Abstract
In different cultural groups, the hemiparasitic plants of the families Loranthaceae and Viscaceae (mistletoes) are frequently used in the treatment of hypertension and/or as diuretic agents. However, it remains unclear as to what commonality makes them diuretic agents or a remedy for hypertension. In this article, the diuretic activity of methanol extracts of Viscum articulatum (VA) Burm. f. and Helicanthus elastica (HE) (Ders.) Dans. in rats is reported. The extracts were administered orally at doses of 100, 200 and 400 mg/kg to rats that had been fasted and deprived of water for 18 hours. Investigations were carried out for diuretic, saluretic and natriuretic effects. The polyphenolic and triterpenoid contents were determined quantitatively using chemical assays and high performance liquid chromatography (HPLC) analysis, respectively. The extracts of VA and HE demonstrated significant and dose-dependent diuretic activity in rats. It was found that while VA mimics the furosemide pattern, HE demonstrated a dose-dependent increase in diuresis, along with an increase in potassium-sparing effects. Phytochemical analysis revealed that polyphenolics and triterpenoids, such as oleanolic acid and lupeol, are the major phytochemicals involved. It was also found that in different combinations, these phytochemicals differed in the way they influenced the electrolyte excretion. A higher content of polyphenolics in association with lower triterpenoid content was found to favor potassium-sparing effects.
Keywords: Diuretic, Helicanthus elastic, polyphenolics, potassium sparing, triterpenoids, Viscum articulatum
INTRODUCTION
Mistletoes (hemiparasitic plants of the families Loranthaceae and Viscaceae) have been widely used in medicine by cultures in almost every continent.[1] An examination of the ethnomedicinal uses of some 52 mistletoe species shows that there is a striking similarity in their traditional uses across cultures. These plants have been used most frequently in the treatment of hypertension or as diuretic agents. [2–7] In addition, an increased diuresis has been recorded as a common consequence of mistletoe infusions used in the treatment of hypertension in folk medicine.[4]
Present study describes the acute diuretic effect of two Asian mistletoes, Viscum articulatum Burm. f. (Viscaceae) and Helicanthus elastica (Ders.) Dans. (Loranthaceae) in laboratory animals. Viscum articulatum (VA), a leafless, hemiparasitic shrub, has been commonly used in Chinese medicine for the treatment of hemorrhage, pleurisy, gout, heart disease, hypertension, epilepsy and arthritis.[8,9] The whole plant of Helicanthus elastica (HE), a hemiparasitic climber, has been used in the Indian system of medicine as a narcotic and diuretic and in the treatment of renal and vesical calculi, kidney afflictions and menstrual disorders. [10] The aqueous extract of HE has been reported to have diuretic activity.[11] However, this report was based on a study conducted with a single dose that was unrealistically high (4000 mg/kg, p.o.). Therefore, for a systematic investigation and for comparative purposes, HE has been included in this study.
MATERIALS AND METHODS
Plant materials and chemicals
Whole plants of HE, which is parasitic on Syzygium cumini L. (Myrtaceae), were collected from a tropical evergreen forest in the Western Ghats. VA, which is parasitic on Cordia macleodii (Grift) Hook and Thoms. (Boraginaceae), was collected from tropical dry deciduous forests of the Satpuda Hills, India. Dr. P. S. N. Rao of the Botanical Survey of India, Pune, authenticated the plant specimens (BSI/WC/822/LOT 1 and BSI/WC/822/LOT 2). Lupeol, betulin, betulinic acid, oleanolic acid, p-coumaric acid, quercetin and catechin were purchased from Sigma Chemicals, USA. Acetonitrile, water and methanol (HPLC grade) and pre-coated silica gel plates were purchased from Merck Ltd., India. Other solvents used for extraction were of analytical grade.
Animals
Twelve-week-old male Wistar rats weighing 170 to 175 g and Swiss albino mice of 5 to 7 weeks age weighing 20 to 25 g were obtained from registered vendors. The animals were housed in separate cages, four animals per cage, and were maintained at 22°C ± 2°C, with a 12-hour light/dark cycle. Food and water were provided ad libitum till the day prior to the study. The Institutional Animal Ethical Committee approved the study (approval no. 652/02/C/CPCSEA).
