Abstract
At laboratory and clinical levels, therapeutic hypothermia has been shown to improve neurologic outcomes and mortality following cardiac arrest. We reviewed each cardiac arrest in our community-based Veterans Affairs Medical Center over a three-year period. The majority of cases were in-hospital arrests associated with initial pulseless electrical activity or asystole. Of a total of 100 patients suffering 118 cardiac arrests, 29 arrests involved comatose survivors, with eight patients completing therapeutic cooling. Cerebral performance category scores at discharge and six months were significantly better in the cooled cohort versus the noncooled cohort, and, in every case except for one, cooling was offered for appropriate reasons. Mean time to initiation of cooling protocol was 3.7 hours and mean time to goal temperature of 33°C was 8.8 hours, and few complications clearly related to cooling were noted in our case series. While in-patient hospital mortality of cardiac arrest was high at 65% mortality during hospital admission, therapeutic hypothermia was safe and feasible at our center. Our cooling times and incidence of favorable outcomes are comparable to previously published reports. This study demonstrates the feasibility of implementing, a cooling protocol a community setting, and the role of neurologists in ensuring effective hospital-wide implementation.
1. Introduction
Comatose survivors of cardiac arrest notoriously have poor outcomes including significant neurological deficits and persistent vegetative state. Although there is variation in incidence and outcomes, epidemiologic studies suggest that between one and five of every 1,000 patients admitted to a hospital in Western countries suffer an in-hospital cardiac arrest [1], with the National Registry of Cardiopulmonary Resuscitation citing an incidence of 0.175 events per bed in a sample of American hospitals [2]. There is a wide range of reported survival to hospital discharge, from 0% to 42%, with larger studies approaching an average of 20% [1].
There is considerable evidence that mild therapeutic hypothermia after cardiac arrest is protective in preventing cerebral injury. Animal and human studies have demonstrated that hypothermia reduces brain metabolism and thus oxygen and ATP consumption [3, 4], reduces the release of excitotoxic glutamate via regulation of transmembrane electrolyte transport [5], protects against oxidative stress and lipid peroxidation [6, 7], and alters gene expression (see also reviews by Holzer [8], Liu and Yenari [9], and Yenari et al. [10]) to promote brain cell survival after cerebral ischemia. These physiologic effects were shown to have clinical significance with the publication in 2002 of two major clinical trials of therapeutic hypothermia in comatose survivors of cardiac arrest. These studies, by Bernard and colleagues [11] in Australia and by the Hypothermia after Cardiac Arrest Study Group [12] in Europe, showed that cooling these patients improved neurological outcomes and reduced rate of death.
Since the publication of these trials, numerous medical centers around the world have replicated the efficacy of therapeutic hypothermia in this patient population and also established its overall feasibility and safety [13–15] (see also review by Sagalyn et al. [16] and meta-analysis by Arrich et al. [17]). Followup from the European multicenter trial also included data from patients who had in-patient cardiac arrests and patients who initially had pulseless electrical activity (PEA) or nonventricular fibrillation or ventricular tachycardia rhythms. While return of spontaneous circulation (ROSC) was faster for in-hospital compared to out-of-hospital patients, there was no statistically significant difference in neurologic outcome or mortality in comparing hypothermia or normothermia within the in-hospital cohort. Within the PEA cohort, mortality was lower for patients who received therapeutic hypothermia [18].
In spite of this growing body of literature, therapeutic hypothermia has not been universally accepted and pursued at all medical centers [19], with cited barriers including lack of institutional protocols, resources, and limited prior experience [20]. Some authors have called upon neurologists to become more active in management of these patients beyond offering prognosis and in helping create hospital-wide policies given that therapeutic hypothermia remains one of the only proven treatments for improving neurologic outcome [21], and a recent report from Prior and colleagues demonstrated the success of planning and implementing a therapeutic hypothermia protocol in a community-based hospital setting [20]. As such, in this retrospective analysis, we report our experience with implementation of therapeutic hypothermia for comatose cardiac arrest survivors and its outcomes in an academically affiliated community-based Veterans Affairs medical center in which the majority of events were in-hospital.
2. Methods
The San Francisco Veterans Affairs Medical Center is a 378-bed hospital that serves more than 310,000 veterans in Northern California. It is a teaching hospital affiliated with the University of California San Francisco medical center. The hospital is staffed 24 hours a day by an inpatient neurology consultation service. In November 2007, a protocol for mild therapeutic hypothermia after cardiac arrest was written and reviewed by neurology attending physicians at the medical center and was discussed with the chiefs of the ICU services in December 2007. In February 2008, all housestaff and ICU nursing staff began in-service training regarding appropriateness and implementation of treatment including training on the use of cooling blankets and devices, monitoring during protocol implementation, and awareness of potential complications. Treating services were asked to consult neurology service in cases where therapeutic hypothermia was being considered.
