Abstract
Anticonvulsant and anxiolytic activities of leaf extracts and fraction of Ocimum gratissimum L. (Lamiaceae) were studied using seizures induced by pentylenetetrazol and open-field tests in mice. The results showed that the extracts and fraction increased the latency of tonic and tonic-clonic seizures and death and elicited 50% protection against mortality. In the open-field test, the extracts and fraction decreased the frequency of line crossing, center square entries, rearing against a wall and grooming, whereas grooming duration and freezing frequency and duration were increased. Acute toxicity test in mice gave an oral LD50 greater than 5000 mg/kg for the methanol extract. These findings suggest that extracts of this plant possess anticonvulsant and anxiolytic-like properties.
Keywords: Anticonvulsant, anxiolytic, Ocimum gratissimum, open field
INTRODUCTION
Ocimum gratissimum L. (Lamiaceae) is a small shrub commonly known as “scent leaf,” “tea bush” or “fever plant.” In Nigeria, it is variously called “Nchuanwu,” “Ahinji,” “Ahigbu” (Igbo), “Efirin” (Yoruba), “Ihiri eziza” (Bini), “Dai doya tagida” (Hausa) or “Ntion” (Efik) and is found in the wild or cultivated throughout the tropics and subtropics. In West Africa, O. gratissimum is commonly found around village huts and gardens[1] and cultivated for medicinal and culinary purposes. The leaves have strong aromatic odor and are popularly used to flavor soup and spice meat, especially game. In southeastern part of Nigeria and beyond, the leaves popularly serve as indispensable flavoring agent in soups, especially “pepper soup,” and other such meals. In traditional medicine practice, it is used in the treatment of diarrhea,[1,2] as a febrifuge and component of anti-malaria remedies,[3] mosquito/insect repellant, stomachic and general tonic, antiseptic, in wound dressing, skin infections, conjunctivitis and bronchitis. An infusion of the leaves, called ‘Ocimum tea,’ is dispensed as a remedy for fever and diaphoresis. [1] The roots are used as sedative for children.[4] Extract of the crushed leaves is an excellent remedy for cough. In southeastern parts of Nigeria, in addition to serving culinary purposes, the leaves are also used for the treatment of convulsive disorders.
Experimental studies showed that extracts of this plant relaxed intestinal smooth muscle,[5] exhibited antinociceptive effect,[6,7] contracted the guinea pig ileum and rat colon, raised mean arterial pressure in rats[1] and lowered blood glucose in diabetic rats.[8] The volatile oil has been credited with antimicrobial, anthelminthic and insect-repellant properties,[1] while the essential oil exhibited sedative and anxiolytic activities.[9] The essential oil also protected mice against tonic seizures induced by maximum electroshock but was not effective against pentylenetetrazol (PTZ)-induced seizures.[9]
Several constituents have been identified in oil from the leaves of this plant. Eugenol, a monoterpene, has been severally identified as the dominant volatile constituent. [10–13] Also present are the monoterpenes-1, 8-cineole,[12] β-pinene, cis-Ocimene, trans-Ocimene, camphor and methyl eugenol; and the sesquiterpenes- trans-Caryophyllene and Germacrene-D,[13] as well as thymol,[11,14] xanthones and lactones.[1]
Although an earlier study has demonstrated the anti -convulsant, sedative and anxiolytic properties[9] of essential oil of the leaves, these activities are not clearly attributable to any specific constituent of the oil. Also, the neuropharmacological effects of non-oil constituents of the leaves are not known. Consequently, in accordance with its use in traditional medicine practice in southeastern Nigeria, we studied the effects of the leaf extracts and fraction on PTZ-induced seizures and paradigms of anxiety and depression in mice.
MATERIALS AND METHODS
Drugs
Diazepam (Valium™, Roche)
Chemicals, solvents and reagents
Ethanol (Fluka, Germany), methanol (Fluka, Germany), pentylenetetrazol (PTZ), petroleum ether (Fluka, Germany), Tween 80.
Equipment
Soxhlet (Staffordshire, ST 150BG; England), rotary evaporator (Staffordshire, ST 150BG; England), open-field apparatus (A plexiglass box measuring 72 × 72 cm with 36-cm high walls. The walls and the floor were painted white. Blue lines, drawn under the clear plexiglass floor with a marker, divided the floor into 16 squares (18 × 18 cm). A central square of equal size was drawn in the middle of the maze) and video camera.
