Abstract
Effective delivery of therapeutic agents into the brain can greatly improve the treatments of neurological and neurodegenerative diseases. Application of focused ultrasound facilitated by microbubbles has shown the potential to deliver drugs across the blood–brain barrier into targeted sites within the brain noninvasively. This review provides a summary of the technological background and principle, highlights of recent significant developments and research progress, as well as a critical commentary on the challenges and future directions in the field. This review also outlines and discusses the tasks that researchers face in order to successfully translate the technology into a clinical reality, including obtaining improved understanding of the mechanisms, demonstration of therapeutic efficacy and safety for specific applications, and development of methodology for rational design to achieve optimized and consistent outcome.
Drug delivery to the brain across the blood–brain barrier
Brain diseases and CNS disorders, such as neurodegenerative diseases and brain cancer, affect millions of Americans and many more people worldwide. These diseases remain one of the world’s leading causes of disability [1], account for numerous hospitalizations and prolonged care, and post enormous financial burden on societies with aging population.
Clinical examples: challenges & significance
As the average age of the population is steadily increasing, more people worldwide are subjected to an increased risk of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS). Progress has been made toward understanding these diseases, but few effective treatments and no cures are currently available [1,2].
Malignancies in the brain, including primary cancer and metastases, are devastating diseases with high mortality and mobility. Primary brain malignancies, which are intrinsically resistant to most chemotherapeutic agents for reasons that are poorly understood, have low survival rate: the 5-year survival rate being approximately 15% for patients aged 45–64 and less than 5% for patients older than 65. The prognosis for patients with glioblastomas, the most common and most aggressive type of primary brain tumors, is particularly poor. Brain metastases are a frequent neurologic complication of many solid tumors and occur in a significant percentage of patients with common malignancies including lung cancer, breast cancer, and colon cancer [3]. As better systemic chemotherapeutic agents have improved clinical outcome in cancer patients with metastatic disease, the incidence of metastases in the CNS is on the rise [4]. Treating the primary and metastatic disease in the CNS continues to be a critical challenge that cancer researchers face, despite the dramatic advances in understanding the molecular basis for carcinogenesis and the development of new targeting agents to treat malignancies [5]. As a growing proportion of patients may experience morbidity and/or mortality from CNS progression with controlled extracranial disease, treatments to improve outcomes in patients with CNS disease are becoming particularly important.
While medical advances have improved the care for individuals with brain and CNS diseases, treatment of these disorders remain challenging and unsatisfactory because of the presence of the blood–brain barrier (BBB), which prevents many drugs in circulation from reaching the brain [1,6]. The BBB possesses specific characteristics that protect the brain from exposure to both endogenous and exogenous toxins. However, this very protective barrier for the brain also blocks most traditional and newer drugs from entering the brain parenchyma from the circulation. While primary brain tumors might have a relatively intact BBB, disease-associated BBB properties may be different from normal BBB. For example, the vasculature in metastasized tumors is leaky compared with normal BBB. However, the leaky BBB associated with tumor pathology is often irregular and highly heterogeneous within the same tumor volume. The disease-associated BBB properties may present additional obstacles to achieve optimal delivery and therapeutic outcome.
The blood–brain barrier
The BBB (Figure 1) [7] is mainly comprised of microvascular endothelial cells, which exhibit many specialized properties: extremely low permeability, high transendothelial electrical resistance and low occurrence of pinocytotic vesicles. The tight junctions (TJs) between the endothelial cells, together with an ensemble of enzymes, receptors, transporters, and efflux pumps of the multidrug resistance (MDR) pathways, control and limit access of molecules in the vascular compartment to the brain by paracellular or transcelllar pathways [6,8]. These morphologic, physiologic and functional characteristics of the BBB ensure that the endogenous and exogenous substrates in the general circulation do not readily and freely reach the brain parenchyma. The BBB also participates in ion regulation, maintains conditions for proper synaptic and axonal signaling by neurotransmitters, and ensures transport of nutrients to the brain [9,10].
Figure 1. The blood–brain barrier.
(A) The blood–brain barrier (BBB) is formed by endothelial cells in the cerebral capillaries. These endothelial cells interact with perivascular elements such as basal lamina and closely associated astrocytic end-feet processes, perivascular neurons and pericytes to form a functional BBB. (B) Cerebral endothelial cells form complex tight junctions (TJ) produced by the interaction of several transmembrane proteins that effectively seal the paracellular pathway. These complex molecular junctions make the brain practically inaccessible for polar molecules, unless they are transferred by transport pathways of the BBB that regulate the microenvironment of the brain. The adherens junctions (AJ) stabilize cell–cell interactions in the junctional zone. The presence of intracellular and extracellular enzymes, such as monoamine oxidase (MAO), γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase, peptidases, nucleotidases and several cytochrome P450 enzymes, endow this dynamic interface with metabolic activity. Large molecules such as antibodies, lipoproteins, proteins and peptides can also be transferred to the central compartment by receptor-mediated transcytosis or nonspecific adsorptive-mediated transcytosis. The receptors for insulin, low-density lipoprotein (LDL), iron transferrin (Tf) and leptin are all involved in transcytosis.
P-gp: P-glycoprotein; MRP: Multidrug resistance-associated protein family.
Reproduced with permission from [7] © Macmillan Publishers Ltd.
The BBB has been recognized as the bottleneck in brain drug development and impermeability of the BBB is the single most important factor limiting the future growth of neurotherapeutics [1,11]. Success in the development of safe and efficient techniques to deliver therapeutic agents across the BBB into the brain interstitium can make significant clinical and societal impact.
Pharmacological treatment strategies for CNS diseases
Treatments that rely on pharmacological methods generally rely on systemic or local drug delivery as the initial route to introduce therapeutic agents into the body.
In conventional systematic delivery, a drug is initially taken either orally or by injection into a vein, the subcutaneous space, or the muscle. The drug is then absorbed into local blood microcirculation in the intestine or near the injected site where the drug molecules enter the systemic blood circulation and are subsequently transported to various organs throughout the body. The drug molecules then pass from the microcirculation to the cells and interstitial space at the organ or tissue location.
The passage of most therapeutic substances from the brain vasculature introduced via systemic delivery is limited by the BBB. In particular, lipid-mediated free diffusion to the brain is limited to molecules with high lipid solubility and low molecular weight (e.g., <400 Da) if they are P-glycoprotein (P-gp) substrates [11,12]. Molecules that have low lipid solubility, or high molecular weight and are substrates for P-gp, including many potent neurologically active substances and drugs, are severely restricted in their ability to reach the brain parenchyma and interstitium from the brain vasculature with systemic administration [1,11].
Furthermore, drugs administered via systemic delivery, if not chemically or biologically targeted, will reach all parts of the body. This is particularly problematic in cancer chemotherapy. While most anticancer agents have the potential to be effective at sufficiently high doses, these doses are often associated with severe systemic side effects. With low tumor targeting efficiency and dose-limiting toxicity, conventional chemotherapy often results in significant patient morbidity and a less desirable outcome. The success of cancer chemotherapy typically hinges upon circumventing dose-limited toxicity; ideally a drug should act as a ‘magic bullet’ that possesses perfect specificity to cancerous cells and has no effect on the rest of the body.
To reduce the systemic toxicity, targeted or site-specific drug delivery through physical targeting (e.g., implantation or local injection) [13] or chemical/molecular targeting strategies [14] have been exploited. In local delivery, drugs are often directly injected or physically implanted at the desired site and then released locally from the site. Targeted delivery can also be achieved via receptor-mediated binding of the drug molecules with specific tissue or cells, even if the drug is injected systemically. However, physical implantation of drug carrier devices is not always possible, and chemical/molecular targeting is often difficult to achieve. As most receptors are also present on more than just the intended targeted site, the circulating drugs will also reach other locations besides the targeted site. Nonspecific binding or uptake can occur outside the intended targets.
Direct delivery techniques usually employ stereotactic positioning and procedures where a 3D coordinate system is used to locate target sites inside the brain in reference to anatomical landmarks. Such neurosurgically-based drug delivery methods bypass the BBB by injection of substances directly into the brain using a needle or catheter. The drug then diffuses in the brain tissue to reach the treatment volume.
For example, convection-enhanced delivery (CED) has emerged as a useful investigational technique for the treatment of brain tumors [15,16]. By administering therapeutic agents directly to the brain interstitium and, more specifically, to tumor-infiltrated parenchyma, this method bypasses the BBB and can also overcome the elevated interstitial pressure produced by brain tumors. Completed and ongoing clinical trials have utilized CED with mixed results [17,18]. Much improved delivery efficiency for various agents than conventional systemic delivery has been achieved. Problems include a potentially limited therapeutic efficacy due to ineffective tissue distribution of the injected drugs from the injection location. The invasive introduction of needles or catheters which traverse untargeted brain tissue can cause unnecessary damage and increase the risk of complication.
The brain delivery techniques clinically used and under research have not been satisfactory. Although direct injection procedures can deliver therapeutic substances into the brain to treat neurological diseases and brain cancer, there is great need for less invasive techniques to transport drugs to the brain via the more advantageous vascular route, which requires efficient drug transport across the BBB.
Strategies for drug delivery to the brain across the BBB
A successful drug delivery system to the brain should be efficient, safe, convenient to use, noninvasive and localized within a specific volume in the brain if needed. These capabilities ensure maximized therapeutic outcome and minimized unwanted side effects. Delivery via the vascular route has obvious advantages but requires efficient drug transport across the BBB. Understanding the function and characteristics of the BBB [6] has contributed greatly in drug discovery to treat diseases in the brain [9] and has guided the development of delivery technologies to overcome and circumvent the barrier [19].
Much effort has been made to develop various approaches for efficient delivery of drugs in the brain across the BBB, although many challenges remain to be overcome in order to achieve a satisfactory outcome. These techniques can be categorized into two basic types: one focuses on modification of drugs and the other exploits modulation of the BBB itself.
Chemical modification of the drugs
Since lipid solubility is a key factor in passive diffusion into the BBB, chemical modification of the drug itself into a more lipophilic form (lipidization) has been pursued. In lipidization, lipid groups are added to the polar ends of drug molecules to increase their permeability. Alternatively, a water soluble drug can be attached to a lipid-soluble drug carrier to achieve the same effect. However, the increased permeability achieved by lipidization (used by several pharmaceutical companies) is not localized, because the permeability of the drug molecules increases not only in a localized region but over the entire brain, and even other organs throughout the body. This limits the use of those drugs that cause deleterious side effects in the brain or other organs throughout the whole body when administration of large amount of drug is needed to achieve required therapeutic concentration and efficacy. In addition, lipidization may bring about undesirable pharmacokinetic effects such as increased uptake by the reticuloendothelial system and increasing nonspecific plasma protein binding when administered intravenously [20]. Increasing lipid solubility may also result in the compound becoming a substrate for active efflux pathways such as P-gp. For these reasons, there are few examples in clinical practice today whereby lipidization strategies of a water soluble drug provide practical solutions to the BBB problem.
Other examples include the development of more hydrophobic drug analogs or linkage of an active compound to a specific carrier of BBB as well as re-engineering of biopharmaceuticals with molecular Trojan horses for delivery to the brain [19]. These strategies take advantage of endogenous transport mechanisms of the BBB. Success of these methods depends on the adaptation to each type of drug molecule and a particular transport mechanism or pathway in the BBB. This usually involves time-consuming and costly processes, and often does not address the issue of localized delivery to targeted regions within the brain.
Disruption of the BBB for drug delivery across the BBB
Temporal and reversible disruption of the BBB either by chemical or physical means has been exploited to allow drugs to penetrate the brain through these disruptions. For example, intra-arterial injection of hyperosmotic mannitol or other hyper-osmotic solutions has been used to open the TJs to reversibly disrupt the BBB [21–23]. A recent report documented the results from a Phase I clinical trial in progress using the osmotic disruption technique, which can be particularly useful for diffuse tumors such as gliomas that cannot be treated surgically [24].
However, disruption of the TJ due to the shrinkage of the endothelial cells caused by hyper-osmotic solution also causes diffusive BBB disruption within the entire tissue volume in the highly perfused brain. The injected solution can reach the brain volume nonselectively supplied by the artery branch where the solution is injected. There is also likely to be a significant expansion of the vascular volume due to the osmotic shift of water into the extracellular/vascular compartment.
In general, techniques that utilize solvents mixed with drugs or adjuvant pharmacological agents attached to drugs to disrupt the BBB through dilation and contraction of the brain blood vessels are not localized and these solvents and adjuvants are potentially toxic.
Ultrasound technology for targeted drug delivery to the brain across the BBB
Following the successful widespread usage of ultrasound (US) imaging as a safe and noninvasive modality for diagnostic radiology and image guided intervention procedures, therapeutic US applications have gained increasing attention in recent years [25,26]. The advantages of US technology include the noninvasiveness and spatiotemporal control of energy deposition, as well as the superior safety profile of nonionizing US exposures, which are devoid of long-term cumulative effects associated with ionizing radiation, and can be usually used repeatedly if needed.
Physical effects generated by US in therapeutic US applications
Therapeutic US techniques utilize the effects produced by the interaction of the acoustic energy with biological systems for therapeutic benefits. The most common, direct and immediate results generated by US exposures are physical effects (i.e., thermal and mechanical effects) which can produce various reversible or irreversible, transient or permanent effects in biological systems.
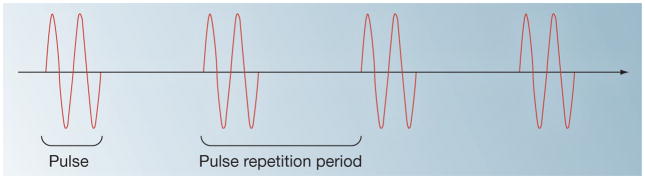
Commonly employing US exposures with center frequencies in the range of 0.5–5 MHz, therapeutic US techniques readily utilize various exposure parameters including the acoustic pressure amplitude or intensity, exposure duration, pulse repetition frequency (PRF) (the inverse of pulse repetition period), or duty cycle (DC) (the ratio of the duration of each pulse over the pulse repetition period) can be used (Figure 2).
Figure 2. Pulsed ultrasound.
The center frequency (Hz) of an ultrasound (US) exposure is the inverse of the period of the pressure cycle. The pulse repetition frequency (PRF) (Hz) is the inverse of the pulse repetition period (PRP); (s), which is the time duration between pulses. Pulse duration (PD); (s), is the duration of each pulse. Duty cycle (DC) is the fraction of time the pulsed US is ‘on’, or DC = PD/PRP. The US intensity (W/cm2) is defined as the temporal average power per unit area of an US exposure, and is related to the square of the amplitude of the acoustic pressure (Pascal).
Thermal effects
A temperature increase in biological tissue exposed to US is due to the thermal conversion of US energy via tissue absorption. Absorption of US energy in biological tissue depends on US exposure parameters (e.g., acoustic intensity and US frequency) and increases with an increasing tissue absorption coefficient [27]. When the rate of US energy deposition surpasses thermal diffusion or conduction in tissue, the temperature increases and can generate cellular and tissue changes.
