Abstract
The levels of some heavy metals in 27 medicinal plant species from Ghana were studied in order to evaluate their health implications. These plant species, especially those used in the treatment of diseases such as hypertension, diabetes and asthma may require long term usage. The metals were copper, zinc, iron, manganese, nickel and cadmium. Atomic Absorption Spectrophotometry (wet digestion) was used for the analyses, and content of metals per sample was expressed as percent µg/g. Daily total intake of these metals is discussed based on the recommended daily intake of the medicinal plants or their corresponding formulations. From the results of the study zinc, copper and cadmium were present in all the plant species examined. Manganese was present in all species except V. amygdalina. Iron was found in all except five species (82%), whilst nickel was (rather rare) detected in only eight (30%) of the plant species. Significant variations in metal content existed (P<0.05) among the medicinal plant species with respect to the heavy metals evaluated. The concentrations of copper, zinc, cadmium and manganese were within their respective maximum permissible daily levels. However, some species, especially Ocimum canum (8), Clausena anisata and Rauwolfia vomitoria had levels of iron higher than the maximum permissible level of 1000 µg/day and may require care to avoid iron toxicity. The results also highlighted the differences in contents of minerals in Lippia multiflora obtained from different locations in Ghana. The findings generally suggest that the use of these plant species for the management of diseases will not cause heavy metal toxicity and may be beneficial to the users in cases of micronutrient deficiency, as these metals were found to be present in readily bioavailable form.
Keywords: Health implications, heavy metals, herbal formulations, medicinal plant
INTRODUCTION
Medicinal plants play a major role in the health care sector of developing nations for the management of diseases. Thus herbal medicines have a prominent role to play in the pharmaceutical markets and health care sector of the 21st century.[1] Heavy metals as micronutrients are important for the proper functioning of vital organs in the body. For example, iron is a component of hemoglobin and other compounds used in respiration. Heavy metals are widespread in soil as a result of geo-climatic conditions and environmental pollution. Therefore, their assimilation and accumulation in plants is obvious. Together with other pollutants, heavy metals are discharged into the environment through industrial activity, automobile exhaust, heavy-duty electric power generators, municipal wastes, refuse burning and pesticides used in agriculture. [2] Human beings, animals and plants take up these metals from the environment through air and water. Heavy metals have the tendency to accumulate in both plants and human organs.
Since plants and animals are essential sources of micronutrients for man, it becomes necessary to monitor the levels in biological materials that are required by man for both dietary and medicinal purposes. This is because deficiency or excess of micronutrients can be factors of disease generation.
Even though a lot of phytochemical and bioactivity studies have been carried out on a number of medicinal plants in Ghana,[3] not much has been reported on the heavy metal contents of these plants. This study therefore sought to establish the presence, quantity and prevalence of six heavy metals (Cu, Zn, Fe, Mn, Ni and Cd) in 27 medicinal plants commonly used for the treatment, prevention and management of diseases in Ghana.
In view of the fact that micronutrients can be good, toxic or lethal depending on the dose, the study also evaluated the health implications of the heavy metals quantified, based on the recommended daily intake of medicinal plant decoctions.[4] The use of atomic absorption spectrophotometry for determination of the amounts of heavy metals in both organic and inorganic samples has been well reported.[5]
MATERIALS AND METHODS
Plant materials
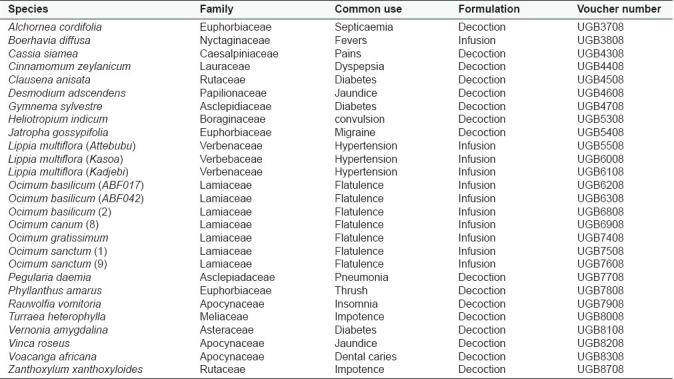
A total of 27 different medicinal plant species used in the study were collected from their natural habitat and authenticated at the Department of Botany, University of Ghana, where voucher specimen of each plant species is deposited [Table 1]. The leaves were air-dried for four days and later pulverized.
Table 1.