Animals
Adult male Swiss albino mice (22-30 g) bred in the laboratory animal facility of the Department of Pharmacology and Toxicology, University of Nigeria, Nsukka, were used for the study. The animals were maintained freely on standard pellets and water and allowed 2 weeks acclimatization period before commencement of studies. All animal experiments were in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals (Pub. No. 85-23, revised 1985).
Collection of plant material and preparation of extract
Fresh leaves of O. gratissimum were collected in May 2007 from Orba, Enugu State, Nigeria. The plant material was identified and authenticated by Mr. A. Ozioko of the International Centre for Ethnomedicines and Drug Development (InterCEDD), Nsukka, Nigeria. The leaves were cut into smaller pieces, dried under shade for 5 days and pulverized to coarse powder using a manual blender. The leaf powder (250 g) was extracted with methanol by continuous extraction in a soxhlet. A fresh batch of leaf powder (750 g) was successively extracted with petroleum ether (40°C-60°C) and methanol in a soxhlet. The extracts were concentrated in a rotary evaporator at 40°C-50°C under reduced pressure to afford the methanol extract (ME: 19.5 g; 7.8% w/w), petroleum ether extract (PE: 9.48 g; 1.26% w/w) and methanol fraction (MF: 11.31 g; 1.51% w/w). The extracts and fraction were subjected to phytochemical analysis for constituent identification using standard procedures.[15,16]
Acute toxicity test
The acute toxicity and lethality of ME were determined in mice using the method described by Lorke.[17] Briefly, nine mice randomly divided into three groups (n = 3) were orally administered 10, 100 and 1000 mg/kg of ME, respectively, and observed for 24 hours for death. Since no death occurred, 1600, 2900 and 5000 mg/kg of ME was administered to a fresh batch of animals (n = 1) and the number of deaths in 24 hours recorded. The LD50 was calculated as the geometric mean of the highest nonlethal dose and the lowest lethal dose.[17]
Pentylenetetrazol-induced seizure test
The anticonvulsant activity of ME, PE and MF was evaluated using the pentylenetetrazol-induced seizure in mice. Adult male albino mice (22-30 g) were randomly divided into eight groups (n = 6). Animals in groups I-VI received oral administration of ME, PE and MF (200 and 400 mg/kg), respectively, while groups VII and VIII received diazepam (1 mg/kg p.o.) and 10% Tween 80 (10 mL/kg p.o.), respectively. Thirty minutes later, pentylenetetrazol (PTZ) (70 mg/kg i.p.) was administered to each animal. The animals were observed for 60 minutes for seizures; an episode of clonic spasm that persisted for a minimum of 30 seconds was taken as a threshold convulsion. Animals devoid of threshold convulsion and without subsequent death during the 60 minutes of observation were considered protected.[18] The onset and duration of seizures, as well as quantal protection, were recorded for each group.
Open field test
The effects of ME, PE and MF on locomotor and exploratory activities, as well as grooming behavior in the open field,[19] were investigated. Adult male Swiss albino mice (19-30 g) were selected at random and divided into groups (n = 6). Each group received oral administration of one of ME, PE or MF (200 or 400 mg/kg) suspended in Tween 80 (10% v/v). The control groups received the vehicle (10 mL/kg) or diazepam (1 mg/kg). Thirty minutes later, each mouse was placed in the center square of the open field and observed for 5 minutes with the aid of video camera. The behavioral parameters recorded included line crossing, center square entries, rearing and grooming behaviors. The floor of the open field was cleaned with 70% ethanol and allowed to dry between tests.
Statistical analysis
Data obtained was analyzed using one-way ANOVA, and the results were expressed as mean ± SEM. Means were compared using LSD post hoc test and differences between treatment and control groups accepted as significant at P < 0.05.
RESULTS
Acute toxicity and lethality (LD50) of methanol extract
The acute toxicity test showed that oral administration of ME caused no death in the two stages of the test. The oral LD50 of ME in mice was therefore greater than 5000 mg/kg.