For example, thermal ablation using focused continuous wave (CW) US exposure with intensity of 1000 W/cm2 or higher can produce rapid (e.g., <3 s) local temperature increase and generate irreversible tissue modification, for example, cell destruction, protein denaturation, and coagulation necrosis. Focused US thermal ablation has been used for tumor destruction [28], hemostasis [29] and other applications [30]. For hyperthermia or heating without permanent thermal tissue damage, lower US intensities can be used to generate a controlled, modest temperature increase.
Mechanical effects & acoustic cavitation
Ultrasound field generates a rapid change of pressure and fluid velocity in the medium (e.g., tissue) where the US wave travels, thus can produce shear stress [31–33] and other mechanical effects in the medium. The US-induced mechanical activities and effects are particularly pronounced when acoustic cavitation occurs, which is the formation, volume oscillation, growth, and/or collapse of gaseous bubbles driven by an US field. Acoustic cavitation is often categorized as inertial cavitation [34–37] or stable cavitation [38,39].
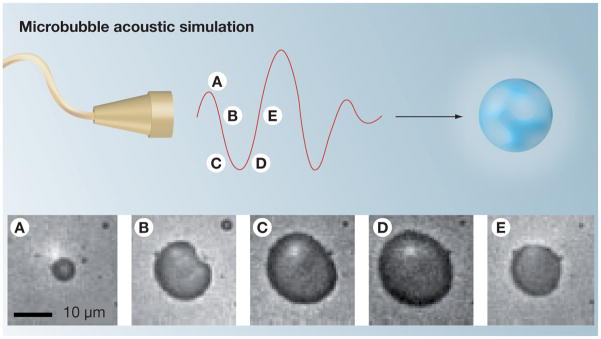
Growth of bubbles occurs with sufficiently large amplitude of the negative acoustic pressure. Due to the large difference in acoustic impedance between gas and liquid, US interacts with bubbles very efficiently [40,41]. Under the influence of the alternating positive and negative pressure of an US field, microbubbles undergo rapid and large volume pulsation (Figure 3) [42] and can collapse violently (inertial cavitation).
Figure 3. Ultrasound interaction with microbubbles.
The microscopy images obtained 330 ns apart demonstrate volumetric oscillation of a microbubble during exposure to ultrasound (500 KHz) that occurs during high- and low-pressure phases. Microbubble images (A–E) were at a constant magnification.
Reproduced with permission from [42] © Macmillan Publishers Ltd.
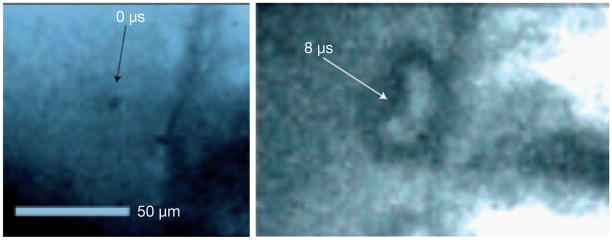
While both types of cavitation describe US-driven dynamic bubble activities, inertial cavitation involves large bubble oscillation and violent collapse dominated by the inertia of the surrounding fluid. For US with MHz center frequency, the oscillation and violent collapse of micron-size bubbles in inertia cavitation generates localized, yet significant mechanical effects [34,43] on nearby cells or tissue (Figure 4), capable of increasing the permeability of cell membrane [44,45] and endothelial barriers [46]. The mechanical effects such as shear stress from micro-streaming by bubble oscillations [31,33,38], shock waves from bubble collapsing [34,43,47], chemical effects [48–50], as well as fluid microjets [34,43] have been identified as important factors (Figure 5) involved in therapeutic US applications [25,34].
Figure 4. Ultrasound-driven microbubble cavitation near a cell membrane.
(A) A frame acquired before ultrasound (US) application showing a quiescent 4-μm-diameter microbubble trapped 17 μm from a cell membrane. (B) At t = 8 μs after US application, the microbubble has developed an involution (arrowed) (indicative of a fluid microjet), which is in contact with the membrane over a region 15 μm.
Reproduced with permission from [34] © Macmillan Publishers Ltd.
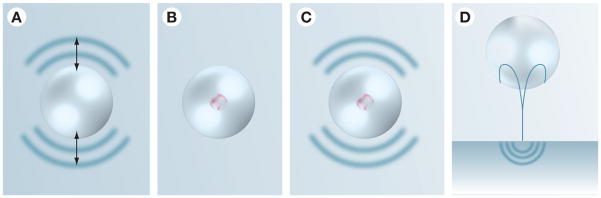
Figure 5. Mechanisms of cavitation-based ultrasound therapies.
(A) Acoustic streaming: bubble volume pulsation around their equilibrium size can generate velocities that induce shear stresses on nearby cells and tissues.
(B) Sonochemistry: rapid collapse of bubbles generates momentary high temperatures in the bubble core. The hot bubble can induce chemical changes in the surrounding medium, including free-radical generation. (C) Shock waves: rapid collapse of cavitation bubbles leads to the formation of shock waves that are capable of disrupting the tissues and enhancing drug transport (implicated in sonophoresis and sonoporation). (D) Liquid microjets: collapsing bubbles near a surface experience nonuniformities in their surroundings that results in the formation of high-velocity microjets. The microjet can penetrate into the tissue or generate secondary stress waves in the tissue (implicated in sonophoresis).
Reprinted by permission from [25] © Macmillan Publishers Ltd.
Acoustic cavitation threshold & microbubble US contrast agents
Inception of acoustic cavitation occurs when the acoustic pressure amplitude surpasses a threshold [27,51–53]. The cavitation threshold increases with increasing US frequency and also depends on other factors such as gas saturation in the liquid. Particularly, cavitation threshold is greatly affected by the availability and the size of cavitation nuclei, which are usually in the form of tiny gas bodies or gas pockets carried by impurities that might be present in the liquid medium. The existence and abundance of gaseous cavitation nuclei greatly decreases the cavitation threshold in the medium.
Thus, in order to achieve repeatable cavitation environment with controlled source of cavitation nuclei, preformed microbubbles with narrow size distribution, such as the microbubble contrast agents for US imaging, have been used. US contrast agents (UCA) (Figure 6) [54] are most commonly in the form of gas-filled, often stabilized, gas-filled micro-vesicles by encapsulation [44,55], designed to enhance US imaging of blood flow and perfusion [40] when injected into the bloodstream.
Figure 6. Characteristics of ultrasound contrast agent preparations.
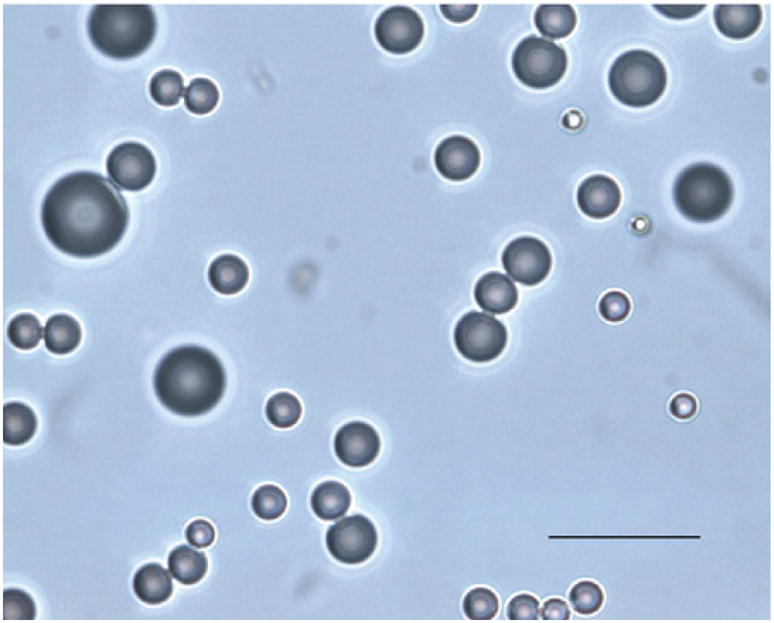
An example of microscopy of a preparation showing typical size distribution (scale bar: 10 μm). Size: microbubble (mean diameter 1–4 μm); nanoparticle (mean diameter <1 μm). Gas composition: air or nitrogen; sulfur hexafluoride; perfluorocarbons (C3F8, C4F10). Shell composition: lipid or other lipid-like surfacant; protein (albumin); biocompatible polymers.
Reproduced with permission from [54] © Elsevier.
The UCAs that are currently available commercially in the United States are Optison® (Mallinckrodt Inc., St. Louis, MO), which are albumin-coated, octafluoropropane-filled microbubbles with a diameter of 1–6 μm, and Definity™ (Lantheus Medical Imaging, MA), which contains lipid-coated, octafluoropropane-filled microbubbles with diameters of 1.1–3.3 μm. Both are FDA-approved blood pool US imaging contrast agents for echocardiology applications, in addition to usages in research investigations for other applications such as contrast-enhanced US imaging detection of lesions in the liver [56], US cerebral imaging [57]. Recently, therapeutic applications using these preformed bubbles have been exploited for US-mediated drug and gene delivery [44,55,58–64] because they can readily induce acoustic cavitation and allow efficient production of mechanical effects at much lower acoustic pressure amplitudes.
Spatiotemporal controlled effects by noninvasive focused US application
Since US is unaffected by the optical opacity of soft tissues, and can be readily transmitted into a soft tissue and focused within a desired volume, a focused US beam can noninvasively target a tissue volume (as small as ~20 mm3) at depth inside the body without affecting the intervening and surrounding soft tissue (Figure 7) [28]. By using US transducers and systems that produce focused beam profiles with controllable exposure parameters, the effects generated by US exposures can be both spatially and temporally controlled in a noninvasive fashion.
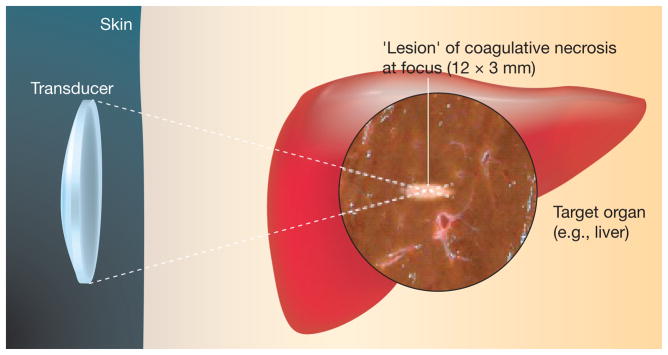
Figure 7. Noninvasive focused ultrasound application.
An extracorporeal ultrasound (US) transducer generates a focused US beam and produced a cigar-shaped focal necrosis lesion at depth within the target tissue (e.g., liver). The volume of ablation (‘lesion’) following a single high-intensity focused US exposure is localized and will vary according to transducer characteristics, but is typically in the order of 1–3-mm wide by 8–15 mm in length along the beam axis. Ablation of larger tissue volumes can be achieved by multiple exposures placed at adjacent locations to cover the entire targeted volume.
Reproduced with permission from [28] © Macmillan Publishers Ltd.
US-induced BBB disruption
With many unique advantages and characteristics, therapeutic US techniques have been pursued to induce disruption of the BBB locally for drug delivery in the brain [65]. Several reviews [65–67] provide detailed accounts of the history and development of the technology, as well as the details of techniques and results used in various experimental studies in different animals.
Focused US-induced BBB disruption in thermal ablation of brain tissue
Disruption of the BBB by US exposures was first observed in studies of high intensity focused ultrasound (HIFU) brain ablation. BBB disruption was observed near the brain tissue subjected to HIFU treatment and was accompanied by neuronal damage. Closely associated with the tissue thermal damage [68–71], the compromised BBB most likely was the result of the pathologic changes of the thermally-damaged tissue region. Although these initial studies demonstrated that BBB disruption can be produced by US at, or near, ablation intensities, the BBB opening was always associated with neuronal damage and thermal tissue necrosis. A thermal window for BBB disruption without irreversible tissue damage has not been identified.
BBB disruption induced by acoustic cavitation
To investigate whether BBB opening can be generated without thermal damage, further experiments using reduced US energy (e.g., lower acoustic intensity and low DC pulsed) were conducted to avoid ablative temperature increase in brain tissue. Encouraging results were obtained by the landmark study led by Hynynen et al. [72], followed by many other studies, and demonstrate that BBB opening can be achieved by US exposures following intravenous administration of preformed microbubbles in a localized and transient fashion in animal models without thermal necrosis lesions [72–76]. These promising results prompted increasing effort in the development of a US technique for targeted delivery of therapeutics into the brain [66,72–75,77,78] after systemic drug introduction in the circulation.
The US-induced BBB opening is believed to be associated with the mechanical effects generated by acoustic cavitation [79,80], that are initiated by the preformed microbubbles in the brain vasculature. Although the detailed mechanism and process are still under investigation, the characteristics and potential advantages of employing microbubbles have been recognized: the presence of microbubbles in the brain microvasculature allows the use of reduced US intensity to generate cavitation without ablative thermal effects to tissue; containment of the disruptive cavitation events within the microvasculature [66,73,77,78] reduces the likelihood of irreversible neuronal damage [73,81–88]. Microbubbles can be engineered to serve as multifunctional agents for multimodal imaging, drug carrier and activator, and molecular targeting agents [44,55,63,64].
Technical implementation of US technique for BBB opening
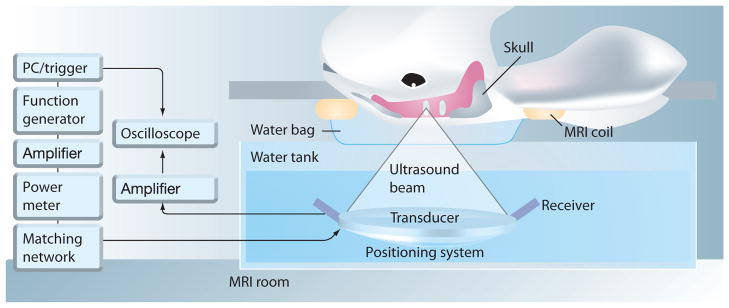
This section describes the general procedures for implementing the US technique to disrupt the BBB, developed and used by many researchers in the field. Figure 8 shows a typical experimental setup [79] for small animals using US to generate BBB opening with MRI guidance. In this experimental configuration, the animal (mouse) was positioned in a supine position. The acoustic coupling allows the US beam to transmit into the brain.
Figure 8. Experimental setup for focused ultrasound application to open the blood–brain barrier in a small animal model with MRI guidance.
A mouse was positioned in a supine position while ultrasound was aimed to a location inside the brain with acoustic coupling.
Reproduced with permission from [79].
Although the US technique for BBB opening has not been reported in human applications, it can be expected that similar principle and procedures can be developed for human applications, as in MRI-guided focused US thermal treatment [89,90]. Parameters and additional considerations specific for human application need to be determined.