This was a retrospective chart review study. In November 2007, a log was kept of every Code Blue on the medical center campus including the Emergency Department. After approval of study design from local IRB, each case was retrospectively reviewed from November 2007 to August 2010. Initial demographic information including age, gender, medical comorbidities, and events leading to the Code Blue alarm was obtained for each case. In those cases of documented cardiac arrest and loss of perfusing rhythm, information regarding the code was obtained including setting and location of the arrest; initial cardiac rhythm; medical cause of arrest if known at time of event or later; treatment during arrest including pharmacologic, electrical, and surgical intervention; duration of code; time at which spontaneous circulation returned; whether the patient survived the code; presence of coma after arrest. In those cases in which the patient was found to be in a coma, the chart was reviewed to determine if neurology service was consulted for possible cooling and any reason why they were not consulted was noted. Appropriate cases were reviewed for initiation of therapeutic hypothermia, and reasons for not starting protocol were also recorded. If started, the time of initiation in relation to return of spontaneous circulation was recorded, as was the cooling method, time at which goal temperature was achieved, total duration of cooling, rewarming time, complications related to cooling (defined as occurring during or up to one week after completion of rewarming), and any technical or unexpected difficulties were noted, including reasons for early termination of protocol. The lowest temperature recorded during treatment was also noted as measure of possible hypothermia overshooting. Cerebral Performance Categories (CPC) Scale scores of outcome (from 1 to 5, with 1 being conscious and alert with good cerebral performance, 2 being conscious with moderate cerebral disability, 3 being conscious with severe disability and dependent on others for activities of daily living, 4 being comatose or persistent vegetative state, and 5 being brain death or death from other causes) [22] were determined for all possible cases at hospital discharge, and, where appropriate, at six-month and two-year followup.
Inclusion criteria for initiation of cooling protocol included age greater than or equal to 18, woman with negative pregnancy test, and coma after cardiac arrest with no eye opening or response to noxious stimulation after resuscitation. Return of spontaneous circulation following loss of perfusing rhythm and subsequent stable cardiac rhythm was also required, with blood pressure greater than 90 mmHg systolic. Any nonperfusing initial rhythm during cardiac arrest including ventricular fibrillation, ventricular tachycardia, PEA, or asystole was deemed appropriate for treatment upon return of spontaneous circulation. Exclusion criteria included noncomatose state or other reasons for coma beyond cardiac arrest, pregnancy, known advanced directive or goals of care that would preclude therapeutic hypothermia and prolonged life support measures, and known severe coagulopathy including administration of full dose tPA during arrest or active bleeding. Cooling was stopped if any of the exclusion criteria developed after initiation.
Under the protocol, surface cooling—with ice packs placed under the axilla and near the neck, torso, and limbs—was initiated once patient was hemodynamically stable after acute treatment for cardiac arrest and if there were no other clear contraindications to initiation of hypothermia. This was done to prevent delay in initiation of cooling. The neurology consult service would then evaluate the patient and history and recommend whether cooling should continue or be discontinued. If cooling was to be continued, a cooling blanket or suit was deployed and nursing staff, primary physicians, and neurology service would work together to achieve goal temperature of 33°C as quickly as possible, ideally within 8 hours of return of spontaneous rhythm. Temperature was then maintained at 32°C to 33°C, and temperature sensing was done with a Foley catheter bladder probe when possible, otherwise, rectal temperatures were recorded. All patients were mechanically ventilated, and midazolam and fentanyl were used for sedation. Vecuronium was sometimes used as a paralytic and to help control shivering, and EEG monitoring was placed if there was concern for seizures under discretion of neurology consult service. All other ICU management and treatment plans were continued as deemed appropriate by the critical care team. Twenty-four hours after initiation of therapeutic hypothermia, passive rewarming was initiated using normal blankets and the removal of cooling measures, with goal of reaching 37°C at a rate of 0.3–0.5°C increase per hour. Paralytics and then sedation would then be discontinued as appropriate, and any subsequent hyperthermia was treated with cooling blanket and antipyretics.
