Abstract
Methanol extracts from S. jambos leaves were tested for antimicrobial activity and toxicity. S. jambos leaf extract inhibited the growth of 4 of the 14 bacteria tested (29%). Both gram-positive and gram-negative bacterial growths were inhibited by S. jambos leaf extract, although gram-positive bacteria appeared more susceptible. Two of the 10 gram-negative bacteria (20%) and 2 of the 4 gram-positive bacteria (50%) tested had their growths inhibited by the extract. The leaf extract also proved to be toxic in the Artemia franciscana bioassay, with a 48-h LC50 of 387.9 ± 38.8 µg/mL, making it slightly more toxic than Mevinphos (505.3± 37.7 µg/mL) and approximately 5-fold less toxic than potassium dichromate (80.4 ± 4.3 µg/mL). Whilst potassium dichromate's LC50 remained constant across the 72-hour test period (24-h LC50, 86.3 ± 5.1; 72-h LC50, 77.9 ± 4.9), the extract and Mevinphos LC50 values decreased by 72 hours (87.0 ± 11.3 µg/mL and 103.9 ± 12.8 µg/mL, respectively), indicating their similar levels of toxicity in the assay.
Keywords: Antibacterial, medicinal plants, methanolic extracts, phytotoxicity, rose apple, Syzygium jambos
INTRODUCTION
Plants have long been used as medicines for treating a variety of different diseases and complaints. Phytotherapy in Asia is particularly widespread. Plant preparations and medications continue to be used in the treatment of numerous disorders, including eczema, malaria, respiratory disorders and infectious diseases.[1] For some of these plant treatments, the antimicrobial activity has been proven; however, for many plant-based antiseptics, the evidence is anecdotal or, at best, epidemiological. Many traditionally used antiseptic agents have yet to be subjected to thorough scientific investigation.
Syzygium jambos Alston (syn. Eugenia jambos L.; Jambosa jambos Millsp.; Jambosa vulgaris DC.; Caryophyllus jambos Stokes) is an evergreen tree of the family Myrtaceae. It is native to Southeast Asia but has been naturalized in India, especially in the state of Kerala, where it is grown both for its fruit and for its medicinal properties. S. jambos has a long history of use in Indian traditional medicine for the treatment of numerous ailments. The fruit has been used as a tonic for the brain and liver and as a diuretic.[2] The flowers are believed to reduce fever, and the seeds were used to treat diarrhea, dysentery and catarrh.[2] In South-American cultures, the seeds have additionally been used as an anesthetic,[2] and recent studies have shown S. jambos extracts to have a similar analgesic efficacy to morphine in rats.[3] S. jambos leaf decoctions were also used traditionally in the treatment of diabetes,[2] although some studies have shown leaf extracts to be ineffective as antihyperglycemic agents.[4] In Indian traditional medicinal systems, S. jambos leaves were also used as a diuretic, an expectorant in the treatment of rheumatism; to treat sore eyes; and as a febrifuge.[2] Bark of the S. jambos tree is used to treat asthma, bronchitis and hoarseness.[2] Cuban healers have also used the root to treat epilepsy.[2] S. jambos leaf extracts have also been shown to possess antiviral activity towards herpes simplex type 1 and type 2 and towards vesicular somatitis virus.[5,6]
The antiseptic properties of some members of the genus Syzygium have been extensively studied. In the commercially most important species Syzygium aromaticum (clove), the antiseptic properties are well known. Numerous studies have reported on the antibacterial[7] and antifungal[8] activities of oils and extracts from this plant. Other Syzygium species from India (Syzygium lineare, Syzygium cumini and Syzygium travancoricum) [9,10] have also been shown to have antimicrobial activity. Two Australian species have also recently been shown to possess antibacterial activity.[11] However, despite its numerous traditional medicinal uses, the antiseptic properties of S. jambos remain largely unexamined. Some studies have demonstrated the antibacterial[12] and antifungal[13] activities of S. jambos bark extracts. To the best of our knowledge, no studies have examined the antibacterial nature of S. jambos leaf extracts. Therefore, the current study reports on the antibacterial properties of S. jambos leaf extracts, as well as examining their toxicity, to determine their potential as antibiotic agents.