Focused US application in the brain
In contrast to soft tissues, the skull that encloses the brain posts a significant obstacle for efficient transmission of US energy into the brain tissue due to the high attenuation of US in bone tissue. For small animals such as rats, the relatively thin skulls present less of a problem compared with larger animals and humans for acoustic access of the brain.
The skull bone has an attenuation coefficient approximately ten times of that in brain tissues (~0.5 dB/cm/MHz) and a higher sound velocity (~3000 m/s) [91] compared with soft tissue (1500 m/s). The skull not only greatly attenuates US to decrease energy deposition inside the brain parenchyma, but also generates unwanted heating in the skull bone. Furthermore, the spatial unevenness of the acoustic impedance of the skull affects both the pressure amplitude and phase of the US wave (aberration). The aberration causes wave front distortion of the US beam, resulting in deteriorated focal beam quality [90,92].
Removing part of the skull (craniotomy) to create an acoustic window can circumvent the problems of wave propagation through the skull bone, although transcranial application without the surgical procedure has obvious advantages. Employing lower frequencies, for example, 0.5 MHz or 1 MHz [73,93], can alleviate the problems of skull attenuation. Increasing the intensity amplitude of the input US can also compensate the attenuation loss, but higher US energy levels generate more heating in the skull. Phased array transducers can allow phase and amplitude correction, potentially capable of compensating the skull’s uneven acoustic impedance and thickness [94–96].
US systems & exposure parameters
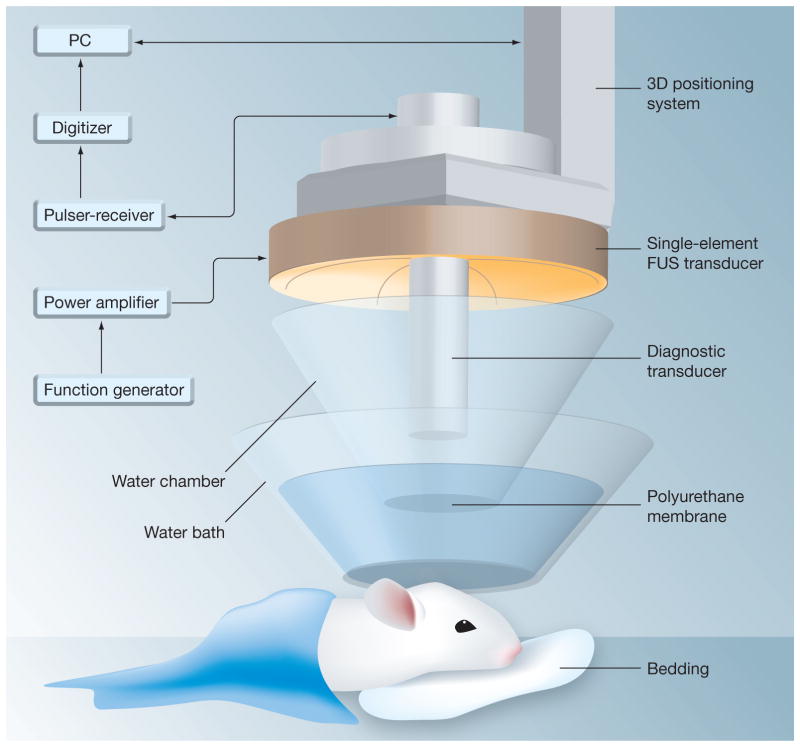
While US array systems provide the most rigorous and may be the ultimate choice for complete noninvasive, transcranial operation in large animals and humans [97], this involves more complex instrumentation and technological implementation. Many studies, especially studies using small animal models with thinner skulls, have used single element-focused US transducers through a craniotomy or intact skull (Figure 9) [81,86]. For the thin skull of mouse, Choi et al. [77], in a simulation and experimental study using wave propagation through μCT-scanned mouse skulls, determined that the attenuation in the skull was not the determining factor for US energy loss. Instead, the energy loss appeared to the consequence of the reflection and refraction at the skull surface.
Figure 9. Trans-cranial application using a single-element spherical segment focused ultrasound transducer with a central aperture (radius: 11.2 mm) that accommodates a collinear and con-focal pulse-echo diagnostic transducer to aid placement of ultrasound focus.
Reproduced with permission from [86] © Elsevier.
US exposure parameters have been mostly empirically determined with the goal to generate cavitation in the brain while avoiding tissue thermal damage. The common choice of the center US frequency for BBB opening is sub- or low MHz (e.g., 0.5–1 MHz). So far, almost all experiments utilize pulsed US exposures with the parameter ranges: total duration of 20–30 s, pulse duration (PD) for each pulse 10 ms and PRF 1–10 Hz (DC of 1–10%), and acoustic pressure of 0.3–0.8 MPa [66,73,77,78].
Animal preparation & microbubble injection
Experiments have been performed with animals under general anesthesia. With a craniotomy or intact skull, the acoustic coupling to the brain is usually accomplished by using a water bath in which the appropriate portion of the cranium is submerged to allow US transmission into the brain (Figures 8 & 9). Intravenous injection of microbubbles into the blood stream is performed before US application. The dose of microbubbles is normally chosen based on animal body weight and extrapolated from manufacturer’s recommendation for human cardiology applications.
Image-guided procedures
MRI has provided the needed guidance for US induced BBB disruption studies [72,98] including the placement of US focus within the brain and assessment of BBB opening. For example, T2-weighted fast spin echo images have been used to delineate the craniotomy if necessary and to select the target location. T-1 weighted imaging following injection of MR contrast agents (e.g., Magnevist, Bayer Healthcare Pharmaceuticals, Montville, NJ) has been used to position US foci prior to treatment and detect the onset and duration of US-induced BBB permeability increase by monitoring the entry of gadolinium into the brain parenchyma (Figure 10) [98].
Figure 10. MRI-guided focused ultrasound (MRIgFUS) increases the permeability of the blood–brain barrier (BBB).
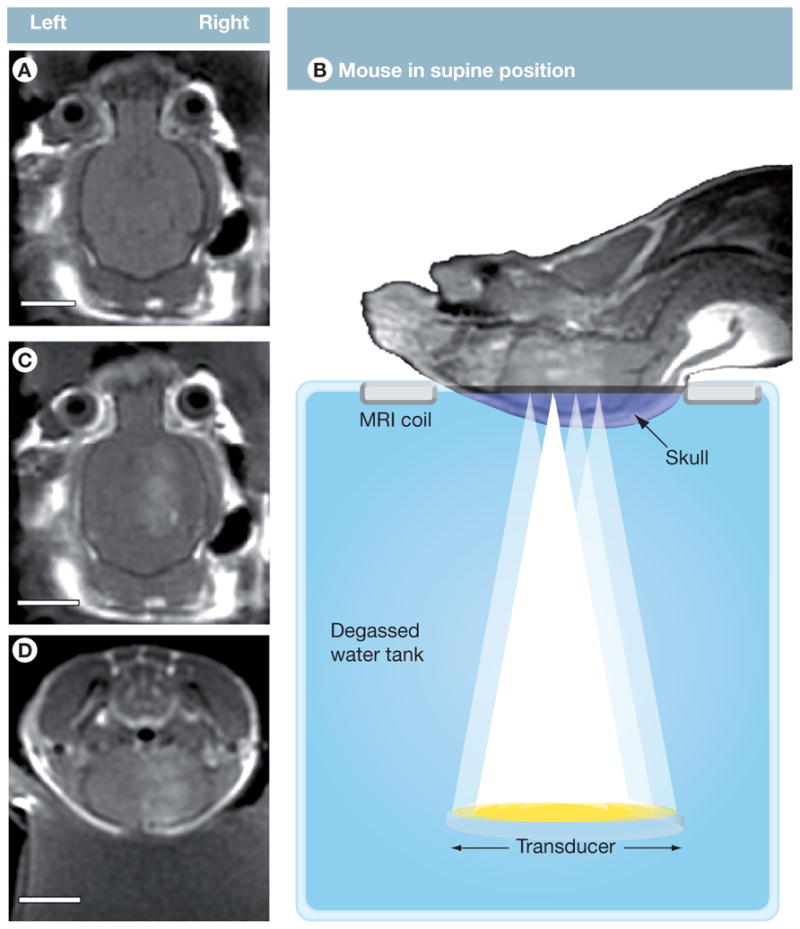
(A) T1-weighted contrast-enhanced MRI scans were used to position ultrasound foci prior to treatment. (B) Mice were positioned in a supine position and injected in the tail vein with microbubbles and gadolinium while US was applied to four aligned spots on the right hemisphere of the brain. Increased BBB permeability was monitored by MRI, visualizing contrast enhancement by the influx of gadolinium (B–D). (A–D: 1286128, TE/TR = 10.4/500.0, FOV = 4 cm, Slice 1 mm, ETL = 4, 3NEX). Scale bars: A, C, D = 0.5 cm.
Reproduced with permission from [98].
Delivery of therapeutic agents
Therapeutic agents are usually systemically injected before or immediately after US application so that these therapeutic agents can be presented in the vascular compartment and can penetrate the brain through the US-induced BBB opening. Assessment of the delivery outcome (e.g., the total amount and location) in animal studies has relied on indirect imaging analysis or post-US histological examination.
Recent development in US-induced BBB disruption for drug delivery into the brain
Following the pioneering work by Hynynen and co-workers [72], which showed the feasibility of US pulses, applied after injection of preformed micron-size gaseous bubbles, to generate transient and localized BBB disruption noninvasively, much progress has been made by these researchers and others in the further development and progress of the technology [80]. This section summarizes and discusses representative, recent, significant findings in the field, including delivery of therapeutics to the brain, demonstration in a large animal model and progress in understanding the mechanisms involved in US BBB opening.
Delivery of therapeutics across the BBB in small animal models
US delivery of therapeutics to the brain represents important and relevant progress of the technique for drug delivery across the BBB.
Delivery of chemotherapeutic drugs to the brain
Doxorubicin (DOX) is an anticancer chemotherapeutic drug and has been widely used clinically as an effective anticancer drug to treat many malignancies including breast cancer and ovarian cancer. However, DOX has not been effective for patients with brain tumors. This is because the BBB prevents DOX from reaching therapeutic concentration in the brain in systematic chemotherapy. In a rat model, Treat et al. demonstrated that MRI guided US technology can disrupt BBB and deliver doxorubicin (DOX) to the brain [87].
Methods
This study [87] used single element-focused US transducers (center frequency of 1.5 or 1.7 MHz) for transcranial application to the rat brain with acoustic pressure of 0.36–2.5 MPa through an intact skull. Under MRI guidance, pulsed US (30 s duration, PRF 1 Hz, and DC 1%) was applied after injection of Optison microbubbles (Malinckrodt, St. Louis, MO) through the tail vein and before the administration of Trypan blue (ICN Biomedical, Aurora, OH). Doxil (Ben Venue Laboratories, Bedford, OH), which is DOX hydrochloride-encapsulated in long-circulating pegylated liposomes, was administered IV in 4 bolus injection immediately after Optison injection at a total dose of 3.0–5.7 mg/Kg. After US exposure, DOX was flushed out from the cerebral vasculature with fresh saline. The tissue block stained with Trypan blue was homogenized to extract the DOX. The amount of DOX within the tissue was measured by the fluorescent signals using a fluorometer (VersaFluor; Bio-Rad Laboratories, Hercules, CA).
Results
Localized BBB opening was confirmed by MRI and Trypan blue examinations at the location of US focal application in all animals and all sites exposed to acoustic power of 0.6W. Therapeutic levels of DOX (886 ± 327 ng/g tissue) were detected in the US-treated brain. The MRI signal strength correlated with the DOX concentration in the brain. No necrotic lesions were observed in locations exposed to US at power of 0.12–1.2 W and 0.1 ml/kg Optison, although macroscopic tissue damage was observed with a higher concentration of Optison (0.5 ml/kg). Other histological findings regarding tissue damage include injury characterized by pronounced vacuolation and local tissue necrosis.
Summary
This study demonstrated that MRI-guided US technology can noninvasively deliver DOX to a targeted region in the brain through an intact skull with a therapeutic concentration. It represents an important step forward in developing techniques to treat primary and metastases in the brain. Although the local permeability increase is transient, further studies to quantify the increase of the permeability of DOX across the BBB induced by US application can help extend this progress for the use of US delivery of DOX to the brain. Additional studies are also needed to demonstrate therapeutic outcomes which may require an adequate level of DOX within the whole tumor in the context of animal survival and other therapeutic benefit, and minimized tissue damage.
In conventional systemic delivery (e.g., injection in blood circulation), entering of DOX into the brain relies on diffusion driven by concentration gradient (higher blood concentration). DOX cannot reach the brain at a sufficient concentration because P-gp (MDR pathways) keeps it out of the brain. In US delivery of DOX through BBB disruption, the entrance of DOX is through the local disruption which is significant for a period of time. During this transient window, a relatively large concentration of DOX can enter the brain. It is possible that the MDR pathways through the P-gp are not sufficient or efficient enough to get the large amount of DOX out of the brain. At a microscopic or cellular/molecular level, the usually extremely low diffusion of molecules across the BBB (observed macroscopically as in measureable levels in bulk tissue) can be the results of various kinds of cellular and molecular mechanisms. It is an intriguing idea that the entrance of DOX via the US-induced BBB disruption may circumvent the MDR pathways via some molecular or cellular mechanisms.
Delivery of monoclonal antibodies
Advances in tumor cell biology have led to the development of new types of anticancer chemotherapeutic agents such as the antibody-based agents. Monoclonal antibodies (mAb) can bind to specific antigens or receptors present on cells to serve as targeted diagnostic and therapeutic agents. mAb are superior to the conventional agents because they can precisely target the signal-transduction pathways that are unique to malignant tumor cells. Antibodies can also be used to guide additional substances or agents such as drugs or radioactive isotopes to the targeted cells. For example, Herceptin (Tratuzmab, Genentech), a humanized mAb that targets human epidermal growth factor receptor 2 (HER2/c-erbB2) expressed in breast cancer cells, has seen remarkable success in treating breast cancer patients [99], but has not been successful in treating brain metastasis resulting from breast cancer. The primary reason is the difficulty in delivering Herceptin across the BBB into the brain efficiently.
Using a similar protocol described in the previous section, Kinoshita et al. demonstrated the feasibility of using the MRI-guided focused US technique to deliver Herceptin to the mouse brain across the BBB [74,75]. The main findings from this study on the delivery of Herceptin through the BBB in a mouse model [74] are summarized below.
Methods
Pulsed US exposures used 0.69 MHz center frequency and 0.6 or 0.8 MPa acoustic pressure. Before US application, 50 μl of Optison was injected. Disruption of the BBB was initiated by US exposures and confirmed by contrast enhanced MRI using Magnevist (Berlex Laboratories, Cedar Knolls, NJ) and post-US examination of Trypan blue leakage in the brain. Localized delivery of Herceptin to the brain was quantified by measuring the amount of human IgG in the tissue homogenized after the experiment.