Fisher's exact test and analysis of variance (ANOVA) were used to look for significant differences across the cardiac arrest groups including baseline demographics, mechanism and duration of cardiac arrest, medical comorbidities, and outcomes at discharge and six months. Within the cohort of patients who completed cooling protocol, correlation analysis between CPC score and time to initiation of protocol and time to goal temperature after ROSC was done by calculating Pearson's correlation coefficient (r) and the variance (r2). Total duration of cooling and time over which rewarming was completed were also noted. All the statistical analysis was performed using a computer software program, R version 2.12.1 (The R Foundation for Statistical Computing, 2010).
3. Results
From November 2007 to August 2010, there were 130 “Code Blue” alerts at the San Francisco Veterans Affairs Medical Center (see Figure 1). Of these, 12 of the alarms were done in error or involved cases in which there was no loss of a perfusing cardiac rhythm. Of the remaining 118 alerts involving 100 patients, 38 cardiac arrests occurred in which the patient did not survive. In these cases, the mean age of the patients was 72 years and 97% of the patients were men. Thirty-four arrests were in-hospital (89%) and the initial cardiac rhythm was PEA or asystole in 95% of arrests, with 5% ventricular tachycardia or fibrillation arrests. The mechanism of arrest involved a primary pulmonary arrest in 8 arrests (21%), primary cardiac in 12 (32%), gastrointestinal bleeding or catastrophe in 5 (13%), intraoperative event in 2 (5%), sepsis or infection in 4 (11%), and unknown or other etiology in 7 (18%). Comorbidities within this cohort included coronary artery disease (CAD) or congestive heart failure (CHF) in 17 patients (45%), cardiovascular risk factors defined as hypertension, diabetes, dyslipidemia, or tobacco use disorder in 21 (55%), cancer diagnosis in 9 (24%), pulmonary disease in 8 (21%), gastrointestinal bleeding or end-stage liver disease in 7 (18%), end-stage renal disease in 5 (13%), human immunodeficiency virus (HIV) or hepatitis infection in 2 (5%), and history or ongoing substance or alcohol abuse in 5 (13%).
Figure 1.
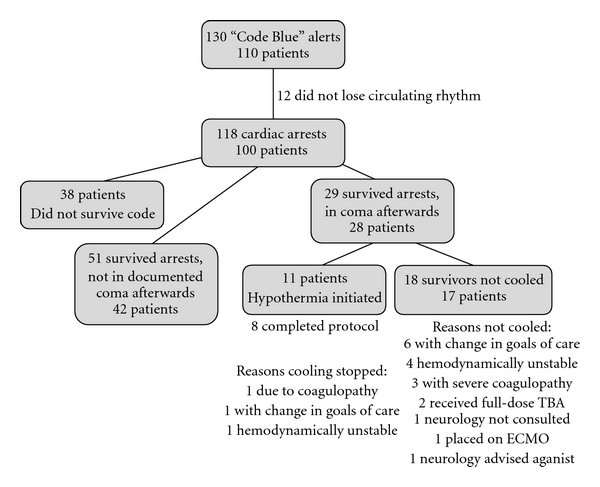
“Code Blue” Alerts at San Francisco VA Medical Center from November 2007 to August 2010. The diagram breaks down the numbers and types of “Code Blue” arrests including brief categorization of reasons of why patients who were comatose after cardiac arrest were not cooled or why cooling was stopped after initiation.
Baseline demographics and characteristics of cardiac arrest are summarized for the 80 cardiac arrests involving 70 unique surviving patients in Table 1. The overall mean age of these cases was 70.7 years, and 95% were men. Ninety-three percent of these arrests occurred in-hospital, and the initial perfusing rhythm was PEA or asystole in 94% of cases and ventricular tachycardia or fibrillation in 6%. Although comparisons were limited by small sample size, return of spontaneous circulation occurred faster in patients who were not in a coma after arrest (F = 29.6, P = 3.9 × 10−9). The 17 patients who were in a coma after arrest and did not undergo therapeutic cooling also had a higher rate of unfavorable CPC at discharge compared to the other groups (P = 7.6 × 10−5) and between the comatose survivor groups at six months after arrest (P = 0.05).
Table 1.
