Abstract
Cinnomomum iners standardized leaves methanolic extract (CSLE) was subjected to analgesic, toxicity and phytochemical studies. The analgesic activity of CSLE was evaluated using formalin, hot plate and tail flick tests at doses of 100, 200 and 500 mg/kg. CSLE showed significant activity (P < 0.05) in the formalin model (late phase) on the rats at doses of 200 and 500 mg/kg. However, CSLE did not show activity in the hot plate and tail flick tests. The results obtained suggest that CSLE acts peripherally to relieve pain. For the toxicity study, CSLE was orally administered to the Swiss albino mice according to the Organization for Economic Co-Operation and Development (OECD) guideline 423. There was no lethality or toxic symptoms observed for all the tested doses throughout the 14-day period. Phytochemical screening of CSLE showed the presence of cardiac glycoside, flavonoid, polyphenol, saponin, sugar, tannin and terpenoid.
Keywords: Analgesic, Cinnomomum iners, toxicity
INTRODUCTION
For centuries, medicinal plants are the basis for the treatment of various diseases.[1] Nearly 80% of people living in developing countries still depend on plant-based traditional medicine for their primary health care and almost three-fourths of the herbal drugs used worldwide are derived from medicinal plants.[2] However, the quality control of herbal medicine remains a challenge owing to the fact that there is a high variability in the active constituents involved.[3] Hence, World Health Organization (WHO) has approved fingerprint technique or standardized extract for quality assurance of herbal medicines.[4] In recent times, the discovery of the physchoactive compounds from plants has provided sufficient information regarding neurochemistry of many central nervous system disorders such as hallucinations, illusions and agnosia.[5] Thus, work has been undertaken in determining the psychoactivity of Cinnomomum iners standardized methanolic extract.
C. iners is a genus of evergreen trees and shrubs belonging to the family Lauraceae. According to Agroforestry Tree Database, C. iners is commonly found in India, Burma (Myanmar), Indo-China, Thailand, Peninsular Malaysia, Sumatra, Java, Borneo, Sulawesi and the southern Philippines. It is a medium-sized evergreen tree found throughout the lowlands and hill forests of Malaysia. It is locally known as kayu manis hutan, medang kemangi and teja with respect to the aroma of crushed leaves. The bitter taste of this plant is due to the presence of terpenes. Due to the bitter characteristic of this plant, it is widely used as a traditional medicine to relieve fever, for digestive system problems and for problems related to appetite.[6] In addition, this plant has been used to relieve headache, breathing problem and cough. The major bioactive compounds of this plant are saponin, terpene, cinnamic aldehyde and eugenol.[7] The ethnobotanical reports offer information on the medicinal properties of C. iners root extract that includes details on their antiplasmodial activity and cytotoxicity.[8] Furthermore, an amylase inhibitor property of this plant has been revealed.[9] Researchers from India have reported that the oil obtained from stem bark of C. iners contains 1,8-cineole as a major component that possesses antinociceptive and anti-inflammatory activities.[10] Recently, the antioxidant activity of essential oil of C. iners has been reported.[11] However, pharmacological activities and mode of action of the plant leaves are yet to be established. Hence, the objective of this study was to screen the chemical constituents, and to test the analgesic activity and toxicity of C. iners leaves methanolic extract.
MATERIALS AND METHODS
Chemicals
All reagents used were of analytical grade. Propylene glycol (propane-1,2-diol) and polysorbate-80 (Tween 80) were purchased from Fisher Scientific (Loughborough, UK). Beta-caryophyllene, aspirin, ferric chloride (FeCl3), thionyl chloride (SOCl2) and sodium chloride (NaCl) were purchased from Sigma Chemicals Co., LTD (St Louis, MO, USA). Morphine was obtained from Hospital Universiti Sains Malaysia (USM, Kelantan, Malaysia).
Plant material
C. inersleaves were collected from USM. The plant leaves were washed with water to remove dirt prior to the drying process. The leaves then crushed into a fine powder. An initial quality evaluation of the dried leaves was carried out to validate their authenticity and also to ensure quality by using techniques adopted from WHO Guidelines on Herbal Quality Control (WHO, 1998). The authenticity work was carried out by a botanist from School of Biological Sciences, USM, where the plant material was deposited. The voucher specimen number is 11014.