Results
The amount of Herceptin delivered in the brain was found to depend on the acoustic power level used and also correlate with MRI signals. Herceptin reached a mean maximum of 1,540 (0.6 MPa) and 3000 ng/g tissue (0.8 MPa), significantly higher than the level in the control group, which was below the detection level of 780 ng/g. No apparent gross damage to the brain was found (at 0.6 MPa), although at the higher US power level (0.8 MPa) used in this study, scattered extravasated red blood cells and a few TUNEL-positive apoptotic cells at the sites of most extravasation were observed.
Summary
This study demonstrated that the US technique can deliver a large molecular agent (Herceptin) to the mouse brain. Future studies are necessary to show successful delivery of antibodies into a tumor site rather than a normal part of the brain, and to demonstrate the delivery of therapeutic level of mAb without damage to the brain. As the US parameters required for delivery and BBB disruption in this study are similar to those used in rabbits [75], it may be possible that the parameters could be species independent. However, this suggestion needs to be further validated.
Delivery of chemotherapeutic drugs for glioblastoma treatment
Glioblastoma multiforme is the most common malignant brain tumor in adults [100] and chemotherapy is an important treatment [101]. For example, 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) is a common chemotherapeutic agent for brain tumors but has had limited success by using traditional means of delivery. Randomized prospective trials document only a small benefit in short-term survival and no benefit in medium- or long-term trials. Toxicity is one of the problems encountered when sufficient concentration in the targeted tumor locations is achieved [102].
A recent study demonstrated the utility of focused US delivery of BCNU across the BBB for tumor treatment [103].
Methods
This study used a rate brain glioma model with an implanted tumor and an MR-compatible single element concave transducer at 0.4 MHz (diameter 60 mm, radius of curvature 80 mm). SonoVue SF6 microbubbles (Bracco Diagnostics, Milan, Italy) were injected before pulsed US exposure (exposure duration 30 s, PRF 1 Hz, DC 1%, pulse duration 10 ms) at different acoustic pressures.
Results
In normal rats, US effectively disrupted the BBB at pressures of 0.48 MPa to 1.35 MPa. The increase of MRI signal at 0.48 MPa was relatively low. As assessed by heavy T-2 weighted MR images, no apparent ICH or brain surface hemorrhage occurred when the pressure was less than 0.62 MPa, although ICH occurred with pressures higher than 0.98 MPa. The local concentration of BCNU in normal brains treated with US (0.62 MPa) increased by 340% (from 150 to 513 μg). BCNU concentration did not increase for US exposures with higher pressures. In tumor-implanted brains treated by US exposures (0.62 MPa), the local BCNU concentration at the tumor site increased by twofold (from 170 to 344 μg).
Examination at day 31, showed that in animals treated with US and BCNU, tumor growth was slower (from 0.03 ± 0.03 to 0.11 cm3 ± 0.11) than tumor progression in animals treated with BCNU alone (from 0.03 ± 0.03 to 0.27 cm3 ± 0.17), animals treated by US alone (from 0.03 ± 0.03 to 0.32 cm3 ± 0.01), and animals without any treatment (from 0.02 ± 0.01 to 0.28 cm3 ± 0.11). The suppression effect of US + BCNU continued beyond the initial period, decreasing the tumor to 0.04 cm3 ± 0.08. In fact four out of six rats survived to day 45 with complete tumor shrinkage.
Survival of the animals was best in the group treated with US and BCNU, with a medium survival of 53 days compared with a medium survival of 33 days for animals treated with BCNU alone, 25.5 days for animals treated by US alone, and 28.5 days for animals without any treatment.
Histologic examination showed that tumor cells in the control group were characterized by dense nuclear distribution, while only tiny areas of gliosis infiltrated with chronic inflammatory cells were found in tumors with shrinkage due to the treatment with US and BCNU.
Summary
This study reported significant initial progress by demonstrating that focused US can noninvasively achieve increased targeted delivery of BCNU across the BBB with chemotherapeutic doses in the tumor region to suppress tumor growth and prolong animal survival. Control of tumor progression as well as improvement of animal survival was explicitly demonstrated as the result of US-enhanced delivery of BCNU to the tumor. Increased delivery of BCNU was achieved in both normal and tumor-implanted rat brains without observed hemorrhage or ICH in the brain when the US pressures were within a range (0.48–0.62 MPa).
The medium survival for the animals treated with US and BCNU is approximately 50 days, significantly longer than the control group (~25 days). It is unclear why the animal survival declined rapidly after 50 days. It is important to investigate and identify other information associated with the US-mediated delivery outcome. For example, careful investigation of the physiology data, animal immunity, and complete biodistribution of chemotherapeutic agents will help to define the index representing the true therapeutic outcome and to better relate the delivery outcome to therapeutic outcome.
US-generated BBB disruption in a larger animal model
A recent study by Xie et al. demonstrated the success of transcranial US and intravenous (IV) injection of microbubbles to transiently increase the BBB permeability in a pig model [104]. This was the first study in a large animal model that demonstrated the feasibility of transcranial US to generate BBB disruption through an intact skull. Compared to previous studies using small animal models with thinner skull bones, this study showed that despite the scattering and attenuation of the US beam by the temporal bone in a large animal model, transcranial US application is capable of generating BBB disruption, representing an important progression in the development of US-induced drug delivery to the brain.
Methods
Under general anesthesia, the animals (pigs with average body weight of 35.9 ± 4.3 kg) were monitored in the experiments using 1.5T MRI and MRI contrast agent Magnevist (Berlex laboratories, Wayen, NJ, USA). Lipid-encapsulated microbubbles (LEMB) from ImaRx Therapeutics, Inc. (Tucson, AZ, USA) and perfluorocarbob-exposed sonicated dextrose albumin (PESDA) microbubbles custom-prepared by the investigators were used. Pulsed US exposures (center frequency 1 MHz, PRF 100 Hz, pulse duration 2 ms) were applied after injection of microbubbles. The peak negative pressure measured in vitro inside a water tank passing through a piece of pig temporal bone was reduced to 0.055 MPa from 0.55 MPa without the temporal bone. Evans blue extravasation measured by color spectrophotometry was used to indicate delivery across the BBB to the brain.
Results
US application combined with injection of LEMB and PESDA microbubbles increased the amount of Evans blue extravasation by approximately 30% (to 17 μg/g) and 60% (to 21 μg/g) respectively, compared with the control groups (13 μg/g) which had US exposures but without injections of microbubbles. No increased extravasation of Evans blue was observed in animals that were injected with Evans blue at 2 h after US, indicating the transient increase of BBB permeability. No local tissue damage was observed.
Summary
The skull bone significantly decreased the acoustic pressure amplitude, resulting in a much lower acoustic pressure required at the tissue site to generate BBB disruption, compared with the values used in small animal experiments and animals with a craniotomy. Thus it was assumed that the BBB disruption was not caused by the collapse of the microbubbles. The bubbles may have undergone stable cavitation instead of inertial cavitation, although this was not directly proven in this study. The BBB disruption in this study appeared to last shorter than previous experiments as well. Further investigation will need to validate the assumption and determine the stable cavitation ‘dose’ required to achieve BBB disruption. This could have important implications since the use of stable cavitation in BBB opening might help to minimize damage to the vasculature and tissue. Systematic studies to correlate US parameters with delivery outcome, and to assess unwanted effects such as hemorrhage and necrosis are needed.
Physical mechanisms & processes in US-generated BBB opening
A complete understanding of the detailed processes and mechanisms involved in US-mediated BBB disruption can greatly facilitate the successful development and translation of the technology. There can be various physical, biophysical, biological and even biochemical processes involved in US-bubble interaction with cells and tissue, and these events can be of different spatial and temporal scales, making it difficult to obtain a coherent understanding of the mechanism. It is a challenging task to study the mechanism of US BBB disruption due to the lack of adequate technology to study the dynamic and microscopic events of acoustic microbubble activities and their biological responses in the brain microvasculature. The major difficulties are: studying the complex dynamic behaviors of US-driven microbubbles in the brain vasculature (physical process) and directly and quantitatively relating the physical causes with the biological outcomes of BBB opening (physiological mechanism).
General hypothesis
Since the BBB opening was found to occur only when US pulses (0.2–0.8 MPa) were applied with IV injection of preformed microbubbles, and the temperature increase was minimal (e.g., < 0.5°C) [77] using low intensity and low DC-pulsed US parameters, it is believed that US-driven microbubble activities within the brain microvasculature are responsible for US-generated BBB disruption. However, the details regarding how these microbubble activities generate BBB disruption are not established.
Microbubble activities driven by US in the brain vasculature
Studies on individual microbubbles driven by US exposures have revealed bubble dynamic behaviors such as stable oscillation (stable cavitation), growth, large amplitude oscillation, or inertia-driven collapse (inertial cavitation). The microbubble behaviors inside the brain micro-vasculature in vivo responsible for BBB opening are still unknown, although the effects of the capillaries on microbubble responses to US impact have been recognized from in vitro model systems [105–107].
Without an adequate technique to obtain spatiotemporal information on US-driven microbubbles on the microscopic level within the in vivo brain vasculature, studies have relied on characterization of ensemble behaviors of microbubbles within a tissue volume and assessment of tissue changes from post US examination. The dynamics of ensemble behaviors of UCAs in the brain microvasculature has also been examined recently [108]. Although largely phenomenological studies, these investigations have provided US parameter ranges that can produce BBB disruption under specific experimental settings. For example, studies have examined the effects of US frequency, acoustic pressure, and exposure duration on BBB disruption [67,80,109] and have identified an acoustic pressure threshold when BBB disruption is detected [80]. In the frequency range of 0.26–2.1 MHz, the threshold for BBB disruption appears to be related to the mechanical index of US exposure [110]. A recent study also investigated the effects of microbubble size on the BBB opening [111] and found that microbubbles of a larger size (4–5-μm diameter) produced larger or longer BBB opening as indicated by the level of increased fluorescence, and required lower acoustic amplitude than the microbubbles with 1–2 μm diameter.
Inertial cavitation versus stable cavitation
Strong microbubble activities such as inertial cavitation from many microbubbles within the brain vasculature can generate cell death or damage to the vasculature [112]. At reduced US pressure levels, the impact of microbubble activities on nearby cells generates milder, reversible or transient responses such as transient cell plasma membrane disruption, which reseals in time to allow cell survival (e.g., sonoporation) [38,113]. McDannold et al. [79] investigated whether US-induced BBB disruption can be generated without wideband emission from US-driven microbubbles. These results suggest that inertial cavitation (or bubble collapse) is not necessarily required for BBB disruption [79]. Although broadband emission is considered a signature of inertial cavitation, the detection of broadband acoustic emission may be limited by the sensitivity of the technique employed (signal to noise ratio as well as frequency response of the detecting transducer) as an indicator for inertial cavitation. Nevertheless, controlling the type and dose of cavitation has been recognized as a means to produce improved results in terms of minimizing tissue damage [114]. Preliminary results [79,104] suggest that the BBB disruption produced by stable cavitation may last for a shorter period of time, but whether the BBB disruption produced by stable cavitation has different mechanisms and characteristics from that produced by inertial cavitation has not been systematically investigated.
US-induced spatiotemporal intracellular calcium activities
The immediate observable bioeffects such as cell lysis and reversible membrane poration generated by US-driven microbubble activities are well recognized from in vitro studies. It is more challenging to ascertain the details regarding the complex, dynamic and micron- or submicron-scale biophysical, biochemical details and the immediate and downstream cellular processes in vivo.
Calcium transients generated by US-driven microbubbles & the potential relevance
Recent investigation revealed that in various cell types, US-driven microbubble activities can generate calcium transients [49,115–117], including increase and recovery of intracellular calcium concentration ([Ca2+]i), temporal [Ca2+]i oscillations, and spatial waves of [Ca2+]i changes in surrounding cells. Calcium waves result in delayed [Ca2+]i changes in cells hundreds of microns away from the initially affected cells that directly interact with microbubbles tens of seconds after US application [115].
These results may have implications in US-mediated cellular responses because [Ca2+]i activities affect cell–cell contact [118], electrical resistance [119,120], ZO-1 TJ protein migration from intracellular sites to the plasma membrane [121], and TJ assembly [122] in epithelial and endothelial cells. In particular, regulation of [Ca2+]i is involved in the disruption of TJs between endothelial cells and BBB permeability [123,124]. Some vasoactive agents (e.g., histamine, bradykinin, endothelin) as well as certain nucleotides (e.g., adenosine triphosphate [ATP], adenosine diphosphate [ADP], uridine triphosphate [UTP]), that increase BBB permeability also cause elevation of [Ca2+]i [123,125]. Decreasing [Ca2+]i changes ZO-1/actin binding and alters the subcellular localization of occluding, while increasing [Ca2+]i from intracellular stores interferes with TJ formation [122].
