An enhanced rate of nuclear export is also observed when GSK-3 phosphorylates cyclin D1 (Diehl et al., 1998). STAT proteins are also targets of serine/threonine protein kinases (Wen and Darnell, 1997). Phosphorylation by MAP kinase regulates nuclear translocation and transcriptional activity of STAT1a and STAT3 (Stephens et al., 1998). The STAT5a and 5b proteins also contain phosphoserine, but this phosphorylation is independent of MAP kinase activity and the identity of the cognate kinase is not yet known. In this paper we identify a GskA phosphorylation site in Dd-STATa and show that this modification enhances export of Dd-STATa from the nucleus. This demonstrates both a novel GSK-3 interaction and a new mechanism of STAT regulation.
Results
Dd-STATa contains potential GSK-3 phosphorylation sites
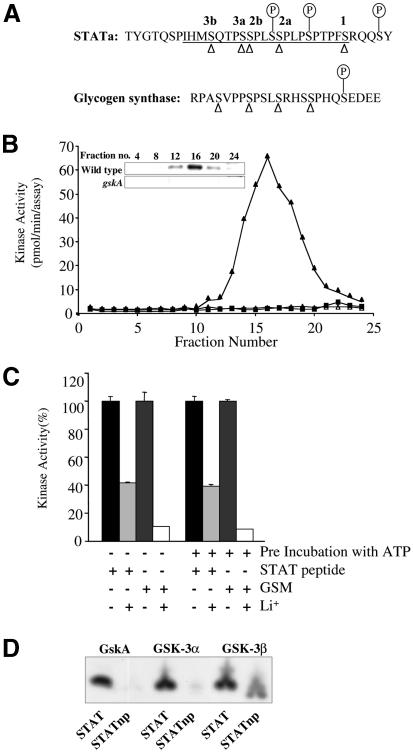
The sequence of Dd-STATa predicts a cluster of GSK-3 sites within a 34 amino acid sequence (amino acids 112–146) in the N-terminal portion of the protein (Figure 1A). A poly(glutamine)-rich region and a poly(asparagine)-rich region delimit this amino acid sequence at its N- and C-terminus, respectively. We refer to this sequence by the abbreviation GPR (GSK-3 phosphorylation region). Most GSK-3 phosphorylation sites are rich in proline and have the consensus S-P-X-X-S(PO4), where the phosphate is transferred to a serine three residues upstream of an existing phosphoserine. The phosphoserine can be formed by a ‘priming’ kinase, such as when protein kinase A modifies CREB or NF-ATc, or by sequential phosphorylation of overlapping GSK-3 sites, as seen in glycogen synthase (Figure 1A). The GPR of Dd-STATa contains five predicted GSK-3 phosphorylation sites: a single site at residue 140 (site 1) and an upstream cluster of four overlapping sites (sites 2a, 2b, 3a and 3b). This cluster is arranged such that GSK-3 phosphorylation of site 2a generates the priming site for site 2b and phosphorylation of the adjacent site 3a generates the priming site for 3b (Figure 1A).
Fig. 1. In vitro evidence for a GSK-3 phosphorylation site within Dd-STATa. (A) A comparison of the cluster of putative GSK-3 phosphorylation sites from Dd-STATa (residues 112–146) to that characterized for bovine glycogen synthase. Open triangles mark serine residues that are potentially phosphorylated by GSK-3 (numbered 1–3). ‘Priming’ phosphoserines, which are required to generate the GSK-3 site, are indicated by P. The sequence of a synthetic peptide, STAT peptide, which contains GSK-3 sites 2a–3b and phosphoserine residues at the putative ‘priming’ sites is underlined. (B) Dictyostelium extracts from wild-type cells (filled symbols) or gskA mutant cells (open symbols) were separated on a P11 phosphocellulose column. Each fraction was tested for its phosphorylation activity on the STAT peptide (triangles) or a peptide containing no phosphoserine residues (STATnp, squares). Activity correlated with the presence of GskA protein as detected by western analysis (see insert). No activity was seen using a STATnp peptide or using a gskA mutant cell extract. (C) Purified GskA [fraction 16 in (B)] was treated with 50 mM LiCl. Lithium inhibited phosphorylation of peptide substrates derived from glycogen synthase (GSM peptide) and the STAT peptide. The same GskA preparation was pre-incubated with ATP for 30 min. Substrate and 50 mM LiCl were added and phosphorylation assayed as before. (D) GskA, GSK-3α and GSK-3β were used to phosphorylate STAT and STATnp peptide substrates, and visualized by Tricine–SDS gel electrophoresis.
To examine whether the GPR is a substrate for GSK-3 phosphorylation, a peptide encompassing sites 2a–3b, termed the STAT peptide, was synthesized (underlined in Figure 1A). The predicted priming phosphates were incorporated during peptide synthesis. Extracts from growing Dictyostelium cells were fractionated on phosphocellulose and tested against both GSM, a peptide based upon glycogen synthase that has been demonstrated to be a good GSK-3 substrate (Ryves et al., 1998), and the STAT peptide. The phosphorylation activities for GSM (data not shown) and the STAT peptide co-purified (Figure 1B). This peak of activity exactly matches the peak of GSK-3 protein concentration as judged by western analysis (Figure 1B, insert). No phosphorylation was seen with either an extract from a gskA mutant strain that lacks all GskA activity or a STAT peptide that lacks the priming phosphates (Figure 1B). The fraction with peak activity was treated with lithium chloride, which is an inhibitor of GSK-3 kinase activity. Lithium inhibited phosphorylation of STAT peptide, although perhaps not to the same degree as GSM (Figure 1C).
As our GskA preparation was not purified to homogeneity, it is possible that a contaminating kinase is activated by GskA and then is responsible for phosphorylation of the STAT peptide. Two experiments suggest that this is not the case. First, we pre-incubated our GskA enzyme preparation with ATP to allow GskA to activate the potential kinase contaminant. STAT peptide substrate and lithium chloride were then added and the reaction was incubated further. Activation of a kinase contaminant would allow STAT peptide phosphorylation in the presence of lithium. This was not observed (Figure 1C). Secondly, we phosphorylated the STAT peptide with GSK-3 kinases from different sources (Figure 1D). We found that the STAT peptide is a good substrate for both GSK-3α, prepared from skeletal muscle, and recombinant GSK-3β, prepared from Escherichia coli. These observations strongly argue that GSK-3 directly phosphorylates the STAT peptide. Interestingly, we found that GSK-3β was able to phosphorylate an unprimed STAT peptide substrate (STATnp) weakly, suggesting that the STAT GPR may not always require a priming phosphate, as observed for other substrates such as axin, β-catenin and tau (Thomas et al., 1999). Using our purified GSK-3 kinase, we calculate a Km of 25 µM for the STAT peptide (data not shown). This value is significantly better than the Km measured for other GSK-3 substrates, including GSM, GS and eIF2B (Welsh et al., 1997; Ryves et al., 1998), further indicating that the GPR of Dd-STATa is a good in vitro GSK-3 substrate.