Standardization of C. iners leaves methanolic extract
Gas chromatography–mass spectrometry conditions
Standardization was carried out by using gas chromatography–mass spectrometry (GC–MS). HP-5MS column (30 m × 0.25 mm × 0.25 μm) was used. The inlet temperature was set at 220 °C and Mass Selective Detector (MSD) transferline heater was set at 225 °C. GC was performed in splitless mode. Flow rate of carrier gas (helium) was maintained at 1.0 ml/min. Initial temperature of oven was 80 °C and then ramped to 170 °C by 10 °C/min and held for 2 min.
C. iners leaves methanolic extract profiling
Powdered dried leaves (100 g) of the plant were macerated in methanol (500 ml) for 3 days. The extract was filtered and concentrated under reduced pressure at 40 °C in a rotary evaporator. The concentrated extract obtained was placed in the oven for 3 days at 40 °C to remove the remaining methanol. Sample solution (1 mg/ml) was prepared by dissolving in methanol. Sample (1 μl) was injected and the leaves’ profiles were acquired using the GC–MS. The peak identification and quantification were carried out using the retention time and mass spectrum provided by the MS library.
Quantification of beta-caryophyllene
For the standardization, chromatogram of C. iners leaves methanolic extract was analyzed. Beta-caryophyllene peak of C. iners leaves methanolic extract was identified using MS library. The peak of purchased standard reference (beta-caryophyllene) was matched with the chromatogram of C. iners leaves methanolic extract for confirmation. Calibration curve of standard reference beta-caryophyllene was prepared in different concentrations (1.5, 3.1, 6.25, 12.5, 25 and 50 ppm) by serial dilution of stock solution. All the concentrations were prepared in triplicate and peak area of each concentration was calculated as a mean of three independent samples. The amount of beta-caryophyllene in C. iners leaves methanolic extract was quantified using the peak area calculated from the equation that was obtained from the calibration curve.
Preliminary phytochemical studies
C. iners standardized leaves methanolic extract (CSLE) was subjected to preliminary phytochemical screening for the identification of chemical constituents according to Brindha and Saraswathy.[12] Essentially, alkaloid was tested with Dragendroff's reagent, cardiac glycosides with Salkowski test, phenols with FeCl3 and K3Fe(CN)6, flavonoids with NaCl and HCl, saponin using frothing test, tannins with FeCl3 and terpenoid with SOCl2.
Analgesic activity
Animals
Experimental animals were obtained from Animal House, USM. Swiss albino mice (20–25 g) and Sprague Dawley rats (200–250 g) were acclimatized to the laboratory for 1 week prior to the experiment. All the experimental animals had free access to water and food pellets. The animals were fasted overnight before use. The experimental procedures were approved by Animal Ethics Committee, USM.
Hot plate test
The method employed here was that of Woolfe and MacDonald.[13] Male Swiss albino mice were orally administered with CSLE (100, 200 and 500 mg/kg), cosolvent (propylene glycol:Tween 80:water = 4:1:4) and morphine (5 mg/kg s.c.). Cosolvent was used as control, whereas morphine was used as a standard. Mice weighing 20–25 g were placed in a hot plate maintained at 55 °C ± 1. A cut-off time of 45 s was selected to avoid tissue damage. The latency (licking or jumping) of nociceptive responses was recorded after 30, 45, 60, 75 and 90 min of administration of the three test groups except the group administered morphine (15 min after the administration).
Tail flick test
The tail flick test was performed according to the method previously described by D’Amour and Smith.[14] CSLE at doses of 100, 200 and 500 mg/kg was administered orally to the experimental group. Cosolvent which acts as control was given orally and morphine (standard) administered s.c. at 5 mg/kg. The tail flick responses (time taken to withdraw its tail from light beam) of mice were recorded by gently placing the mice tail in light beam. The cut-off time was 10 s to avoid tissue damage. The tail flick response was recorded at intervals of 30, 45, 60, 75 and 90 min (except for morphine where it was recorded at 15 min after administration).
