Abstract
Standardization of herbal formulations is essential in order to assess the quality of drugs, based on the concentration of their active principles. This article reports on standardization of Ajmodadi churna, a polyherbal ayurvedic medicine used as a carminative and an antispasmodic, and is a strong wormifuge, and helps in all painful conditions like sciatica and stiffness in back and also restores normal digestive functions. Ajmodadi churna was prepared as per Ayurvedic Formulary of India. In-house preparation and the marketed drug have been standardized on the basis of organoleptic characters, physical characteristics, and physico-chemical properties. The set parameters were found to be sufficient to evaluate the churna and can be used as reference standards for the quality control/quality assurance laboratory of a Pharmaceutical house.
Keywords: Ajmodadi churna, physico-chemical, polyherbal formulation, standardization
INTRODUCTION
The subject of herbal drug standardization is massively wide and deep. There is so much to know and so many seemingly contradictory theories on the subject of herbal medicines and their relationship with human physiology and mental function. For the purpose of research work on standardization of herbal formulations and neutraceuticals, a profound knowledge of the important herbs found in India and widely used in Ayurvedic formulation is of utmost importance. India can emerge as the major country and play the lead role in production of standardized, therapeutically effective ayurvedic formulations. India needs to explore the medicinally important plants. This can be achieved only if the herbal products are evaluated and analyzed using sophisticated modern techniques of standardization. The World Health Organization (WHO) has appreciated the importance of medicinal plants for public health care in developing nations and has evolved guidelines to support the member states in their efforts to formulate national policies on traditional medicine and to study their potential usefulness including evaluation, safety, and efficacy.[1] “Ajmodadi churna” is a polyherbal ayurvedic medicine used as a carminative and an antispasmodic, is a strong wormifuge, and helps in all painful conditions like sciatica and stiffness in back and also restores normal digestive functions.[2] This study reports on the standardization of Ajmodadi churna based on organoleptic characters, physical characteristics, and physico-chemical properties.
MATERIALS AND METHODS
Plant material
Ajmodadi churna consists of 12 ingredients, viz., Trachyspermum ammi, Piper nigrum, Piper longum, Plumbago zeylanica, Terminalia chebula, Cedrus deodara, P. longum (stems), salt (Saindava lavana), Embelia ribes, Zingiber officinale, Argyreia nervosa, and Anethum graveolens.[3] All these ingredients were procured from the local market of Udupi, Karnataka, India, and were authenticated by botanist G.K. Bhatt, Professor of the Department of Botany, P.P.C. College, Udupi, Karnataka. A voucher specimen of the same has been deposited in the museum of Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal, for future reference.
Preparation of Ajmodadi churna
The churna was prepared as per the procedure given in Ayurvedic Formulary of India. All the ingredients were powdered separately, passed through 80 # sieve and then mixed together in specified proportions to get uniformly blended churna.
Marketed samples
The marketed samples of various brands of Ajmodadi churna, i.e., Rajashree Ayurvedic pharmacy (R) and the In-house preparation (I) were standardized based on their organoleptic characters, physical characteristics, and physico-chemical properties.
Organoleptic evaluation
Organoleptic evaluation refers to evaluation of the formulation by color, odor, taste, texture, etc. The organoleptic characters[4] of the samples were evaluated based on the method described by Siddiqui et al.
Physico-chemical investigations
Physico-chemical investigations of formulations were carried out, including the determination of extractive values and ash values.[1,5]
Fluorescence analysis
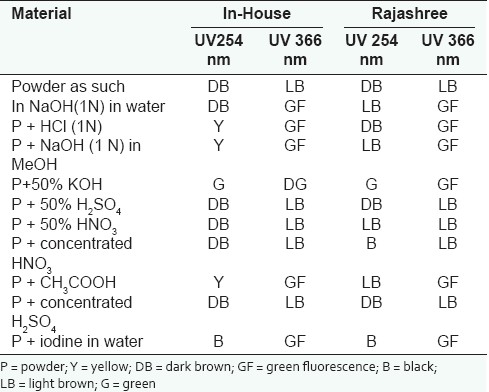
The powdered samples were exposed to ultraviolet light at wavelengths of 254 and 366 nm.[4] Fluorescence analysis was carried out in accordance with the procedure reported by Kokoshi et al. One milligram of powdered drug was placed on a microslide and observed under UV 366, UV 254 and in day light to observe the fluorescent characteristics of powder, if any. One milligram powdered drug was placed on a microslide and treated with 1 ml 1 N HCl and observed under UV 366, UV 254 and in day light while wet. One milligram powdered drug was placed on a microslide and treated with 1 ml 1 N NaOH and observed after a few minutes in day light, under UV 366 and UV 254. One milligram powdered drug was placed on a microslide and treated with 1 ml 1 N NaOH in 1 ml methanol and observed under UV 366, UV 254 and in day light while still wet. One milligram powdered drug was placed on a microslide and treated with 1 ml 50% KOH and observed under UV 366, UV 254 and in day light while still wet. One milligram powdered drug was placed on a microslide and treated with 1 ml of 50% sulfuric acid and observed under UV 366, UV 254 and in day light while still wet. One milligram powdered drug was placed on a microslide and treated with 1 ml of concentratred sulfuric acid and observed under UV 366, UV 254 and in day light while still wet. One milligram powdered drug was placed on a microslide and treated with 1 ml of 50% HNO3 and observed under UV 366, UV 254 and in day light while still wet. One milligram powdered drug was placed on a microslide and treated with 1 ml of concentrated HNO3 and observed under UV 366, UV 254 and in day light while still wet. One mg powdered drug was placed on a micro slide and treated with 1 ml of acetic acid and observed under UV 366, UV 254 and in day light while still wet. One milligram powdered drug was placed on a microslide and treated with 1 ml of iodine and observed under UV 366, UV 254 and in day light while still wet.