Extraction of plant material
Air-dried whole plant material (300 g) was extracted with methanol (3 × 600 mL) by cold maceration for 72 hours at room temperature, with the used solvent being replaced with fresh solvent after every 24 hours. The pooled extracts were concentrated in a rotary vacuum evaporator. The yields of HE and VA extracts were 11.77% and 12.16% w/w, respectively. The extraction was repeated three times to determine the average yield of methanol extract under similar conditions.
Acute toxicity study
The toxicity of both extracts was studied as per organization for economic co-operation and development (OECD) guideline number 425. The limit test was performed initially. Swiss albino mice weighing 20 to 25 g were used in the toxicity study. Six mice were serially administered a 2000-mg/kg dose of extract prepared in water as recommended in the guideline. After dose administration, each animal was observed after every hour for signs of toxicity and abnormality in behavior up to the 48th hour. After this, daily observations for toxicity and mortality were made up to the 14th day. The body weights of the animals were recorded every third day. On the 14th day after dosing, all the mice were sacrificed and processed for gross necropsy.
Diuretic activity
The diuretic activity was assessed using the reported method. [12] Each treatment group contained six male albino rats. The rats were fasted and deprived of water for 18 hours before the experiment. Screening of diuretic activity was started between 7 a.m. and 8 a.m. on the subsequent day. Each rat received an oral load of 2.5 mL of 0.9% NaCl/100 g of body weight.[13] The extract was dissolved in water and administered at doses of 100, 200 and 400 mg/ kg in different groups. Care was taken to keep the volume of this dose equal in all the rats. The rats in the control group were given an equal amount of water in addition to saline. Furosemide (Aventis, India) was given orally as a standard drug at a dose of 10 mg/kg. Immediately after dosing, animals were placed in metabolic cages (one in each cage) specially designed to separate the urine and feces and maintained at room temperature. The urine volumes after 5 hours and after the next 19 hours were recorded for each rat. The cumulative volume was determined for 24 hours. Urine sodium, potassium, chloride, urea and creatinine concentrations were also determined, along with the serum creatinine level. Sodium and potassium concentrations were measured using a flame photometer (Systronic-128, India). Urine chloride, urea and creatinine were estimated using commercially available kits (Accurex Biomedical, India; Ranbaxy RFCL, India; Beacon Diagnostic, India, respectively). Blood samples were collected through a retro-orbital puncture at the end of 24 hours, and the serum was rapidly separated and processed for determination of the serum creatinine level using a commercially available kit (Beacon Diagnostic, India). The sum of the Na+ and Cl- excretions was calculated as a measure of the saluretic activity, and the Na+/K+ ratio was calculated for assessing the natriuretic activity. The diuretic index and glomerular filtration rate (GFR) were determined using the following equations.[14,15]

Phytochemical analysis
The extracts were analyzed for their electrolyte content to ascertain the interference with urinary excretion, if any, caused by sodium and potassium present in the extract. The concentrations of potassium and sodium in a solution of the extract (1 mg/mL) prepared in distilled water were determined using a flame photometer (Systronic-128, India).
The total phenolic content was determined using Folin-Ciocalteu reagent as described in the literature.[16] The phenolic content was determined from a standard curve of p-coumaric acid recorded at 660 nm using a spectrophotometer (Shimadzu, Japan) and expressed as w/w p-coumaric acid equivalents (PCAE). The extract was further analyzed for total flavonoids and proanthocyanidins. [17] The flavonoid and proanthocyanidin contents were determined using a standard curve established with quercetin at 415 nm and catechin at 500 nm, respectively. The total flavonoids and proanthocyanidins are expressed as percent w/w quercetin equivalents (QE) and catechin equivalents (CE), respectively. Further, the gross phenolic content was determined using the following formula.
Gross phenolic content (g %) = PCAE × extractive/100
Qualitative analysis of triterpenoids was performed using high performance thin layer chromatography (HPTLC) on pre-coated silica gel plates using appropriate reference standards. After application of spots, each plate was run in benzene: Ethyl acetate as the solvent system. (Various combinations of the given solvent system were used in detection of betulin, betulinic acid, oleanolic acid and lupeol.) After development, the plate was heated and maintained at 100°C for 3 minutes, sprayed with anisaldehyde-H2SO4 and evaluated at 366 nm densitometrically using the win CAT software package. Quantitative analysis of the triterpenoids in the methanol extract was performed using a YOUNGLIN (Acme-9000) series HPLC system. Chromatographic separation was performed on an RP C-18 column (250 cm × 4.6 mm, 5 µm particle size) in isocratic mode with acetonitrile: Water (90:10 v/v) as the solvent system. The temperature of the column was kept constant at 30°C, and the mobile phase was delivered at a flow rate of 0.2 mL/min. A photodiode array detector set at 215 nm for oleanolic and betulinic acids and at 230 nm for lupeol was used to monitor the elution. The extract was sampled, dissolved in methanol (1 µg/mL) and analyzed. The gross triterpenoid content was determined using the following formula.