List of experimental plants used and their voucher numbers
Mineral analysis
An acid digest of each plant species was prepared by oxidizing 0.2 g of the powdered plant sample with a nitric/perchloric acid (2:1) mixture (10 ml). Aliquots of the mixture were used to estimate Fe, Cu, Zn, Mn, Ni and Cd by Analyst 400 Atomic Absorption Spectrometer. Each sample was analyzed thrice, and the data were reported as mean ± SD in µg/g.
Statistical analysis
The data were based on three replicates and subjected to analysis of variance. Standard errors of each individual nutrient of the samples were computed, and variations among the species were evaluated by least significance difference (LSD) at 5% level of probability (P = 0.05). Data analysis was conducted using the SAS Statistical Computer Package.
RESULTS AND DISCUSSION
From the study, zinc, copper and cadmium were present in all plant species examined. Manganese was present in all species except V. amygdalina. Iron was found in all except five species (82%), while nickel was (rather rare) detected in only eight of the plant species (30%) [Table 2].
Table 2.
Concentrations of heavy metals present in the medicinal plants
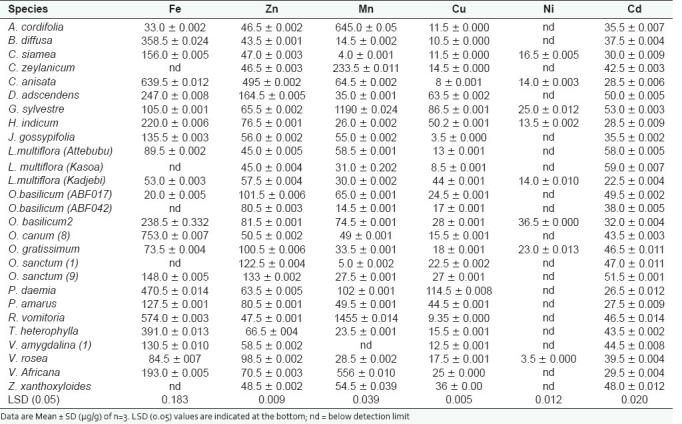
The iron content found in all the plant species examined was generally relatively high (20-753 µg/g), with the three highest concentrations found in O. canum 8, C. anisata and R. vomitoria, respectively. The maximum permissible level (MPL) of iron is 1000 µg/day.[6] In this study, however, majority of the plant species examined (60%) had iron content above the maximum permissible level per 10 g daily dose of plant material or its equivalent formulation. Iron salts have an astringent action resulting in irritation of the gastrointestinal mucosa, which gives rise to gastric discomfort, nausea, vomiting and diarrhea or constipation.[7,8] Therefore, the presence of these symptoms commonly associated with the intake of some medicinal plants may be due to iron toxicity.
Zinc content ranged from 43.5 µg/g in B. diffusa to 495.0 µg/g in C. anisata. Zinc is a component of many metalloenzymes, especially some enzymes which play a central role in nucleic acid metabolism.[9] Zinc is also a membrane stabilizer and a stimulator of the immune response.[10] Manifestations of acute zinc poisoning include nausea, vomiting, diarrhea, fever and lethargy.[7] The estimated safe and adequate daily intake of zinc is between 10,000 and 20,000 µg/day.[6] The zinc levels per 10 g of plant species were not more than 4950 µg, hence lower than the maximum permissible level and regarded as safe.
Manganese content was disproportionately very high in only a few species, including R. vomitoria (1455 µg/g) followed by G. sylvestre (1190 µg/g), A. cordifolia (645 µg/g) and V. africana (556 µg/g). The estimated safe and adequate daily dietary intake of manganese in adults is 11,000 µg/ day. [11] Therefore, most of the plant species examined (93%) can be said to have acceptable manganese content. Deficiency of manganese in humans may lead to immunodeficiency disorder, rheumatic arthritis in adults, disorder of bony cartilaginous growth in infants, as well as myocardial infarction and other cardiovascular diseases.[12]
Copper content was variable among the plant species, ranging from 8.0 to 114.5 µg/g, with G. sylvestre recording the highest amount. Copper is essential to the human body since it forms a component of many enzyme systems, such as cytochrome oxidase, lysyl oxidase and ceruloplasmin, an iron-oxidizing enzyme in blood. The observation of anemia in copper deficiency may probably be related to its role in facilitating iron absorption and in the incorporation of iron in hemoglobin.[6] However, copper could be toxic depending on the dose and duration of exposure. [7] The maximum permissible level (MPL) of copper is 12,000 µg/ day.[6] Therefore, the suggested average intake of about 10 g of plant material or its equivalent formulation gives a maximum of 1145 µg of copper per day.[4] This implies that the 27 medicinal plants evaluated contained safe levels of copper.