Formalin test
Formalin test was done by using previously described method by Hunskaar et al.[15] and Miranda et al.[16] For this test, five rats per group were treated with different doses of CSLE, cosolvent and standard. CSLE was administered orally at doses of 100, 200 and 500 mg/kg. Aspirin (100 mg/kg) and morphine sulfate (5 mg/kg) were used as standards. After 30 min treatment of all tested drugs (15 min treatment for morphine), 20 μl of 2.5% formalin was s.c. injected in hind paw of rats. The time spent in licking the injected paw in early phase (0–5 min) and late phase (15–30 min) was recorded.
Acute toxicity
An acute toxicity study was carried out by using OECD guideline 423.[17] CSLE was administered orally to Swiss albino mice at doses of 300, 2000 and 5000 mg/kg. The behavioral changes (abdominal constriction, hyperactivity, sedation, grooming), mortality and body weight were observed for 14 days.
Statistical analysis
Data were expressed as mean ± SEM and were analyzed by Sigmastat 3.5 software program. Statistically significant difference between groups was calculated by the application of analysis of variance (ANOVA) followed by Dunnett's test. Tukey test was used for independent pairwise comparison between two groups. A difference was considered significant at P < 0.05.
RESULTS
Standardization of C. iners leaves methanolic extract
Analysis of the chromatogram
There were 24 common peaks in the chromatogram of C. iners leaves extract. The major peak was vitamin E with percentage area of 16.86%. Eleven peaks were identified as corresponding to hydrocarbons (32.86%), five peaks were identified as those of fatty acids (20.16%) and three peaks that of amino acids (12.83%). The rest of the peak was phytol (10.38%), beta-caryophyllene (1.46%) and stigmasterol (5.45%). Beta-caryophyllene and stigmasterol were identified as the unique bioactive compounds present in C. iners leaves extract. Stigmasterol reference standard was not commercially available, so beta-caryophyllene was chosen to be a marker compound for the standardization purpose.
Quantification of beta-caryophyllene
Beta-caryophyllene in C. iners leaves extract chromatogram was identified by matching of retention time and mass spectra provided by MS library and purchased marker standard (beta-caryophyllene) [Figures 1 and 2]. Beta-caryophyllene gives well-distinguished peaks at retention time of 6.078 minutes as depicted in Figure 1. As for the standardization, the quantitation of beta-caryophyllene was based on the peak area calculated from the calibration curve equation (y = 11544x – 15831, r2 = 0.997). The amount of beta-caryophyllene present was found to be 0.048 mg (4.8%) in 1 mg of C. iners leaves methanolic extract.
Figure 1.
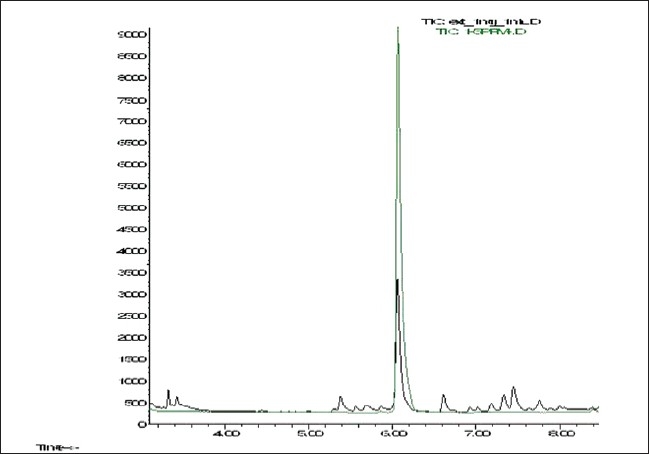
An overlay GC–MS chromatogram of C. iners extract and standard beta-caryophyllene
Figure 2.
