Abstract
Leaf extracts of Ficus exasperata P. Beauv. (Moraceae) are commonly used in Ghanaian traditional medicine for the treatment of several pathological states including inflammatory disorders. The present study was undertaken to evaluate the antiarthritic effect of an ethanolic extract of F. exasperata (FEE) in the Freund's adjuvant-induced arthritis model in rats. Since free radicals and reactive oxygen species are implicated in inflammatory joint diseases such as rheumatoid arthritis, the antioxidant potential of the extract was investigated in in vitro experimental models. FEE as well as the positive controls, dexamethasone and methotrexate, showed significant dose-dependent antiarthritic properties when applied to established adjuvant arthritis. Oral administration of FEE (30–300 mg/kg p.o.) significantly reduced the arthritic edema in the ipsilateral paw of rats with a maximal inhibition of 34.46 ± 11.42%. FEE (30–300 mg/kg p.o.) also significantly prevented the spread of the edema from the ipsilateral to the contralateral paws indicating inhibition of systemic spread. The disease-modifying antirheumatic drug methotrexate (0.1–1 mg/kg i.p.) and the steroidal anti-inflammatory agent dexamethasone (0.3–3 mg/kg i.p.) also reduced very significantly the total polyarthritic edema as well as the spread of the arthritis from the ipsilateral to the contralateral paws of the treated animals. The extract also exhibited reducing activity (EC50 = 8.105 ± 18.49), scavenged 2,2-diphenyl-1-picrylhydrazyl (DPPH, EC50 = 0.499 ± 0.302) and prevented lipid peroxidation (IC50 = 1.283 ± 0.923) in rat brain homogenates. Phenols were detected in the extract. These results suggest that ethanolic extract of the leaves of F. exasperata exerts antiarthritic activity after oral administration and also has antioxidant properties which may contribute to its activity.
Keywords: DPPH, Ficus exasperata, Freund's adjuvant-induced arthritis, lipid peroxidation, total phenol
INTRODUCTION
Ficus exasperata Vahl, family Moraceae, is a terrestrial Afro-tropical shrub or tree that grows up to about 20 m in height and prefers evergreen and secondary forest habitats.[1,2] The whole plant is known to have several medicinal properties in African traditional medicine. The leaf extract has been used to treat high blood pressure, rheumatism, arthritis, intestinal pains and colics, epilepsy, bleeding and wounds.[3] The roots are also used to manage asthma,[4] dyspnoea[4] and venereal diseases. Previous work on F. exasperata shows that an aqueous leaf extract produced a dose-related reduction in mean arterial blood pressure[5] as well as significant antiulcerogenic effect in aspirin-induced ulcerogenesis.[6] Macfoy[7] demonstrated that the methanolic and hot and cold aqueous extracts of F. exasperata were inactive against three Gram-negative and three Gram-positive bacteria species: Aerobacter aerogenes, Agrobacterium tumefaciens, Bacillus subtilis, Clostridium sporogenes, Escherichia coli and Staphylococcus aureus. Recently, the anti-inflammatory, antipyretic and antinociceptive effects were also established.[8] It is, however, yet to be scientifically proven if the traditional use of F. exasperata in the treatment of arthritis is justified.
Rat adjuvant-induced arthritis (AIA) is a chronic, polyarticular, erosive type of arthritis induced by an injection of killed mycobacteria.[9] AIA in rat is an experimental model that shares some features with human rheumatoid arthritis (RA) and has been widely used for studying the pathogenesis of RA and for searching new drugs for treatment of rheumatoid disease.[10,11] One of the most important features of AIA is the chronic synovitis, including inflammatory cell infiltration, pannus formation, cartilage destruction and bone erosion. The wide utilization of this model seems, amongst other reasons, to be due to the strong correlation between the efficiency of therapeutic agents in this model and in RA in humans.[12]
The importance of free radicals and reactive oxygen species in the pathogenesis of RA has been identified with increasing incidence.[13–15] Polymorphonuclear leukocytes and macrophages are stimulated, which results in the production of inflammatory mediators including large amount of superoxide and hydrogen peroxide that can cause significant impairment and destruction of synovial fluid, cartilage and other articular constituents.[16,17] Free radicals and, in particular, superoxide radicals cause cellular disruption due to peroxidation of membrane lipids.[17,18] The production of free radicals is essential for normal metabolism but they can be destructive if their toxicity is not controlled by cellular defense mechanisms. Failure of antioxidant defense system has been reported in many pathological conditions including RA.[17] It is therefore desirable that potential antiarthritic agents be investigated for their possible contribution to the strengthening of the antioxidant defense system.
This study evaluates the antiarthritic and antioxidant properties of the F. exasperata leaf extract in the Freund's AIA and in vitro antioxidant assay models, respectively. This would help provide scientific justification for the use of this plant locally for treating arthritic conditions and as well provide an alternative for the drugs currently being used for controlling arthritis.
MATERIALS AND METHODS
Plant material
Leaves of F. exasperata were collected from the campus of Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, in June 2007. Leaves were authenticated in the Department of Pharmacognosy of the university, where a voucher specimen (No.FP/08/023) has been kept.
Preparation of extract
The powdered bark was Soxhlet-extracted with 70% v/v of ethanol. The hydro-alcoholic extract was then evaporated to a syrupy mass under reduced pressure in a rotary evaporator, air-dried and kept in a dessicator. This is referred to subsequently as FEE or extract.