MATERIALS AND METHODS
Plant material
Collection of plant samples
Syzygium jambos leaves were a gift from Mervyn Cooper of the Queensland Tropical Fruit Association. Leaves were obtained from a single tree, washed in deionized water and processed within 4 hours of collection.
Preparation of crude extracts
Syzygium jambos leaves were dried in a Sunbeam food dehydrator, and the dried material was ground to a coarse powder. One gram of each of the dried plant materials was extracted extensively in 50 mL methanol (Ajax, AR grade) for 24 hours at 4°C with gentle shaking. The extract was filtered through filter paper (Whatman no. 54) under vacuum, followed by drying by rotary evaporation in an Eppendorf concentrator 5301. The resultant pellet was dissolved in 10 mL 20% methanol. The extract was passed through 0.22-µm filter (Sarstedt) and stored at 4°C.
Antibacterial screening
Test microorganisms
All microbial strains were obtained from Michelle Mendell and Tarita Morais, Griffith University, Australia. Stock cultures of Aeromonas hydrophilia, Alcaligenes faecalis, Bacillus cereus, Citrobacter freundii, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas fluorescens, Salmonella newport, Serratia marcescens, Shigella sonnei, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pyogenes were subcultured and maintained in nutrient broth at 4°C.
Evaluation of antimicrobial activity
Antimicrobial activity of all plant extracts was determined using a modified Kirby-Bauer[14] disc diffusion method. Briefly, 100µL of the test bacteria was grown in 10 mL of fresh media until they reached a count of approximately 108 cells/mL. One hundred microliters of microbial suspension was spread onto nutrient agar plates.
The extract components were tested using 5-mm sterilized filter paper discs. Discs were impregnated with 10µL of the test sample, allowed to dry and placed onto inoculated plates. The plates were allowed to stand at 4°C for 2 hours before incubation with the test microbial agents. Plates inoculated with Alcaligenes faecalis, Aeromonas hydrophilia, Bacillus cereus, Citrobacter freundii, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas fluorescens, Serratia marcescens were incubated at 30°C for 24 hours, and then the diameters of the inhibition zones were measured in millimeters. Plates inoculated with Escherichia coli, Salmonella newport, Shigella sonnei, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pyogenes were incubated at 37°C for 24 hours, and then the diameters of the inhibition zones were measured. All measurements were to the closest whole millimeter. Each antimicrobial assay was performed in at least triplicate. Mean values are reported in this study. Standard discs of ampicillin (2µg) and chloramphenicol (10µg) were obtained from Oxoid Ltd. and served as positive controls for antimicrobial activity. Filter discs impregnated with 10µL of distilled water were used as a negative control.
Minimum inhibitory concentration determination
The minimum inhibitory concentration (MIC) of the S. jambos extract was determined by the disc-diffusion method across a range of doses. The plant extracts were diluted in deionized water across a concentration range of 5 mg/mL to 0.1 mg/mL. Discs were impregnated with 10µL of the test dilutions, allowed to dry and placed onto inoculated plates. The assay was performed as outlined above, and graphs of the zone of inhibition versus concentration were plotted for each extract. Linear regression was used to calculate the MIC values.
Toxicity screening
Reference toxins for toxicity screening
Potassium dichromate (K2Cr2O7) (AR grade, Chem-Supply, Australia) was prepared as a 1.6 mg/mL solution in distilled water and was serially diluted in artificial seawater for use in the Artemia franciscana nauplii bioassay. Mevinphos (2-methoxycarbonyl-1-methylvinyl dimethyl phosphate) was obtained from Sigma-Aldrich as a mixture of cis (76.6%) and trans (23.0%) isomers and prepared as a 4 mg/ mL stock in distilled water. The stock was serially diluted in artificial seawater for use in the bioassay.