GSK-3 targets Dd-STATa in vivo
Two forms of tyrosine-phosphorylated Dd-STATa with different electrophoretic mobilities are detected by western analysis (Araki et al., 1998). The faster migrating tyrosine-phosphorylated species appears after 10 s stimulation with extracellular cAMP and its level reaches a plateau at 1 min. Its appearance correlates with the nuclear localization of Dd-STATa and with specific DNA-binding activity. The lower mobility tyrosine-phosphorylated species first appears 5 min after extracellular cAMP stimulation and levels reach a maximum at 15 min, a profile that matches the activation of GskA by cAMP (Plyte et al., 1999). To investigate the potential link between GskA and Dd-STATa, we examined the Dd-STATa protein in a gskA mutant that lacks all GskA protein and activity. The lower mobility form of Dd-STATa was absent in the gskA mutant, suggesting that this form arises through GskA phosphorylation (Figure 2). The higher mobility form could still be detected using antiserum specific for the tyrosine-phosphorylated Dd-STATa, indicating that GskA activity is not required for tyrosine phosphorylation.
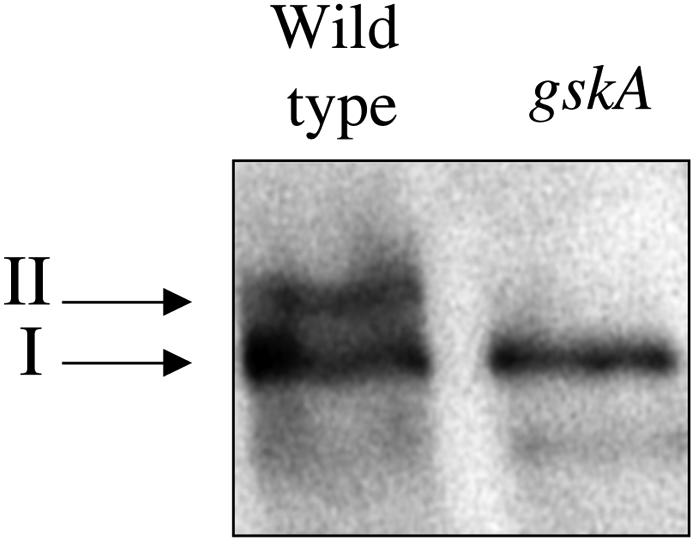
Fig. 2. Western analysis of Dd-STATa in wild-type and gskA mutant cells. Extracts of cells at 8 h development were investigated by western analysis using affinity-purified SC7-pTyr antiserum, which is specific for tyrosine-phosphorylated Dd-STATa (Araki et al., 1998). The high mobility form of Dd-STATa (I) is present in both strains, but the slow mobility form (II) is not present in a gskA mutant. Proteins with higher mobilities than band I result from proteolysis of Dd-STATa.
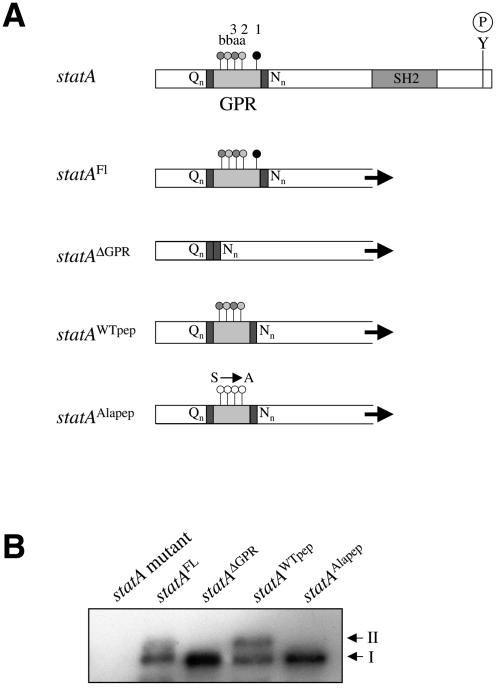
We next deleted the GPR to confirm that this region is required to generate the lower mobility form of Dd-STATa. Both the full-length Dd-STATa (Dd-STATaFL) and deleted (Dd-STATaΔGPR) proteins were expressed from the semi-constitutive actin 15 promoter in a mutant lacking Dd-STATa (Figure 3A). The lower mobility form was absent in the Dd-STATaΔGPR transformed cells (Figure 3B). A sequence corresponding to the STAT peptide was reintroduced into Dd-STATaΔGPR. This gene, Dd-STATaWTpep, contains four of the five GSK-3 phosphorylation sites present in the GPR and its introduction restores the lower mobility form (Figure 3B). A mutated sequence was made where alanine residues replace all of the serine residues predicted to be phosphorylated by GskA. This was introduced into Dd-STATaΔGPR to make Dd-STATaALApep. This mutant Dd-STATa again lacks the lower mobility form (Figure 3B). These results indicate that the cluster of GskA phosphorylation sites identified in our in vitro biochemical assay is a likely in vivo target of GskA.
Fig. 3. In vivo analysis of the GskA phosphorylation sites of Dd-STATa. (A) The putative GskA phosphorylation site was deleted from the Dd-STATa protein to make Dd-STATaΔGPR. A sequence that corresponds to the STAT peptide was inserted into Dd-STATaΔGPR to make Dd-STATaWTpep. A peptide sequence with alanine substitutions for those serine residues predicted to be phosphorylated by GskA was also inserted into Dd-STATa to make Dd-STATaALApep. (B) Western analysis indicates that Dd-STATa proteins which lack the GPR or its GskA sites do not possess the lower mobility form of the Dd-STAT protein (II).
