Abstract
Solanum trilobatum is a widely used plant in the Indian indigenous systems of medicine. It is mainly used in the treatment of respiratory diseases like bronchial asthma. In our present study, we report that the aqueous and alcoholic extracts of S. trilobatum exhibited inhibition of mast cell degranulation. Further, aqueous and alcoholic extracts of S. trilobatum significantly decreased the release of IL1α and increased the release of IL8 from the cultured keratinocytes. Oral administration of the aqueous and alcoholic extracts of S. trilobatum stabilized mast cells in experimental rats.
Keywords: Histamine, immunomodulation, interleukin, mast cell, medicinal plants, Solanum trilobatum
INTRODUCTION
Solanum trilobatum belongs to the family Solanaceae, which is commonly known as Alarka in Sanskrit. Several reports document the diverse properties of this plant. S. trilobatum possesses antioxidant activity,[1] hepatoprotective activity[2] and protects against UV-induced damage and radiation-induced toxicity in mice.[3] Aqueous and solvent extracts of S. trilobatum were found to possess anti-inflammatory and antimicrobial properties.[4–6]
Atopic allergy implies a familial tendency to manifest conditions such as asthma, rhinitis, urticaria and eczematous dermatitis, alone or in combination. However, individuals without an atopic background may also develop hypersensitivity reactions, particularly urticaria and anaphylaxis, associated with IgE antibody. Mast cell is the key effector cell of the biologic response in allergic reaction. The use of synthetic antihistamines to control atopic allergy over a prolonged period of time could lead to potential side effects, and the relief offered by them is mainly symptomatic and short-lived. A safe and effective management of atopy through plant resources has received much attention in recent years. This study was undertaken to evaluate the efficacy of aqueous and solvent extracts of S. trilobatum in regulating the mast cell degranulation event and inflammatory responses (IL1α and IL8) through in vitro and in vivo studies. Further, the determination of efficacy of these extracts in preventing the disease progression in experimental model was also attempted.
MATERIALS AND METHODS
Extraction procedure
S. trilobatum plant was collected from Chennai, south India, and authenticated by the Department of Plant Biology and Biotechnology, The New College, Chennai. Nondestructive cold-process extraction was employed with different solvents (ethanol, ethyl acetate and chloroform); and in the case of aqueous extraction, heat distillation process was used. Shade-dried powdered leaves of S. trilobatum were soaked separately in ethanol, ethyl acetate and chloroform in a ratio of 1:5 (solute versus solvent) in a conical flask. The entire setup was kept at room temperature for 24 hours with intermittent shaking. After 24 hours, the mixture was filtered through Whatman no.1 filter paper, and the filtrate was dried to evaporate the solvent. The extract settled at the bottom was used for the experiment at varying concentrations. In the case of aqueous extraction, the leaves were boiled in water at 1:5 ratio at 100°C for 30 minutes.[7] After 30 minutes, the mixture was filtered and the filtrate was stored in a refrigerator until use. All animal experiments were approved by the Institutional Animal Ethics Committee.
Collection of peritoneal mast cells
Cold normal saline (2 mL) was injected into the peritoneal cavity of the BALB/c mice under mild ether anesthesia. After a gentle massage, the peritoneal fluid was collected and transferred into siliconized test tubes containing 3 mL of RPMI-1640 medium (pH, 7.2-7.4). The cells were washed twice by low-speed centrifugation (400-500 rpm) and suspended in RPMI-1640 medium (Himedia, India).[8]
Degranulation of mast cells
The peritoneal exudate cells containing, among other things, mast cells were incubated for 15 minutes at 37°C with antigen (0.1 mg egg albumin/mL). After incubation, the cells were stained with 1% toluidine blue[9] and observed under high-power microscope. The total number of peritoneal exudate cells and the number of mast cells (degranulated and granulated) were counted in the complete field of the microscope.
Effect of S. trilobatum on rat mesenteric mast cells
Male albino Wistar rats weighing 150-200 g were sensitized with egg albumin (1 mg/rat) intramuscularly. After 10 days another dose of egg albumin was given as above. The rats were then divided into five groups (six rats in each group). The aqueous and ethanolic extracts of S. trilobatum in 5% Tween 80 were given orally for 3 consecutive days, following the second dose of antigen administration. The aqueous extract was administered at a dose of 5 and 10 mg/kg body weight to groups I and II, respectively. The ethanolic extract was administered at 5 and 10 mg/ kg body weight to groups III and IV, respectively. Group V animals treated with cetrizine hydrochloride at 0.5 mg/kg body weight were used as control. Twenty-four hours after the last dose of drug or 5% Tween 80 treatment, rats were sacrificed by cervical dislocation and intestinal mesentery was collected.