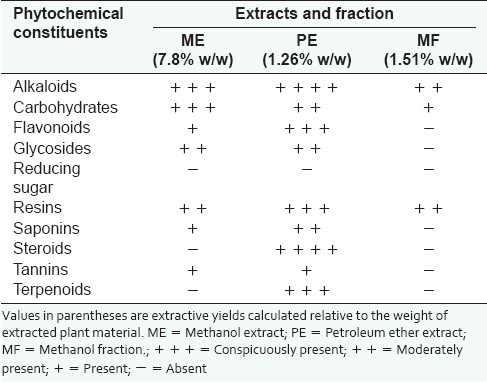
Phytochemical constituents of extracts and fraction
Phytochemical tests showed that ME tested positive to alkaloids, carbohydrates, flavonoids, glycosides, resins, saponins and tannins. Petroleum ether extract (PE) gave positive reactions for steroids and terpenoids, in addition to all the constituents of ME. The methanol fraction (MF) tested positive to alkaloids, carbohydrates and resins [Table 1].
Table 1.
Phytochemical constituents of the extracts and fraction
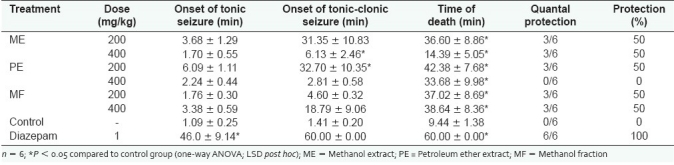
Effect of extracts and fraction on pentylenetetrazol-induced seizures
The extracts and fraction elicited a non-dose-related increase in the latency of tonic and tonic-clonic seizures and death. They also offered 50% protection of treated mice against seizure-induced mortality [Table 2].
Table 2.
Effect of extracts and fraction on pentylenetetrazol-induced seizures
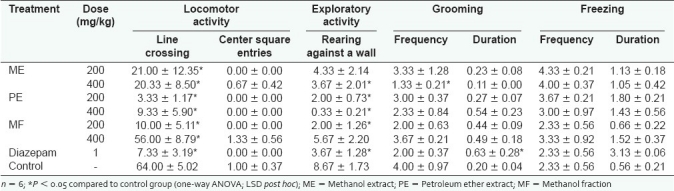
Effect of extracts and fraction on locomotion and exploratory activities
The extracts and fraction significantly (P < 0.05) decreased the frequency of line crossing in a non-dose-related manner. The frequencies of center square entries, rearing against a wall and grooming were also reduced, whereas grooming duration and freezing frequency and duration were increased [Table 3].
Table 3.
Effect of extracts and fraction on behavior in the open field
DISCUSSION
Although the popularity of O. gratissimum is derived mainly from its culinary uses, the medicinal value has long been recognized. In this study, extracts of the leaves exhibited anticonvulsant activity by delaying the onset of PTZ-induced seizures and protecting treated mice from mortality induced by seizures. PTZ induces convulsion by antagonizing the γ-aminobutyric acid (GABA)A receptor chloride (Cl)-channel complex[20] to attenuate GABA-dependent inhibition. Drugs protecting against tonic-clonic seizures induced by PTZ are considered useful in controlling myoclonic and absence seizures in humans.[21] Thus, demonstration of activity in this seizure model suggests that the plant possesses anticonvulsant activity, which may underlie its traditional use in the treatment of convulsive disorders. Although the effect of the extracts and fraction on GABA was not evaluated in this study, antagonism of PTZ-induced seizures suggests possible enhancement of GABAergic transmission consistent with general depression of the central nervous system (CNS). An earlier study showed that the essential oil was not effective in protecting against PTZ seizures. In this study, however, the result has shown that constituents of the extracts and fraction protected treated mice against PTZ seizures. Thus, it is likely that constituents other than those of the essential oil are responsible for the anticonvulsant activity of leaves of this plant.