Characteristics of cardiac arrests in which patient survived.
| Not in coma after arrest (n = 51 arrests, 42 patients) | In coma after arrest, not cooled (n = 18 arrests, 17 patients) | In coma after arrest, cooling started (n = 11 arrests, 11 patients) | Statistical test for significance | |
|---|---|---|---|---|
| Sex | ||||
| Male | 40 (95) | 17 (100) | 10 (91) | P = 0.53e |
| Age, in yrs | 70.4 ± 11.1 | 72.4 ± 12.4 | 69.5 ± 13.0 | F = 0.24, P = 0.79f |
| In-hospital Arrest | 47 (92) | 16 (94) | 11 (100) | |
| Initial cardiac rhythm | ||||
| PEA/asystole | 47 (92) | 18 (100) | 10 (91) | P = 0.51e |
| VTach/VFib | 4 (8) | 0 (0) | 1 (9) | |
| ROSC in minutes | 5.2 ± 4.6 range (0.1–20)a | 33.5 ± 21.2 range (5–60)b | 21.9 ± 11.9 range (5–35)c | F = 29.6, P < 0.001f |
| Mechanism of arrest | ||||
| 1° Cardiac | 24 (47) | 4 (22) | 2 (18) | P = 0.07e |
| 1° Pulmonary | 13 (25) | 8 (44) | 3 (27) | P = 0.33e |
| 1° Gastrointestinal | 1 (2) | 2 (11) | 1 (9) | P = 0.18e |
| Sepsis/infection | 5 (10) | 2 (11) | 0 (0) | P = 0.72e |
| Intraoperative | 2 (4) | 2 (11) | 0 (0) | P = 0.30e |
| Unknown/other | 6 (12) | 0 (0) | 5 (45) | P = 0.005 |
| Comorbidities | ||||
| CAD or CHF | 24 (57) | 9 (53) | 7 (64) | P = 0.83e |
| Vascular RFs | 26 (62) | 13 (76) | 6 (55) | P = 0.47e |
| Cancer | 8 (19) | 5 (29) | 3 (27) | P = 0.66e |
| Pulmonary disease | 8 (19) | 3 (18) | 1 (9) | P = 0.91e |
| GI/liver | 5 (12) | 2 (12) | 1 (9) | P = 1.0e |
| ESRD | 6 (14) | 3 (18) | 0 (0) | P = 0.41e |
| HIV, hepatitis | 3 (7) | 1 (6) | 0 (0) | P = 1.0e |
| Substance abused | 3 (7) | 1 (6) | 2 (18) | P = 0.48e |
| Expired before discharge | 13 (31) | 16 (94) | 6 (55) | P < 0.001e |
| CPC at discharge | ||||
| Favorable (1-2) | 27 (64) | 1 (6) | 5 (45) | P < 0.001e |
| Unfavorable (3–5) | 15 (36) | 16 (94) | 6 (55) | |
| CPC at 6 Months | ||||
| Favorable (1-2) | 19 (45) | 0 (0) | 3 (27) | P = 0.001e |
| Unfavorable (3–5) | 23 (55) | 17 (100) | 8 (73) |
Values as whole number and (percentage), except in case of continuous variable, given as mean ± standard deviation.
ROSC: return of spontaneous circulation; 1°: primary; VTach, ventricular tachycardia; VFib: ventricular fibrillation; vascular RFs: cardiovascular risk factors.
Values as whole number and (percentage), except in case of continuous variable, given as mean ± standard deviation.
aData available for 34 of 51 arrests; bavailable for 10 of 18 arrests; cavailable for 8 of 11 arrests; dsubstance abuse includes alcohol abuse; eFisher's exact test; fanalysis of variance (ANOVA).
Amongst the 51 cardiac arrests in which the survivor was not comatose afterwards, 14 were treated with attempted electrical cardioversion (27%) and one patient received full-dose tPA (2%). In one case, a chest tube was placed due to hemothorax. Neurology was consulted in one case for consideration of therapeutic hypothermia but patient was noted to be moving extremities and was not comatose; the service was consulted for six other patients later in their hospital course typically for prognosis or management of other complications. Of note, one patient was initially noted to not be in a coma by primary team but was noted to be comatose by neurology service 72 hours after arrest and has since remained in persistent vegetative state.
Of the 18 cardiac arrests in which the survivor was comatose but not cooled, one patient was treated with attempted electrical cardioversion (6%) and three with full-dose tPA (17%). One patient was taken to the operating room urgently for cardiac surgery and was placed on extracorporeal membrane oxygenation. Neurology service was consulted for five cases regarding initiation of therapeutic hypothermia and two times for other reasons including prognosis. The reasons why patients were not cooled are listed in Figure 1. Of note, neurology was not called initially by primary team for one patient due to prolonged duration of code (30 minutes before ROSC); that patient was later declared brain dead by neurology service. In addition, one patient was felt to have a focal examination concerning for focal cerebral process after cardiac arrest rather than global hypoxia and so neurology service advised against cooling; that patient remained neurologically devastated and was eventually transitioned to hospice care and died one month later.