GskA phosphorylation of the GPR inhibits Dd-STATa nuclear localization
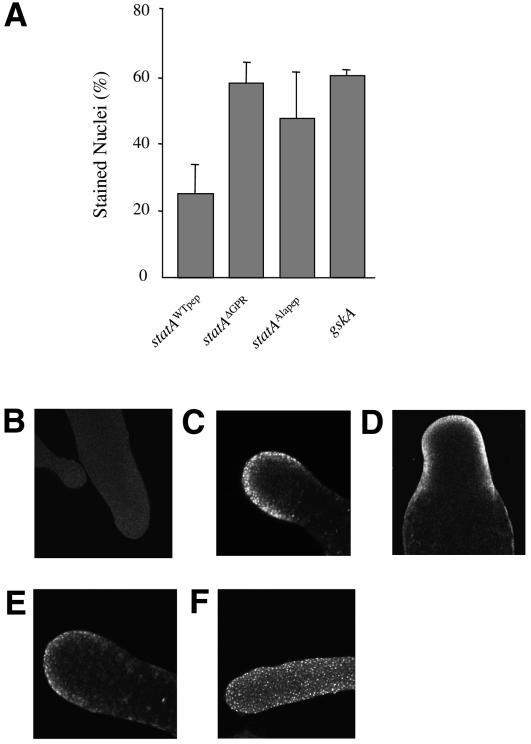
We investigated the function of the GPR and its phosphorylation by GskA. The subcellular distribution of Dd-STATa was examined at various stages of development using an antiserum specific for the tyrosine-phosphorylated form of Dd-STATa. Mutant cells lacking the Dd-STATa gene show no staining, but this is restored by expression of the Dd-STATaWTpep gene. As in wild-type cells, the number of stained nuclei increases during early development to peak at 10 h after the beginning of starvation. Expression of the Dd-STATaΔGPR mutant, which lacks the GPR, again restores cell staining, but this protein has an enhanced rate of nuclear accumulation. This is best seen at 8 h development, when nuclear accumulation of Dd-STATa is undergoing its major increase. At this point, cells expressing Dd-STATaΔGPR possess approximately twice as many stained nuclei as cells expressing Dd-STATaWTpep (Figure 4A). This suggests that the GPR acts to limit nuclear accumulation of Dd-STATa. To investigate whether GskA phosphorylation is required, we examined cells expressing the Dd-STATaALApep mutant. The number of strongly stained nuclei is also increased to approximately double that in Dd-STATaWTpep-expressing cells (Figure 4A). In addition, we examined the distribution of endogenous Dd-STATa protein in the gskA null mutant. Again, an increased number of stained nuclei was observed (Figure 4A).
Fig. 4. GskA phosphorylation and nuclear localization. (A) Dictyostelium cells at 8 h development were fixed and immunostained for Dd-STATa. The number of stained nuclei is expressed as a percentage of the total number. In both a gskA mutant and cells expressing Dd-STATaΔGPR or Dd-STATaALApep, significantly higher numbers of stained nuclei are observed. (B–E) Confocal images of slug structures (formed after 16 h development) using anti-Dd-STATa antiserum: (B) STATa mutant; (C) STATa mutant cells expressing Dd-STATaWTpep; (D) STATa mutant cells expressing Dd-STATaΔGPR; (E) STATa mutant cells expressing Dd-STATaALApep. (F) A confocal image of a 16 h structure from a gskA mutant using anti-Dd-STATa antiserum; these cells rarely form slug structures, but form an elongated finger-like projection.
These observations suggest that during early development GskA phosphorylation of Dd-STATa inhibits its nuclear accumulation. This is not, however, the case during the later stages of development. After tip formation, Dd-STATaΔGPR- and Dd-STATaALApep-expressing cells exhibit the same pattern of nuclear staining as wild-type or Dd-STATaWTpep-expressing cells (Figure 4C–E). Enriched nuclear staining is, however, retained in all gskA mutant cells during these later developmental stages (Figure 4F). The reason for this difference is unclear, but could be an indirect consequence of the aberrant cell type patterning that occurs in the gskA mutant.
Aspartate for serine substitutions within the GPR reduce nuclear localization
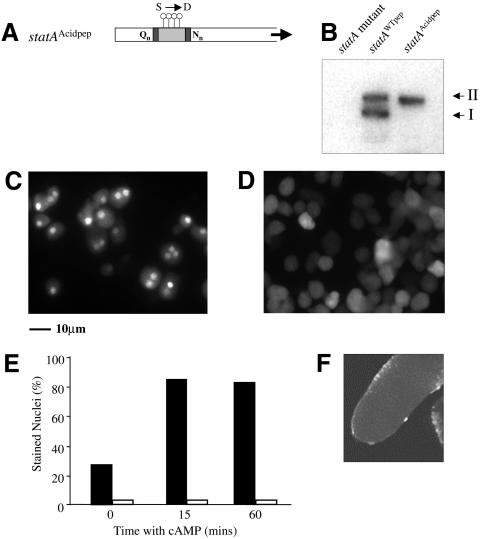
Phosphorylation increases the net negative charge of a protein and in some cases its effects can be mimicked by substitution of the target amino acid with an acidic residue. We made an acidified GPR by replacing the serines of each GskA site with aspartate residues (Figure 5A). This protein was expressed in a STATa null mutant and its behaviour examined. As expected for a protein lacking its GskA phosphorylation sites, this mutant, Dd-STATaAcidpep, has only a single mobility form (Figure 5B). Dd-STATaAcidpep protein, however, is slower migrating than the Dd-STATaALApep mutant and has a mobility that is close to that of the GSK-3 phosphorylated form. The mutant Dd-STATaAcidpep protein was detected using antiserum specific for the tyrosine-phosphorylated form of Dd-STATa and the kinetics of this tyrosine phosphorylation appeared to be the same as those of wild-type protein (data not shown). In contrast to cells that express other mutant Dd-STATa proteins, we found very few nuclei in which Dd-STATaAcidpep protein had accumulated after 8 h development (Figure 5C and D). To confirm that this is a cell autonomous effect, we incubated cells after 8 h development with 1 mM cAMP. This chronic application of cAMP induces nuclear staining in nearly all Dd-STATaWTpep cells. However, even after prolonged cAMP stimulation, we found no accumulation of Dd-STATaAcidpep in the nucleus (Figure 5E).
Fig. 5. Ser→Asp substitution within the GPR. (A) A peptide sequence in which the serine residues of the GSK-3 site are replaced by aspartates was inserted into Dd-STATaΔGPR to make Dd-STATaAcidpep. (B) Western analysis indicates that a Dd-STATa protein with the aspartate substitutions is present as a single band with reduced mobility. (C and D) The distribution of tyrosine-phosphorylated Dd-STATa was examined in disaggregated cells after 8 h development. Dd-STATaWTpep cells (C) exhibit strong nuclear staining, whereas Dd-STATaAcidpepcells (D) show little distinct nuclear staining, but in many cases show strong cytoplasmic staining. (E) Disaggregated cells after 8 h development were treated with 5 mM cAMP. This increased the number of Dd-STATaWTpep cells with nuclear staining but had no effect on Dd-STATaAcidpep cells. (F) A confocal image of a slug structure formed by Dd-STATaAcidpep cells using anti-Dd-STATa antiserum. Very few nuclei are stained in comparison with Dd-STATaWTpep (see Figure 4C).