Artemia franciscana nauplii toxicity screening
Toxicity was tested using the Artemia franciscana nauplii lethality assay developed by Meyer et al.[15] for the screening of active plant constituents with the following modifications. Artemia franciscana Kellogg cysts were obtained from North American Brine Shrimp, LLC, USA (harvested from the Great Salt Lake, Utah). Synthetic seawater was prepared using Reef Salt, AZOO Co., USA. Seawater solutions at 34 g/L distilled water were prepared prior to use. Two grams of A. franciscana cysts were incubated in 1 L synthetic seawater under artificial light at 25°C, 2000 lux with continuous aeration. Hatching commenced within 16-18 hours of incubation. Newly hatched A. franciscana (nauplii) were used within 10 hours of hatching. Nauplii were separated from the shells and remaining cysts and were concentrated to a suitable density by placing an artificial light at one end of their incubation vessel, and the nauplii-rich water closest to the light was removed for biological assays. Four hundred microliters of seawater containing approximately 40 (mean, 39.8; n = 175; SD, 19.0) nauplii was added to wells of a 48-well plate and immediately used for bioassay. The plant extracts were diluted to 2 mg/mL in seawater for toxicity testing, resulting in a 1 mg/mL concentration in the bioassay. Four hundred microliters of diluted plant extracts and the reference toxins were transferred to the wells and incubated at 25 ± 1°C under artificial light (1000 lux). A negative control (400µL seawater) was run in at least triplicate for each plate. All treatments were performed in at least triplicate. The wells were checked at regular intervals and the number of dead was counted. The nauplii were considered dead if no movement of the appendages was observed within 10 seconds. After 72 hours, all nauplii were sacrificed and counted to determine the total number per well. The LC50 with 95% confidence limits for each treatment was calculated using probit analysis.[16]
RESULTS
Antibacterial activity
One gram of powdered dried S. jambos leaves was extensively extracted with methanol and dried under vacuum, resulting in 656 mg of dried extracted material. Resuspension of the dried fraction in 10 mL of 20% methanol resulted in the crude test extract concentration of 65.6 mg/mL. The extract was diluted to a concentration of 15 mg/mL for testing for antimicrobial activity. Ten microliters of extract (150µg) was tested in the disc-diffusion assay against 14 bacteria [Table 1]. The S. jambos leaf extract inhibited the growth of 4 of the 14 bacteria tested (28.6%).
Table 1.
Antibacterial activity of Syzygium jambos leaf extract
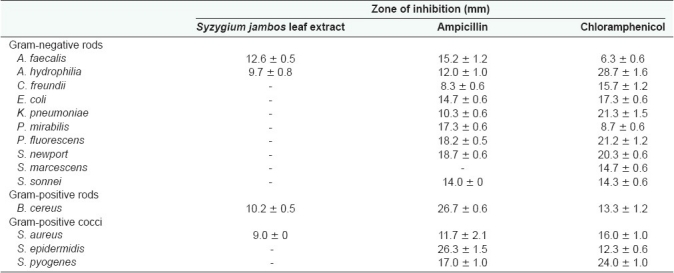
Numbers indicate the mean diameters of inhibition (mm) of triplicate experiments ± standard deviation. ‘-’ indicates no growth-inhibition. Chloramphenicol (10µg) and ampicillin (2µg) were used as positive controls.
Both gram-positive and gram-negative bacteria were affected by S. jambos leaf extract, although the gram-positive bacteria appeared more susceptible. Of the 10 gram- negative bacteria tested, only A. faecalis and A. hydrophilia (20%) were inhibited by S. jambos leaf extract. The leaf extract also inhibited the growth of 2 (B. cereus and S. aureus) of the gram-positive bacteria tested (50%).