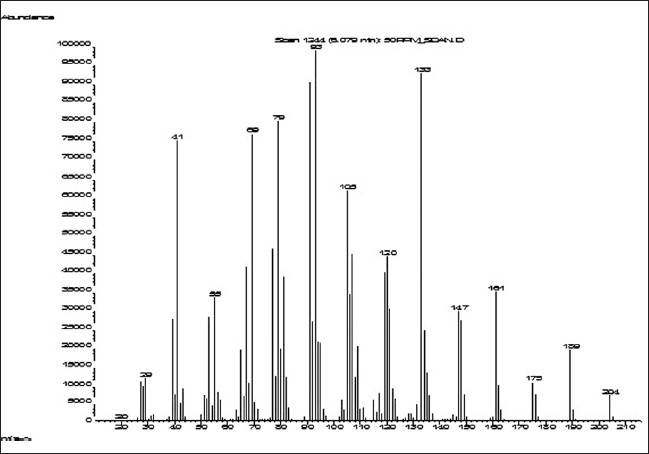
Mass spectrum of beta-caryophyllene
Acute toxicity study
CSLE at doses of 300, 2000 and 5000 mg/kg had no adverse effect on the behavioral responses of the tested mice up to 14 days of observation. There was no mortality observed at all the tested doses. None of the used doses affected the weight of the mice. The LD50 of CSLE was therefore estimated to be more than 5000 mg/kg. As the highest dose used in the analgesic study was 10-fold lesser than the dose used in acute toxicity study, it suggests that the doses of 100, 200 and 500 mg/kg given to the mice and rats in this study are very safe.
Analgesic activity
Hot plate test
CSLE was not capable of increasing the latency period of pain induced by heating of the plate. This result indicates that CSLE does not show any significant difference compared with control, at doses of 100, 200 and 500 mg/kg [Figure 3]. As expected, morphine (5 mg/kg) significantly prolonged the latency of nociceptive response at 30, 45 and 60 min.
Figure 3.
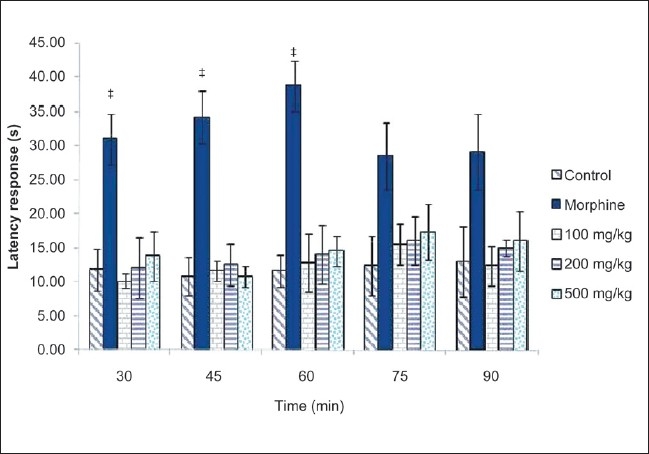
Effect of CLSE (100, 200 and 500 mg/kg) and morphine on the latency response of mice in the hot plate test. Values are presented as the mean ± SEM (n = 5) *P < 0.05, significant increase from control
Tail flick test
CSLE did not show any difference compared with control, at all the tested doses [Figure 4]. Morphine showed a significant increase of latency time at 45, 60 and 75 min.
Figure 4.
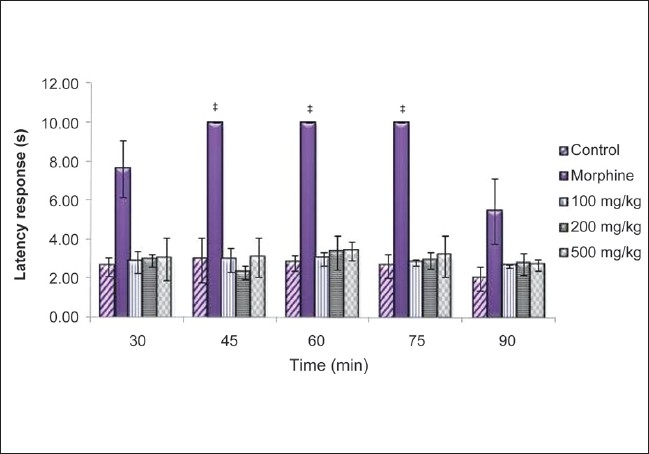
Effect of CSLE (100, 200 and 500 mg/kg) and morphine on the latency response of mice in the tail flick test. Values are presented as the mean ± SEM (n = 5) *P < 0.05, significant increase from control
Formalin test
CSLE at doses of 200 and 500 mg/kg showed significant decrease compared with control at late phase [Figure 5]. CSLE possessed analgesic activity in a dose-dependent manner in the formalin test. A nonsteroidal anti-inflammatory drug (NSAID), aspirin, showed significant activity at inflammatory phase (late phase). As expected, morphine inhibited both early and late phases.