Drugs and chemicals
Dexamethasone sodium phosphate was purchased from Pharm-Inter (Brussels, Belgium) and methotrexate sodium from Dabur Pharma (New Delhi, India). Ascorbic acid, ammonium molybdate, ferric chloride, n-propyl gallate, potassium ferricyanide, tannic acid, thiobarbituric acid (TBA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic acid (TCA) and Folin-Ciocalteau reagent were also purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Animals
Sprague–Dawley rats (200–250 g) of both sexes were purchased from Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, and maintained in the animal house of the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi. The animals were housed in groups of six in stainless steel cages (34 × 47 × 18 cm3) with soft wood shavings as bedding, fed with normal commercial pellet diet (GAFCO, Tema, Ghana), given water ad libitum and maintained under laboratory conditions (temperature 24–28°C, relative humidity 60–70% and 12 h light–dark cycle). All procedures and techniques used in these studies were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH, Department of Health and Human Services publication no. 85-23, revised 1985) and were approved by the Departmental Ethics Committee.
Induction of arthritis
Adjuvant arthritis was induced as previously described.[19] Briefly, animals were injected intraplantar with 0.1 ml Complete Freund's Adjuvant (CFA) into the right hind paw of each rat. CFA was prepared by triturating heat-killed Mycobacterium tuberculosis [strains C, DT and PN (mixed) obtained from the Ministry of Agriculture, Fisheries and Food, UK] in paraffin oil to make a 5 mg/ml suspension.
Foot volume was measured by water displacement plethysmography[20] for both the ipsilateral (injected paw) and the contralateral paw (noninjected paw) before intraplantar injection of CFA (day 0) and every other day (days 2, 4, 6, 8, … ,28). The edema component of inflammation was quantified by measuring the difference in foot volume between day 0 and the various time points. Raw scores for ipsilateral and contralateral paw volumes were individually normalized as percentage of change from their values at day 0, then averaged for each treatment group.
Effect of drugs on AIA
The effects of FEE and standard drugs were investigated on established arthritis. Arthritis was induced by intraplantar injection of 0.1 ml CFA and drugs were then administered on day 9 with the onset of arthritis.
The animals were grouped (n = 5) randomly to receive various treatments as follows:
-
Group I:
arthritic control/CFA (intraplantar injection of 0.1 ml CFA)
-
Group II:
nonarthritic control/Incomplete Freund's adjuvant (IFA) (intraplantar injection of 0.1 ml of IFA)
-
Groups III–V:
treated with dexamethasone (0.3–3 mg/kg i.p.) from day 9 after intraplantar injection of CFA and every other day
-
Groups VI–VIII:
treated with methotrexate (0.1–1 mg/kg i.p.) from day 9 after intraplantar injection of CFA and every 4 days
-
Groups IX–XI:
treated with FEE (30–300 mg/kg p.o.) from day 9 after intraplantar injection of CFA and every day.
Doses of drugs used for this study were selected based on previous literature data and preliminary studies done in our laboratory.[21,22] The extract was suspended in 2% tragacanth mucilage whilst the reference drugs (methotrexate and dexamethasone) were dissolved in normal saline. All test drugs were freshly prepared and administered in volumes not exceeding 10 ml/kg. Inflammed control animals were given 2% tragacanth mucilage (10 ml/kg p.o.).
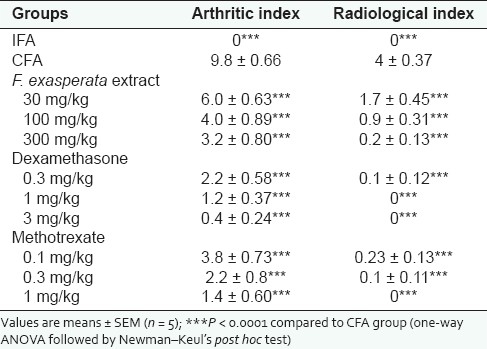
Arthritic index
Inflammation in each paw was graded blindly by the same person in all rats on day 29 according to the extent of erythema and edema of the periarticular tissues,[23,24] using a scale of 0–4, where 0 = no inflammation, 1 = unequivocal inflammation of one joint of the paw, 2 = unequivocal inflammation of at least two joints of the paw or moderate inflammation of one joint, 3 = severe inflammation of one or more joints and 4 = maximum inflammation of one or more joints in the paw. The scores for each paw were then added to get the total arthritis score (maximum possible score 16 per animal). The total score for each rat was designated as the arthritic index. The arthritis score of each rat on day 0 was determined to be 0. Photographs of rats were also taken on day 29 with a camcorder (Everio™ model GZ-MG1300, JVC, Tokyo, Japan).
The hind paw volume and arthritis index were used as the measurement parameters of inflammation and arthritis.
Radiological index
Radiographs of hind limbs of animals were taken on day 29 with a UMB type-2 X-ray unit (Softex Ltd., Tokyo, Japan) and industrial X-ray film (Fuji Photo Film, Tokyo, Japan) after animals were anesthetized with chloroform. The X-ray apparatus was operated at 30-kV peak and 10-s exposure with a 45-cm tube-to-film distance for lateral projections. Using the radiographs, the severity of bone and joint destruction was scored blindly by the same person for each hind limb according to the method described by Hoffman et al.[25] Briefly, radiographic scoring was performed by assessing soft tissue swelling, periosteal new bone formation, joint space narrowing, periarticular osteoporosis and bone destruction on a scale of 0 (normal) to 3 (maximum) per hind limb. The maximum radiographic score was 6 per animal. The radiological score for normal control rats was determined to be 0. The radiological score was termed the radiological index.