Evaluation of the effect of the extracts and fraction on paradigms of depression and anxiety in the open field showed decreased locomotor and exploratory activities and increased grooming and freezing behaviors. Reduction in locomotor and exploratory activities may derive from reduced excitability of the central nervous system[22–24] due likely to central depression. Grooming behavior is a displacement response expected to be displayed in a novel environment. Reduced grooming behavior connotes reduced stress. Therefore, the reduction in grooming frequency and increase in the duration may indicate reduced stress consistent with anxiolytic-like effect. Anxiety is also associated with augmented autonomic activity resulting in increased defecation and urination.[24] Although the extracts and fraction caused varying effects on defecation and urination (data not shown), the validity of these parameters as appropriate measures of emotionality remains controversial.[19,25,26]
Solvent-guided extraction was performed to relate neurological activity to constituents revealed by phyto chemical analysis. However, the extent of separation of the leaf constituents achieved by solvent-guided extraction in this study was not distinct enough to permit the assignment of activity to any constituent or group of constituents. Earlier identification of high levels of eugenol in the essential oil of this plant coupled with the demonstration of its anticonvulsant and sedative properties[27] fuelled speculations that it could account for the anticonvulsant activity. Despite its high content of eugenol, the essential oil was not effective in protecting mice against PTZ-induced seizures.[9] Synergism among different constituents such as linalool[28,29] and myrcene,[30,31] shown to possess sedative and/or anticonvulsant effects, and sesquiterpenes has been suggested.[9] Thus, the anticonvulsant activity is not entirely due to the essential oil, which suggests a possible role for other phytochemical constituents such as those present in the leaf extracts and fraction.
The high LD50 value from acute toxicity studies showed that constituents of the leaves may be generally regarded as safe. This high degree of relative safety is consistent with the use of the plant's leaves in food. It is however not known if consumption of the leaves in food predisposes to interaction with sedatives/anxiolytics or anticonvulsants on concurrent use.
CONCLUSION
Findings from this study showed that leaves of O. gratissimum contain constituents which possess anticonvulsant and anxiolytic-like activities. Studies aimed at isolating the anticonvulsant constituents are ongoing.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Iwu MM. Handbook of African medicinal plants. Florida: CRC Press Inc. Boca Raton; 1993. [Google Scholar]
- 2.Dalziel JM. Useful plants of West tropical Africa. London: Crown Agents for Overseas Governments; 1956. [Google Scholar]
- 3.Oliver B. Medicinal plants in Nigeria. Ibadan, Nigeria: Nigerian College of Arts, Science and Technology; 1960. [Google Scholar]
- 4.Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz M, Jr, Tien OS, Kakinami SH, et al. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia. 2002;73:69–91. doi: 10.1016/s0367-326x(01)00362-8. [DOI] [PubMed] [Google Scholar]
- 5.Madeira SV, Matos FJ, Leal-Cardoso JH, Criddle DN. Relaxant effects of the essential oil of Ocimum gratissimum on isolated ileum of the guinea-pig. J Ethnopharmacol. 2002;81:1–4. doi: 10.1016/s0378-8741(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 6.Aziba PI, Bass D, Elegbe Y. Pharmacological investigation of Ocimum gratissimum in rodent for analgesic activity. Phytother Res. 1999;13:427–9. doi: 10.1002/(sici)1099-1573(199908/09)13:5<427::aid-ptr467>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Rabelo M, Souza EP, Soares PM, Miranda AV, Matos FJ, Criddle DN. Antinoceptive properties of the essential oil of Ocimum gratissimum L.(Labiatae) in mice. Braz J Med Biol Res. 2003;36:521–4. doi: 10.1590/s0100-879x2003000400016. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed A, Tanko Y, Okasha MA, Magaji RA, Yaro AH. Effects of aqueous leaves extract of Ocimum gratissimum on blood glucose levels of streptozocin-induced diabetic Wistar rats. Afr J Biotech. 2007;6:2087–90. [Google Scholar]