US-generated calcium transients in brain microvascular endothelial cells
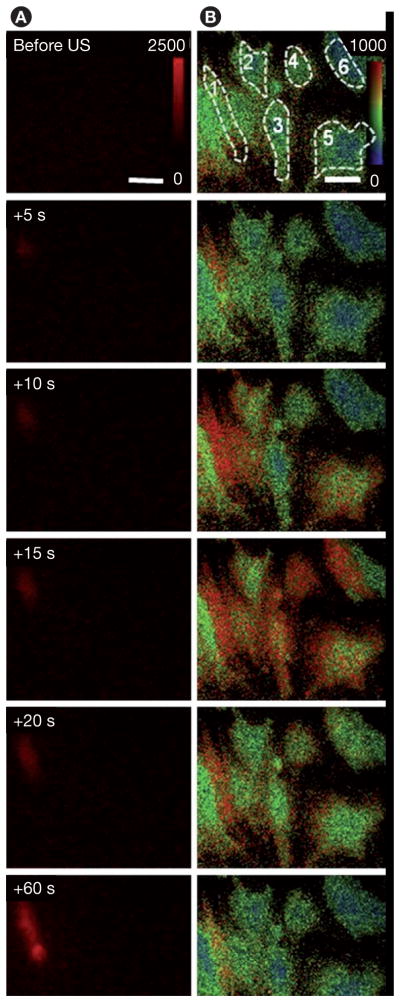
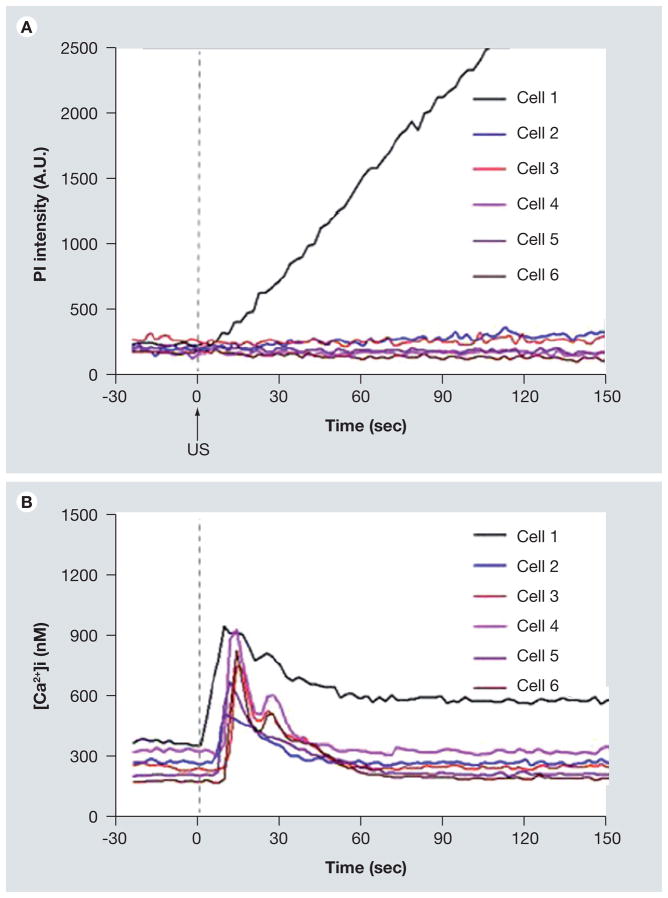
Regulation of [Ca2+]i in brain microvascular endothelial cells may be important in US-mediated BBB opening. A recent study [126] demonstrated that US-driven microbubbles can generate [Ca2+]i activities in cultured brain microvascular endothelial cells (Figures 11 & 12) with a change in [Ca2+]i from 180 to 1000 nM [127]. This is similar to the range of the observed elevation in brain endothelial cells treated by bradykinin, histamine, mannitol and ATP, which are known to increase the permeability of the BBB [123,125,128]. [Ca2+]i waves were also observed, initiating changes of [Ca2+]i in cells nearby the cell in contact with a microbubble (Figures 11 & 12).
Figure 11. Calcium waves in cultured b. End3 cells.
(A) Time-lapse images of propidium iodide (PI) fluorescence showing cell 1 with membrane disruption, (B) [Ca2+]i changes in cell 1 with membrane disruption and the surrounding cells without membrane disruption. US tone burst is 1.25 MHz with 10 cycles and peak negative pressure of 0.24 Mpa. The color bars in each column indicate the PI fluorescence intensity in arbitrary units and the [Ca2+]i in nM.
Reproduced with permission from [126] © Elsevier.
Figure 12. Propidium iodide (PI) fluorescence intensity and [Ca2+]i showing immediate and delayed changes of [Ca21]i in b.End3 cells.
(A) Mean PI fluorescence as a function of time for cells 1–6 where cell 1 had irreversible membrane disruption, (B) mean [Ca21]i as a function of time for cells 1–6, showing the immediate change in calcium (cell 1) and delayed calcium change (cells 2–6). The regions-of-interest for cell 1–6 are shown in Figure 11B.
Reproduced with permission from [126] © Elsevier.
Summary
This study showed that US-driven microbubbles can generate down-stream [Ca2+]i activities that extend beyond the initial isolated and discrete impact from the cells affected by an individual microbubble. This can be an important aspect in US-induced BBB disruption, although whether the US-generated [Ca2+]i activities play an important role in the molecular mechanisms of BBB disruption remains to be elucidated. Further study is needed to determine whether the US generated calcium changes are correlated with the molecular changes in the BBB and its permeability.
Molecular mechanisms in US induced BBB opening
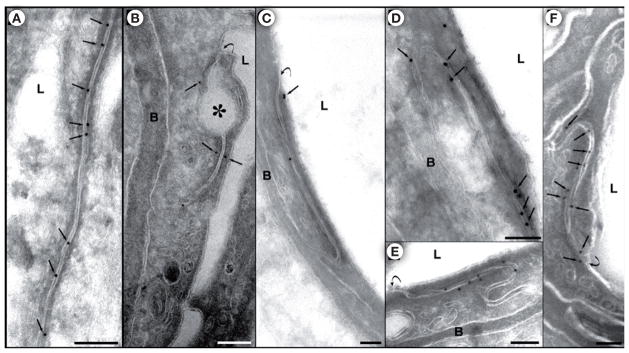
Histological examination has shown tissue damage and cellular changes in response to the mechanical impact of US-driven microbubble activities. Ultrastructural studies [82,129] employing electron microscopy (EM) and immuno-electron microscopy (IEM) have revealed more details and insights regarding the molecular changes in the BBB and the routes through which materials pass into the brain across the US-induced BBB disruption.
Paracellular & transcellular transport
An initial ultrastructure study [82] examined the rabbit brain exposed to US after injection of microbubbles. (The BBB disruption was confirmed by contrast-enhanced MRI as well as trypan blue leakage at the sonicated locations.) EM examination showed transcellular and intracellular passages of molecules approximately 1–2 h after US application. Transcellular passages involve transcytosis, endothelial cell cytoplasmic opening with fenestration and channel formation, opened TJ, as well as injured endothelium that occurred with high levels of US intensity.
As shown in Figure 13A, at low US intensities (0.5 W), the tight junctional complexes appeared intact but some interendotherlial clefts were lightened. The basement membrane had no irregularities or splitting. Numerous caveolae attached to the basement membrane and free caveolae in the endothelial cell cytoplasm were seen. At higher US power (3 W), moderate to severe damage to the brain vasculature was found. As shown in Figure 13B, the endothelial cytoplasm (EC) appeared very dark with poorly distinguishable organelles, tortuous basement membrane, and leakage of blood plasma outside the membrane. Detachment of the endothelial lining was observed and sometimes with discontinued membrane.
Figure 13.
(A) Ultrastructural view of part of a microvessel exposed to low US intensity (0.55 W). Numerous caveolae (arrows) attached to the basement membrane, b, and also free caveolae (thick arrow) present in the endothelial cell cytoplasm (EC); caveolae formation is seen on the luminal surface of the cell (arrowheads). A lightened interendothelial cleft with no apparent tight-junctional complexes is shown (double arrows). (B) A damaged blood vessel from the central part of a location sonicated at 3 W. Very dark, infolded endothelial cell cytoplasm, (EC), tortuous basement membrane, (b), with blurred outlines (arrow) and leakage of blood plasma outside the membrane (★) are shown. Edematous astrocytes (A) and damaged neuropil (NP) are present, and two astrocyte nuclei (N) with agglomerated chromatin are seen at the bottom of the microphotograph. L: Lumen; R: Red blood cell in the lumen; P: Pericyte.
Reproduced with permission from [82] © Elsevier.
The results obtained from this study suggest four mechanisms of passage of materials through the BBB: transcytosis, transendothelial openings, widening of interendothelial clefts and opening of TJs, and free passage through the damaged endothelial lining.
Permeability & cellular/molecular changes in the BBB
A further ultrastructural study [129] revealed additional details and information regarding permeability and cellular/molecular changes in rat brains treated with US in the presence of microbubbles in the brain microvasculature.
Using rabbit polyclonal anti-occludin antibody, rabbit polyclonal anti-claudin-1, anti-claudin-5 antibodies, as well as rabbit polyclonal anti-ZO-1 antibody (Tight Junction Antibody Sampler Pack, Zymed Laboratories Inc., CA, USA), changes in junctional proteins were examined using EM and IEM examinations. Immunosignals of Claudin-1, Claudin-5, and ZO-1, representing Occludin, were all found to be significantly reduced at 1 and 2 h after sonication compared with controls, indicating disassembling of the tight junctional complexes. For example, Figure 14 shows the immuno-gold expression of occludin. In nonsonicated regions, the occluding-labeled gold particles were mostly present in the interendothelial clefts (Figure 14A). The number of immunosignals was reduced at 1 and 2 h after US (Figures 14B & C). The localization of the occludin immunosignals was also atypical, indicating a disassembling of the junctional complexes. At 4 and 6 h after US, the density of the signals was higher, and most of the particles were again located inside the cleft (Figures 14D & E). At 24 h, the expression and localization appeared normal (Figure 14F).
Figure 14. Expression of junctional antigens: occludin-labeled gold particles.
(A) The interendothelial cleft is labeled with numerous immunosignals represented by the grains of colloidal gold. The signals (arrows) are located inside the cleft, over the cell membranes or very close to the membranes. (B) 1 h after sonication. A few signals are present in this cleft, three of which are located more distantly from the cell membrane (arrows). A detachment of the membranes forming a large dilation of the first third of the cleft is indicated by an asterisk. (C) 2 h after sonication. Reduction of the immune expression of occluding is evident: only three signals are present in this long junctional cleft. Two of the signals are atypically located at a distance from the cleft (arrow). (D) 4 h and (E) 6 h after sonication. The immunosignals in both micrographs are more numerous, compared with those in (B) and (C) and they are typically located between or over the cell membranes of the cleft. (F) 24 h after sonication. The immunosignals (arrows) are seen along the cleft, showing a normal density and localization.
L: Lumen; B: Basement membrane; Curved arrows show the luminal entrance of the interendothelial cleft. Scale bars: A, B, D, E 100 nm; C, F 200 nm.
Reproduced with permission from [129] © Elsevier.
Increase of the BBB permeability was assessed by the transport of horseradish perroxidase (HRP); (Type IV, m.w. 40,000 Da, effective hydrate radius 29.8 Å, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and Lanthanum chloride in the brain. Lanthanum chloride is a low molecular weight marker (~139 Da, effective hydrate radius 3.1 Å) which normally does not penetrate the BBB. Both vesicular transendothelial and paracellular passage through jucntional cleft were seen 1 or 2 h after sonication, indicating the paracellular passage of the tracer molecules to be the result of junctional complex disintegration. The absence of the tracer passage after 4 h indicated the transient nature of BBB permeability enhancement. For example, Figure 15 shows that increase at both 1 and 2 h after US and cessation of the tracer leakage of Lanthanum chloride, consistent with the reversible changes of the immunosignals at approximately 4 h after US, indicating the restoration of barrier function of the TJs in addition to other cellular changes responsible for transcellular and paracellular pathways.
Figure 15. Blood–brain barrier permeability for lanthanum.
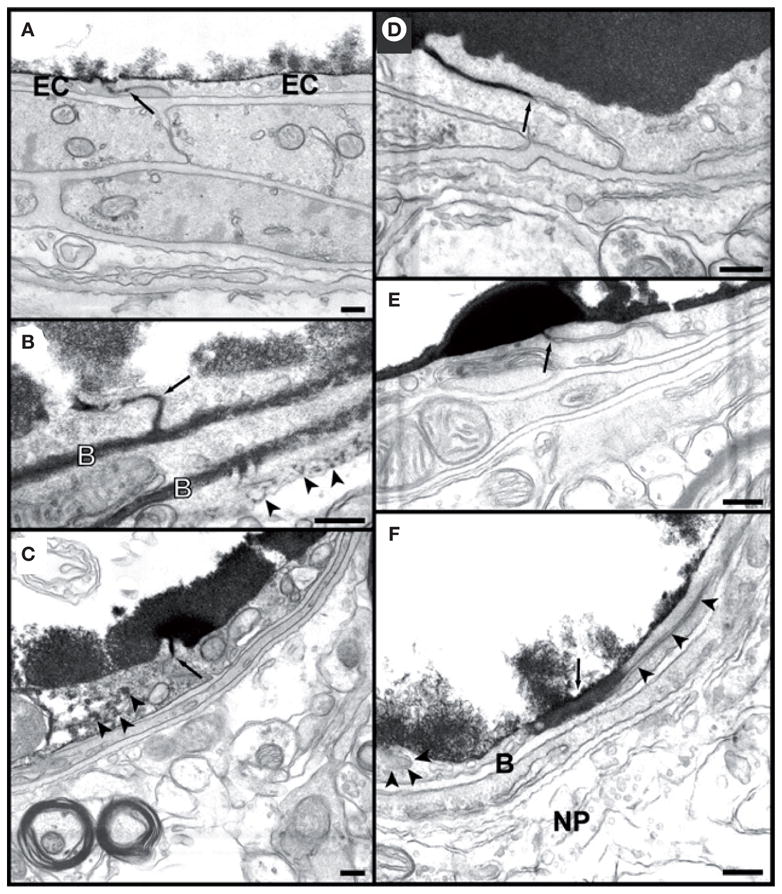
(A) Photomicrograph of a microvessel from a nonsonicated area. The tracer has entered the interendothelial cleft but is stopped at the first tight junction (arrow). The endothelium and subendothelial structures are free from lanthanum. Deposits of the tracer are seen only on the luminal surface of the endothelial cells (EC).
(B) 1 h after sonication. Lanthanum has passed the entire length of the interendothelial cleft and has infiltrated both sheets of the basement membrane (B). Deposits of lanthanum are also seen beyond the basement membrane (arrowheads). (C) and (D). 4 h after sonication. In (C), the tracer is stopped at the first tight junction and does not pass through (arrow). In (D), a longer part of the cleft is labeled but again the penetration was stopped (arrow). In both vessels the subendothelial structures appear free of lanthanum deposits. Note the diffusion of a small amount of lanthanum in endothelial cell cytoplasm in (C) (arrowheads). (E) 24 h after sonication. No passage of the tracer beyond the first tight junction (arrow). Endothelium and all subendothelial structures appear free from lanthanum. (F) 24 h after sonication. Part of one of endothelial cell’s cytoplasm is diffusely stained by the tracer (large arrow). The interendothelial cleft (arrowheads) does not appear to be filled with lanthanum, and neither is the basement membrane (B) or the neutropil (NP). Scale bars: 200 nm.
Reprinted with permission from [129] © Elsevier.
Summary
Results from the ultrastructural studies showed for the first time that US-induced BBB disruption involved a disintegration of the TJ molecular complexes of the cerebral microvascular endothelium with both paracellular and transcellular transport mechanism. These results provide important knowledge for the mechanism of US-induced BBB disruption, although no details are currently available to gauge the relative importance of these pathways in the final delivery outcome through the BBB. In addition, these observations from the ultrastructural studies provide some assurance, at the molecular level, of the transient nature of the US-induced BBB disruption.
Since no information is available regarding cavitation events involved in these particular studies, a direct correlation cannot be established to quantitatively and specifically relate the cavitation activities with the post-US examination results of molecular and cellular changes in the BBB. Nevertheless, these molecular and cellular changes detected in the ultrastructural studies probably represent a general outcome from experiments using typical and similar US exposure conditions.