Amongst the 11 cases where cooling was initiated, 3 were treated with attempted electrical cardioversion (28%), and 2 were treated with bedside cardiac massage. Two were treated with bedside cardiac massage. Table 2 displays data specific to this group of patients, including description of discharge and six-month outcomes and details regarding cessation of cooling therapy in three cases. For the 8 patients that completed the cooling protocol, mean time to initiation of cooling protocol was 3.7 hours (range 2 to 6 hours) and mean time to goal temperature of 33°C after ROSC was 8.8 hours (range 3 to 17 hours). Cooling was continued for an average of 23.6 hours (range 22 to 24 hours). Rewarming occurred over an average of 13.9 hours (range 4 to 24 hours). Five of these 8 patients had favorable CPC score of 1 or 2 after 72-hour evaluation (63%) and three expired before discharge (38%). Three patients continued to have CPC score of 1 or 2 at six months after cardiac arrest (38%). Complications noted during therapeutic cooling or during the subsequent week after cardiac arrest included cardiac arrhythmia requiring treatment with medication in 3 patients (38%), pneumonia in 3 patients (38%), another infection in 1 patient (13%), and deep venous thrombosis or subsegmental pulmonary embolus in 2 patients (25%). There were no instances of acute kidney injury, skin breakdown or rash, or bleeding or hemorrhage during this same time period.
Table 2.
Comatose survivors of cardiac arrest that were treated with therapeutic hypothermia.
| Patient no. | Date of arrest | Age | Mechanism of arrest | Duration pulseless | Time to initiation | Time to 33°C | Time cooled | Lowest temp. | Rewarming time | Comments/CPC at discharge |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11/22/2007 | 55 | Suicide attempt, hanging | 35 mins | 3.5 hrs | 11 hrs | 24 hrs | N/A | 16 hrs | At 72 hrs, intact brainstem reflexes but persistent coma, care withdrawn (CPC 5) |
| 2 | 1/26/2008 | 83 | Unknown | 20 mins | — | — | — | — | — | Cooling stopped when goals of care changed after family arrived; died next day (CPC 5) |
| 3 | 5/2/2008 | 59 | Variceal bleeding, aspiration | 20 mins | — | — | — | — | — | Cooling stopped due to coagulopathy and severe GI bleeding; died next day (CPC 5) |
| 4 | 5/27/2008 | 85 | Aspiration | 30 mins | 6 hrs | 9 hrs | 24 hrs | 32.1°C | 12 hrs | Neurology called late due to concern for coagulopathy; persistent coma at 72 hrs; care withdrawn a week later (CPC 5) |
| 5 | 7/27/2008 | 83 | Cardiac Arrhythmia | 35 mins | 2 hrs | 6 hrs | 23 hrs | 31.3°C | 24 hrs | Discharged to rehab unit with short-term memory deficits, required assistance with walking (CPC 2); later diagnosed with gastric cancer, deceased 8 months later |
| 6 | 8/20/2008 | 61 | Pericardial tamponade | N/A | 4 hrs | 9 hrs | 24 hrs | 32.4°C | 9 hrs | Initially treated in OR for tamponade; at 72 hrs in persistent coma with myoclonic status epilepticus; ethics consult called and care withdrawn (CPC 5) |
| 7 | 2/21/2009 | 57 | Hyperkalemia, postextubation | N/A | 4 hrs | 6 hrs | 22 hrs | 33.0°C | 10 hrs | At 72 hr exam, neurologically normal examination; discharged to rehab. unit, still alive and neurologically intact (CPC 1) |
| 8 | 2/24/2009 | 85 | Possible vagal hyperactivity | 5 mins | 2.5 hrs | 3 hrs | 24 hrs | 32.3°C | 14 hrs | At 72 hrs, awake and alert, neurologically intact aside from mild confusion (CPC 2); had another arrest 3 weeks later and died |
| 9 | 5/29/2009 | 59 | Pulmonary embolus, sepsis | 5 mins | 0.5 hrs | 4 hrs | 5 hrs | N/A | — | Cooling stopped due to persistent hypotension and multiorgan failure; deceased two days later (CPC 5) |
| 10 | 2/20/2010 | 78 | COPD, aspiration | N/A | 5 hrs | 9 hrs | 24 hrs | 30.5°C | 22 hrs | At 96 hrs, alert, briskly following commands; remained ventilator dependent, transferred to rehab. unit (CPC 2); died 6 months later |
| 11 | 8/10/2010 | 59 | Unknown | 25 mins | 2.5 hrs | 17 hrs | 24 hrs | 32.0°C | 4 hrs | Myoclonus while cooled; rewarming occurred quickly and febrile afterwards; despite normal neuroexamination at 72 hrs; discharged to home, still alive (CPC 1) |
Neither the time to initiation of cooling protocol (r = 0.48, r2 = 0.23) nor the time to goal temperature of 33°C (r = 0.05, r2 = 0.003) was associated with CPC scores at discharge.