The MPL of nickel is 100 µg/day.[10] Although nickel was present in only eight species, all except V. rosea had nickel content higher than the permissible level. This however constitutes only 26%; the majority (74%) had nickel content within acceptable limits. Nickel exerts a potent toxic effect on peripheral tissues and on the reproductive system.[10] It also causes dose-related decreases in bone marrow cellularity and in granulocytomacrophage and pluripotent stem cell proliferative responses.
Cadmium was also found in all the plant species, and its concentration ranged from 22.5 µg/g in L. multiflora (Kadjebi) to 59.0 µg/g in L. multiflora (Kasoa). Cadmium is a nonessential trace element with uncertain direct functions in both plants and humans.[13] It is however reported that the lowest level of cadmium which can cause yield reduction in plants is 5 to 30 µg/g, and the maximum acceptable concentration for foodstuff is about 1 µg/g.[14] The results of this study indicated that about 80% of the plant species had cadmium content above 30 µg/g, which is essential for improved yield.
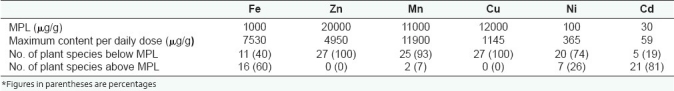
The overall results indicated clearly that heavy metals are present in Ghanaian medicinal plants and that the contents of these metals except iron were within acceptable and safe limits [Table 3]. Therefore, herbal formulations of these plant species can also be beneficial sources of appropriate and essential trace elements, though care must be taken to avoid iron toxicity, especially in higher doses.
Table 3.
Comparison of the maximum permissible levels and maximum contents of the heavy metals assessed in the plant species
CONCLUSIONS
Most of the plant species contained safe levels of the heavy metals analyzed and hence may have no adverse effects normally associated with heavy metal toxicity on people who patronize these products for their health needs.
Acknowledgments
We would like to thank the technical staff of the Ecological Laboratory, University of Ghana, Legon for their assistance in this work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Annan K, Houghton PJ. Antibacterial, antioxidant and fibroblast growth stimulation of aqueous extracts of Ficus asperifolia Miq. and Gossypium arboreum L.; wound healing plants from Ghana. J Ethnopharmacol. 2008;119:141–4. doi: 10.1016/j.jep.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 3.Bayor MT, Gbedema SY, Annan K. Croton membranaceus used in herbal formulations for measles in Ghana has potent antimicrobial properties. J Pharmacog Phytother. 2009;1(4):47–51. [Google Scholar]
- 4.Ghana Herbal Pharmacopoeia. Accra, Ghana: Science and Technology Policy Research Institute (STEPRI); 2007. [Google Scholar]
- 5.Llobet JM, Falcó G, Casas C, Teixidó A, Domingo JL. Concentrations of arsenic, cadmium, mercury and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. J Agric Food Chem. 2003;29:51–6. doi: 10.1021/jf020734q. [DOI] [PubMed] [Google Scholar]
- 6.Food and Nutrition Board NRC. 9th ed. Washington, DC: National Academy of Sciences; 1980. NRC Recommended dietary allowances. [Google Scholar]
- 7.Obi E, Akunyili DN, Ekpo B, Orisakwe OE. Heavy metal hazards of Nigerian herbal remedies. Sci Total Environ. 2006;369:35–41. doi: 10.1016/j.scitotenv.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Bourman WC, Rand MJ. Textbook of Pharmacology. 2nd ed. London: Blackwell Scientific Publications; 1980. [Google Scholar]
- 9.Atukorala TM, Waidyanatha US. Zinc and copper content of some common foods. J Nat Sci Coun. 1987;15:61–9. [Google Scholar]
- 10.Das KK, Dasgupta S. Effect of nickel sulphate on testicular steroidogenesis in rats during protein restriction. Environ Health Perspect. 2002;110:923–6. doi: 10.1289/ehp.02110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell RM. New micronutrient dietary reference intakes from the National Academy of Sciences. Nutr Today. 2001;36:163–71. [Google Scholar]
- 12.Dey S, Saxena A, Dan A, Swarup D. Indian medicinal herb: A source of lead and cadmium for humans and animals. Arch Environ Occup Health. 2009;4:164–7. doi: 10.1080/19338240903240525. [DOI] [PubMed] [Google Scholar]
- 13.Ano AO, Ubochi CI. Nutrient composition of climbing and prostrate vegetable cowpea accessions. Afr J Biotechnol. 2008;7:3795–6. [Google Scholar]
- 14.Linder M, editor. Nutritional Biochemistry and Metabolism. New York: Elsevier Science Publishing Co., Inc; 1985. [Google Scholar]