Total phenol
Total phenols present in FEE were determined with the Folin–Ciocalteau reagent.[26,27] Various concentrations (0.01, 0.03, 0.1, 0.3 mg/ml) of FEE (1 ml) were mixed with the Folin–Ciocalteau phenol reagent (1 ml diluted 1:10 with distilled water). This mixture was incubated at room temperature (28°C) for 5 min. Na2CO3 (2% w/v, 1 ml) was added and incubated for 2 h. It was then centrifuged (650 g for 10 min) to get a clear solution and the absorbance measured at 760 nm using spectrophotometer (Cecil CE 7200 UV/VIS spectrophotometer, Cecil Instrument Ltd., Milton Technical Centre, England). Tannic acid was used as reference, and the total phenols in FEE expressed as milligrams per milliliter of tannic acid equivalents (TAEs).
Reducing power
The reducing capacity of the extract was determined by the reduction fo Fe3+ to Fe2+[28] as previously described in Ref. 29 with some modifications.
One millilitre (1 ml) of various concentrations of FEE (0.1, 0.3, 1, 3 mg/ml, in methanol) was mixed with 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 ml 1% potassium ferricyanide solution (K3Fe[CN]6) in a test tube. The mixture was incubated at 50°C for 20 min. TCA (10%; 1.5 ml) was then added to the mixture and centrifuged at 650 g for 10 min. Two-and-a-half milliliters of the supernatant was mixed with 2.5 ml distilled water and 0.5 ml of 0.1% ferric chloride solution. The absorbance was then measured at 700 nm. Blank samples were prepared as follows: 1 ml distilled water was added to 2.5 ml sodium phosphate buffer and 2.5 ml potassium ferricyanide and the mixture processed as above. The n-propyl gallate (0.001, 0.003, 0.01, 0.03 mg/ml in methanol) was used as standard antioxidant. Each test was done in triplicate. The greater the reducing power, the higher the absorbance.
Data were presented as concentration–absorbance curves and the EC50 (concentration that gives 50% of maximal response) was computed.
Scavenging of DPPH
The test is based on decolorization of the purple colored methanolic solution of DPPH free radical to yellow by free radical scavengers.[30] One millilitre methanolic solution of FEE (0.1, 0.3, 1.0 and 3.0 mg/ml) was added to 3 ml methanolic solution of DPPH (20 mg/l) in a test tube. The reaction mixtures were kept at 25°C for 1 h. The absorbance of the residual DPPH was then determined at 517 nm in a Cecil UV/VIS spectrophotometer (Model CE 7200, Milton, England). The scavenging action of FEE (0.1, 0.3, 1.0 and 3.0 mg/ml in methanol) was compared with that of the standard, n-propyl gallate (0.00083, 0.0025, 0.0083, 0.025 mg/ml in methanol). One millilitre methanol (99.8%) added to 3.0 ml DPPH solution, incubated at 25°C for 1 h served as control and methanol (99.8%) was used as blank. The absorbance decreases with increasing free radical scavenging activity. Results were expressed as percentages of blank and the concentration of extracts required to cause a 50% decrease in the absorbance was calculated (IC50).
Lipid peroxidation
For lipid peroxidation determination, rats (200–250 g) were euthanized and whole brain (except cerebellum) was dissected out and homogenized (100 mg/ml) in ice-cold phosphate buffer (0.1 M, pH 7.4) and used as a source of polyunsaturated fatty acids. The extract (0.3–3 mg/ml) and n-propyl gallate (0.01–0.3 mg/ml) were dissolved in methanol. The extent of lipid peroxidation in brain tissue was assayed by measuring the thiobarbituric acid-reactive substances (TBARS) using the method of Ohkawa et al.[31] with some modifications. Briefly, 2.5 ml of brain homogenate was mixed with 1 ml of phosphate buffer and 0.5 ml of test drug. Lipid peroxidation was then initiated by the addition of 0.5 ml of 0.1 mM ascorbic acid and 0.5 ml of 5 mM ferric chloride. The mixture was incubated in a shaking water bath at 37 °C for 1 h after which 0.1 ml of the reaction mixture was transferred to a test tube containing 1.5 ml of 10% TCA. The tubes were allowed to stand for 10 min before centrifugation at 1150 g for 10 min.
The supernatants were separated and mixed in a tube containing 1.5 ml of 0.67% TBA in 20% acetic acid. The reaction mixtures were heated in a hot water bath at 85°C for 1 h, allowed to cool and absorbance determined at 535 nm in a Cecil UV/VIS spectrophotometer (Model CE 7200, Milton, England). Phosphate buffer was used as blank in the experiments. Test results were determined in triplicates.
Percentage inhibition of lipid peroxidation by the test drugs was assessed, comparing the absorbance of the drug test with that of the control (homogenate mixture without any drug). Data were presented as percentage inhibition of lipid peroxidation against concentration.