Determination of pH
The pH of different formulations in 1% w/v and 10% w/v of water soluble portions was determined using pH paper (range 3.5–6) and (6.5–1.4) with standard glass electrode at 240°C.
Estimation of sodium content
Sodium content was estimated by using a flame photometer.[6,7] A stock solution 100 μg/ml of NaCl was prepared in distilled water and further dilutions were made to get 1, 2, 3, 4, 5, and 10 μg/ml for preparing the standard graph. Sodium content of the formulations was estimated by flame photometric method based on the measurement of emission intensity in a nanometer. The method was validated for linearity, precision, and accuracy. The method obeyed Beer's law in the concentration range1–10 μl/ml. The standard drug solution was assayed repeatedly (n=6) and the mean error and relative standard deviation (precision) was calculated.
Determination of physical characteristics of powder formulation
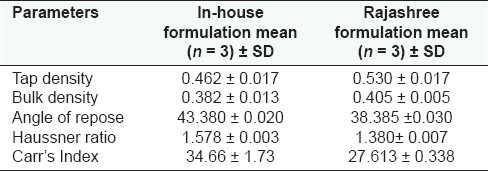
Physical characteristics like bulk density, tap density, angle of repose, Haussner ratio, and Carr's index were determined for different formulations.[8,9] The term bulk density refers to packing of particles or granules. The equation for determining bulk density (Db) is Db = M/Vb where M is the mass of particles and Vb the total volume of packing. The volume of packing can be determined in an apparatus consisting of graduated cylinder mounted on mechanical tapping device (jolting volumeter) that has a specially cut rotating can. Hundred grams of weighed formulation powder was taken and carefully added to cylinder with the aid of a funnel. The initial volume was noted and sample was then tapped until no further reduction in volume was noted. The initial volume gave the bulk density value and after tapping the volume reduced, it gives the value of tapped density.
Angle of repose has been used as an indirect method quantifying powder flowability because of its relationship with interparticle cohesion. The fixed funnel and the free standing cone method employs an apparatus that is secured with its tip at a given height (H) above the glass paper that is placed on a flat horizontal surface. Powder or granules were carefully poured through the funnel until the apex of the conical pile just touched the tip of funnel. Thus, with R being the radius of the conical pile, tan a = H/R or a = arc tan H/R, where a is the angle of repose. Haussner ratio is related to interparticle friction and as such can be used to predict the powder flow properties. The equation for measuring the Haussner ratio is Df/D0, where Df is the tapped density and D0 the bulk density.
Carr's index is another indirect method of measuring the powder flow from bulk density. The equation for measuring Carr's index is
I=(Df-D0/Df) × 100
where Df is the tapped density and D0 the bulk density.
RESULTS
In-house formulation was prepared in accordance with the Ayurvedic Formulary of India.[3] Water soluble and alcohol soluble extractive values are given in Table 1 and ash values (total ash and acid insoluble ash) in Table 2. The ash values of the samples were estimated based on the method as described by the WHO guidelines for medicinal plant materials. The physico-chemical and organoleptic comparisons between in-house formulations and marketed formulations are given in Tables 3 and 4, respectively. The results obtained with the market formulations and the in-house formulations were found to be comparable and variation was insignificant. Acid insoluble ash value for in-house formulation was found to be 0.596 ± 0.067 (average value along with standard deviation); in case of marketed formulation this was found to be 0.573 ± 0.108. The pH of 1% w/v and 10% w/v solutions revealed that pH values of both the formulations were comparable and was slightly acidic for both the formulations (i.e., in-house and Rajashree Ayurvedic pharmacy). The various physical characteristics are presented in Table 5.