Gross triterpenoid content (g %) = Total triterpenoid content × extractive/100
Statistical analysis
The data were analyzed using one-way ANOVA, followed by Dunnett's test using the GraphPad Prism 4.0 software package. The level of significance was determined in comparison with the control group.
RESULTS
Acute toxicity study
In the acute toxicity study, neither death nor any observable neurobehavioral effects were observed in the limit test. No significant alterations in the histology of the vital organs were observed in the necropsy after euthanasia. Hence, as stated in OECD guideline number 425, these compounds were classified as globally harmonized system (GHS) category-5 substances. Due to lack of any observable toxicity at the 2000-mg/kg dose, LD50 was not determined. The observed lack of toxicity of the two plants is consistent with their traditional use as fodder.[18,19]
Diuretic activity
The results of diuretic study indicate that HE and VA extracts had a significant effect on the urine excretion volume in comparison with the control group in the tested range of 100 to 400 mg/kg [Figure 1]. The results also indicate that HE and VA extracts had dose-dependent effects on the urine output and that a dose of 400 mg/kg at 24 hours had a diuretic index of 70.37% and 60.49%, respectively, in comparison with the furosemide-treated group [Table 1]. In contrast to the acute diuretic effect of furosemide, which was limited to the first 5 hours, both extracts caused consistent diuresis up to 24 hours. The results of urine electrolyte analysis further indicate that VA caused diuresis through significant sodium, potassium and chloride loss, mimicking the pattern of furosemide. However, unlike furosemide, VA had less potent effects on potassium, chloride and urea loss [Figure 2]. VA caused an obvious dose-dependent increase in saluretic (Na+ + Cl–) and natriuretic (Na+/K+) effects [Table 1]. Further, in case of VA at doses of 100, 200 and 400 mg/kg, the GFR increased to 324%, 355% and 365% (P < 0.001) of the control value, respectively [Table 1]. Furosemide produced a large increase (465%) in the GFR (P < 0.001). Like VA, HE exerted a significant and dose-dependent sodium loss but concurrently had limited effects on the urinary excretion of potassium, chloride and urea. Consistent with these effects, HE had higher natriuretic (Na+/K+) and lower saluretic (Na+ + Cl-) effects than did furosemide [Table 1]. Similar to VA, the GFR increased to 322%, 382% and 419% (P < 0.001) of the control value with HE at doses of 100, 200 and 400 mg/kg, respectively.
Figure 1.
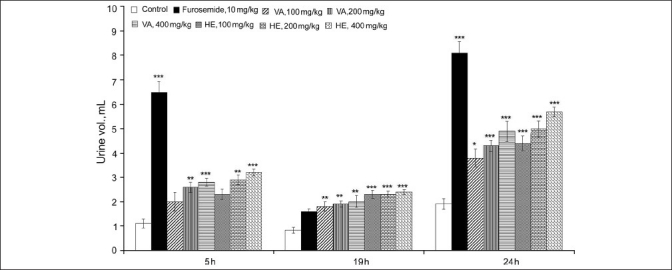
Effect of oral administration of methanol extracts of V. articulatum and H. elastica on urine excretion volume measured separately at 5 and 19 hours and cumulative volume determined at 24 hours; (Data expressed as mean ± SEM, n = 6. The data were analyzed using one-way ANOVA followed by Dunnet's test. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control group)
Table 1.
Effect of oral administration of methanol extracts of V. articulatum and H. elastica on electrolyte excretion and glomerular filtration rate in rats
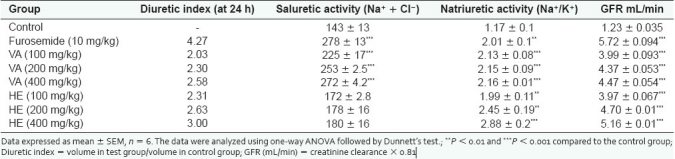
Figure 2.