Analysis of data
For the arthritic experiment, the time–course curves for foot volume were subjected to two-way (Treatment × Time) repeated measures analysis of variance (ANOVA) with Bonferroni's post hoc t test. Total foot volume for each treatment was calculated in arbitrary units as the area under the curve (AUC) and to determine the percentage inhibition for each treatment, the following equation was used:
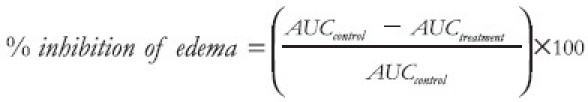
Differences in AUCs were analyzed by ANOVA followed by Student Newman–Keuls’ post hoc test.
ED50, EC50and IC50 (dose/concentration responsible for 50% of the maximal effect) values were determined using an iterative computer least squares method, with the following nonlinear regression (three-parameter logistic) equation:
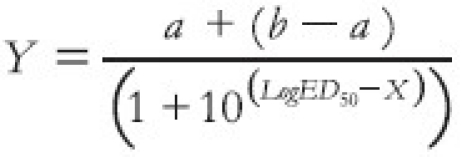
where X is the logarithm of dose and Y is the response. Y starts at a (the bottom) and goes to b (the top) with a sigmoid shape.
The fitted midpoints (ED50) of the curves were compared statistically using F test.[32,33] GraphPad Prism for Windows version 4.03 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses and ED50 determinations. P < 0.05 was considered statistically significant.
RESULTS
AIA in rats
Intraplantar injection of CFA into the right foot pad of rats induced an inflammatory response characterized by paw swelling in both the ipsilateral and the contralateral paws. The response on the injected paw was biphasic. It consists of the acute and polyarthritic/chronic phases corresponding to days 0–10 and 10–28 post-CFA inoculation, respectively. The acute phase response was characterized by unilateral inflammatory edema of the ipsilateral paw peaking around days 4–6, followed by subsequent polyarthritic/chronic phase response which began around day 10 characterized by inflammatory edema of the contralateral paw. Throughout the 28-day experiment, there was no significant change in the paw volume of the noninflamed control groups injected with IFA.
FEE, dexamethasone and methotrexate significantly suppressed the time–course of ipslateral and contralateral paw edema in rats. Two-way ANOVA (Treatment × Time) revealed a significant effect of drug treatments on the edema (FEE: F3,144 = 4.97, P < 0.05; dexamethasone: F3,144 = 85.58, P < 0.001 and methotrexate: F3,144= 13.10, P < 0.001).
FEE (30–300 mg/kg p.o.) dose-dependently and significantly reduced the total ipslateral paw edema response over the 19 days of treatment with a maximal inhibition of 34.46 ± 11.42% [Figure 1b]. Similarly, the DMARD methotrexate (0.1–1 mg/kg i.p.) and the steroidal anti-inflammatory agent dexamethasone (0.3–3 mg/kg i.p.) profoundly reduced the total ipslateral paw edema by 53.09 ± 8.87% [Figure 1f] and 91.59 ± 2.06% [Figure 1d], respectively. FEE (30–300 mg/kg) also significantly (F3,144 = 5.38, P = 0.0094) reduced the extent of spread of edema form the ipsilateral to the contralateral paws indicating inhibition of systemic spread [Figure 1a, b]. Dexamethasone (F3,144 = 32.23, P < 0.0001) and methotrexate (F3,16 = 19.54, P < 0.0001) also completely prevented the spread of the arthritis from the ipsilateral to the contralateral paws of the treated animals [Figure 1d, f].
Figure 1.
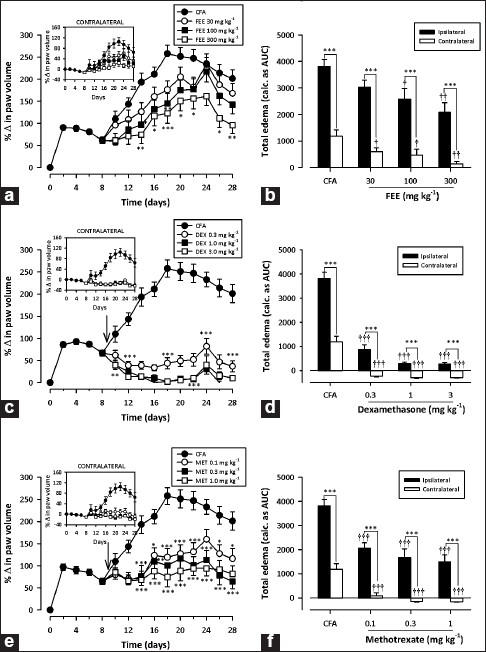
Effect of FEE (30–300 mg/kg p.o.), dexamethasone (0.3–3 mg/kg i.p.) and methotrexate (0.1–1 mg/kg i.p.) on time–course curve (a, c and e, respectively) and the total edema response (b, d and f, respectively) in AIA in rats. The total edema was calculated as AUCs over the 19-day period of drug treatment. Values are means ± SEM (n = 5). ***P < 0.001; **P < 0.01; *P < 0.05 compared to vehicle-treated group (two-way ANOVA followed by Bonferroni's post hoc test). †††P < 0.001; ††P < 0.01; †P < 0.05 compared to vehicle-treated group (oneway ANOVA followed by Newman–Keul's post hoc test)
Dose–response curves for the inhibition of foot edema are shown in Fig. 2. FEE (ED50 531.3 ± 237.83 mg/kg) was the least potent compared to methotrexate (ED50 0.41 ± 0.14 mg/kg; F1,28 = 52.45, P < 0.0001) and dexamethasone (ED50 0.11 ±0.02 mg/kg; F1,25 = 337.1, P < 0.0001) while dexamethasone was the most potent [Figure 2 and Table 1].