It is important to note that the nucleus is not devoid of Dd-STATa staining, indicating that some Dd-STATaAcidpep does enter the nucleus. Interestingly, Dd-STATaAcidpep transformed cells exhibit higher levels of cytoplasmic staining than wild-type cells (Figure 5D). As an antiserum specific for tyrosine-phosphorylated Dd-STATa was used in these experiments, this cytoplasmic accumulation represents the tyrosine-phosphorylated form of Dd-STATa. Examination of Dd-STATaAcidpep at later developmental stages demonstrates that the failure to accumulate Dd-STATa in the nucleus persists during development (Figure 5F).
The GPR sequence regulates nuclear export
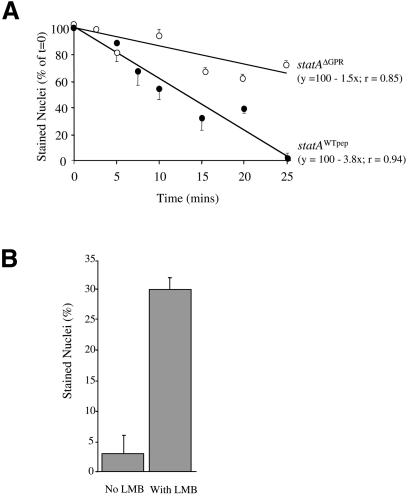
The GPR sequence could, in principle, regulate nuclear accumulation of Dd-STATa by either reducing the rate of nuclear import or increasing the rate of nuclear export. Two experiments indicate that the GPR enhances nuclear export. In the first experiment, cells were developed in shaking suspension for 8 h and then stimulated with 1 mM cAMP to induce maximal nuclear enrichment of Dd-STATa. cAMP was then removed and the decrease in nuclear staining monitored by immunocytochemistry. After cAMP removal, Dd-STATa is progressively lost from the nuclei of cells expressing Dd-STATaWtpep, with a half-maximal number of cells with stained nuclei reached after ∼15 min (Figure 6A). The rate of nuclear exit from cells expressing Dd-STATaΔGPR is 2.5 times lower, with a half-maximal number of cells with stained nuclei reached after ∼40 min (Figure 6A). Removal of the GPR therefore reduces the export rate of Dd-STATa.
Fig. 6. GskA regulates nuclear export of Dd-STATa. (A) Cells were developed in shaking culture for 8 h and Dd-STATa nuclear localization induced by treatment with 5 mM cAMP for 15 min. cAMP was removed and timed samples were fixed and immunostained for Dd-STATa. The rate of loss is plotted as stained nuclei, expressed as a percentage of the initial number, against the elapsed time after cAMP removal. Linear regression was used to fit each line; equations and correlation coefficients are shown in parentheses. (B) Dd-STATaAcidpep-expressing cells were developed for 8 h and then incubated with cAMP in the presence or absence of the nuclear export inhibitor LMB (20 nM). This treatment leads to nuclear localization, indicating that Dd-STATaAcidpep exits the nucleus at a higher rate.
None of our GPR mutants appears to affect the tyrosine phosphorylation of Dd-STATa and we find no apparent difference between the loss of tyrosine phosphorylation after cAMP removal for Dd-STATaWtpep and Dd-STATaΔGPR mutants (data not shown). This suggests that GskA phosphorylation may affect the interaction of Dd-STATa with the nuclear export machinery. To address this question, we further investigated the behaviour of Dd-STATaAcidpep, as failure to accumulate in the nucleus could be due to a high rate of nuclear export. Cells were treated with the nuclear export inhibitor leptomycin B (LMB), which in many species binds and inhibits the protein CRM-1/exportin 1 (Fornerod et al., 1997; Fukuda et al., 1997; Ossareh-Nazari et al., 1997; Stade et al., 1997). To date, the use of LMB has not been reported in Dictyostelium; however, we confirmed that the Dictyostelium homologue of CRM-1/exportin 1 contains the conserved cysteine that is alkylated by LMB (Kudo et al., 1999), thus making it LMB sensitive. Cells expressing Dd-STATaAcidpep were developed in shaking culture for 8 h and then induced with 1 mM cAMP for 1 h in the presence or absence of LMB. Exposure to LMB increased the number of cells with nuclear localized Dd-STATaAcidpep from 2 to 30% (Figure 6B). This dramatic increase suggests that, in the absence of LMB, Dd-STATaAcidpep enters the nucleus but is rapidly exported back to the cytoplasm. It was not possible to obtain a degree of nuclear localization >30%, as concentrations of >20 nM LMB inhibited nuclear import. Taken together, these two sets of experiments suggest very strongly that GskA phosphorylation of GPR enhances nuclear export.
GPR is not a nuclear export signal
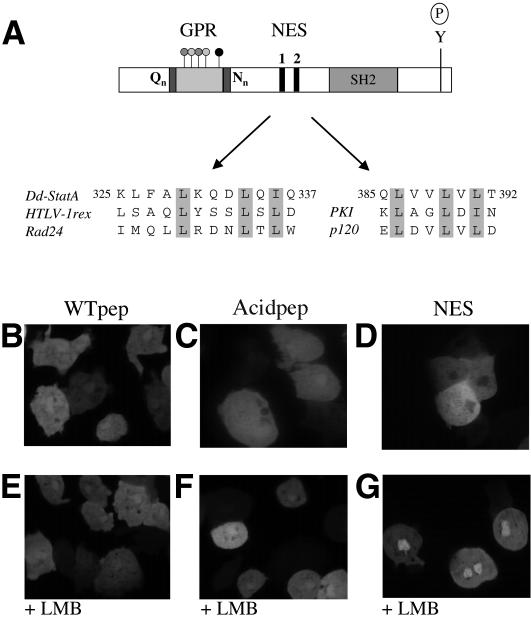
The GPR sequence does not conform to that of any known nuclear export signal (NES) (Figures 1A and 7A), but to examine whether the GPR may nonetheless possess NES activity we fused it to green fluorescent protein (GFP). GFP is a small protein that is able to enter the nucleus by diffusion and it hence equilibrates throughout the cell: although in our hands a slight nuclear enrichment is seen (data not shown). Fusion of either the wild-type or the aspartate-substituted form of the STAT peptide to GFP had no effect on its nuclear localization (Figure 7B and C). This argues that the GPR sequence alone does not have intrinsic NES activity. We examined the rest of the Dd-STATa sequence for NES sequences. These have a distinctive pattern of leucine residues, although substitutions by isoleucine and valine residues are permitted (van Hengel et al., 1999). Two potential NES sequences were found in the Dd-STATa sequence. The first (at positions 329–336) conforms to the less frequent LXXXLXL pattern seen in the NES sequences of HTLV-1rex and Rad24, but contains an isoleucine substitution for the third leucine (Figure 7A). The second (at positions 386–391) conforms to the more general LXXLXL pattern seen for the majority of NES sequences (Figure 7A). A region of Dd-STATa (positions 288–444) containing both potential NES sequences was fused to GFP. In contrast to STAT peptide–GFP fusions, this protein was excluded from the nucleus (Figure 7D). Blocking nuclear export with LMB caused nuclear accumulation of this GFP fusion protein (Figure 7E–G), suggesting that this region also contains a weak nuclear import signal. No nuclear accumulation of GFP was observed for the STAT peptide–GFP fusions when the cells were treated with LMB (Figure 7E and F). These results indicate the presence of NES sequences within Dd-STATa that utilize an exportin/CRM-1-dependent nuclear export mechanism but that are distinct from the GPR.