Collection and preparation of mesentery
This was done essentially as described by Rathinum et al.[10] The intestinal mesentery of overnight-fasted rats was collected, washed in normal saline, separated along with gut and suspended in RPMI-1640 medium. The mesentery was then cut into pieces of 1 cm2 each and used for the experiment. Two pieces from each rat were incubated in 5 mL RPMI-1640 medium with or without 0.1 mg/mL egg albumin, for 15 minutes at 37°C. The mesentery was removed, spread on a clean glass slide, dried in air and separated from the gut using a sharp blade. The dried slide was stained with toluidine blue and counter-stained with light green stain[9] and observed under the microscope.
Effect of release of histamine from mast cells
Mast cells were isolated from peritoneal cavity of albino Wistar rats and maintained in RPMI-1640. Substance P (neuromediator) was used as mast cell degranulation substance. Substance P prepared at a concentration of 3.10-5 M was used for the test. Different solvent extracts of S. trilobatum were tested for their effect on histamine release. After treatment with the respective extracts, the cells were incubated at 37°C for 20 minutes. After incubation the reaction was stopped by changing the temperature to 4°C.[11] Later, reaction mixture was centrifuged and the supernatant was assayed for histamine by Immunotech kit (Beckman Coulter Cat. No. 2562).
Isolation of keratinocytes
Primary cultures of human keratinocytes were established from the skin remaining of abdomen surgery from healthy donors. Skin pieces of 1 cm2 were exposed to dispase 2.4 U/ mL (Sigma) overnight at 4°C. The epidermis was removed from the dermal layer and incubated in 0.25% EDTA-trypsin (Sigma) for 10 minutes at 37°C. After incubation, enzymatic activity of trypsin was stopped to adding Dulbecco's Modified Eagle Medium (DMEM)medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gibco) and was homogenized by repeated aspirations. Cell suspension was centrifuged at 1500 rpm for 10 minutes, and the pellet was resuspended in serum-free medium for keratinocytes (Gibco). Cells were seeded into 75-cm2 culture flasks and kept at 37°C at 5% CO2. The culture medium was renewed every 3 days.[12]
Release of IL1α from keratinocytes
Keratinocytes were incubated in keratinocyte serum-free medium (KSFM) (Gibco, BRL - France) at 37°C with 5% CO2. All the extracts at all the concentrations were tested for the effect of release of IL1α. Keratinocytes were irradiated with UV A and B at 0.15 J/cm2. After irradiation, the cell culture was kept at 37°C for 24 hours in KSFM; the supernatant was collected and assayed for IL1α by ELISA kit (Beckman Coulter Cat. No. IM0755)
Release of IL8 from keratinocytes
To study the release of IL8, a procedure similar to the one mentioned above was employed. Subsequently PMA (Phorbol-12- Myristate 13-Acetate) was used as IL8 stimulant. IL8 was assayed by ELISA kit (Beckman Coulter Catalog No. IM227)
Dust inhalation assay
Twelve healthy New Zealand white rabbits (6 animals per group) orally pretreated with aqueous extract of S. trilobatum at 10 mg/kg body weight for 14 days were exposed to house dust on day 15 through a nebulizer in a dust inhalation chamber for 1 hour. Four extract-pretreated animals each unexposed to house dust challenge were maintained as control. The amounts of histamine released in test and control animals were assayed using standard procedure.[13]
Statistical analysis
The results are expressed as mean ± SD. Significant differences between two experimental groups were analyzed by ANOVA. Values of P < 0.05 were considered significant.
RESULTS
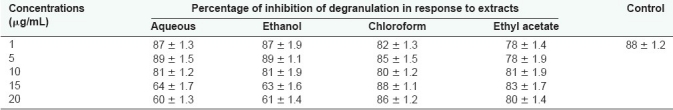
The aqueous and alcoholic extracts of S. trilobatum exhibited mast cell degranulation inhibition activity at 15 µg/mL. Increase in activity with increase in concentration with the above extracts was seen. Chloroform and ethyl acetate extracts of the above plant did not show any activity at all concentrations [Table 1].
Table 1.