Figure 2.
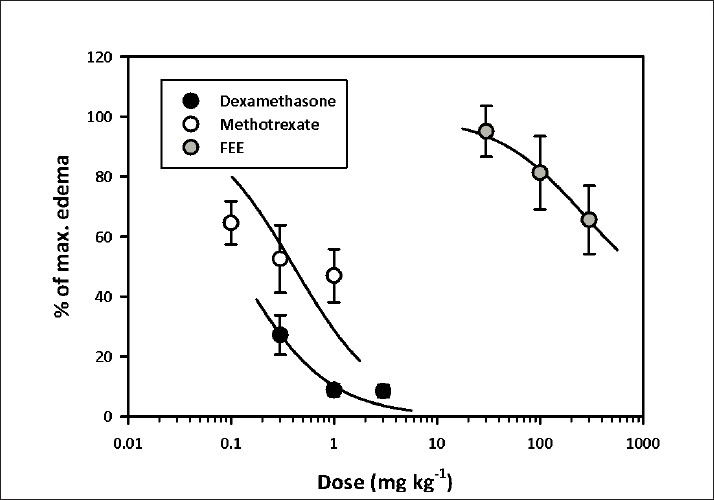
Dose–response curves for FEE (30–300 mg/kg p.o.), methotrexate (0.1–1 mg/kg i.p.) and dexamethasone (0.3–3.0 mg/kg i.p.) on AIA in rats
Table 1.
ED50 values for AIA

From the arthritic indices, FEE (30–300 mg/kg), dexamethasone (0.3–3 mg/kg) and methotrexate (0.1–1 mg/kg) showed significant (F10, 44 = 20.09, P < 0.0001) dose-dependent clinical improvement in arthritis. FEE reduced the arthritic index by a maximum of 67.35% whilst dexamethasone and methotrexate similarly inhibited the same by 95.91 and 85.71%, respectively [Table 2].
Table 2.
Arthritic/radiological indices of rats in the AIA
Comparing the radiographs of the hind limbs from each group [Figure 3], the CFA group demonstrated most severe bone destruction displaying reduced bone density and focal areas of excessive bone resorption. FEE (30–300 mg/kg) dose-dependently suppressed the pathological changes in bone with maximal inhibition of radiological index of 95%, compared with that of the CFA group. Similarly, dexamethasone (0.3–3 mg/kg) and methotrexate (0.1–1 mg/kg) almost totally prevented bone destruction in AIA radiographically [Figure 3 and Table 2] both reducing the radiological index by 100%.
Figure 3.
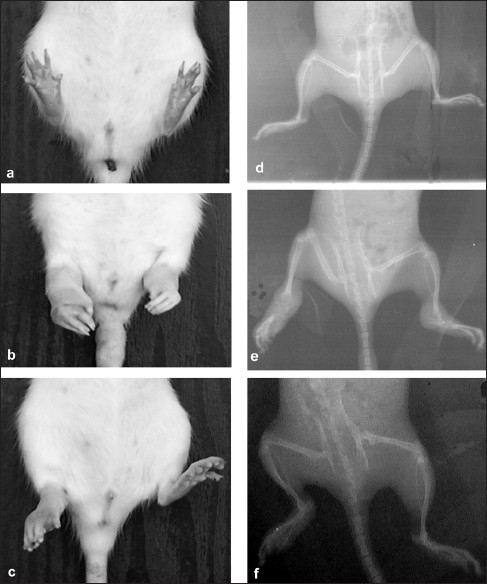
Photographs and radiographs of rats pretreated with IFA/non-arthritic control (a, d), CFA/arthritic control (b, e) and FEE (c, f) in the rat AIA
Total phenol
The total phenol content determination revealed a significant concentration-dependent increase (F3,7= 1687, P < 0.0001) in the total phenolics in FEE [Figure 4a] when expressed in tannic acid equivalents.
Figure 4.
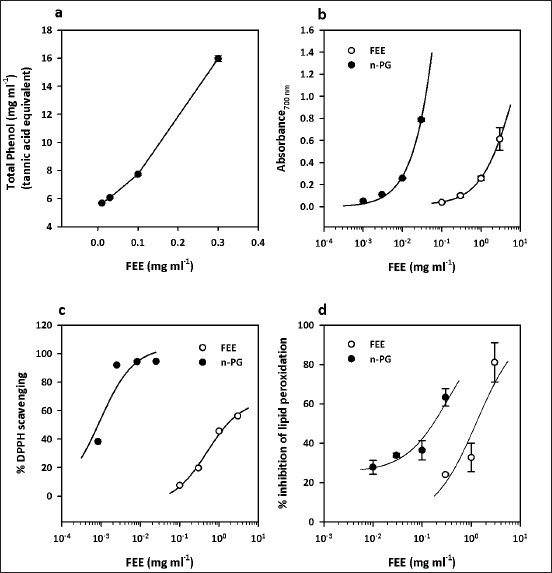
Total phenols present in various concentrations of FEE (0.01–0.3 mg/ml), expressed as tannic acid equivalent (a), reducing power of FEE (0.1–3 mg/ml) compared to n-propyl gallate (0.001–0.03 mg/ml) (b), free radical scavenging ability of FEE (0.1–3 mg/ml) compared to n-propyl gallate (0.00083–0.025 mg/ml) in the DPPH radical assay (c) and percentage inhibition of lipid peroxidation by FEE (0.3–3 mg/ml) compared with that of n-propyl gallate (0.01–0.3 mg/ml) (d). Values are mean ± SEM (n = 3)
Reducing power
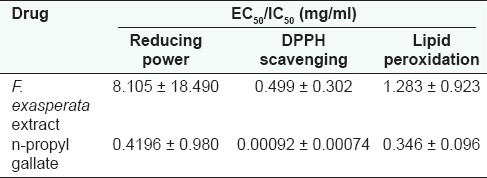
There was a concentration-dependent reducing activity by both FEE and n-propyl gallate with EC50 values of 8.105 ± 18.49 and 0.4196 ± 0.98 mg/ml, respectively [Figure 4b and Table 3]. The n-propyl gallate was however more potent, exhibiting a 19-fold reducing power compared to the extract (F1,21 = 827.1, P < 0.0001) [Table 3].