Fig. 7. The Dd-STATa NES is separated from the GPR. (A) Schematic diagram showing the relative position of the GPR and two putative NESs within Dd-STATa. An alignment is shown for each NES with those established for other proteins. Numbering corresponds to the amino acid position within the Dd-STATa protein. (B–G) Living cells expressing GFP fusions with the GPR, Wtpep (B and E) and Acidpep (C and F) or the region of Dd-STATa that contains both NESs. Both GPR–GFP fusions are present in both the cytoplasm and nucleus, although they are excluded from vesicles and the contractile vacuole. The NES–GFP fusion is excluded from the nucleus (D). Treatment with LMB (20 nM) has no effect on cells expressing GPR–GFP fusions (E and F), but causes nuclear accumulation of the NES–GFP fusion (G).
Discussion
Dd-STATa is a substrate of GSK-3
We have identified a sequence within Dd-STATa that contains several overlapping GSK-3 phosphorylation sites, which we named the GPR. Biochemical analysis demonstrates that this sequence is an excellent substrate for GskA. These results explain the observations of Araki et al. (1998), who discovered a lower mobility form of the Dd-STATa protein. Our analysis indicates that this lower mobility form is generated by GskA phosphorylation, strongly suggesting that Dd-STATa is an in vivo substrate for GskA. The incorporation of up to eight phosphate moieties at this position could explain the significant change in mobility observed by SDS–PAGE. This is the first target of GskA to be positively identified in Dictyostelium and this is, to our knowledge, the first report of GSK-3 phosphorylation of a STAT protein, increasing the list of nuclear targets known to be phosphorylated by GSK-3.
Phosphorylation by GskA enhances nuclear export of Dd-STATa
Removal of the GPR reduces the rate of nuclear export and increases the number of cells with nuclear enrichment of Dd-STATa during early development. This effect is not, however, an absolute block as even in its absence Dd-STATa protein does eventually exit the nucleus. This indicates that other mechanisms also act on Dd-STATa and the role of the GPR is to enhance an intrinsic level of nuclear export. Specific mutation of the GskA phosphorylation sites within the GPR has the same effect as its removal. Furthermore, serine to aspartate substitution within the GPR creates a protein that accumulates in the cytoplasm rather than the nucleus, the opposite behaviour to GPR removal. The behaviour of the aspartate substitution mutant can be reversed by inhibition of nuclear export using LMB, indicating that the mutant enters the nucleus but is then rapidly exported. Collectively, these observations argue strongly that GskA phosphorylation is required for the effect of the GPR on nuclear export of Dd-STATa.
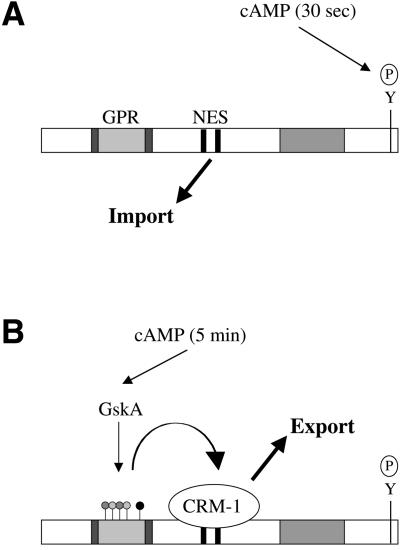
GSK-3 phosphorylation has previously been shown to regulate nuclear export of NF-ATc and cyclin D1 (Beals et al., 1997; Diehl et al., 1998), but the mechanism by which GSK-3 regulates nuclear export of these proteins is unclear. Our results suggest a mechanism for Dd-STATa. The GPR sequence does not contain a leucine-rich NES motif and we can find no evidence for it acting as an NES by itself. As fusions between the STAT peptide and GFP are not exported from the nucleus, we can also eliminate the role of an additional protein that possesses a NES and promotes export by binding to the Dd-STATa GPR, as seen when the 14.3.3 protein Rad24p binds to Cdc25p after Chk1 phosphorylation (Lopez-Girona et al., 1999). We have identified two potential NES sequences within Dd-STATa, and the GPR could interact, either positively or negatively, with one or both of these. We can, however, exclude the possibility of the GPR ‘masking’ the activity of these NES sequences because GPR removal leads to reduced rather than enhanced nuclear export. The mechanism we favour is for phosphorylation of the GPR by GskA to cause an intramolecular change within Dd-STATa that enhances its intrinsic NES activity (Figure 8).
Fig. 8. A scheme for GskA regulation of Dd-STATa. (A) Stimulation by extracellular cAMP leads to rapid (within 30 s) tyrosine phosphorylation of Dd-STATa and nuclear import. (B) cAMP stimulation also leads to slower (>5 min) phosphorylation at the GPR by GskA. This causes a conformational change that enhances exportin/CRM-1 binding to the NES and nuclear export.
A complex regulation of Dd-STATa by extracellular cAMP
The appearance of a lower mobility form of STATa correlates well with the regulation of GskA activity, suggesting that GskA is the major kinase active on this site. GskA activity has previously been shown to be both developmentally regulated and to be induced by extracellular cAMP. The pattern of GskA activation matches that of the appearance of the lower mobility form of Dd-STATa; it increases during early development (6–10 h) and occurs after 5 min of cAMP stimulation.