Molecular weight of deliverable molecules by US-induced BBB opening
The delivery of many otherwise nonpermeable molecules by US-induced BBB opening has been demonstrated in previous studies. The list of deliverable imaging and therapeutic agents includes MRI contrast agents Omniscan (573 Da) and Magnevist (938 Da) [72,77,86], Evans Blue [74], Trypan Blue [130], Herceptin (148 kDa) [74], horseradish peroxidase (40 kDa) [129], doxorubicin (544 Da) [87] and rabbit anti-Aβ antibodies [130]. These studies showed that molecules with a molecular weight greater than the BBB’s natural exclusion size threshold of 400 Da can be delivered across the BBB by US. More importantly, a recent study by Choi et al. [88] investigated the dependence of molecular delivery on a molecular weight of up to 2000 kDa to a specific region of the brain such as the hippocampus.
Methods
In a mouse model, the delivery of fluorescently labeled dextrans of molecular weight of 3, 70 and 2000 kDa by US-induced BBB opening was determined using fluorescent microscopy. The experiments used an intact skull transmission configuration (Figure 9) and a UCA (Sonovue; Bracco Diagnostics, Inc., Milan, Italy) consisting of microbubbles with mean diameters of 3–4.5 μm in stoke concentration of 5.0–8.0 × 108 bubbles/ml. Fluorescent microscopy examination of the brain allows analysis of the spatial deposition pattern of molecules in the brain with respect to the underlying vasculature.
Results
The increase in spatially averaged fluorescence and the total area of fluorescence are examined to analyze the extent of fluorescence markers delivered to the mouse hippocampus. The mice receiving 3 kDa dextran exhibited a high increase in average fluorescence. The animals receiving 70 kDa also had an increase in fluorescence although the average fluorescence intensity is relatively low, indicating the smaller amount of 70 kDa compared with the 3 kDa dextran molecules. No significant increase in fluorescence was detected for 2000 kDa dextran, indicating an upper limit in the size of molecules that can be delivered by US to the hippocampus of the mouse. The percentage area of the targeted hippocampus with fluorescence is similar for 3 kDa and 70 kDa dextran molecules, 54.2 ± 24.6% and 45.6 ± 12.6% respectively.
The analysis of the spatial distribution of dextrans of different molecular weight showed that the 3 kDa dextrans were present throughout the targeted (left) hippocampus and part of its immediate surroundings, with greater signals detected near the internal and external hippocampal vessels. The 70 kDa dextrans were also observed to be diffusely distributed, although at a lower level. In addition, isolated regions of higher fluorescence were distributed along the internal transverse hippocampal vessels. However, no significant fluorescence was detected for 2000 kDa dextrans. These results suggest a size of the US induced BBB opening. They also indicate that the spatial distribution of delivered agents depend on the molecular weight or size of the agents as well as the vessel location.
Summary
This study provides important information about US-mediated delivery of molecules across the BBB and beyond. The study determined that the US microbubble-generated BBB opening has a size threshold for the deliverable molecules between 70 kDa and 2000 kDa using dextrans as model molecules. Higher concentration of molecules with smaller sizes (e.g., 3 kDa) can be delivered than of the larger molecules. The larger molecules, the 70 kDa dextrans, had a more heterogeneous overall distribution.
It is worth nothing that various therapeutic agents may have different chemical properties than dextran. The shape and chemical properties of the agents may also influence their transport through the US-induced BBB opening in addition to molecule weight.
Current status, challenges & future directions
While US-mediated drug delivery across the BBB has not yet translated into clinical usage, studies in small animal models have obtained promising results that show the feasibility and potential of the technology. Exciting future results can be expected with continuing effort and progress in the field.
Important tasks
The main tasks needed to be accomplished to facilitate the translation of the US BBB delivery technology for successful clinical applications include:
Further technology development for controlled and efficient US energy transmission into the brain, optimal microbubbles or agents for BBB opening and drug transport, and adequate monitoring strategies;
Demonstration of therapeutic efficacy and safety of the technology in specific applications.
Complete understanding of the mechanism involved in US-induced BBB disruption. It is particularly important to make progress on the aspect of fundamental science behind the technology, which is the key to ensure the translational efforts are rationally designed, guided, and efficiently executed.
US transducers and systems for focused US exposure in the brain
For human applications, transcranial US application for BBB opening requires US transducers and systems that can overcome the problems presented by the skull such as the defocused beam and heating in the skull bone. With the improvement of multichannel electronics, US transducer arrays operated at sub- to low-MHz frequencies using piezo-ceramic and piezocomposite material. These array transducer systems are capable of producing high acoustic pressure. Phase aberration corrections techniques based on the time-reversal method [92,131,132] or phase conjugation have been proven to restore good focusing with array transducers. By correcting both phase and amplitude using spatiotemporal inverse filtering and data from 3D CT scans [92], focusing quality can be improved. These techniques will need to be validated and may need to be further improved for eventual application in humans. Also, for MRI image guided focused US procedures, US transducers must be MRI compatible [133].
Image-guided US intervention & delivery quantification
To monitor the US-induced BBB disruption in vivo, standard imaging of BBB integrity that is performed with small, water soluble, contrast agents with short plasma half lives can be employed. For example, enhancement in the brain on computed tomography (CT) scans produced by iodinated contrast agents indicates loss of BBB integrity. Such enhancement is often found in malignant tumors or other lesions that cause vasogenic edema. The degree of enhancement on CT scans increases linearly with the amount of contrast agents entering the brain. It is important to distinguish the disease-associated (pre-existing) BBB permeability from the BBB-induced permeability change.
Widely used in noninvasive US ablation applications [90], MRI has provided capability for in vivo target tissue visualization within the brain. Contrast-enhanced MRI has provided assessment of BBB disruption (Figure 10) [72,98] as well as hemorrhage during US-induced BBB opening [134,135].
Important progress has been made by Choi et al. [77] on the molecular rate and path of diffusion using sequential, slow-contrast diffusion, high field MRI. The shape of the BBB opening was determined to be not only associated with US beam shape, but also the underlying vasculature of the sonicated region. This study provided insightful findings regarding the spatiotemporal variations of the model drug delivered in the brain by US at a specific location, and particularly recognized that the type of vasculature in the US-targeted region influences how the drug will be delivered. The larger vessels or their immediate vicinity are more sensitive to US, resulting in BBB opening, perhaps due to the fact that more microbubbles were present in the larger vessels or more microbubble activities were available in these locations. Although high field MRI may not be implemented in human application, this study underscored the importance of image monitoring of US BBB disruption and delivery, and revealed valuable mechanistic knowledge not available otherwise.
A recent study [136] demonstrated the use of micro-SPECT/CT for detecting 99mTc-DTPA to quantitatively assess the BBB disruption in a rat model. 99mTc diethylenetriamine pentaacetate (99mTc-DTPA) is a nondiffusible radiotracer widely used in nuclear medicine to measure the permeability of BBB in humans.
Although the leakage of MRI contrast agents or the radiotracer in the SPECT/CT study into the brain parenchyma from the vasculature can be related to BBB opening, different drugs will have different pharmacokinetics, presenting a difficult challenge, which remains to be addressed. New approaches need to be developed for pharmacokinetic analysis or quantification of specific drugs that cross the BBB into the brain.
Demonstration of therapeutic efficacy & safety for specific applications
The success of US drug delivery across the BBB for human applications depends upon the efficacy and safety of the technology. Although reported results indicate that it is possible that damage can be contained to minimal hemorrhage [85,134,135], safety needs to be assessed for any specific application under consideration.
In general, US exposure may produce unwanted effects such as tissue injury and generation of reactive oxygen species. Different applications may have different requirements regarding efficacy and safety. Tumor treatment requires sufficient drug concentration in the required tissue volume and may tolerate some degree of side effects. However, it is important to avoid tissue damage and hemorrhage in the brain where neuroimaging agents or drugs are delivered to treat Alzheimer’s disease.
A variety of US-induced bioeffects including tissue mechanical injury, hemorrhage, extravsation, the dilation of vessels, temporary ischemia, mechanically-induced opening of the TJs, activation of various transport mechanisms [76,82,129], spread depression (SD) [67], intracellular calcium activities [126], necrosis, and apoptosis may have different implications in different applications and need to be determined in the context of a specific application.
It is important to keep in mind the fact that delivery through opening of the BBB can permit entry of toxins along with therapeutic agents. Thus, therapeutic index and pharmacokinetics need to be carefully studied. Other toxicity such as overall BBB function, neural impairments, and cell membrane damage also need to be evaluated, in the context of the specific application that is being considered.
Although US exposures are devoid of the long-term cumulative effects often associated with ionizing radiation, it is unknown whether US-induced BBB disruption produces any long term adversary effects on the CNS. Further studies are needed to investigate what cumulative effects on the CNS are produced if US is repeatedly used to open the BBB in the same region multiple times. These questions regarding the safety of the technique are important for eventual human applications.
Microbubbles
Commercially available, preformed microbubbles such as Optison and Definity have been used in most experimental studies to facilitate US BBB disruption. Since these microbubbles were developed for contrast-enhanced US imaging of the microvasculature and perfusion to assess cardiac functions and morphology [137] in echocardiography, they may not possess the optimal characteristics for inducing BBB opening for drug and gene delivery to the brain. New agents that are specifically tailored and optimized for BBB disruption may be more desired and necessary. For example, if stable cavitation is preferred to inertial cavitation, nano-sized bubbles may be more desirable since their acoustic resonant frequency [40] for the smaller size is further away from the 1 MHz US frequency usually used for brain application. Novel designs of microbubbles with the potential capability to carry drug load as well as targeting ligands may provide improved performance and outcome. Efficacy of these preformed microbubbles or agents requires validation and FDA approval for application in humans.
Characteristics of BBB under pathological conditions & delivery outcome
It is known that BBB breakdown or alterations in transport systems play an important role in the pathogenesis of many CNS diseases such as HIV-1 encephalitis, Alzheimer’s disease, ischemia, tumors, MS and Parkinson’s disease [10]. Disease-associated brain pathology often results in an increased BBB permeability and abnormal BBB function. Proinflammatory substances and specific disease-associated proteins often mediate such BBB dysfunction. Therefore, it is important to understand whether these pathologically-comprised BBB characteristics affect the outcome of US delivery of therapeutic agents to the brain to treat these conditions.
For example, in brain tumors (especially metastasized tumors), the vasculature is leaky compared with normal BBB, but this leaky BBB or increased permeability associated with tumor pathology is often irregular, highly heterogeneous, varies widely within the same tumor volume and does not necessarily correlate with histological characteristics, tumor size or location. This causes noncontrolled and nonuniform transportation of chemotherapeutic agents from the blood circulation to reach the entire tumor volume, limiting the overall treatment success. Thus, special consideration of the disease-associated BBB properties may need to be considered in order to develop the US technique to achieve the required drug concentration throughout the entire tumor volume for successful tumor treatment.
Quantification of the US-induced permeability increase across the BBB
Many studies have demonstrated that US application in the presence of microbubbles can increase the delivery of therapeutic agents to the brain and the total delivered amount of agents determined in post-US examinations. While delivery results from the enhanced permeability, quantification of the increased permeability (e.g., permeability coefficient) by US exposures has not been obtained. Such quantification is highly desirable in order to establish a general knowledge base for extending and improving the application of US technology for delivery of therapeutic agents to the brain. Since the permeability increase induced by US is often local (by focused US exposure) and transient and may depend on the properties of the specific agents, systematic quantification of the US-induced permeability of different agents across the BBB is a challenging task.
Transport & diffusion of drug in the brain after crossing the BBB
The overall therapeutic efficacy of a drug treatment will be affected by the drug diffusion characteristics in the brain tissue as well as the clearance of the drug away from the brain. Sufficient US-facilitated BBB transport is required to balance the drug transport within the brain parenchyma and clearance after passing across the BBB in order to maintain sufficient drug distribution for the desired therapeutic outcome. Thus, whether adequate rate of drug delivery across the BBB by US exposure can be achieved using US technology needs to be determined.
Complete mechanism & processes involved in US-induced BBB disruption
With a diameter of 1–3 μm, typical micro-bubbles are smaller than endothelial cells but much larger than molecular and cellular structures. Thus it is unlikely that microbubbles can directly and precisely interact with cellular and molecular structures in the BBB to induce BBB opening. Whether the BBB opening is a downstream cellular response following the initial impact from the US-driven microbubble activities remains to be fully elucidated.
Many studies reported US parameters that were capable of generating BBB opening in animal models, but it is difficult to derive mechanistic insights from these largely phenomenological results, which may not always be consistent with each other. Future efforts are needed to reveal mechanism-based information on how US exposures combined with microbubbles to induce BBB disruption. This will help to determine the optimal parameters and conditions for US exposures and microbubble concentrations to achieve efficient, consistent and controllable outcomes of US BBB disruption without tissue damage and other side effects.
Challenges, limitations & unmet needs
Several factors specific to the US BBB delivery that may limit the future development and application of the technology are listed below.
Narrow parameter window
The opening of the BBB was shown to be due to US-driven microbubbles [77,83,86], but irreversible damage to the blood vessels and its surrounding cells also occurred [112]. Since US-driven microbubble activities are complex and affected by a large variety of parameters that may be difficult to control, the therapeutic window (e.g., US parameters and microbubble properties) within which to achieve efficient and safe delivery outcome may be intrinsically narrow, thus difficult to indentify.
Controlled US interaction with microbubbles
The US microbubble-induced mechanical effects have been recognized as the initiating factors in BBB disruption [77,83,86], but more details are needed to directly relate the microbubble activities to the resulting bioeffects.
US microbubble interaction depends on US exposure parameters as well as the bubble size and property (e.g., gas content and shell material). It is also affected by the specific geometry and environment of the microbubbles. The size dispersion of microbubbles and their relative locations in the US beam will result in different responses from individual bubbles. Besides US-driven inertial and stable cavitation, translation microbubble movement (by the primary and secondary acoustic radiation forces [138,139]) can also occur. The actual bubble activities in an experiment could be a combination of all of these dynamic phenomena, thus complex and difficult to control, especially when multiple bubbles are present and longer duration of US exposure is used.
It is currently unknown whether one or more of these activities are the main determining factors in BBB disruption or whether an optimal BBB disruption with minimal damage to the microvasculature can be achieved, and if so, how these more desired conditions can be achieved consistently.
Discrete presence & effects of microbubbles
Microbubbles are discrete entities with a relatively large size distributed in the blood within the vasculature compared with typical drug molecules. The maximum in vivo concentration of the microbubbles in the circulation after a typical bolus injection is in the range of 103–106 bubbles/ml. Therefore the presence of the microbubbles in the brain microvasculature is discrete and very likely to be sparse. Consequently, the endothelium will not be uniformly and homogenously subjected to the effects of these discrete microbubbles, even with reperfusion of fresh microbubbles to the tissue volume after a bolus injection. The scarcity and discrete nature of microbubbles could result in limited efficiency of BBB opening in a nonuniform manner.
Toxicity of US-induced BBB opening
As in other methods for BBB disruption, molecules, both desired and not desired, which the BBB is designed to keep out of the CNS are now allowed entrance through the disruption of the BBB. In addition, US-driven microbubble activities can cause damage to the endothelial cells, which can be a source of neurotoxins released into the brain. Damage to the endothelial cells can also impede the ability of brain endothelial cells to deliver glucose and other nutrients to the brain. Thus, such toxicity associated with US exposures and US-induced BBB opening on brain function needs to be investigated along with the therapeutic index and pharmacokinetics of intended agents.