Table 3.
EC50 /IC50 of F. exasperata extract and n-propyl gallate in the reducing power, DPPH scavenging and lipid peroxidation assays
DPPH scavenging
The DPPH assay determines the ability of an agent to scavenge free radicals. FEE showed a concentration-dependent scavenging activity in a manner similar to n-propyl gallate [Figure 3c]. The EC50 values (in milligrams per milliliter) of 0.499 ± 0.302 and 0.00092 ± 0.00074 for FEE and n-propyl gallate, respectively [Table 3], suggest that FEE has lesser ability to scavenge free radicals compared to n-propyl gallate (F1,4 = 13.94, P < 0.05).
Lipid peroxidation
The result of the lipid peroxidation determination [Figure 4d] showed a concentration-dependent inhibitory activity by both FEE and the standard, n-propyl gallate, with IC50 values (in milligrams per milliliter) of 1.283 ± 0.923 and 0.346 ± 0.096, respectively [Table 3]. The n-propyl gallate was more potent when compared to FEE (F1,17 = 5.174, P < 0.05).
DISCUSSION AND CONCLUSIONS
The purpose of this study was to evaluate the antiarthritic and antioxidant properties of F. exaperata leaf extract with the aim of establishing the scientific basis of its traditional medicinal uses in inflammatory conditions. The results of the study clearly indicate that the leaf extract exhibits antiarthritic and antioxidant effects.
Rat AIA is an experimental model that shares some features (such as swelling, cartilage degradation and loss of joint function) with human RA and has been widely used for studying the pathogenesis of RA and for searching new drugs for treatment of rheumatoid disease.[10,11] One of the most important features of AIA is the chronic synovitis, including inflammatory cell infiltration, pannus formation, cartilage destruction and bone erosion. The development of rat AIA, just like human RA, can be divided into three phases starting with the induction phase without evidence of synovitis, followed by early synovitis, and finally late synovitis with progressive joint destruction.[25] Any effective antiarthritic agent should be able to exert its activity in one or more of these phases.
In this study, oral administration of F. exasperata leaf extract caused a significant reduction in joint inflammation, synovitis as well as systemic spread of the adjuvant arthritis. The extract, as evidenced from X-ray pictures [Figure 3], also protected against bone loss due to reduced bone formation and increased resorption which are the causes of bone loss in AIA in rats.[34,35] These effects of the plant extract may be attributed to immunological protection rendered by at least one of the active principles of the plant extract. Indeed, FEE has been shown by phytochemical analysis to contains alkaloids, flavonoids, tannins and saponins,[5,36,37] and one of them may be responsible for the antiarthritic effect especially as a lot of these secondary plant metabolites identified have been shown to exhibit antiarthritic properties.[38–42]
Free radicals have long been implicated as mediators of tissue damage in RA patients, which are released in large amounts into the surrounding tissue.[17,43] Several reports have also shown that during inflammatory joint diseases, such as AIA, phagocytes accumulate in the joints and produce superoxide radicals and hydrogen peroxide, which in the presence of traces of iron salts found in synovial fluids interact to form the highly reactive hydroxyl radical.[44] Consequently, when the endogenous antioxidant defenses are overcome, the resulting production of free radicals induces impairment and destruction of the synovial fluid, cartilage and other articular constituents[17,43] through a number of independent mechanisms including the initiation of lipid peroxidation, inactivation of a variety of enzymes and depletion of glutathione.[45] Thus, inhibition of the deleterious effects of free radicals in AIA may be one of the mechanisms by which antiarthritic drugs may work. The antioxidant activity of FEE has been evaluated in this study by four different assays: the total phenol content, reducing power, DPPH scavenging ability and lipid peroxidation inhibition.
Phenolic antioxidants are potent free radical terminators.[46] The high potential of phenolic compounds to scavenge radicals may be explained by their phenolic hydroxyl groups.[47] Detection of phenols in FEE therefore strongly suggested a possible antioxidant activity.
The reductive capacity of FEE, in terms of Fe3+–Fe2+transformation, is a significant indicator of its antioxidant activity. The result clearly shows that FEE has significant concentration-dependent reducing activity but with less potency compared to standard n-propyl gallate.
DPPH is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule The reduction in DPPH radical as seen in the scavenging experiment shows that both n-propyl gallate and FEE have free radical scavenging activity.