The relative level of antibacterial activity was evaluated by determining the MIC values for each extract against the bacteria which were shown to be susceptible by disc- diffusion assays. MICs were evaluated in the current studies by disc diffusion across a range of concentrations. This has previously been determined to be a valid method of MIC determination, as MIC values determined by disc diffusion correlate well with those determined by broth-dilution assays.[17] The antibacterial activity was strongest against gram-positive bacteria, especially B. cereus (as determined by the minimum inhibitory concentration) [Table 2].
Table 2.
Minimum inhibitory concentrations (µg/mL) of Syzygium jambos extract against susceptible bacteria
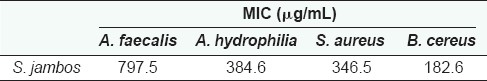
Numbers indicate the mean MIC values of at least triplicate determinations.
Quantification of toxicity
The S. jambos leaf extract was diluted to 2000 mg/mL in artificial seawater for toxicity testing, resulting in a 1000-mg/ mL concentration in the Artemia franciscana lethality bioassay [Figure 1a]. For comparison, the reference toxins potassium dichromate (800µg/mL) [Figure 1b] and Mevinphos (2000µg/mL) [Figure 1c] were also tested in the Artemia franciscana lethality bioassay. Both reference toxins were more rapid in their induction of the onset of mortality than the S. jambos leaf extract at the concentrations tested. For the reference toxins, the induction of mortality was seen within the first 3 hours of exposure. One hundred percent mortality was evident following 4 hours of exposure. In contrast, a period of 6 hours was required for S. jambos extract to induce the onset of mortality, and 24 hours was required to kill 100% of the brine shrimp.
Figure 1.
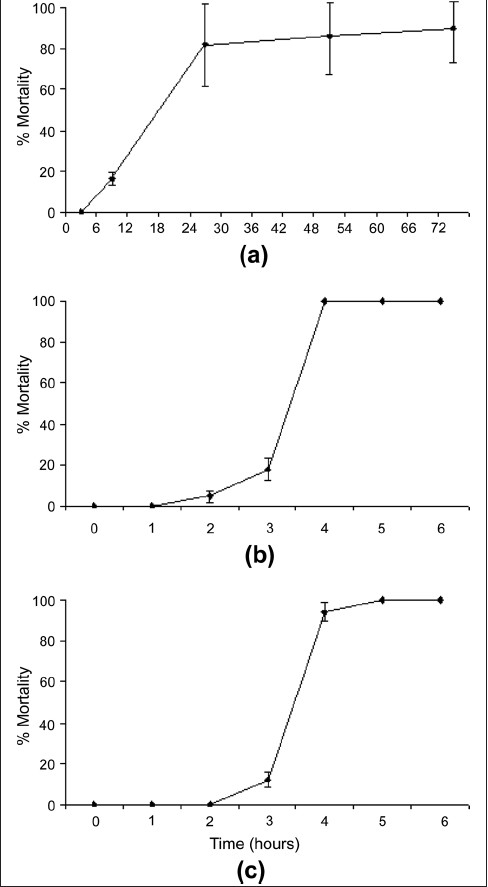
Brine shrimp lethality of (a) S. jambos leaf extract (1000 µg/ mL), (b) potassium dichromate (800 µg/mL) and (c) Mevinphos (2000 µg/mL). All bioassays were performed in at least triplicate and are expressed as mean ± standard deviation
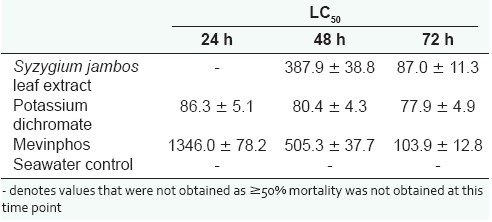
To determine the effect of toxin concentration on the induction of mortality, the extract was diluted in artificial seawater to test across the concentration range 1000 µg/ mL to 15µg/mL in the Artemia nauplii bioassay at 24, 48 and 72 hours [Figure 2]. Table 3 shows the LC50 values of S. jambos extracts towards A. franciscana. No 24- hour LC50 values are reported for the S. jambos extracts as less than 50% mortality was seen by this time for all concentrations tested. The S. jambos leaf extract was slightly more toxic than Mevinphos at 48 hours, with LC50 values of 387.9 ± 38.8 and 505.3 ± 37.7, respectively. Potassium dichromate was substantially more toxic (80.4 ± 4.3) at 48 hours. The extract and both reference toxins showed similar toxicity at 72 hours as seen by their LC50 values.