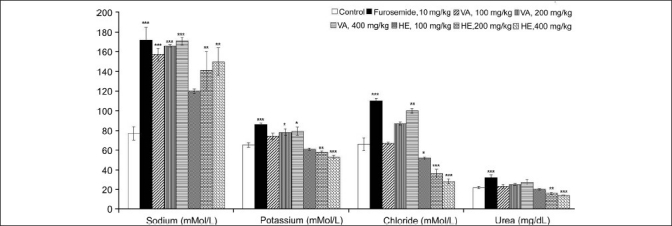
Effect of oral administration of methanol extracts of V. articulatum and H. elastica on urine excretion of sodium, potassium, chloride and urea in rats; (Data expressed as mean ± SEM, n = 6. The data were analyzed using one-way ANOVA followed by Dunnet's test. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control group)
Phytochemical analysis
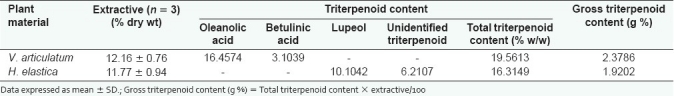
The sodium and potassium levels in HE and VA extracts were 94.56 mMol/L, 40.51 mMol/L; and 131 mMol/L, 51.92 mMol/L, respectively. Further, the quantitative chemical assays revealed a preponderance of polyphenolic compounds in the HE extract in comparison with the VA extract. The gross phenolic content in HE and VA was 5.02 ± 0.61 g % and 0.39 ± 0.02 g %, respectively [Table 2]. Among the polyphenolic compounds, flavonoids constituted a major part, followed by proanthocyanidin compounds. The results of the HPLC analysis show that oleanolic acid is the major triterpenoid of the VA extract, with a small betulinic acid content [Figure 3 and Table 2]. The results further indicate that neither oleanolic acid nor betulinic acid is present in HE and that lupeol is the major triterpenoid. However, one additional peak representing an unidentified triterpenoid was also detected [Figure 4]. Quantification further suggests that, in contrast to the polyphenolics, the triterpenoid allocation is the opposite, with the VA extract having a 19% higher triterpenoid content in comparison with the HE extract [Table 2].
Table 2.
Phenolic content of methanol extracts of V. articulatum and H. elastica

Figure 3.
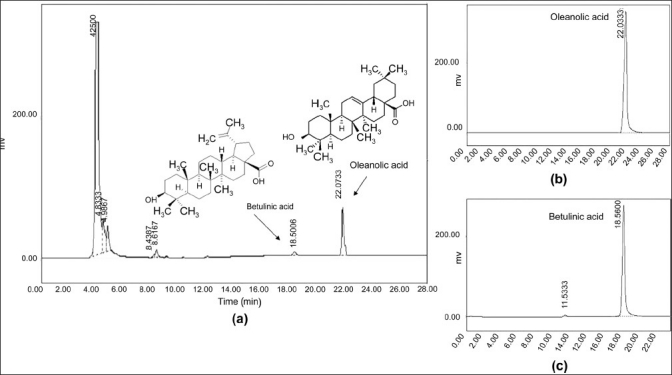
HPLC analysis of methanol extract of (a) Viscum articulatum and (b) marker compounds oleanolic and (c) betulinic acid
Figure 4.
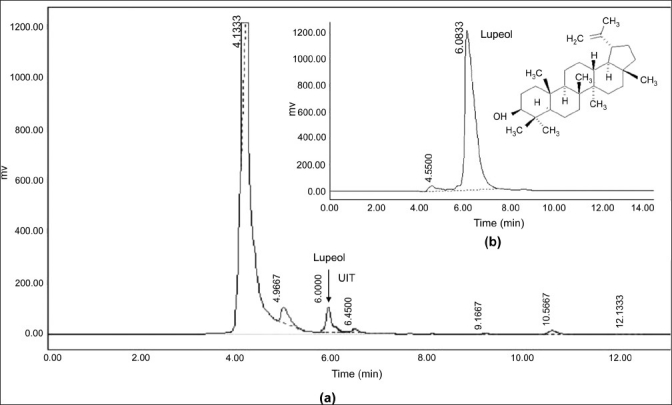
HPLC analysis of methanol extract of Helicanthus elastica (a) and marker compound lupeol (b) unidentified triterpenoid, UIT
DISCUSSION
The study shows that the methanol extracts of VA and HE had significant and dose-dependent diuretic activity in rats [Table 1]. It further shows that while VA mimicked the furosemide pattern, HE brought about a dose-dependent increase in diuresis along with an increase in potassium-sparing effects [Figures 1 and 2]. These observations suggest that although HE and VA had more or less similar diuretic potentials, their mechanisms were disparate. Phytochemical analysis of the methanol extracts of VA and HE further suggests that these are predominantly composed of carbon-based secondary chemicals (CBSCs) such as triterpenoids and polyphenolic compounds; however, they differed qualitatively as well as quantitatively [Figures 3 and 4]. VA has a higher content of triterpenoids (such as oleanolic acid) and a lower content of polyphenolics, whereas HE has a lower content of triterpenoids (such as lupeol) and a higher content of polyphenolics. As triterpenoids and many polyphenolics are well known to affect the kidney's physiology,[20,21] it can be presumed that these constituents of the mistletoes are responsible for the observed diuretic effect.