Effect of S. trilobatum on mast cell degranulation
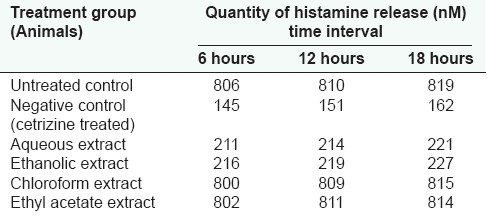
Ten-day administration of the aqueous and alcoholic extracts of S. trilobatum showed mast cell stabilization in animals at 10 mg/kg body weight. The histamine release in these animals was as low as that for cetrizine hydrochloride (0.5 mg/kg body weight)-treated animals. Increase in activity with increase in concentration of the above extract was not seen [Tables 2 and 3].
Table 2.
Effect of S. trilobatum in the release of histamine from mast cells

Table 3.
Effect of S. trilobatum in the release of histamine in animals exposed to dust (dust inhalation test/extract administration at 10 mg/kg body weight)
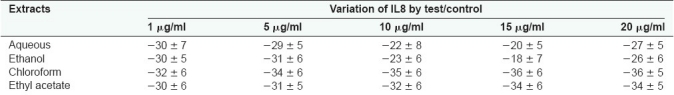
Aqueous and alcoholic extracts of S. trilobatum at 10 and 15 µg/mL, respectively, significantly decreased the release of IL1α and increased the release of IL8 from the cultured keratinocytes [Tables 4 and 5].
Table 4.
Effect of S. trilobatum in the release of IL1α from keratinocyte culture

Table 5.
Effect of S. trilobatum in the release of IL8 from keratinocyte culture
DISCUSSION
With the advent of urbanization and industrialization, allergic manifestation, especially the state of atopy, has increased significantly. As per WHO, 20% of the global population is considered to be hypersensitive/atopic in nature. Management of atopy is widely achieved either through immune-suppressive drugs such as steroids or through neutralization of the end product of allergic process — histamine with antihistamine drugs.[14] Therefore, for the management of allergy, there arises the need for either histamine inactivation or mast cell stabilization. In the current study, we have evaluated the role of S. trilobatum by addressing the preliminary mechanism of response in the allergic manifestation, i.e., mast cell degranulation inhibition.
The aqueous and ethanolic extracts of S. trilobatum showed mast cell degranulation inhibition property, whereas none of the other extracts of the same plant showed any significant activity. This was reconfirmed by quantifying the release of histamines in mesenteric mast cells. The aqueous and ethanolic extracts of S. trilobatum showed activity for in vitro assay for inhibition of mast cell degranulation, whereas other extracts of the same plant did not show any activity. This plant if properly exploited can serve as an effective drug in the management of various allergic and inflammatory conditions.
The efficacy of S. trilobatum in the inhibition of mast cell degranulation was reconfirmed by dust inhalation study. In the S. trilobatum-treated animals, the release of histamine was very low in response to dust exposure. This could be due to the mast cell stabilization event, resulting in relatively low release of histamine. The in vitro-in vivo concordance in the result proves that S. trilobatum has anti-allergic properties. Studies have been carried out in the past to establish anti-inflammatory and anti-oxidant properties of S. trilobatum,[15–20] but its antihistaminic property, in particular, is a novel finding of this present investigation.
Keratinocytes are the chief cells which play a vital role in the skin during allergic responses. They release a variety of interleukins; IL1α and IL8, in particular, are pro-inflammatory mediators. There is a dearth of studies on the effect of S trilobatum extract over the cultured keratinocytes and the release of interleukins and hence this study assumes prime importance.