Figure 2.
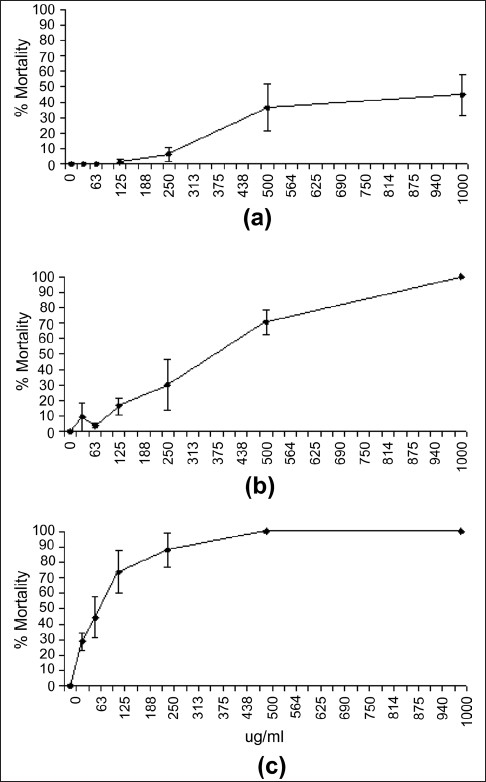
Dependence of A. franciscana mortality on S. jambos leaf extract concentration following exposure for (a) 24 h, (b) 48 h and (c) 72 h. All bioassays were performed in at least triplicate and are expressed as mean ± standard deviation
Table 3.
LC50 (95% confidence interval) for brine shrimp nauplii exposed to S. jambos leaf extract, the reference toxins potassium dichromate and Mevinphos and a seawater control
DISCUSSION
The current study reports on the antimicrobial activity and toxicity of S. jambos leaf methanolic extracts. The ability of S. jambos leaf extract to inhibit the growth of both gram-positive and gram-negative bacteria is in agreement with previous reports of the antibacterial activity of other Syzygium species[7–11] and with the reported antibacterial activity of S. jambos bark extracts.[12] The greater susceptibility of gram-positive bacteria seen in this study is in agreement with previously reported results for south-american,[18] African[19,20] and Australian[21] plant extracts. Results in this laboratory[11] have also confirmed the greater susceptibility of gram-positive bacteria towards other Australian plant extracts. The gram-negative bacterial cell wall outer membrane is thought to act as a barrier to many substances, including antibiotics.[22] The uptake of the S. jambos extract antibiotic agents by gram-negative bacteria is presumably affected by the cell wall outer membrane of some bacteria.
Individual S. jambos leaf components responsible for the extract's antiseptic potential were not identified in the current study. However, other reports have identified various bioactive components from S. jambos. Scientific studies have demonstrated the anti-inflammatory activity of leaf extracts in rats[23] and have indicated that the flavanoids myricetin and quercetin 3-O-b-D-xylopyranosyl (1-2) a-L-rhamnopyranosides are likely to be responsible for this activity.[24,25] Indeed, myricetin and quercetin 3-O-b-D-xylopyranosyl (1-2) a-L-rhamnopyranosides isolated during these studies proved to be more effective anti-inflammatory agents than phenylbutazone and indomethacine. S. jambos leaves have also been shown to contain 3,3%,4%-tri-O-methylellagic acid-4-O-b-D-glucopyranoside and 3,3%,4%-tri-O-methylellagic acid,[26] as well as several ellagitannins (pedunculagin, casuarinin, tellimagrandin I, strictinin, casuarictin, 2,3-HHDP-glucose and tellimagrandin II),[27] and a number of triterpenes such as friedelin,β-amyrin acetate, betulinic acid and lupeol.[13] Future studies need to focus on the purification and identification of the antibacterial and toxic components of S. jambos leaf extracts.