The most efficacious diuretic, furosemide, acts on sodium reabsorption in the ascending limb of the loop of Henle. However, it produces significant hypokalemia and metabolic alkalosis.[22] Consistent with these observations, in our study, the furosemide-treated group showed a significantly higher potassium loss (P < 0.001) in comparison with the control group [Figure 2]. Like furosemide, oleanolic acid has also been reported to induce diuresis associated with significant potassium loss.[21] Thus, the furosemide-like pattern of VA is consistent with this observation since it has oleanolic acid as a major triterpenoid. However, unlike furosemide and oleanolic acid, VA brings about potassium loss to a lesser extent, implying that the potassium-sparing effect may be attributed to the presence of polyphenolic compounds. Similar observation has also been reported regarding the methanol extract of Viscum angulatum Heyne ex DC (Viscaceae), which consists of triterpenoids and polyphenolic compounds.[23] Further, animals treated with HE displayed a dose-dependent increase in the potassium-sparing activity. This observation further supports the above conclusion, as HE extract contains higher levels of polyphenolics and lower levels of triterpenoids in comparison with VA extract [Tables 2 and 3]. These results clearly suggest that in the presence of polyphenolics, potassium loss caused by triterpenoids is markedly inhibited. Moreover, at elevated levels of polyphenolics, the potassium sparing increased significantly. To confirm whether electrolyte content of the extract itself is responsible for this disparity in potassium sparing, we also estimated the electrolyte content in the extracts under investigation. We found that the potassium content in the HE extract does not correlate with the urine potassium content, which makes the possibility of interference doubtful. Whereas most commercial diuretics typically act through a single mechanism,[22] a combination of different compounds such as triterpenoids and polyphenolics has a favorable effect on a physiological process, making the observed results important. The data of this study indicate that HE and VA have a significant diuretic potential but that this potential is lower than that of furosemide. At similar doses, these extracts have a more or less similar diuretic potency. However, their diuretic mechanisms are qualitatively different, and more than one mechanism seems to be involved. It is proposed that these differences in the diuretic mechanisms are attributable to the qualitative and quantitative variation in the triterpenoids and polyphenolic compounds.
Table 3.
HPLC analysis of triterpenoid content in methanol extracts of V. articulatum and H. elastica
It has been observed that induction of diuresis in herbivores is a defensive mechanism found in plants.[24] Mistletoes are regarded as a keystone resource of forest and woodland communities since these plants interact with a wide variety of wildlife.[25] Because of their high nutritional quality and the absence of major structural defenses, a wide variety of mammals, birds, insects and fungi consume mistletoes. [26] These observations suggest that in the absence of major structural defenses, mistletoes are very reliant on chemical defenses. Further, these plants are known to retain several primitive characters in their morphology as well as in defensive chemistry, in which latter is restricted mostly to primitive CBSCs such as polyphenolics and triterpenoids. [27–30] Thus, in the absence of direct evidence of diuretic effects of consumption of mistletoes by a herbivore, an indirect evidence generated on laboratory animals in this study suggests that induction of diuresis in animals is one of the defensive mechanisms in mistletoes, which is attributed to CBSCs such as triterpenoids and polyphenolic compounds. However, further studies are required to ascertain the ecological significance of these effects in mistletoe plants.