- 9.Freire CM, Marques MO, Costa M. Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L.essential oil. J Ethnopharmacol. 2006;105:161–6. doi: 10.1016/j.jep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura CV, Ueda-Nakamura T, Bando E, Melo AF, Cortez DA, Dias Filho BP. Antibacterial activity of Ocimum gratissimum L.essential oil. Mem Inst Oswaldo Cruz. 1999;94:675–8. doi: 10.1590/S0074-02761999000500022. [DOI] [PubMed] [Google Scholar]
- 11.Iwalokun BA, Gbenle GO, Adewole TA, Akinsinde KA. Shigellocidal properties of three Nigerian medicinal plants: Ocimum gratissimum, Terminalia avicennoides and Momordia balsamina. J Health Popul Nutr. 2001;19:331–5. [PubMed] [Google Scholar]
- 12.Vieira RF, Grayer RJ, Paton A, Simon JE. Genetic diversity of Ocimum gratissimum L based on volatile oil constituents, flavonoids and RAPD markers. Biochem Syst Ecol. 2001;29:287–04. doi: 10.1016/s0305-1978(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 13.Matasyoh LG, Matasyoh JC, Wachira FN, Kinyua MG, Muigai AWT, Mukiama TK. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L growing in Eastern Kenya. Afr J Biotech. 2007;6:760–5. [Google Scholar]
- 14.Keita SM, Vincent C, Jean-Pierre S, Belanger A. Essential oil composition of Ocimum basilicum L, O. gratissimum L, and O. suave L in the Republic of Guinea. Flav Frag. 2000;15:339–41. [Google Scholar]
- 15.Harborne JB. Phytochemical methods. London: Chapman and Hall; 1973. [Google Scholar]
- 16.Trease GE, Evans WC. Textbook of Pharmacognosy. 12th ed. United Kingdom: Balliere Tindall; 1983. [Google Scholar]
- 17.Lorke D. A new approach of practical acute toxicity testing. Arch Toxicol. 1983;54:272–89. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 18.Akah PA, Sampson A, Gamaniel K, Wambebe C. Effect of coconut water on the activity of some centrally acting drugs. Indian Drugs. 1998;35:693–5. [Google Scholar]
- 19.Archer J. Tests for emotionality in rats and mice: A review. Anim Behav. 1973;21:205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 20.Corda MG, Giorgi O, Longoni B, Orlandi M, Biggio G. Decrease in the function of γ-aminobutyric acid coupled chloride channel produced by repeated administration of pentylenetetrazole in rats. J Neurochem. 1990;55:1216–21. doi: 10.1111/j.1471-4159.1990.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 21.Nisar M, Khan I, Simjee SU, Gilani AH, Obaidullah, Perveen H. Anticonvulsant, analgesic and antipyretic activities of Taxus wallichiana Zucc. J Ethnopharmacol. 2008;116:490–4. doi: 10.1016/j.jep.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Ozturk Y, Aydine S, Baser KH, Berberoglu H. Effects of Hypericum perforatum L.and Hypericum calycinum L. extracts on the central nervous system in mice. Phytomed. 1996;3:139–46. doi: 10.1016/S0944-7113(96)80027-4. [DOI] [PubMed] [Google Scholar]
- 23.Perez RM, Perez JA, Garcia LM, Sossa H. Neuropharmacological activity of Solanum nigrum fruit. J Ethnopharmacol. 1998;62:43–8. doi: 10.1016/s0378-8741(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 24.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;46:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 25.Walsh RN, Cummins RA. The open-field test: A critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 26.Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–60. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 27.Dallmeier K, Carlini EA. Anesthetic, hypotermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogues. Pharmacology. 1981;22:113–27. doi: 10.1159/000137479. [DOI] [PubMed] [Google Scholar]
- 28.Elisabetsky E, Brum LF, Souza DO. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine. 1999;6:107–13. doi: 10.1016/s0944-7113(99)80044-0. [DOI] [PubMed] [Google Scholar]
- 29.Brum LF, Elisabetsky E, Souza D. Effects of linalool on MK801 and [(3)H] muscimol binding in mouse cortical membranes. Phytother Res. 2001;15:422–5. doi: 10.1002/ptr.973. [DOI] [PubMed] [Google Scholar]
- 30.Viana GS, do Vale TG, Silva CM, Matos FJ. Anticonvulsant activity of essential oil and active principles from chemotypes of Lippia alba (Mill) NE Brown. Biol Pharm Bull. 2000;23:1314–7. doi: 10.1248/bpb.23.1314. [DOI] [PubMed] [Google Scholar]
- 31.do Vale TG, Furtado EC, Santos JG, Jr, Viana GS. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill) NE Brown. Phytomedicine. 2002;9:709–14. doi: 10.1078/094471102321621304. [DOI] [PubMed] [Google Scholar]