The two effects of extracellular cAMP stimulation on Dd-STATa suggest a biphasic regulation of nuclear translocation. Extracellular cAMP rapidly stimulates tyrosine phosphorylation of Dd-STATa and promotes its nuclear import. Activation of GskA and phosphorylation of the GPR sequence promote nuclear export with slower kinetics. Under chronic cAMP stimulation, this would be expected to drive a dynamic equilibrium, with Dd-STATa molecules shuttling between the cytoplasm and nucleus. The major developmental activation of GskA is via carC, which accounts for >80% of the GskA activation seen at the mound stage (Plyte et al., 1999). This receptor has a slightly lower affinity than carA (Johnson et al., 1992), the receptor shown to mediate tyrosine phosphorylation of Dd-STATa, and this may lead to differential regulation of Dd-STATa nuclear import and export with varying external cAMP concentrations. carC is also differentially expressed during multicellular stages of development (Yu and Saxe, 1996), suggesting further possibilities for the regulation of Dd-STATa translocation.
Although our observations clearly demonstrate a role of GskA in the regulation of Dd-STATa, mutations of the GPR alone have very little effect on gene expression or morphogenesis. All of the Dd-STATa GPR mutants described in this report are able to rescue the Dd-STATa null mutant to give apparent wild-type patterns of gene expression and development, at least under standard laboratory conditions (data not shown). In part, this may reflect the additional complexity we observe in the regulation of Dd-STATa during later stages of development. We find that elimination of the GskA phosphorylation sites only affects nuclear enrichment of Dd-STATa during early developmental stages, arguing for additional mechanisms that affect nuclear translocation during later development. One such mechanism could be stimulation of nuclear import, an obvious prerequisite for export. As stimulation by extracellular cAMP is required for tyrosine phosphorylation and nuclear import, reduced signalling would negate the effects of loss of GskA phosphorylation. Preliminary evidence suggests that there is indeed a reduced level of extracellular cAMP in the posterior part of the slug and that this is responsible for the lower levels of nuclear Dd-STATa observed in the cells of this region (T.Abe, D.Dorman, K.Weijer and J.G.Williams, unpublished observation).
As no nuclear enrichment of the aspartate-substituted GPR is seen in the slug tip, GskA regulation of Dd-STATa is likely to be present throughout development. This means that the reduced amount of Dd-STATaAcidpep in the nucleus at any given time is either sufficient for development or that Dd-STATa is functionally redundant to other STAT proteins. The most significant role for GskA phosphorylation of the GPR could be in early development when GskA activity levels begin to rise during late aggregation. Preliminary observations suggest that under certain conditions our Dd-STATa GPR mutants may affect cell adhesion (data not shown). Finally, these observations indicate that failure to phosphorylate Dd-STATa is not a major cause of the strong phenotype observed for the loss of gskA (Harwood et al., 1995).
GSK-3 and STAT signal transduction
Our results demonstrate an interaction between GSK-3- and STAT-mediated signal transduction in Dictyostelium and raise the possibility that similar interactions exist in metazoa. Although no direct interactions have been reported, there are a number of potential situations where they may occur. In Drosophila both the Wg and JAK-STAT pathways act in parallel to establish cell polarity in the developing eye (Strutt and Strutt, 1999). Both pathways converge to regulate expression of the four jointed gene (Zeidler et al., 1999) and hence could directly interact within the four jointed promoter. Components of the Wnt-1 pathway are active during mammalian lymphocyte development. In mammalian lymphocytes the transcription factors LEF-1 and TCF-1 regulate transcription and, consistent with an active Wnt-1 pathway, they form nuclear complexes with β-catenin (Schilham and Clevers, 1998; Prieve and Waterman, 1999; Staal et al., 1999). Furthermore, T-cell activation leads to a decrease in GSK-3 activity (Staal et al., 1999). These events alone, however, are not sufficient to regulate transcription in native peripheral T lymphocytes, suggesting the requirement for additional signal pathways (Prieve and Waterman, 1999). Further support for a role of GSK-3 signalling in lymphocytes is suggested by frequent loss of the GSK-3 inhibitor FRAT-1 in lymphoblastomas (Yost et al., 1998) and by regulation of the lymphoid transcription factor NF-ATc by GSK-3 (Beals et al., 1997; Diehl et al., 1998). GSK-3 therefore is situated at a potentially significant junction point in the signal transduction network of lymphocytes. Extrapolation of our results suggests that STAT transcription factors could be an additional component of this signalling network. In support of this interaction, potential GSK-3 phosphorylation sites are present in the N-termini of mammalian STATs 4, 5B and 6 and in the Drosophila STAT homologue Marelle (A.J.Harwood, unpublished observations). There are, however, no reports to date of serine phosphorylation in the N-terminal region of a metazoan STAT molecule.
In summary, we have demonstrated that Dd-STATa is a substrate for GSK-3 in Dictyostelium and that its phosphorylation enhances its nuclear export. This form of regulation is consistent with the paradigm established for NF-ATc, whereby GSK-3 phosphorylation regulates the rate of nuclear export. As both tyrosine phosphorylation and GSK-3 activity are determined by extracellular cAMP stimulation, this suggests a complex regulation of Dd-STATa nuclear translocation.
Materials and methods
Growth and development of cells
Wild-type (DH1) and mutant (Dd-STATa and gskA) strains were grown in HL5 medium at 22°C (Watts and Ashworth, 1970). Cells were transformed by electroporation (Howard et al., 1988) and grown in HL5 medium supplemented with 20 µg/ml G418. For development, cells growing at 2–3 × 106/ml were washed twice in KK2 (16.5 mM KH2PO4, 3.8 mM K2HPO4 pH 6.2) and either plated onto KK2 agar (KK2, 2% agar, 1 M MgSO4) or resuspended in KK2 (1 × 107 cells/ml) and shaken at 150 r.p.m. and 22°C for 8 h.
DNA constructs
PCR was carried out on the Dd-STATa cDNA using the primers 5′-CCGCTCGAGCGGTTAACTATTTAAAGGTTCATAACCAC-3′ and 5′-GCATATGGTAACAACAACAATAGTAATAATACTTCATCA-3′. This reaction incorporated a NdeI restriction enzyme fragment at nucleotide 941 and a XhoI site at nucleotide 2634, immediately 3′ of the termination codon. This fragment was subcloned into NdeI and XhoI double-digested pAct15-STATa (Mohanty et al., 1999) to create a deletion mutant of Dd-STATa (Dd-STATaΔGPR) that lacks amino acids 112–146 (the GPR). The deletion site contained a NdeI restriction enzyme into which a double-stranded oligonucleotide could be subcloned. Oligonucleotides encoding wild-type and mutant versions of the STAT peptide were inserted to give constructs that express Dd-STATaWtpep, Dd-STATaALApep or Dd-STATaAcidpep from the actin15 promoter.