Further, the diuretic and antihypertensive effects of triterpenoids and polyphenolic compounds have been conclusively demonstrated in the preclinical and clinical studies.[20,21] These observations, therefore, support the contention that plants synthesize a wide variety of secondary compounds that can exert diuretic effect in mammals. Interestingly, this diversity of secondary chemicals is consistent with the diversity of chemical structures present among commercial diuretics.[22] Despite this fact, bioprospecting of phytochemicals as scaffolds in discovering new diuretic agents is not attempted, and almost all currently used diuretics are derived by a synthetic route.[31] The results of the present investigation, therefore, propose that CBSCs such as triterpenoids and polyphenolic compounds are interesting molecular scaffolds for discovering the new class of diuretic agents since their safety and potential as a scaffold in discovering new drugs for different therapeutic classes have already been established.[32–33]
CONCLUSION
This study provides scientific evidence for the diuretic effect of the methanol extracts of VA and HE in rats. It further shows that the observed activity is attributable to triterpenoids such as oleanolic acid and lupeol, along with polyphenolic compounds. It was also found that in different combinations, these phytochemicals differed in the way they influenced the electrolyte excretion. Further, the study proposes that prevalence of CBSCs such as polyphenolics and triterpenoids in the mistletoes might be responsible for observed similarity in their traditional uses across cultures.
Acknowledgments
The authors are grateful to the All India Council for Technical Education, New Delhi, for their generous grant (8023/BOR/RID/RPS/16/7/8). The authors also acknowledge the help by taxonomists Dr. C. B. Salunkhe and Dr. Milind Sardesai.
Footnotes
Source of Support: All India Council for Technical Education, New Delhi, for their generous grant (8023/BOR/RID/RPS/16/7/8)
Conflict of Interest: None declared.
REFERENCES
- 1.Arndt B. Introduction: History of mistletoe uses. In: Arndt B, editor. Mistletoe: The genus Viscum. Singapore: Hardwood Academic Publishers; 2000. pp. 1–6. [Google Scholar]
- 2.Aleykutty NA, Srinivasan KK, Rao GP, Udupa AL, Keshavamurthy KR. Diuretic and anti-lithiatic activity of Dendrophthoe falcata. Fitoterapia. 1993;64:325–31. [Google Scholar]
- 3.Adsersen A, Adsersen H. Plants from Reunion Island with alleged antihypertensive and diuretic effects: An experimental and ethnobotanical evaluation. J Ethnopharmacol. 1997;58:189–206. doi: 10.1016/s0378-8741(97)00100-1. [DOI] [PubMed] [Google Scholar]
- 4.Deliorman D, Caliş I, Ergun F, Doğan BS, Buharalioğlu CK, Kanzik I. Studies on the vascular effects of the fractions and phenolic compounds isolated from Viscum album spp. album. J Ethnopharmacol. 2000;72:323–9. doi: 10.1016/s0378-8741(00)00251-8. [DOI] [PubMed] [Google Scholar]
- 5.Lohézic-Le Dévéhat F, Bakhtiar A, Bézivin C, Amoros M, Boustie J. Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia. 2002;73:400–5. doi: 10.1016/s0367-326x(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 6.Varela BG, Fernández T, Ricco RA, Zolezzi PC, Hajos SE, Gurni AA, et al. Phoradendron liga (Gill. ex H. et A.) Eichl. (Viscaceae) used in folk medicine: Anatomical, phytochemical, and immunochemical studies. J Ethnopharmacol. 2004;94:109–16. doi: 10.1016/j.jep.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Ojewole JA, Adewole SO. Hypoglycemic and hypotensive effects of Globimetula cupulata (DC) Van Tieghem (Loranthaceae) aqueous leaf extract. Cardiovasc J South Afr. 2007;18:9–15. [PubMed] [Google Scholar]
- 8.Chiu ST. Flora of Taiwan. II. Taipei: Editorial Committee of the Flora of Taiwan; 1996. [Google Scholar]
- 9.Chiu ST. Proceedings of International Symposium on Plant Biodiversity and Development of Bioactive Natural Products. Taichung, Taiwan: 2001. Nov 18-20, Medicinal resource of Formosan mistletoes. [Google Scholar]
- 10.Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd ed. Dehradun: International Book Distributors, Booksellers and Publishers; 1999. [Google Scholar]