Figure 5.
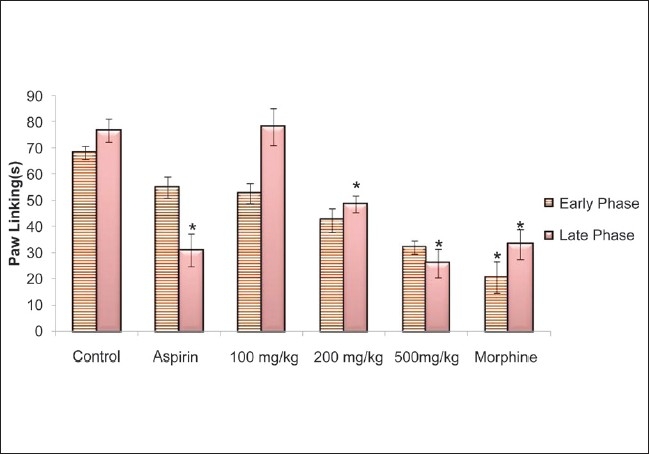
Effect of CLSE (100, 200 and 500 mg/kg), morphine and aspirin on the reaction time of rat in the formalin test. Values are presented as the mean ± SEM (n = 5) *P < 0.05, significant increase from control, early phase (0–5 min) and late phase (15–30 min)
Qualitative phytochemical study
The result of qualitative phytochemical screening is presented in Table 1. CSLE showed abundant occurrence of chemical constituents such as polyphenol, saponin, tannin, terpenoid, and trace amounts of cardiac glycoside and flavonoid were noticed. Alkaloid and steroids were completely absent in CSLE.
Table 1.
Qualitative phytochemical screening of secondary metabolites of CSLE

DISCUSSION
For the standardization, beta-caryophyllene was chosen as a marker compound since many studies suggested that beta-caryophyllene is the active chemical constituent presence in many plants. The presence of beta caryophyllene in a plant contributes to its diuretic, antioxidant, antifungal, antimycotic and antimicrobial activities.[18–21] Furthermore, beta-caryophyllene has been reported to have anti-inflammatory, antibiotic, anticarcinogenic and local anesthetic activities.[22] It was used as a marker compound for the standardization of Humulus lupulus L. essential oil.[23] A group of researchers from Dublin had perceived beta-caryophyllene as being responsible for green aromas of leafy green vegetables, hence it can be used as a biomarker for quality control purpose.[24]
The potential toxic effects that follow prolonged use of some of the more popular herbal remedies, are also a cause of alarm. So, toxicity assays are also an important part of the research. The acute toxicity (LD50) of CSLE was estimated to be >5000 mg/kg, suggesting that the extract was very safe since its LD50 value was >1000 mg.[25] This finding is in agreement with the fact that so far no side effects of using this plant in treating various ailments have been reported.
The analgesic effect of CSLE was investigated using hot plate, tail flick and formalin tests. In the present study, our results revealed that neither the hot plate nor tail flick test of CSLE significantly affected the pain response. Since the analgesic action in hot plate and tail flick tests involves supraspinal and spinal components, respectively, it means that CSLE probably does not act at supraspinal and spinal levels of central nervous system.[26,27] Morphine, a centrally acting analgesic drug produces an inhibitory effect on the nociceptive response in the hot plate and tail flick tests.