4. Discussion
We report our experience of therapeutic cooling in comatose survivors at our academically affiliated community-based Veterans Affairs medical center. We show that it is possible to implement such a cooling protocol in this setting where the majority of arrests occurred in hospital. Of the patients who completed the therapeutic hypothermia protocol, 63% (5 of 8) had a favorable neurologic outcome at the time of hospital discharge. Although this is a small sample size, this rate is similar to those reported in the original trials (49% and 55%) [11, 12] and in subsequent series [20]. This percentage of favorable outcomes is also consistent with larger registries that have noted that 60 to 85% of patients who survive in-hospital arrests tend to have a favorable neurologic outcome [2, 23].
In terms of the initiation of the cooling protocol from time of ROSC, our average of 3.7 hours is comparable to another series from a community hospital [20] and the average time to goal temperature of 8.8 hours was also comparable to previous reports, which ranged from 5.0 to 9.2 hours [15, 18, 20]. Given that most of the arrests were in-hospital, it would be hoped that time to initiation would be even shorter. However, there was no association with time from ROSC to initiation of protocol or time to goal temperature and CPC score at discharge, although the small sample size likely contributed to the lack of statistical significance.
In comparing the survivors of cardiac arrest, there was a significantly faster ROSC in the cases where the survivor was not in a coma afterwards. Although this data was not available in all cases, faster return of circulating rhythm was also associated with higher probability for favorable CPC given that more patients who were not in a coma were neurologically intact at discharge and at six months compared to the patients who were comatose. Although these noncomatose survivors were similar in age and comorbidities, and had high rates of initial PEA or asystole during arrest compared to the comatose survivors, there was a trend towards a higher proportion of primary cardiac etiologies (P = 0.07). Similarly, only one patient out of 18 arrests in the noncooled comatose survivor group was treated with electrical cardioversion, compared to more than 25% of the arrests in the noncomatose and cooled comatose survivor groups, suggesting increased evolution of rhythm to ventricular fibrillation or tachycardia in these latter arrests. Thus, there were some differences in the baseline characteristics in these patient groups which likely affected the duration of arrest. This also meant that, as expected, patients with longer periods of impaired cerebral perfusion were more likely to become comatose.
We should also point out that, within this sample, 38 patients did not survive the arrest itself and overall 65 patients (65%) died before hospital discharge. This rate is higher than reported in previous registries of in-hospital cardiac arrest [1, 2] and may be related to the large majority of patients having an initial rhythm at time of arrest of PEA or asystole (94% overall), which is associated with poorer outcome and has been noted to be the predominant initial rhythm in other in-hospital arrest series as well [24, 25]. Among patients who received cooling, 2 died within the following six months (18%). These observations are also in line with a recent study that demonstrated decreased chances of good outcome from cooling in patients with such nonshockable rhythms [26]. It is possible that patients in the latter category may have more severe disease, but this study did not suggest that such patients were harmed by cooling.
Of the 18 cases in which the survivor was comatose and therapeutic hypothermia was not initiated, these patients were not felt to be candidates for therapeutic hypothermia due to change in goals of care status, hemodynamic instability, or severe coagulopathy and hemorrhage. There were 2 cases where patients might have benefitted from cooling and neurology was not consulted initially. These cases occurred before a formal cooling protocol was adopted by our hospital and relevant staff were educated in its implementation. Since staff training occurred, no further cases were identified. Moving forward, one way to prevent this in the future would be to have every case in which the patient was comatose after cardiac arrest discussed with the on-call neurologist, even in cases where there is a clear contraindication. Other measures to improve our current protocol might include providing a dedicated neurology code pager in code blue protocols and staff training and reorientation at regular intervals.