Lipid peroxidation is considered as a critical mechanism of the injury that occurs during RA,[48] and its inhibition in RA is highly desired. FEE significantly inhibited lipid peroxidation rat brain tissue and this could be attributed to the scavenging of hydroxyl or superoxide radicals, the changing of Fe3+/Fe2+ state, the reduction of the rate of conversion of ferrous to ferric or the chelation of the iron itself.
From the powerful effects shown by FEE with reference to free radical scavenging, reducing capacity and inhibition of lipid peroxidation, it is clear that FEE is an antioxidant. The mechanism of antioxidant activity of FEE can be stipulated from the above findings as being related to the reduction of free radicals as well as scavenging of reactive oxygen species and other free radicals. Though the present study did not investigate the exact mechanism by which FEE exerts its antiarthritic effects, it is proposed that its antioxidant effects may be one of the mechanisms. FEE may be inhibiting the deleterious effects of free radicals released in AIA which cause synovitis and joint destruction.
In many patients, current therapies for arthritis (corticosteroids, non-steroidal anti-inflammatory drugs and DMARDs) are often effective in relieving symptoms, although this benefit is attended by a significant risk of toxicity. There is also little evidence that anti-rheumatic drugs, such as gold, sulfasalazine and methotrexate, can change the long-term disease outcome with regard to joint destruction,[24] making it necessary, therefore, to develop new agents that are effective in preventing joint destruction as well as synovial inflammation in RA. FEE, due to the effects on synovitis and joint destruction as shown in this study, is proposed to be a promising candidate for the treatment of RA. However, its toxicity needs to be studied.
In conclusion, F. exasperata leaf extract used in this study possesses antiarthritic and antioxidant effects and validates its traditional use in the treatment of chronic inflammatory conditions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Berg CC. Classification and distribution of Ficus. Experientia. 1989;45:605–11. [Google Scholar]
- 2.Berg CC, Wiebes JT. African fig trees and fig wasps. 1 ed. Amsterdam: Koninklijke Nederlandse Akademie van Wetenschappen; 1992. [Google Scholar]
- 3.Irvine FR. Woody plants of Ghana. 1 ed. London: Oxford University Press; 1961. [Google Scholar]
- 4.Chhabra SC, Mahunnah RL, Mshiu EN. Plants used in traditional medicine in eastern Tanzania. IV. Angiosperms (Mimosaceae to Papilionaceae) J Ethnopharmacol. 1990;29:295–323. doi: 10.1016/0378-8741(90)90041-q. [DOI] [PubMed] [Google Scholar]
- 5.Ayinde BA, Omogbai EK, Amaechina FC. Pharmacognosy and hypotensive evaluation of Ficus exasperata Vahl (Moraceae) leaf. Acta Pol Pharm. 2007;64:543–6. [PubMed] [Google Scholar]
- 6.Akah PA, Orisakwe OE, Gamaniel KS, Shittu A. Evaluation of Nigerian traditional medicines: II. Effects of some Nigerian folk remedies on peptic ulcer. J Ethnopharmacol. 1998;62:123–7. doi: 10.1016/s0378-8741(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 7.Macfoy CA, Cline EI. In vitro antibacterial activities of three plants used in traditional medicine in Sierra Leone. J Ethnopharmacol. 1990;28:323–7. doi: 10.1016/0378-8741(90)90083-6. [DOI] [PubMed] [Google Scholar]
- 8.Woode E, Poku RA, Ainooson GK, Boakye-Gyasi E, Abotsi WKM, Mensah TL, et al. An evaluation of the anti-inflammatory, antipyretic and antinociceptive effects of Ficus exasperata (Vahl) leaf extract. J Pharmaco Toxicol. 2009;4:138–51. [Google Scholar]
- 9.Pearson CM, Wood FD. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheum. 1959;2:440–59. [Google Scholar]
- 10.Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–84. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald RA. Animal models for evaluation of arthritis drugs. Methods Find Exp Clin Pharmacol. 1991;13:75–83. [PubMed] [Google Scholar]
- 12.Andersen ML, Santos EH, Seabra Mde L, da Silva AA, Tufik S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund's adjuvant-induced arthritis in rats. J Ethnopharmacol. 2004;91:325–30. doi: 10.1016/j.jep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Biemond P, Swaak AJ, Koster JF. Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1984;27:760–5. doi: 10.1002/art.1780270706. [DOI] [PubMed] [Google Scholar]
- 14.Lunec J, Halloran SP, White AG, Dormandy TL. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981;8:233–45. [PubMed] [Google Scholar]
- 15.Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7–13. [PubMed] [Google Scholar]