- 11.Aleykutty NA, Srinivasan KK, Rao GP, Keshavamurthy KR, Udupa AL. Study of the diuretic activity of Helicanthes elastica. Fitoterapia. 1991;62:439–40. [Google Scholar]
- 12.Lipschitz WL, Hadidian Z, Kerpcsar A. Bioassay of diuretics. J Pharmacol Exp Ther. 1943;79:97–110. [Google Scholar]
- 13.Wiebelhaus VD, Weinstock J, Maass AB, Brennan FT, Sosnowski G, Larsen T. The diuretic and natruretic activity of Triamterene and several related pteridines in the rat. J Pharmacol Exp Ther. 1965;149:397–403. [PubMed] [Google Scholar]
- 14.Martín-Herrera D, Abdala S, Benjumea D, Pérez-Paz P. Diuretic activity of Withania aristata: An endemic Canary Island species. J Ethnopharmacol. 2007;113:487–91. doi: 10.1016/j.jep.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Manotham K, Booranalertpaisarn V, Eiam-Ong S, Chusil S, Praditpornsilpa K, Tungsanga K. Accurately simple estimation of glomerular filtration rate in kidney transplant patients. Transplant Proc. 2002;34:1148–51. doi: 10.1016/s0041-1345(02)02788-4. [DOI] [PubMed] [Google Scholar]
- 16.Gelareh M, Zahra E, Karamatollah R. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–8. [Google Scholar]
- 17.Liu CT, Wu CY, Weng YM, Tseng CY. Ultrasound assisted extraction methodology as a tool to improve the antioxidant properties of herbal drug Xiao-chia-hu-tang. J Ethnopharmacol. 2005;99:293–300. doi: 10.1016/j.jep.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Green SM, Minkowski K. The lion-tailed macaque and its south Indian rainforest habitat. In: Prince Rainier HSH III, Bourne GH, editors. Primate Conservation. New York: Academic Press; 1977. pp. 290–337. [Google Scholar]
- 19.Thapa B, Walker DH, Sinclair FL. Indigenous knowledge of the feeding value of tree fodder. Anim Feed Sci Technol. 1997;67:97–114. [Google Scholar]
- 20.Christie S, Walker AF, Lewith GT. Flavonoids-A new direction for the treatment of fluid retention? Phytother Res. 2001;15:467–75. doi: 10.1002/ptr.1011. [DOI] [PubMed] [Google Scholar]
- 21.Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–21. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- 22.Jackson EK. Diuretics. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw Hill; 1996. pp. 685–713. [Google Scholar]
- 23.Jadhav RB, Bhatnagar SP, Surana SJ. Diuretic activity of squamate mistletoe, Viscum angulatum. Pharm Biol. 2010 doi: 10.3109/13880200903150427. (In press) [DOI] [PubMed] [Google Scholar]
- 24.Dearing MD, Mangione AM, Karasov WH. Plant secondary compounds as diuretics: An overlooked consequence. Am Zoo. 2001;41:890–901. [Google Scholar]
- 25.Watson DM. Mistletoe-A keystone resource in forests and woodlands worldwide. Ann Rev Ecol Syst. 2001;32:219–49. [Google Scholar]
- 26.Ehleringer JR, Ullman I, Lange OL, Farquhar GD, Cowan IR, Schulze ED, et al. Mistletoes: A hypothesis concerning morphological and chemical avoidance of herbivory. Oecologia. 1986;70:234–7. doi: 10.1007/BF00379245. [DOI] [PubMed] [Google Scholar]
- 27.Lin JH, Lin YT. Flavonoids from the leaves of Loranthus kaoi (Chao) Kiu. J Food Drug Anal. 1999;7:185–90. [Google Scholar]
- 28.Kim YK, Kim YS, Choi SU, Ryu SY. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch Pharm Res. 2004;27:44–7. doi: 10.1007/BF02980044. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CA, Calvin CL. Development, taxonomic significance and ecological role of the cuticular epithelium in the Santalales. IAWA Journal. 2003;24:129–38. [Google Scholar]
- 30.Jadhav RB, Anarthe SJ, Surana SJ, Gokhale SB. Host-hemiparasite transfer of the C-glucosyl xanthone mangiferin between Mangifera indica and Dendrophthoe falcate. J Plant Interactions. 2005;1:171–7. [Google Scholar]
- 31.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 32.Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M, Biedermann D, et al. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep. 2006;23:394–411. doi: 10.1039/b515312n. [DOI] [PubMed] [Google Scholar]
- 33.Sporn MB, Liby K, Yore MM, Suh N, Albini A, Honda T, et al. Platforms and networks in triterpenoid pharmacology. Drug Dev Res. 2007;68:174–82. [Google Scholar]