As for the formalin test, experimental evidence obtained in the present study indicates that CSLE possessed analgesic activity in the tested experimental animals (rats). Formalin model (chemical model) of nociception involves intraplantar injection of formalin which causes flinching/shaking of the affected hind paw of the tested animal model.[28] This model of nociception involves two phases, that is, neurogenic and inflammatory phases. It had been reported that nociceptive activity of the first phase is mediated by the effect on peripheral nociceptors activating primary afferent fibers to transmit the pain signals. Second phase of the formalin test occurs through the activation of ventral horn neurons at the spinal cord levels.[1] This phase of formalin model involves inflammatory mediators such as histamine, prostaglandin, serotonin and bradykinin.[29] CSLE at doses of 200 and 500 mg/kg was effective in decreasing the time spent in licking paw of formalin test at late phase. The activity observed in the formalin-induced pain test leads us to conclude that CSLE acts peripherally. Furthermore, peripherally acting analgesic is inactive against thermal stimuli exert by hot plate and tail flick tests.[30] This result suggests that CSLE is effective as an antinociceptive agent in nonopioid way due to its lack of effect in hot plate and tail flick tests. Morphine suppresses the formalin response in both the phases. This is in agreement with previous studies which state that narcotic drug inhibits both the phases of formalin test.[31,32]
Aspirin, an NSAID, inhibits the late phase on formalin model by inhibiting the enzyme cyclooxygenase and reducing the prostaglandin synthesis.[33,34] The peripheral analgesic effect of CSLE extract may also be mediated via inhibition of cyclooxygenase or any other inflammatory mediator. CSLE shows analgesic activity of the inflammatory phase (late phase) in formalin model and this might be due to the presence of terpenoid (beta-caryophyllene). This is in agreement with Gertsch et al.[35] who showed that beta-caryophyllene exerts significant anti-inflammatory effects in tested mice. These results justify the traditional usage of this plant in folk medicine to relieve fever and headache.
CONCLUSION
CSLE has demonstrated promising analgesic activity in chemical (but not thermal) model of nociception. The finding reported here highlights the fact that CSLE exerts analgesic activity that is mediated peripherally. In addition, our present investigation shows that CSLE is safe for consumption with high LD50 value. The qualitative phytochemical screening of CSLE indicates that there is absence of alkaloid. This study paves the way for further attention in investigating the mechanism of action of CSLE and its participation in the pain inhibitory mechanisms of peripheral nervous system.
Acknowledgments
This project was funded by Research University Grant from Universiti Sains Malaysia. F.M. is supported by USM Fellowship Scheme from Institute of Postgraduate Studies of Universiti Sains Malaysia. Finally, we would like to thank Mr. Hilman for expert technical assistance.
Footnotes
Source of Support: Research University Grant from Universiti Sains Malaysia. F.M. is supported by USM Fellowship Scheme from Institute of Postgraduate Studies of Universiti Sains Malaysia
Conflict of Interest: None declared.
REFERENCES
- 1.Ridtitid W, Sae-Wong C, Reanmongkol W, Wongnawa M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J Ethnopharmacol. 2008;118:225–30. doi: 10.1016/j.jep.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Singh SP. Current and future status of herbal medicine. Veterinary World. 2008;1:347–50. [Google Scholar]
- 3.Lijuan M, Xuezhu Z, Haiping Z, Yiru G. Development of a fingerprint of Salvia miltiorrhiza Bunge by high-performance liquid chromatography with a coulometric electrode array system. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:139–46. doi: 10.1016/j.jchromb.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 4.WHO Guidelines for the Assessment of Herbal Medicine. Munich: World Health Organization; 1991. [Google Scholar]
- 5.Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Pengelly A. Constituents of medicinal plants. Cambridge, USA: CABI Publishing; 2004. [Google Scholar]
- 7.Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, et al. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003;73:471–85. doi: 10.1016/s0024-3205(03)00311-4. [DOI] [PubMed] [Google Scholar]
- 8.Wan O, Ngah Z, Zaridah M, Noor R. In vitro and In Vivo antiplasmodial properties of some Malaysian plants used in traditional medicine. Inf Dis J Pak. 2007;16:97–101. [Google Scholar]