At our institution, hypothermia was instituted using surface cooling methods and cooling blankets or suits. While our time to target temperature was comparable to previously published studies, it might be possible to further shorten the cooling time by induction with chilled intravenous saline solution. Further, there has also been some concern that surface cooling has been associated with overshooting of goal temperatures which could lead to higher complication rates. Studies have shown that endovascular catheter-assisted hypothermia allows for better control of temperature and faster cooling rates [27]. As such, the incorporation of endovascular cooing catheters might help achieve cooling at a faster rate and prevent overshooting of mild hypothermia goals. However, placement of these catheters requires a trained physician and this can often delay the initiation of cooling if the physician is not readily available [28]. Many community hospitals, including ours, do not always have such trained individuals on staff. Thus, our experience demonstrates that it is still possible to implement a cooling protocol where advanced technologies are not available. It should be noted that, in our series, there were two cases of overshooting beyond 32°C but both of these patients had a favorable CPC score at discharge.
Complications that were noted during the cooling phase and in the week after induction of hypothermia included pneumonia, cardiac arrhythmia requiring administration of medication, deep venous thrombosis, and, in one case, subsegmental pulmonary embolus. It is unclear whether these complications were necessarily related to cooling, as these are also common complications that occur in noncooled cardiac arrest patients. However, a recent study of therapeutic cooling in stroke patients did report a somewhat higher incidence of pneumonia that did not adversely affect eventual patient outcome [29]. Our rates were similar to those described by Prior and colleagues in their series at a community-based hospital [20]. There were no complications associated with rewarming, and although one patient was warmed faster than expected, this was likely related to underlying fever leading to rapid increase in temperature once therapeutic hypothermia was completed.
Limitations of this study are largely related to the retrospective nature of this analysis. Some data points were not recorded in each case, and determination of timing often involved estimates based on ICU nursing charting and by extrapolating from time of arrest indicated by treating physicians. The objective of this study was not to show evidence of the efficacy of therapeutic hypothermia which has been well established but the implementation of a cooling protocol in this particular hospital setting. The lack of a true control group makes it difficult to interpret the overall significance of therapeutic hypothermia beyond comparison of results with previous trials. Although there were patients who were comatose and not cooled, the baseline characteristics of these arrests and the fact that they were actively not cooled precludes direct comparison with the patients who were cooled.
5. Conclusion
This work describes our experience over the last three years with therapeutic hypothermia for comatose survivors of cardiac arrest. In contrast to previous reports, the vast majority of our arrests occurred in-hospital, the initial rhythm was PEA or asystole, and many of these patients died prior to hospital discharge. With the exception of 2 cases, therapeutic hypothermia was appropriately started for patients who were comatose and had no contraindication to cooling and the rate of favorable CPC scores at hospital discharge were comparable in our small series to previous reports and the original landmark trials. Clearly, the mortality of in-hospital cardiac arrest is high but we were able to offer a treatment where prior clinical trials showed clear benefit in terms of neurologic recovery. We describe our experience with this treatment which was readily implemented and completed. Reviewing these cases will also help in improving our protocols in the future and demonstrate the role of neurologists in initiating and managing therapeutic hypothermia.
Disclosures
The authors have no relationships or other items to disclose.
Acknowledgments
This study was supported by the Department of Veterans Affairs and NIH NINDS grant NS40516 (MAY). The authors would like to thank the nursing and ancillary staff at the San Francisco Veteran Affairs Medical Center who helped in initiating and enacting our treatment protocol. The authors would also like to thank our colleagues in the medical, surgical, and neurology departments who provided care for these patients over the last three years. Lastly, the authors would like to acknowledge the patients and their family members described in this case review and are hopeful that we will continue to improve our ability to care for them in these times of significant illness.