GFP was also expressed from the actin15 promoter. Constructs for expression of GFP fusion proteins were constructed by an in-frame insertion into an EcoRI restriction site 5′ of the gfp gene. WTpep–GFP and Acidpep–GFP fusions encoded the sequences HMSQTPSSP LSSPLPSPTPF and HMDQTPDDPLDDPLPDPTPF, respectively. The Dd-STATa–NES fusion encoded protein sequence from position 288 to 444.
Measuring GSK-3 activity
Cell extracts were prepared from 1 × 109 Dictyostelium cells by sonication/lysis (3 × 5 s bursts on ice with a Vibratome probe sonicator at setting 4) in homogenization buffer [25 mM Tris–HCl pH 7.5, 250 mM sucrose, 10 mM EGTA, 2 mM EDTA, 100 µg/ml leupeptin, 0.1 U/ml aprotinin, 10 mM dithiothreitol, 1 µM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 100 µM sodium vanadate, 10 mM benzamidine] and centrifugation at 100 000 g (30 min). The supernatant was loaded onto a base/acid-treated phosphocellulose P11 column (10 ml volume) equilibrated in buffer A (25 mM Tris–HCl pH 8.0, 40°C) connected to an FPLC apparatus (Pharmacia). After washing with buffer A, a linear salt gradient was initiated with buffer A + 500 mM NaCl and 1 ml fractions collected. The GSK-3 activity assay was performed on each fraction as described in Ryves et al. (1998) using synthetic peptide substrates (Zinsser Analytical Ltd). Assays were conducted in duplicate and means ± SEM of separate experiments are shown. Peptides were separated by Tricine–SDS gel electrophoresis as described by Schagger and von Jagow (1987). GSK-3α and GSK-3β were supplied from Upstate Biotechnology and New England Biolabs, respectively.
Western analysis
Cells were lysed at 1 × 107cells/ml in NP-40 buffer (phosphate-buffered saline pH 7.4, 50 mM sodium fluoride, 1% NP-40, 2 mM EDTA pH 7.2, 1 mM sodium pyrophosphate, 1.6 µg/ml leupeptin, 4 µg/ml aprotinin, 10 mM benzamidine, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride) and cleared by centrifugation. Supernatant equivalent to 5 × 105 cells was separated by 7.5% SDS–PAGE and transferred to nitrocellulose (Hybond-C Extra; Amersham Pharmacia Biotech) using standard procedures. Membranes were blocked overnight at 4°C in 10% milk in 1× TBS-T (0.02 M Tris, 0.137 M NaCl, 0.05% Triton X-100 pH 7.6), washed in 1× TBS-T and incubated at 4°C overnight with a 1:500 dilution of affinity-purified SC7-pTyr antiserum, which is specific for tyrosine-phosphorylated Dd-STATa (Araki et al., 1998). Membranes were washed in 1× TBS-T and incubated with horseradish peroxidase-coupled anti-rabbit IgG (1:1000) for 1 h at room temperature. Antibody binding was visualized by chemiluminescence (Supersignal; Pierce & Warriner Ltd). Total STATa was detected using the monoclonal antibody D4 (Araki et al., 1998) and GskA by a monoclonal antibody raised against the Shaggy protein (Harwood et al., 1995).
Immunohistochemical staining
Specimens were fixed in cold methanol, then rehydrated for 5 min in 70% methanol/KK2, followed by 30 min in KK2 containing 10 mg/ml bovine serum albumin. Specimens were incubated overnight at 4°C with a 1:200 dilution in KK2 of affinity-purified SC7-pTyr antiserum, followed by 1:500 anti-rabbit IgG–Texas red for 4 h at room temperature. Specimens were dehydrated using 70% ethanol followed by 100% ethanol and then mounted in Mowiol. Fluorescence microscopy was carried out using either a Nikon Optiphot II confocal microscope with Bio-Rad Lasersharp software or a Zeiss Axioskop fluorescence microscope with an attached Sony camera.
Nuclear export assay
Cells were developed for 8 h in shaking culture and then treated with 1 mM cAMP for 15 min. Cells were allowed to settle onto microscope cells, washed twice in KK2 to remove the cAMP and fixed in cold methanol at timed intervals. Samples were stained using D4, an anti-DdSTATa monoclonal antibody, and scored for nuclear staining. The numbers of cells with stained nuclei were normalized so that the cell count at 2.5 min was taken as 100% and linear regression lines fitted using the program StatView 5.0.1 (SAS Institute Inc., Cary, NC).
Acknowledgments
Acknowledgements
This work was supported by an MRC project grant (G9412338MB) to R.S.G., a Wellcome project grant (048928) to W.J.R., a Wellcome Trust Programme Grant (050.801709) to J.G.W. and a Wellcome Trust Senior Fellowship to A.J.H. Leptomycin B was a kind gift of Dr Sonia Lane.