Biological activity of IL1α is pleotropic. Many of its effects are to increase the production of other cytokines. Chemotaxis, transendothelial migration, induction of lysosomal enzyme release, respiratory burst and generation of superoxide anions are the chief effects of IL8 on neutrophils. In order to confirm the effect of S. trilobatum in controlling inflammatory responses, the extracts were studied for their effect on the release of IL1α and IL8. The keratinocytes were treated with extracts in vitro and were exposed to UV radiation. The findings revealed that the release of IL1α significantly decreased while that of IL8 increased in the treated cells. Interestingly, this is the first report revealing the anti-inflammatory property S. trilobatum by modulating the release of interleukins by keratinocytes. S. trilobatum showed activity in controlling allergic responses. The aqueous and ethanolic extracts of S. trilobatum exhibited anti-inflammatory activities. The above activities could be attributed to the presence in these plants of specific active compounds capable of producing pharmacological effects. The analysis of the crude aqueous and ethanolic extracts showed an alkaloid content of 0.42% and the presence of tannins, saponins, flavonoids, steroids, terpenoids and cardiac glycosides (data not included). The immunomodulatory and anti-inflammatory properties of S. trilobatum could be attributed to these constituent ingredients in the extracts. In the present study, an attempt was made to investigate the effect of whole extract as used in the traditional systems of therapy, such as Ayurveda, rather than studying the activity of specific isolated active principles. Pure phytochemicals, besides their pharmacological benefits, may also possess toxicity when used individually. However, their toxic activities may get nullified/neutralized while present together with other compounds during therapeutic use.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Shahjahan M, Vani G, Shyamaladevi CS. Effect of Solanum trilobatum on the antioxidant status during diethyl nitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rat. Chem Biol Interact. 2005;156:113–23. doi: 10.1016/j.cbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Shahjahan M, Sabitha KE, Jainu M, Shyamala Devi CS. Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res. 2004;120:194–8. [PubMed] [Google Scholar]
- 3.Mohanan PV, Devi KS. Effect of Sobatum on tumour development and chemically induced carcinogenesis. Cancer Lett. 1997;112:219–23. doi: 10.1016/s0304-3835(96)04574-0. [DOI] [PubMed] [Google Scholar]
- 4.Doss H, Mubarack M, Dhanabalan R. Antibacterial activity of tannins from the leaves of Solanum trilobatum Linn. Indian J Sci Technol. 2009;2:41–3. [Google Scholar]
- 5.Govindan S, Viswanathan S, Vijayasekaran V, Alagappan R. A pilot study on the clinical efficacy of Solanum xanthocarpum and Solanum trilobatum in bronchial asthma. J Ethnopharmacol. 1999;66:205–10. doi: 10.1016/s0378-8741(98)00160-3. [DOI] [PubMed] [Google Scholar]
- 6.Govindan S, Viswanathan S, Vijayasekaran V, Alagappan RJ. Antimicrobial activity and phytochemicals of Solanum trilobatum Linn. Cancer Lett. 2004;127:135–40. [Google Scholar]
- 7.Ignácio SR, Ferreira JL, Almeida MB, Kubelka CF. Nitric oxide production by murine peritoneal macrophages in vitro and in vivo treated with Phyllanthus tenellus extracts. J Ethnopharmacol. 2001;74:181–7. doi: 10.1016/s0378-8741(00)00371-8. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PP, Srimal RC, Avasthi K, Garg N, Chandre T, Bhakuni DS. Anti-allergic activity of alkyl substituted pyrazolo[3,4-d]pyrimidine (compound 88-765) Ind J Exp Biol. 1995;33:38–40. [PubMed] [Google Scholar]
- 9.Gupta RJ, Skelton FR. The role of mast cells in cadmium chloride-induced injury in mature rat testis. Arch Pathol. 1968;85:89–93. [PubMed] [Google Scholar]
- 10.Rathinum K, Mohanan PV, Lizzy M. Comparative studies on mesenteric mast cells of rats, mice, rabbits, guinea pigs, chicken and frogs. Biomedicine. 1991;11:17–9. [Google Scholar]
- 11.Taylor M, Reide P. London: Mosby; 1998. Immune system; Allergic disorders and drug therapy. [Google Scholar]
- 12.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 13.Van Dyke K, Head RJ. Modern Pharmacology. 5th ed. Little Brown and Company; 1998. Histamine and histamine antagonists. [Google Scholar]
- 14.Rabson A, Roitt IM, Delves PJ. Really Essential Medical Immunology. 2nd ed. Blackwel Publishing; 2005. [Google Scholar]
- 15.Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53:121–33. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 16.Wagner H, Bladt S. Plant Drug Analysis-A Thin Layer Chromatographic Atlas. Springer-Verlag Berlin Heidelberg; 1996 [Google Scholar]
- 17.Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP. Anti-inflammatory activity of natural products: I. Triterpenoids. Eur J Pharmacol. 1969;6:67–70. doi: 10.1016/0014-2999(69)90067-3. [DOI] [PubMed] [Google Scholar]
- 18.Pandurangana A, Khosaa RL, Hemalathab S. Evaluation of Anti-inflammatory and Analgesic Activity of Root Extract of Solanum trilobatum Linn. Iranian J Pharma Res. 2008;7:217–21. [Google Scholar]
- 19.Sini H, Devi KS. Antioxidant activities of the chloroform extract of Solanum trilobatum. Pharm Biol. 2004;42:462–6. [Google Scholar]
- 20.Emmanuel S, Ignacimuthu S, Perumalsamy R, Amalraj T. Anti-inflammatory activity of Solanum trilobatum. Fitoterapia. 2006;77:611–2. doi: 10.1016/j.fitote.2006.09.009. [DOI] [PubMed] [Google Scholar]