Since the toxicity associated with US-induced BBB disruption may have different implications in applications with different therapeutic goals (e.g., tumor treatment vs Alzheimer’s disease), it is probably best investigated within the context of specific medical applications under consideration. While the US-induced BBB disruption is local and transient, and tissue and cell damage can be kept minimal, developing approaches to reduce the toxicity of US-induced BBB disruption might be necessary to address these aspects of US-induced BBB opening, which may ultimately limit the range and types of use of the technology.
Other barriers of translation
As in noninvasive US ablation applications [90], target tissue visualization and exposure quantification can be a barrier for widespread use of focused US technology for brain delivery. With many unique advantages, US-induced BBB disruption is a physical method and the technique does not directly address the biological mechanisms associated with transport across the BBB. In addition, as it usually requires imaging guidance of MRI for US application and BBB opening, disadvantages such as claustrophobia and discomfort for some patients with the closed bore, and the cost associated with MRI scanners could be a limiting factor that may restrict the widespread use of the technology.
Future directions & opportunities
With continuing interest and effort in US drug BBB delivery, exciting opportunities may be expected in the future. A number of possibilities are outlined below as examples.
Concerted research efforts and methodology
A standardized research methodology will undoubtedly have a great positive impact on the technology development. More systematic and rigorous characterization of US systems and experimental conditions will lead to easier comparison of results from different studies, thus better identification of critically important parameters. This will facilitate progress in the investigation of mechanism and establish US exposure regimes for efficient and safe clinical applications.
Smart carriers & therapeutic microbubbles
The majority of studies thus far have utilized either systemic co-injection of microbubbles and drugs, or introduction of drug after microbubble injection. Development of new vehicles such as multifunctional microbubble-based carriers can open new grounds and possibilities for the technology.
As illustrated in Figures 16 & 17, multifunctional microbubble-based vehicles can carry the required drug load, be activated by US for controlled release of drug (Figure 17) [140], and interact with US to open the BBB. The carriers can also be fabricated to serve as imaging agents for multimodal imaging such as MRI, positron emission tomography (PET), SPECT, CT, US imaging, or optical imaging [141] in preclinical and clinical applications. In addition, they can possess specific ligands allowing binding to receptors on the cell surface to achieve more desirable bio-distribution at the targeted tissue site in the brain. Thus improved US BBB opening may be expected from the development of multifunctional agents, which will provide interdisciplinary opportunity and stimulate innovation.
Figure 16. Microbubble constructed for drug delivery.
Gas-filled microspheres can be designed so that their interior is loaded with drugs and gas. A stabilizing material, in this case a lipid, surrounds the perfluorocarbon bubble. Drugs can be incorporated alone, or if insoluble, in water, or in an oil layer. The microsphere can be targeted to specific tissue by incorporating protein ligands on the surface. The microbubble is capable of three functions: visualization at US, site-specific targeting, and drug transportation.
Reproduced with permission from [140] © BMJ Publishing Group Ltd.
Figure 17. Enhanced gene delivery with ultrasound and microbubbles.
Presence of gas in gene-filled microbubble allows ultrasound energy to ‘pop’ the bubble. An energetic wave is then created that allows genetic material to enter surrounding cells.
Reproduced with permission from [140] © BMJ Publishing Group Ltd.
Synergy with research in other fields
Great synergy is expected in combining knowledge and approaches from different fields in the effort to develop novel technology for brain drug delivery. For example, multifunctional nanomaterials promise to achieve improved delivery to the brain with improved pharmacokinetics, reduced systemic toxicity and increased therapeutic index [142]. Progress in ongoing research regarding the modification of drugs or drug carriers with targeting and transport-enhancing agents is emerging. Improved identification of disease-associated changes of the BBB properties in brain cancers can provide new directions in developing microbubble agents to achieve better targeting and delivery outcomes. New results may be expected with US technology combined with new vectors that involve small molecules as BBB-targeting/transport enhancing agents, stable in biological media and versatile for synthesis purposes, and able to carry a large drug payload. These efforts and possibilities will likely promote new innovations in the field that may address the current challenges and unmet needs.
Conclusion
With the unique capability to penetrate biological tissue and focus the energy of action into a focal volume in a noninvasive and noncontact fashion, the US technique presents an attractive strategy for delivery of therapeutics into the brain across the BBB. By selectively increasing the BBB permeability transiently in a spatially and temporally controlled manner, the technology has shown great promise and potential for improving treatment of CNS diseases.
The basic framework and principle of the technology have been established in various animal models. Studies have demonstrated the feasibility and potential of the technology for enhanced delivery of chemotherapeutic agents, antibodies, and other agents into the brain. Progress has been made in understanding the physical and physiological mechanism of US-mediated BBB opening. However, much further studies are needed to reveal the complete biophysical and biological mechanisms involved, and to demonstrate the efficacy and safety of the technology in specific applications.
Future perspective
Ultrasound targeted drug delivery to the brain across the BBB is a novel strategy that has advantageous characteristics over other techniques. Successful development and translation of the technology will have a significant impact on the treatment of various diseases in the CNS. Continuing effort in obtaining improved understanding of the mechanisms involved and developing new interdisciplinary approaches will facilitate faster translation and widespread adoption of this technology in clinical applications.
Executive summary.
Ultrasound (US) can penetrate soft tissue and be readily focused at a targeted tissue site at depth without affecting intervening and surrounding tissue.
Focused US provides a noninvasive and nonionizing means to generate localized, reversible and permanent tissue changes through thermal and mechanical mechanisms.
Development of improved US array systems and techniques can improve the transmission of US through the skull, avoiding skull bone heating and defocusing.
US interacts with microbubbles efficiently and can generate significant mechanical effects including fluid streaming, shear stress, and other mechanical impacts on nearby cells and tissue structures.
US driven microbubble activities have been demonstrated to be able to produce transient opening of the blood–brain barrier (BBB).
MRI guided focused US has been demonstrated in animal models to increase the permeability of the BBB which allow the delivery of therapeutic agents such as chemotherapeutic drugs antibodies to the brain.
Development of novel, multifunctional microbubble based agents could lead to molecularly targeted, image-guided US technology for brain delivery across the BBB.
Continuing technology development and research to demonstrate the safety and efficacy of the US technology for brain delivery will facilitate translation of the technology into clinical application.
Key Terms
- Neurodegenerative diseases
Neurodegenerative diseases describe the progressive loss of structure or function of neurons, including death of neurons. Neurodegenerative diseases, including Parkinson’s, Alzheimer’s, and Huntington’s diseases, occur as a result of neurodegenerative processes and may be related to one another on a sub-cellular level
- Blood–brain barrier
Formed by tight junctions of endothelial cells lining cerebral blood vessels and capillaries, the blood–brain barrier effectively limits the transport of many molecules from the circulation to the brain
- Focused ultrasound
A focused ultrasound beam is achieved when the energy of the propagating acoustic wave is concentrated into a small focal volume with gain of orders of magnitude
- Acoustic cavitation
Acoustic cavitation is the formation, volume oscillation, growth, and/or collapse of gaseous bubbles driven by an acoustic field. Acoustic cavitation is often categorized as inertial cavitation when collapse of bubbles is dominated by the inertia of the surround fluid or stable cavitation when the bubble exhibits periodic volume oscillation by the periodic acoustic pressure field
Footnotes
Financial & competing interests disclosure
Financial support from the University of Michigan and the United States National Institutes of Health is duly acknowledged. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Pardridge WM. The blood–brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neugroschl J, Sano M. An update on treatment and prevention strategies for alzheimer’s disease. Curr Neurol Neurosci Rep. 2009;9(5):368–376. doi: 10.1007/s11910-009-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 4.Tosoni A, Ermani M, Brandes AA. The pathogenesis and treatment of brain metastases: a comprehensive review. Crit Rev Oncol Hematol. 2004;52(3):199–215. doi: 10.1016/j.critrevonc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Deeken JF, Loscher W. The blood–brain barrier and cancer: transporters, treatment, and trojan horses. Clin Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 6.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Cecchelli R, Berezowski V, Lundquist S, et al. Modelling of the blood–brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6(8):650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 8.Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13(23–24):1099–1106. doi: 10.1016/j.drudis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Abbott NJ. Dynamics of cns barriers: Evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: Structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 11.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem. 1980;23(6):682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- 13.Fung LK, Shin M, Tyler B, Brem H, Saltzman WM. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13(5):671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- 14.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Vogelbaum MA. Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review. J Neurooncol. 2007;83(1):97–109. doi: 10.1007/s11060-006-9308-9. [DOI] [PubMed] [Google Scholar]
- 16.Bidros DS, Liu JK, Vogelbaum MA. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 2010;6(1):117–125. doi: 10.2217/fon.09.135. [DOI] [PubMed] [Google Scholar]
- 17.Sampson JH, Archer G, Pedain C, et al. Poor drug distribution as a possible explanation for the results of the precise trial. J Neurosurg. 2009 doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 18.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular trojan horses. Bioconjug Chem. 2008;19(7):1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 20.Greig NH, Daly EM, Sweeney DJ, Rapoport SI. Pharmacokinetics of chlorambucil-tertiary butyl ester, a lipophilic chlorambucil derivative that achieves and maintains high concentrations in brain. Cancer Chemother Pharmacol. 1990;25(5):320–325. doi: 10.1007/BF00686230. [DOI] [PubMed] [Google Scholar]
- 21.Jahnke K, Kraemer DF, Knight KR, et al. Intraarterial chemotherapy and osmotic blood–brain barrier disruption for patients with embryonal and germ cell tumors of the central nervous system. Cancer. 2008;112(3):581–588. doi: 10.1002/cncr.23221. [DOI] [PubMed] [Google Scholar]
- 22.Doolittle ND, Abrey LE, Bleyer WA, et al. New frontiers in translational research in neuro-oncology and the blood–brain barrier: report of the tenth annual blood–brain barrier disruption consortium meeting. Clin Cancer Res. 2005;11(2 Pt 1):421–428. [PubMed] [Google Scholar]
- 23.Kroll RA, Neuwelt EA. Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–1099. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 24.Riina HA, Fraser JF, Fralin S, Knopman J, Scheff RJ, Boockvar JA. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. J Exp Ther Oncol. 2009;8(2):145–150. [PubMed] [Google Scholar]
- 25.Mitragotri S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4(3):255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 26.Campbell P, Prausnitz MR. Future directions for therapeutic ultrasound. Ultrasound Med Biol. 2007;33(4):657. doi: 10.1016/j.ultrasmedbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng CX, Xu Q, Apfel RE, Holland CK. In vitro measurements of inertial cavitation thresholds in human blood. Ultrasound Med Biol. 1996;22(7):939–948. doi: 10.1016/0301-5629(96)00104-4. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 29.Vaezy S, Martin R, Crum L. High intensity focused ultrasound: a method of hemostasis. Echocardiography. 2001;18(4):309–315. doi: 10.1046/j.1540-8175.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 30.Vaezy S, Fujimoto VY, Walker C, Martin RW, Chi EY, Crum LA. Treatment of uterine fibroid tumors in a nude mouse model using high-intensity focused ultrasound. Am J Obstet Gynecol. 2000;183(1):6–11. doi: 10.1067/mob.2000.105347. [DOI] [PubMed] [Google Scholar]
- 31.Wu J. Shear stress in cells generated by ultrasound. Prog Biophys Mol Biol. 2007;93(1–3):363–373. doi: 10.1016/j.pbiomolbio.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Collis J, Manasseh R, Liovic P, et al. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics. 50(2):273–279. doi: 10.1016/j.ultras.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Vanbavel E. Effects of shear stress on endothelial cells: possible relevance for ultrasound applications. Prog Biophys Mol Biol. 2007;93(1–3):374–383. doi: 10.1016/j.pbiomolbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Prentice P, Cuschieri A, Dholakia K, Prausnitz M, Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nature Physics. 2005;1(2):107–110. [Google Scholar]
- 35.Okada K, Kudo N, Niwa K, Yamamoto K. A basic study on sonoporation with microbubbles exposed to pulsed ultrasound. J Med Ultrasonics. 2005;32:3–11. doi: 10.1007/s10396-005-0031-5. [DOI] [PubMed] [Google Scholar]
- 36.Hallow DM, Mahajan AD, Mccutchen TE, Prausnitz MR. Measurement and correlation of acoustic cavitation with cellular bioeffects. Ultrasound Med Biol. 2006;32(7):1111–1122. doi: 10.1016/j.ultrasmedbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Cui J, Deng CX. Dynamics of sonoporation correlated with acoustic cavitation activities. Biophys J. 2008;94(7):L51–53. doi: 10.1529/biophysj.107.125617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Wamel A, Kooiman K, Harteveld M, et al. Vibrating microbubbles poking individual cells. Drug transfer into cells via sonoporation. J Control Release. 2006;112(2):149–155. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Forbes MM, Steinberg RL, O’brien WD., Jr Examination of intertial cavitation of optison in producing sonoporation of chinese hamster ovary cells. Ultrasound Med Biol. 2008;34:2009–2018. doi: 10.1016/j.ultrasmedbio.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Deng CX, Lizzi FL. A review of physical phenomena associated with ultrasonic contrast agents and illustrative clinical applications. Ultrasound Med Biol. 2002;28(3):277–286. doi: 10.1016/s0301-5629(02)00475-1. Review of the physical principles and phenomena in ultrasound interaction with micorbubbles. [DOI] [PubMed] [Google Scholar]
- 41.Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54(6):R27–57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3(6):527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 43.Ohl CD, Arora M, Ikink R, et al. Sonoporation from jetting cavitation bubbles. Biophys J. 2006;91(11):4285–4295. doi: 10.1529/biophysj.105.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 45▪.Ferrara KW. Driving delivery vehicles with ultrasound. Adv Drug Deliv Rev. 2008;60(10):1097–1102. doi: 10.1016/j.addr.2008.03.002. Thorough review regarding the utility and opportunities using microbubbles in both ultrasound (US) imaging and therapeutic applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98(13):1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 47.Postema M, Van Wamel A, Lancee CT, De Jong N. Ultrasound-induced encapsulated microbubble phenomena. Ultrasound Med Biol. 2004;30(6):827–840. doi: 10.1016/j.ultrasmedbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy--a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11(6):349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Juffermans LJ, Dijkmans PA, Musters RJ, Visser CA, Kamp O. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2006;291(4):H1595–H1601. doi: 10.1152/ajpheart.01120.2005. [DOI] [PubMed] [Google Scholar]
- 50.Okada K, Kudo N, Kondo T, Yamamoto K. Contributions of mechanical and sonochemical effects to cell membrane damage induced by single-shot pulsed ultrasound with adjacent microbubbles. J Med Ultrason. 2008;35(4):169–176. doi: 10.1007/s10396-008-0192-0. [DOI] [PubMed] [Google Scholar]
- 51.Deng CX, Xu Q, Apfel RE, Holland CK. Inertial cavitation produced by pulsed ultrasound in controlled host media. J Acoust Soc Am. 1996;100(2 Pt 1):1199–1208. doi: 10.1121/1.416304. [DOI] [PubMed] [Google Scholar]
- 52.Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17(2):179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- 53.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88(5):2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007;18(1):11–16. doi: 10.1016/j.copbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60(10):1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuber-Jerger I, Schacherer D, Woenckhaus M, Jung EM, Scholmerich J, Klebl F. Contrast-enhanced ultrasound in diagnosing liver malignancy. Clin Hemorheol Microcirc. 2009;43(1):109–118. doi: 10.3233/CH-2009-1225. [DOI] [PubMed] [Google Scholar]
- 57.Powers J, Averkiou M, Bruce M. Principles of cerebral ultrasound contrast imaging. Cerebrovasc Dis. 2009;27(Suppl 2):14–24. doi: 10.1159/000203123. [DOI] [PubMed] [Google Scholar]
- 58.Phillips LC, Klibanov AL, Bowles DK, Ragosta M, Hossack JA, Wamhoff BR. Focused in vivo delivery of plasmid DNA to the porcine vascular wall via intravascular ultrasound destruction of microbubbles. J Vasc Res. 2010;47(3):270–274. doi: 10.1159/000258905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otani K, Yamahara K, Ohnishi S, Obata H, Kitamura S, Nagaya N. Nonviral delivery of sirna into mesenchymal stem cells by a combination of ultrasound and microbubbles. J Control Release. 2009;133(2):146–153. doi: 10.1016/j.jconrel.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 60.Li YS, Davidson E, Reid CN, Mchale AP. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: Potential applications for gene therapy of cancer. Cancer Lett. 2009;273(1):62–69. doi: 10.1016/j.canlet.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 61.Vandenbroucke RE, Lentacker I, Demeester J, De Smedt SC, Sanders NN. Ultrasound assisted sirna delivery using peg-siplex loaded microbubbles. J Control Release. 2008;126(3):265–273. doi: 10.1016/j.jconrel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Zarnitsyn VG, Kamaev PP, Prausnitz MR. Ultrasound-enhanced chemotherapy and gene delivery for glioma cells. Technol Cancer Res Treat. 2007;6(5):433–442. doi: 10.1177/153303460700600509. [DOI] [PubMed] [Google Scholar]
- 63.Deng CX. Sonoporation and gene delivery. In: Frenkel V, editor. Therapeutic Ultrasound: Mechanisms to Applications. Nova Science Publishers; NY, USA: 2010. [Google Scholar]
- 64.Mayer CR, Geis NA, Katus HA, Bekeredjian R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv. 2008;5(10):1121–1138. doi: 10.1517/17425247.5.10.1121. [DOI] [PubMed] [Google Scholar]
- 65▪▪.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60(10):1209–1217. doi: 10.1016/j.addr.2008.03.010. Review with details of experimental studies and progress in US induced blood–brain barrier (BBB) opening for drug delivery to the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hynynen K. Macromolecular delivery across the blood–brain barrier. Methods Mol Biol. 2009;480:175–185. doi: 10.1007/978-1-59745-429-2_13. [DOI] [PubMed] [Google Scholar]
- 67.Vykhodtseva N, Mcdannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48(4):279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Summary and insightful commentary of progress and problems in US BBB opening including many.