References
- Araki T., Gamper,M., Early,A., Fukuzawa,M., Abe,T., Kawata,T., Kim,E., Firtel,R.A. and Williams,J.G. (1998) Developmentally and spatially regulated activation of a Dictyostelium STAT protein by a serpentine receptor. EMBO J., 17, 4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals C.R., Sheridan,C.M., Turck,C.W., Gardner,P. and Crabtree,G.R. (1997) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science, 275, 1930–1934. [DOI] [PubMed] [Google Scholar]
- Binari R. and Perrimon,N. (1994) Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev., 8, 300–312. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., Smeal,T., Defize,L.H., Angel,P., Woodgett,J.R., Karin,M. and Hunter,T. (1991) Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell, 64, 573–584. [DOI] [PubMed] [Google Scholar]
- Cadigan K.M. and Nusse,R. (1997) Wnt signaling: a common theme in animal development. Genes Dev., 11, 3286–3305. [DOI] [PubMed] [Google Scholar]
- Diehl J.A., Cheng,M., Roussel,M.F. and Sherr,C.J. (1998) Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev., 12, 3499–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol C.J., Williams,J.S., Chou,C.H., Wang,Q.M., Roach,P.J. and Andrisani,O.M. (1994) A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J. Biol. Chem., 269, 32187–32193. [PubMed] [Google Scholar]
- Fornerod M., Ohno,M., Yoshida,M. and Mattaj,I.W. (1997) CRM1 is an export receptor for leucine-rich nuclear export signals. Cell, 90, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Asano,S., Nakamura,T., Adachi,M., Yoshida,M., Yanagida,M. and Nishida,E. (1997) CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature, 390, 308–311. [DOI] [PubMed] [Google Scholar]
- Harrison D.A., Binari,R., Nahreini,T.S., Gilman,M. and Perrimon,N. (1995) Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J., 14, 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood A.J., Plyte,S.E., Woodgett,J., Strutt,H. and Kay,R.R. (1995) Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell, 80, 139–148. [DOI] [PubMed] [Google Scholar]
- Howard P.K., Ahern,K.G. and Firtel,R.A. (1988) Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res., 16, 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J.N. and Kerr,I.M. (1995) Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet., 11, 69–74. [DOI] [PubMed] [Google Scholar]
- Johnson R.L., Van Haastert,P.J., Kimmel,A.R., Saxe,C.L.,III, Jastorff,B. and Devreotes,P.N. (1992) The cyclic nucleotide specificity of three cAMP receptors in Dictyostelium. J. Biol. Chem., 267, 4600–4607. [PubMed] [Google Scholar]
- Kawata T., Shevchenko,A., Fukuzawa,M., Jermyn,K.A., Totty,N.F., Zhukovskaya,N.V., Sterling,A.E., Mann,M. and Williams,J.G. (1997) SH2 signaling in a lower eukaryote: a STAT protein that regulates stalk cell differentiation in Dictyostelium. Cell, 89, 909–916. [DOI] [PubMed] [Google Scholar]
- Kim L., Liu,J. and Kimmel,A.R. (1999) The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell, 99, 399–408. [DOI] [PubMed] [Google Scholar]
- Kudo N., Matsumori,N., Taoka,H., Fujiwara,D., Schreiner,E.P., Wolff,B., Yoshida,M. and Horinouchi,S. (1999) Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA, 96, 9112–9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A., Furnari,B., Mondesert,O. and Russell,P. (1999) Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature, 397, 172–175. [DOI] [PubMed] [Google Scholar]
- Mohanty S., Jermyn,K.A., Early,A., Kawata,T., Aubry,L., Ceccarelli,A., Schaap,P., Williams,J.G. and Firtel,R.A. (1999) Evidence that the Dictyostelium Dd-STATa protein is a repressor that regulates commitment to stalk cell differentiation and is also required for efficient chemotaxis. Development, 126, 3391–3405. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Bachelerie,F. and Dargemont,C. (1997) Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science, 278, 141–144. [DOI] [PubMed] [Google Scholar]
- Plyte S.E., Hughes,K., Nikolakaki,E., Pulverer,B.J. and Woodgett,J.R. (1992) Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim. Biophys. Acta, 1114, 147–162. [DOI] [PubMed] [Google Scholar]
- Plyte S.E., O’Donovan,E., Woodgett,J.R. and Harwood,A.J. (1999) Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development, 126, 325–333. [DOI] [PubMed] [Google Scholar]
- Prieve M.G. and Waterman,M.L. (1999) Nuclear localization and formation of β-catenin–lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol. Cell. Biol., 19, 4503–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel L., Bourouis,M., Heitzler,P., Pantesco,V. and Simpson,P. (1993) Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature, 362, 557–560. [DOI] [PubMed] [Google Scholar]
- Ryves W.J., Fryer,L., Dale,T. and Harwood,A.J. (1998) An assay for glycogen synthase kinase 3 (GSK-3) for use in crude cell extracts. Anal. Biochem., 264, 124–127. [DOI] [PubMed] [Google Scholar]
- Schagger H. and von Jagow,J. (1987) Tricine–SDS PAGE for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem., 166, 368–397. [DOI] [PubMed] [Google Scholar]
- Schilham M.W. and Clevers,H. (1998) HMG box containing transcription factors in lymphocyte differentiation. Semin. Immunol., 10, 127–132. [DOI] [PubMed] [Google Scholar]
- Schindler C. and Darnell,J.E.,Jr (1995) Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem., 64, 621–651. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Wilder,E.L. and Perrimon,N. (1994) Components of Wingless signaling in Drosophila. Nature, 367, 76–80. [DOI] [PubMed] [Google Scholar]
- Staal F.J., Burgering,B.M., van de Wetering,M. and Clevers,H.C. (1999) Tcf-1-mediated transcription in T lymphocytes: differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int. Immunol., 11, 317–323. [DOI] [PubMed] [Google Scholar]
- Stade K., Ford,C.S., Guthrie,C. and Weis,K. (1997) Exportin 1 (Crm1p) is an essential nuclear export factor. Cell, 90, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Stephens J.M., Lumpkin,S.J. and Fishman,J.B. (1998) Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M and interferon-γ in adipocytes. J. Biol. Chem., 273, 31408–31416. [DOI] [PubMed] [Google Scholar]
- Strutt H. and Strutt,D. (1999) Polarity determination in the Drosophila eye. Curr. Opin. Genet. Dev., 9, 442–446. [DOI] [PubMed] [Google Scholar]
- Thomas G.M., Frame,S., Goedert,M., Nathke,I., Polakis,P. and Cohen,P. (1999) A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and β-catenin. FEBS Lett., 458, 247–251. [DOI] [PubMed] [Google Scholar]
- van Hengel J., Vanhoenacker,P., Staes,K. and van Roy,F. (1999) Nuclear localization of the p120(ctn) Armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc. Natl Acad. Sci. USA, 96, 7980–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.J. and Ashworth,J.M. (1970) Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J., 119, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh G.I., Patel,J.C. and Proud,C.G. (1997) Peptide substrates suitable for assaying glycogen synthase kinase-3 in crude cell extracts. Anal. Biochem., 244, 16–21. [DOI] [PubMed] [Google Scholar]
- Wen Z. and Darnell,J.E.,Jr (1997) Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res., 25, 2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.G., Duffy,K.T., Lane,D.P., McRobbie,S.J., Harwood,A.J., Traynor,D., Kay,R.R. and Jermyn,K.A. (1989) Origins of the prestalk–prespore pattern in Dictyostelium development. Cell, 59, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Yost C., Farr,G.H., Pierce,S.B., Ferkey,D.M., Chen,M.M. and Kimelman,D. (1998) GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell, 93, 1031–1041. [DOI] [PubMed] [Google Scholar]
- Yu Y. and Saxe,C.L., (1996) Differential distribution of cAMP receptors cAR2 and cAR3 during Dictyostelium development. Dev. Biol., 173, 353–356. [DOI] [PubMed] [Google Scholar]
- Zeidler M.P., Perrimon,N. and Strutt,D.I. (1999) The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr. Biol., 9, 1363–1372. [DOI] [PubMed] [Google Scholar]