The findings reported here also indicate that S. jambos extracts display toxicity. This would impact on the usefulness of the extract as a medicinal antiseptic agent. Toxicity was assessed in this study with the test organism Artemia franciscana. Whilst toxicity towards A. franciscana has previously been shown to correlate well with toxicity towards human cells for some toxins,[28] further studies are required to determine whether this is also true for the S. jambos extracts. Toxic antibacterial extracts may be useful as nonmedicinal antibacterial agents (e.g., surface disinfectants). Likewise, toxic plant extracts may also still have medicinal potential even if they are not antimicrobial. McLaughlin et al.[28] have demonstrated that toxicity in the A. franciscana bioassay may indicate anticancer potential. Toxic extracts such as the S. jambos leaf extract should therefore also be tested against human cancer cell lines to determine their potential as anticancer drugs.
CONCLUSION
The results of this study support the traditional use of decoctions of S. jambos leaves as antiseptic agents. Furthermore, these findings indicate that S. jambos leaf extracts are worthy of further study due to their antibacterial activity. Further evaluation of the antibacterial properties of these extracts against a more extensive panel of microbial agents is warranted. Likewise, purification and identification of the bioactive components is needed to examine the mechanisms of action of these agents. Whilst the extracts examined in this report are promising as antimicrobial agents, caution is needed before these compounds can be applied for medicinal purposes and as food additives to inhibit spoilage. In particular, further toxicity studies using human cell lines are needed to determine the suitability of these extracts for these purposes.
Acknowledgments
Financial support for this work was provided by the School of Biomolecular and Physical Sciences, Griffith University, Australia. The authors would like to thank Mervyn Cooper of the Queensland Tropical Fruit Association for providing the S. jambos leaf specimens used in these studies.
Footnotes
Source of Support: School of Biomolecular and Physical Sciences, Griffith University, Australia.
Conflict of Interest: None declared.
REFERENCES
- 1.Hoareau L, DaSilva EJ. Medicinal plants: A re-emerging health aid. Electron J Biotechnol. 1999;2:56–70. [Google Scholar]
- 2.Morton J. Rose Apple. USA: Fruits of Warm Climates Florida Flair Books; 1987. [Google Scholar]
- 3.Avila-Peña D, Peña N, Quintero L, Suárez-Roca H. Antino ciceptive activity of Syzygium jambos leaves extract on rats. J Ethnopharmacol. 2007;112:380–5. doi: 10.1016/j.jep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira CC, Weinert LS, Barbosa DC, Ricken C, Esteves JF, Fuchs FD. Syzygium cumini (L.) Skeels in the treatment of type 2 diabetes. Results of a randomized, double-blind, doubledummy, controlled trial. Diabetes Care. 2004;27:3019–20. doi: 10.2337/diacare.27.12.3019-a. [DOI] [PubMed] [Google Scholar]
- 5.Athikomkulchai S, Lipipun V, Leelawittayanont T, Khanboon A, Ruangrungsi N. Anti-herpes simplex virus activity of Syzygium jambos. J Health Res. 2008;22:49–51. [Google Scholar]
- 6.Abad MJ, Bermejo P, Villar A. Antiviral activity of medicinal plant extracts. Phytother Res. 1997;11:198–202. [Google Scholar]
- 7.Arora DS, Kaur GJ. Antibacterial activity of some Indian medicinal plants. J Nat Med. 2007;61:313–7. [Google Scholar]