Table 1.
Extractive values of individual ingredients present in Ajmodadi churna
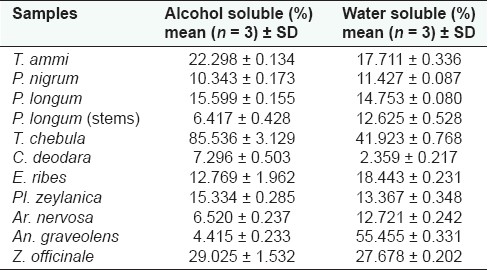
Table 2.
Percent ash values of individual ingredients present in Ajmodadi churna (w/w)
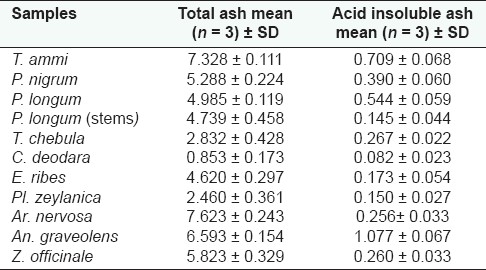
Table 3.
Physico-chemical characteristics of Ajmodadi churna formulations
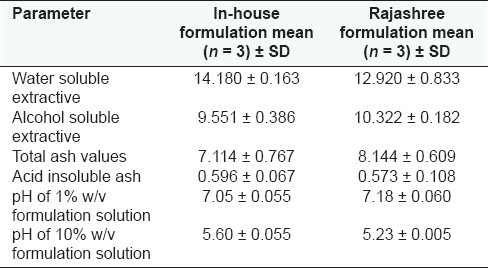
Table 4.
Organoleptic properties of different Ajmodadi churna formulations
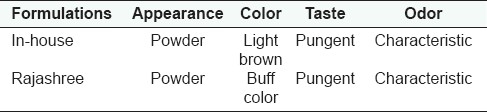
Table 5.
Physical characteristics of different Ajmodadi churna formulations
Estimation of sodium content shows that the sodium content in both the formulations were also comparable. The standard plot for sodium content is given in Table 6 and the sodium content was found to be higher in Rajashree Ayurvedic pharmacy formulation compared to in-house (average value along with standard deviation) (n = 3) and the results are shown in Table 7. In Table 8 fluorescent analysis of different Ajmodadi churna formulations is reported.
Table 6.
Standard graph of sodium by flame photometry method

Table 7.
Sodium content in different Ajmodadi churna formulations

Table 8.
Powder fluorescence test of different Ajmodadi churna formulations
CONCLUSION
The physicochemical standardization of polyherbal formulation Ajmodadi churna was carried out. The individual ingredients of the formulation were authenticated and standardized as per WHO guidelines and Indian Herbal Pharmacopoeia. The in-house formulation was prepared and studied for various physicochemical properties in comparison with the marketed sample.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Organisation Mondiale De La Sante, Quality control methods for medicinal plant materials, 559, rev. 1. Original English, World Health Organisation; 1992. p. 159. [Google Scholar]
- 2. [cited in 2009]. Available from: http://www.ayurvedicdietsolution.com .
- 3.Government of India, Ministry of Health and family welfare. 2nd revised edition. New Delhi: Department of ISM and H; 2003. The Ayurvedic Formulary of India, Part-I; p. 106. [Google Scholar]
- 4.Siddiqui, Hakim MA. Format for the pharmacopoeial analytical standards of compound formulation, workshop on standardization of Unani drugs, (appendix) New Delhi: Central Council for Research in Unani Medicine (CCRUM); 1995. Jan 24-25, [Google Scholar]
- 5.Indian Pharmacopoeia, Ministry of Health and Family Welfare. New Delhi: Government of India; 1996. [Google Scholar]
- 6.Skoog AD, West DM, Holler FJ. Fundamentals of analytical chemistry. 17th ed. New York, USA: Saunders College Publishing; 1991. pp. 613–5. [Google Scholar]
- 7.Mendham J, Denney RC, Bames JD, Thomas M. Vogel's text book of quantitative chemical analysis. 6th ed. Singapore: Pearson Education Pvt. Ltd; 2002. pp. 605–13. [Google Scholar]
- 8.Lachman L, Lieberman H A, Kanig JL. The theory and practice of industrial pharmacy. 3rd eds. Mumbai: Verghese Publishing House; 1987. pp. 183–316. [Google Scholar]
- 9.Aulton ME. Pharmaceutics, The science of dosage forms designs. 2nd ed. New Delhi: Churchill Livingstone; 2002. pp. 205–21. [Google Scholar]