- 16.Ostrakhovitch EA, Afanas’ev IB. Oxidative stress in rheumatoid arthritis leukocytes: suppression by rutin and other antioxidants and chelators. Biochem Pharmacol. 2001;62:743–6. doi: 10.1016/s0006-2952(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 17.Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003;5:122–31. doi: 10.1186/ar748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui K, Luo X, Xu K, Ven Murthy MR. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:771–99. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956;91:95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 20.Fereidoni M, Ahmadiani A, Semnanian S, Javan M. An accurate and simple method for measurement of paw edema. J Pharmacol Toxicol Methods. 2000;43:11–4. doi: 10.1016/s1056-8719(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 21.Woode E, Ainooson G, Boakye-Gyasi E, Ansah C, Obiri D, Koffour G, et al. Anti-arthritic and antioxidant properties of the ethanolic stem bark extract of Newbouldia laevis (P. Beauv.) Seaman ex Bureau (Bignoniaceae) J Med Plants Res. 2008;2:180–8. [Google Scholar]
- 22.Woode E, Boakye-Gyasi E, Danquah C, Ansah C, Duwiejua M. Anti-arthritic effects of palisota hirsuta. Schum. Leaf extract in Freund's adjuvant-induced arthritis in rats. Int J Pharmacol. 2009;5:181–90. [Google Scholar]
- 23.Kinne RW, Schmidt-Weber CB, Hoppe R, Buchner E, Palombo-Kinne E, Nürnberg E, et al. Long-term amelioration of rat adjuvant arthritis following systemic elimination of macrophages by clodronate-containing liposomes. Arthritis Rheum. 1995;38:1777–90. doi: 10.1002/art.1780381211. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Shuto T, Hirata G, Iwamoto Y. Aminobisphosphonate (YM175) inhibits bone destruction in rat adjuvant arthritis. J Orthop Sci. 2000;5:397–403. doi: 10.1007/pl00021456. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann JC, Herklotz C, Zeidler H, Bayer B, Rosenthal H, Westermann J. Initiation and perpetuation of rat adjuvant arthritis is inhibited by the anti-CD2 monoclonal antibody (mAb) OX34. Ann Rheum Dis. 1997;56:716–22. doi: 10.1136/ard.56.12.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 27.Slinkard K, Singleton VL. Total phenol analyses: Automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 28.Oyaizu M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 29.Woode E, Ansah C, Ainooson GK, Abotsi WM, Mensah AY, Duwiejua M. Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (Forsk.) Vahl (Apocynaceae) J Sci Tech. 2007;27:6–15. [Google Scholar]
- 30.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Motulsky HJ, Christopoulos A. A practical guide to curve fitting. San Diego, CA: GraphPad Software Inc; 2003. Fitting model to biological data using linear and nonlinear regression. [Google Scholar]
- 33.Miller JR. Step-by-step examples. San Diego, CA: GraphPad Software Inc; 2003. GraphPad Version 4.0. [Google Scholar]
- 34.Aota S, Nakamura T, Suzuki K, Tanaka Y, Okazaki Y, Segawa Y, et al. Effects of indomethacin administration on bone turnover and bone mass in adjuvant-induced arthritis in rats. Calcif Tissue Int. 1996;59:385–91. doi: 10.1007/s002239900144. [DOI] [PubMed] [Google Scholar]
- 35.Findlay DM, Haynes DR. Mechanisms of bone loss in rheumatoid arthritis. Mod Rheumatol. 2005;15:232–40. doi: 10.1007/s10165-005-0412-z. [DOI] [PubMed] [Google Scholar]
- 36.Ijeh II, Ukwemi AI. Acute effect of administration of ethanol extracts of Ficus exasperata vahl on kidney function in albino rats. J Med Plants Res. 2007;1:27–9. [Google Scholar]
- 37.Woode E, Poku R, Ainooson G, Boakye-Gyasi E, Abotsi W, Mensah T, et al. An evaluation of the anti-inflammatory, antipyretic and antinociceptive effects of Ficus exasperata (Vahl) leaf extract. J Pharmaco Toxicol. 2009;4:138–51. [Google Scholar]
- 38.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 39.Rotelli AE, Guardia T, Juarez AO, de la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003;48:601–6. doi: 10.1016/s1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]
- 40.Barbosa-Filho J, Piuvezam M, Moura M, Silva M, Lima K, da-Cunha E, et al. Anti-inflammatory activity of alkaloids: A twenty-century review. Rev Bras Farmacogn. 2006;16:109–39. [Google Scholar]
- 41.Clavin M, Gorzalczany S, Macho A, Munoz E, Ferraro G, Acevedo C, et al. Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J Ethnopharmacol. 2007;112:585–9. doi: 10.1016/j.jep.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Whitehouse M, Fairlie D, Thong Y. Anti-inflammatory activity of the isoquinoline alkaloid, tetrandrine, against established adjuvant arthritis in rats. Inflamm Res. 1994;42:123–7. doi: 10.1007/BF01983477. [DOI] [PubMed] [Google Scholar]
- 43.Bauerova K, Bezek A. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys. 1999;18:15–20. [PubMed] [Google Scholar]
- 44.Fahim AT, Abd-el Fattah AA, Agha AM, Gad MZ. Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacol Res. 1995;31:73–9. doi: 10.1016/1043-6618(95)80051-4. [DOI] [PubMed] [Google Scholar]
- 45.Jung HJ, Nam JH, Choi J, Lee KT, Park HJ. Antiinflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund's complete adjuvant-induced rats. J Ethnopharmacol. 2005;97:359–67. doi: 10.1016/j.jep.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 46.Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 47.Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem. 1999;47:397–402. doi: 10.1021/jf980765e. [DOI] [PubMed] [Google Scholar]
- 48.Narendhirakannan RT, Subramanian S, Kandaswamy M. Free radical scavenging activity of Cleome gynandra L. leaves on adjuvant induced arthritis in rats. Mol Cell Biochem. 2005;276:71–80. doi: 10.1007/s11010-005-3234-6. [DOI] [PubMed] [Google Scholar]