- 8.Park MJ, Gwak KS, Yang I, Choi WS, Jo HJ, Chang JW, et al. Antifungal activities of the essential oils of Syzygium aromaticum (L.) Mer. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J Microbiol. 2007;45:460–5. [PubMed] [Google Scholar]
- 9.Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafi PM, Rosamma MK, Jamil K, Reddy PS. Antibacterial activity of Syzygium cumini and Syzygium tra_ancoricum leaf essential oils. Fitoterapia. 2002;73:414–6. doi: 10.1016/s0367-326x(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 11.Cock IE. Antibacterial activity of selected Australian native plant extracts. Internet J Microbiol. 2008;4:2. [Google Scholar]
- 12.Djipa CD, Delmée M, Quetin-Leclercq J. Antimicrobial activity of bark extracts of Syzygium jambos(L.) Alston (Myrtaceae) J Ethnopharmacol. 2000;71:307–13. doi: 10.1016/s0378-8741(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 13.Kuiate JR, Mouokeu S, Wabo HK, Tane P. Antidermatophytic Triterpenoids from Syzygium jambos (L.) Alston (Myrtaceae) Phytother Res. 2007;21:149–52. doi: 10.1002/ptr.2039. [DOI] [PubMed] [Google Scholar]
- 14.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 15.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 16.Finney DJ. Probit Analysis. 3rd ed. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- 17.Gaudreau C, Girouard Y, Ringuette L, Tsimiklis C. Comparison of disc diffusion and agar dilution methods for erythromycin and ciprofloxacin susceptibility testing of Campylobacter jejuni subsp. Jejuni. Antimicrob Agents Chemother. 2007;52:1524–6. doi: 10.1128/AAC.00905-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso Paz E, Cerdeiras MP, Fernandez J, Ferreira F, Moyna P, Soubes M, et al. Screening of Uruguayan medicinal plants for antimicrobial activity. J Ethnopharmacol. 1995;45:67–70. doi: 10.1016/0378-8741(94)01192-3. [DOI] [PubMed] [Google Scholar]
- 19.Kudi AC, Umoh JU, Eduvie LO, Gefu J. Screening of some Nigerian medicinal plants for antibacterial activity. J Ethnopharmacol. 1999;67:225–8. doi: 10.1016/s0378-8741(98)00214-1. [DOI] [PubMed] [Google Scholar]
- 20.Vlietinck AJ, Van Hoof L, Totté J, Lasure A, Vanden Berghe D, Rwangabo PC, et al. Screening of a hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol. 1995;46:31–47. doi: 10.1016/0378-8741(95)01226-4. [DOI] [PubMed] [Google Scholar]
- 21.Palombo EA, Semple SJ. Antibacterial activity of traditional Australian medicinal plants. J Ethnopharmacol. 2001;77:151–7. doi: 10.1016/s0378-8741(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 22.Tortora GJ, Funke BR, Case CL. San Francisco: Benjamin Cummings; 2001. Microbiology: An Introduction. [Google Scholar]
- 23.Slowing K, Carretero E, Villar A. Anti-inflammatory activity of leaf extracts of Eugenia jambos in rats. J Ethnopharmacol. 1994a;43:9–11. doi: 10.1016/0378-8741(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 24.Slowing K, Söllhuber M, Carretero E, Villar A. Flavonoid glycosides from Eugenia jambos. Phytochemistry. 1994;37:255–8. doi: 10.1016/0031-9422(94)85036-4. [DOI] [PubMed] [Google Scholar]
- 25.Slowing K, Carretero E, Villar A. Anti-inflammatory compounds of Eugenia jambos. Phytother Res. 1996;10:S126–7. [Google Scholar]
- 26.Chakravarty AK, Das B, Sarkar T, Masuda K, Shiojima K. Ellagic acid derivatives from the leaves of Eugenia jambos Linn. Indian J Chem. 1998;37B:1316–8. [Google Scholar]
- 27.Okuda T, Yoshida T, Hatano T, Yazaki K, Ashida M. Ellagitannins of the Casuarinaceae, Stachyuraceae and Myrtaceae. Phytochemistry. 1982;21:2871–4. [Google Scholar]
- 28.McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf J. 1998;32:513–24. [Google Scholar]