References
- 1.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Medicine. 2007;33(2):237–245. doi: 10.1007/s00134-006-0326-z. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 3.McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Annals of Thoracic Surgery. 1999;67(6):1895–1899. doi: 10.1016/s0003-4975(99)00441-5. [DOI] [PubMed] [Google Scholar]
- 4.Laptook AR, Corbett RJT, Sterett R, Garcia D, Tollefsbol G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P AND 1H magnetic resonance spectroscopy. Pediatric Research. 1995;38(6):919–925. doi: 10.1203/00006450-199512000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Hachimi-Idrissi S, Van Hemelrijck A, Michotte A, et al. Postischemic mild hypothermia reduces neurotransmitter release and astroglial cell proliferation during reperfusion after asphyxial cardiac arrest in rats. Brain Research. 2004;1019(1-2):217–225. doi: 10.1016/j.brainres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK. Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiology of Disease. 2002;11(1):28–42. doi: 10.1006/nbdi.2002.0513. [DOI] [PubMed] [Google Scholar]
- 7.Lei B, Tan X, Cai H, et al. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke. 1994;25(1):147–152. doi: 10.1161/01.str.25.1.147. [DOI] [PubMed] [Google Scholar]
- 8.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. New England Journal of Medicine. 2010;363(13):1256–1264. doi: 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Frontiers in Bioscience. 2007;12(3):816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- 10.Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection? Stroke. 2008;39(10):2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New England Journal of Medicine. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 12.Holzer M, Sterz F, Darby JM, et al. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. New England Journal of Medicine. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 13.Belliard G, Catez E, Charron C, et al. Efficacy of therapeutic hypothermia after out-of-hospital cardiac arrest due to ventricular fibrillation. Resuscitation. 2007;75(2):252–259. doi: 10.1016/j.resuscitation.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Hay AW, Swann DG, Bell K, Walsh TS, Cook B. Therapeutic hypothermia in comatose patients after out-of-hospital cardiac arrest. Anaesthesia. 2008;63(1):15–19. doi: 10.1111/j.1365-2044.2007.05262.x. [DOI] [PubMed] [Google Scholar]
- 15.Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. International Journal of Cardiology. 2009;133(2):223–228. doi: 10.1016/j.ijcard.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Sagalyn E, Band RA, Gaieski DF, Abella BS. Therapeutic hypothermia after cardiac arrest in clinical practice: review and compilation of recent experiences. Critical Care Medicine. 2009;37(7):S223–S226. doi: 10.1097/CCM.0b013e3181aa5c7c. [DOI] [PubMed] [Google Scholar]
- 17.Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database of Systematic Reviews. 2009;(4) doi: 10.1002/14651858.CD004128.pub2. Article ID CD004128. [DOI] [PubMed] [Google Scholar]
- 18.Arrich J, Sterz F, Roine RO, et al. Clinical application of mild therapeutic hypothermia after cardiac arrest. Critical Care Medicine. 2007;35(4):1041–1047. doi: 10.1097/01.CCM.0000259383.48324.35. [DOI] [PubMed] [Google Scholar]
- 19.Brooks SC, Morrison LJ. Implementation of therapeutic hypothermia guidelines for post-cardiac arrest syndrome at a glacial pace: seeking guidance from the knowledge translation literature. Resuscitation. 2008;77(3):286–292. doi: 10.1016/j.resuscitation.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Prior J, Lawhon-Triano M, Fedor D, Vanston VJ, Getts R, Smego RA. Community-based application of mild therapeutic hypothermia for survivors of cardiac arrest. Southern Medical Journal. 2010;103(4):295–300. doi: 10.1097/SMJ.0b013e3181d3cedb. [DOI] [PubMed] [Google Scholar]
- 21.Froehler MT, Geocadin RG. Hypothermia for neuroprotection after cardiac arrest: mechanisms, clinical trials and patient care. Journal of the Neurological Sciences. 2007;261(1-2):118–126. doi: 10.1016/j.jns.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Brain Resuscitation Clinical Trial I Study Group. A randomized clinical study of cardiopulmonary-cerebral resuscitation: deisgn, method and patient characteristics. American Journal of Emergency Medicine. 1986;4:72–86. [PubMed] [Google Scholar]
- 23.Weil MH, Fries M. In-hospital cardiac arrest. Critical Care Medicine. 2005;33(12):2825–2830. doi: 10.1097/01.ccm.0000191265.20007.9d. [DOI] [PubMed] [Google Scholar]
- 24.Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K. A comparison of antecedents to cardiac arrests, deaths and eMergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study. Resuscitation. 2004;62(3):275–282. doi: 10.1016/j.resuscitation.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. Journal of the American Medical Association. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 26.Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients? Insights from a large registry. Circulation. 2011;123(8):877–886. doi: 10.1161/CIRCULATIONAHA.110.987347. [DOI] [PubMed] [Google Scholar]
- 27.Flint AC, Hemphill JC, Bonovich DC. Therapeutic hypothermia after cardiac arrest: performance characteristics and safety of surface cooling with or without endovascular cooling. Neurocritical Care. 2007;7(2):109–118. doi: 10.1007/s12028-007-0068-y. [DOI] [PubMed] [Google Scholar]
- 28.Kupchik NL. Development and implementation of a therapeutic hypothermia protocol. Critical Care Medicine. 2009;37(7):S279–S284. doi: 10.1097/CCM.0b013e3181aa61c5. [DOI] [PubMed] [Google Scholar]
- 29.Hemmen TM, Raman R, Guluma KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41(10):2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]