- 9.Iida N, Ishii R, Hakamata J, Myamoto S, Oozeki H. Amylase inhibitors for food and pharmaceutical. Japan: Kok Tok Ko; 1997. [Google Scholar]
- 10.Baruah A, Nath S, Hazarika A. Stem bark oil of Cinnomomum iners Reinw. Indian Perfumer. 2001;45:261–3. [Google Scholar]
- 11.Phutdhawong W, Kawaree R, Sanjaiya S, Sengpracha W, Buddhasukh D. Microwave-assisted isolation of essential oil of Cinnamomum iners Reinw. ex Bl.: comparison with conventional hydrodistillation. Molecules. 2007;12:868–77. doi: 10.3390/12040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brindha P, Saraswathy A. In: Proceeding National Seminar on Recent Trends in Natural Products Chemistry. Bharathidasan University, Tiruchirappalli, India: 1981. Phytochemical comparison of Pentatropis, Oldenlandia and plumeria. [Google Scholar]
- 13.Woolfe G, Donald AM. The evaluation of the analgesic action of pethidine hydrochloride (DEMEROL) J Pharm Exp Ther. 1994;80:300–7. [Google Scholar]
- 14.D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharm Exp Ther. 1941;72:74–9. [Google Scholar]
- 15.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;4:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 16.de Miranda FG, Vilar JC, Alves IA, Cavalcanti SC, Antoniolli AR. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001;1(6) doi: 10.1186/1471-2210-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OECD guideline 423. OECD guideline for testing of chemicals. Acute Oral Toxicity-Acute Toxic Class Method. 2001 [Google Scholar]
- 18.Ratnasooriya WD, Pieris KP, Samaratunga U, Jayakody JR. Diuretic activity of Spilanthes acmella flowers in rats. J Ethnopharmacol. 2004;91:317–20. doi: 10.1016/j.jep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Sabitha RA, Murty US. Antifungal potential of flower head extract of Spilanthesa acmella Linn. Afr J Biom Res. 2006;9:67–9. [Google Scholar]
- 20.Kawaree R, Okonogi S, Chowwanapoonpohn S, Phutdhawong W. Chemical composition and antioxidant evaluation of volatile oils from Thai medicinal plants. Acta Horticulturae (ISHS) 2008;786:209–6. [Google Scholar]
- 21.Rai MK, Acharya D. Screening of some Asteraceous plants for antimycotic activity. Compositae Newsletter. 1999;34:37–43. [Google Scholar]
- 22.Ríos MY, Castrejón F, Robledo N, León I, Rojas G, Navarro V. Chemical Composition and Antimicrobial Activity of the Essential Oils from Annona cherimola (Annonaceae) Rev de la Soc Quí de Méx. 2003;47:139–42. [Google Scholar]
- 23.Jorge K, Trugo L. Discrimination of different hop varieties using headspace gas chromatographic data. J Braz Chem Soc. 2003;14:411–5. [Google Scholar]
- 24.Lonchamp J, Ryan CB, Devereux M. Identification of volatile quality markers of ready-to-use lettuce and cabbage. Food Res Int. 2009;42:1077–86. [Google Scholar]
- 25.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 26.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–8. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 27.Mayer DJ, Liebeskind JC. Pain reduction by focal electrical stimulation of the brain: An anatomical and behavioral analysis. Brain Res. 1974;68:73–93. doi: 10.1016/0006-8993(74)90534-4. [DOI] [PubMed] [Google Scholar]
- 28.Dubuisson D, Dennis SG. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–74. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 29.Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, et al. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003;73:471–85. doi: 10.1016/s0024-3205(03)00311-4. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Raviprakash V, et al. Antinociceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res. 2003;17:259–64. doi: 10.1002/ptr.1126. [DOI] [PubMed] [Google Scholar]
- 31.Hunskaar S, Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–14. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 32.Campos AR, Albuquerque FA, Rao VS, Maciel MA, Pinto AC. I Investigations on the antinociceptive activity of crude extracts from Croton cajucara leaves in mice. Fitoterapia. 2002;73:116–20. doi: 10.1016/s0367-326x(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 33.Waller D, Renwick A. Principle of medical pharmacology. London: Saunders Company Ltd; 1994. [Google Scholar]
- 34.Trongsakul S, Panthong A, Kanjanapothi D, Taesotikul T. The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegata Kruz. J Ethnopharmacol. 2003;85:221–5. doi: 10.1016/s0378-8741(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 35.Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 2008;105:9099–104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]