- 69.Ballantine HT, Jr, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg. 1960;17:858–876. doi: 10.3171/jns.1960.17.5.0858. [DOI] [PubMed] [Google Scholar]
- 70.Patrick JT, Nolting MN, Goss SA, et al. Ultrasound and the blood–brain barrier. Adv Exp Med Biol. 1990;267:369–381. doi: 10.1007/978-1-4684-5766-7_36. [DOI] [PubMed] [Google Scholar]
- 71.Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol. 1995;21(7):969–979. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]
- 72.Sheikov N, Mcdannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood–brain barrier. Ultrasound Med Biol. 2006;32(9):1399–1409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 73▪▪.Hynynen K, Mcdannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood–brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. Landmark study demonstrating the BBB opening by US activated microbubbles. [DOI] [PubMed] [Google Scholar]
- 74.Hynynen K, Mcdannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 75▪.Kinoshita M, Mcdannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc Natl Acad Sci USA. 2006;103(31):11719–11723. doi: 10.1073/pnas.0604318103. Delivery of monoclonal antibody Herceptin to the mouse brain by using US technique to open the BBB is demonstrated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinoshita M, Mcdannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340(4):1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 77.Mesiwala AH, Farrell L, Wenzel HJ, et al. High-intensity focused ultrasound selectively disrupts the blood–brain barrier in vivo. Ultrasound Med Biol. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 78▪.Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood–brain barrier using focused ultrasound. Phys Med Biol. 2007;52(18):5509–5530. doi: 10.1088/0031-9155/52/18/004. Spatio-temporal distribution of molecules by US induced BBB opening provided informaton on the dependence on the underlying vasculature. [DOI] [PubMed] [Google Scholar]
- 79.Ng KY, Liu Y. Therapeutic ultrasound: its application in drug delivery. Med Res Rev. 2002;22(2):204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- 80.Mcdannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51(4):793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 81.Mcdannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood–brain barrier disruption. Ultrasound Med Biol. 2008;34(6):930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hynynen K, Mcdannold N, Martin H, Jolesz FA, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (optison) Ultrasound Med Biol. 2003;29(3):473–481. doi: 10.1016/s0301-5629(02)00741-x. [DOI] [PubMed] [Google Scholar]
- 83.Sheikov N, Mcdannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood–brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30(7):979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Mcdannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood–brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51(5):913–923. doi: 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- 85.Mcdannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood–brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol. 2005;31(11):1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Hynynen K, Mcdannold N, Vykhodtseva N, et al. Focal disruption of the blood–brain barrier due to 260-khz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105(3):445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 87.Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood–brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33(1):95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 88.Treat LH, Mcdannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 89▪.Choi JJ, Wang S, Tung YS, Morrison B, 3rd, Konofagou EE. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood–brain barrier opening in vivo. Ultrasound Med Biol. 2010;36(1):58–67. doi: 10.1016/j.ultrasmedbio.2009.08.006. Size of US induced BBB opening was determined. The distribution of different sized molecules was also investigated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mcdannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50(2):221–229. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 92.Fry FJ, Barger JE. Acoustical properties of the human skull. J Acoust Soc Am. 1978;63(5):1576–1590. doi: 10.1121/1.381852. [DOI] [PubMed] [Google Scholar]
- 93.Marquet F, Pernot M, Aubry JF, et al. Non-invasive transcranial ultrasound therapy based on a 3D ct scan: Protocol validation and in vitro results. Phys Med Biol. 2009;54(9):2597–2613. doi: 10.1088/0031-9155/54/9/001. [DOI] [PubMed] [Google Scholar]
- 94.White PJ, Clement GT, Hynynen K. Local frequency dependence in transcranial ultrasound transmission. Phys Med Biol. 2006;51(9):2293–2305. doi: 10.1088/0031-9155/51/9/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hynynen K, Sun J. Trans-skull ultrasound therapy: the feasibility of using image-derived skull thickness information to correct the phase distortion. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46(3):752–755. doi: 10.1109/58.764862. [DOI] [PubMed] [Google Scholar]
- 96.White J, Clement GT, Hynynen K. Transcranial ultrasound focus reconstruction with phase and amplitude correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52(9):1518–1522. doi: 10.1109/tuffc.2005.1516024. [DOI] [PubMed] [Google Scholar]
- 97.Clement GT, Hynynen K. Correlation of ultrasound phase with physical skull properties. Ultrasound Med Biol. 2002;28(5):617–624. doi: 10.1016/s0301-5629(02)00503-3. [DOI] [PubMed] [Google Scholar]
- 98.Hynynen K, Clement GT, Mcdannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52(1):100–107. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- 99.Jordao JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the tgcrnd8 mouse model of alzheimer’s disease. PLoS One. 2010;5(5):e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baselga J. Current and planned clinical trials with trastuzumab (herceptin) Semin Oncol. 2000;27(5 Suppl 9):27–32. [PubMed] [Google Scholar]
- 101.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 102.Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin Oncol. 2004;31(5):635–644. doi: 10.1053/j.seminoncol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Grossman SA, O’neill A, Grunnet M, et al. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: eastern cooperative oncology group trial 2394. J Clin Oncol. 2003;21(8):1485–1491. doi: 10.1200/JCO.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 104▪▪.Liu HL, Hua MY, Chen PY, et al. Blood–brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 255(2):415–425. doi: 10.1148/radiol.10090699. Results obtained in a animal model show delivery of chemotherpeutic drugs to the brain across the BBB and the therapeutic outcome of tumor control and animal survival. [DOI] [PubMed] [Google Scholar]
- 105▪.Xie F, Boska MD, Lof J, Uberti MG, Tsutsui JM, Porter TR. Effects of transcranial ultrasound and intravenous microbubbles on blood brain barrier permeability in a large animal model. Ultrasound Med Biol. 2008;34(12):2028–2034. doi: 10.1016/j.ultrasmedbio.2008.05.004. First demonstration of US disruption of BBB in a large animal model. [DOI] [PubMed] [Google Scholar]
- 106.Sassaroli E, Hynynen K. Cavitation threshold of microbubbles in gel tunnels by focused ultrasound. Ultrasound Med Biol. 2007;33(10):1651–1660. doi: 10.1016/j.ultrasmedbio.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sassaroli E, Hynynen K. Forced linear oscillations of microbubbles in blood capillaries. J Acoust Soc Am. 2004;115(6):3235–3243. doi: 10.1121/1.1738456. [DOI] [PubMed] [Google Scholar]
- 108.Qin S, Ferrara KW. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys Med Biol. 2006;51(20):5065–5088. doi: 10.1088/0031-9155/51/20/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goertz DE, Wright C, Hynynen K. Contrast agent kinetics in the rabbit brain during exposure to therapeutic ultrasound. Ultrasound Med Biol. 2010;36(6):916–924. doi: 10.1016/j.ultrasmedbio.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood–brain-barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci. 2010;1(5):391–398. doi: 10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mcdannold N, Vykhodtseva N, Hynynen K. Blood–brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008;34(5):834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi JJ, Feshitan JA, Baseri B, et al. Microbubble-size dependence of focused ultrasound-induced blood–brain barrier opening in mice in vivo. IEEE Trans Biomed Eng. 2010;57(1):145–154. doi: 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tu J, Hwang JH, Matula TJ, Brayman AA, Crum LA. Intravascular inertial cavitation activity detection and quantification in vivo with optison. Ultrasound Med Biol. 2006;32(10):1601–1609. doi: 10.1016/j.ultrasmedbio.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 114.Zhou Y, Shi J, Cui J, Deng CX. Effects of extracellular calcium on cell membrane resealing in sonoporation. J Control Release. 2008;126(1):34–43. doi: 10.1016/j.jconrel.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tung YS, Choi JJ, Baseri B, Konofagou EE. Identifying the inertial cavitation threshold and skull effects in a vessel phantom using focused ultrasound and microbubbles. Ultrasound Med Biol. 2010;36(5):840–852. doi: 10.1016/j.ultrasmedbio.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumon RE, Aehle M, Sabens D, et al. Spatiotemporal effects of sonoporation measured by real-time calcium imaging. Ultrasound Med Biol. 2009;35(3):494–506. doi: 10.1016/j.ultrasmedbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumon RE, Aehle M, Sabens D, Parikh P, Kourennyi D, Deng CX. Ultrasound-induced calcium oscillations and waves in chinese hamster ovary cells in the presence of microbubbles. Biophys J. 2007;93(6):L29–31. doi: 10.1529/biophysj.107.113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan Z, Kumon RE, Park J, Deng CX. Intracellular delivery and calcium transients generated in sonoporation facilitated by microbubbles. J Control Release. 2010;142(1):31–39. doi: 10.1016/j.jconrel.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 120.Nigam SK, Rodriguez-Boulan E, Silver RB. Changes in intracellular calcium during the development of epithelial polarity and junctions. Proc Natl Acad Sci USA. 1992;89(13):6162–6166. doi: 10.1073/pnas.89.13.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shasby DM, Shasby SS. Effects of calcium on transendothelial albumin transfer and electrical resistance. J Appl Physiol. 1986;60(1):71–79. doi: 10.1152/jappl.1986.60.1.71. [DOI] [PubMed] [Google Scholar]
- 122.Stuart RO, Sun A, Panichas M, Hebert SC, Brenner BM, Nigam SK. Critical role for intracellular calcium in tight junction biogenesis. J Cell Physiol. 1994;159(3):423–433. doi: 10.1002/jcp.1041590306. [DOI] [PubMed] [Google Scholar]
- 123.Stuart RO, Sun A, Bush KT, Nigam SK. Dependence of epithelial intercellular junction biogenesis on thapsigargin-sensitive intracellular calcium stores. J Biol Chem. 1996;271(23):13636–13641. doi: 10.1074/jbc.271.23.13636. [DOI] [PubMed] [Google Scholar]
- 124.Abbott NJ. Role of intracellular calcium in regulation of brain endothelial permeability. In: Pardridge WM, editor. Introduction to the Blood–Brain Barrier: Methodology, Biology and Pathology. Cambridge University Press; NY, USA: 1998. pp. 345–351. [Google Scholar]
- 125.Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood–brain barrier disruption after stroke. Stroke. 2002;33(6):1706–1711. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- 126.Revest PA, Abbott NJ, Gillespie JI. Receptor-mediated changes in intracellular [Ca2+] in cultured rat brain capillary endothelial cells. Brain Res. 1991;549(1):159–161. doi: 10.1016/0006-8993(91)90614-2. [DOI] [PubMed] [Google Scholar]
- 127.Park J, Fan Z, Kumon RE, El-Sayed ME, Deng CX. Modulation of intracellular Ca2+ concentration in brain microvascular endothelial cells in vitro by acoustic cavitation. Ultrasound Med Biol. 2010;36(7):1176–1187. doi: 10.1016/j.ultrasmedbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paemeleire K, De Hemptinne A, Leybaert L. Chemically, mechanically, and hyperosmolarity-induced calcium responses of rat cortical capillary endothelial cells in culture. Exp Brain Res. 1999;126(4):473–481. doi: 10.1007/s002210050755. [DOI] [PubMed] [Google Scholar]
- 129.Abbott NJ. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell Mol Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130▪▪.Sheikov N, Mcdannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34(7):1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. Ultrastructual results from electron microscopy and immunoelectron microscopy demonstrated the details of molecular changes of the blood–brain barrier correlated with the increase of blood–brain barrier permeability generated by US exposures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raymond SB, Treat LH, Dewey JD, Mcdannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in alzheimer’s disease mouse models. PLoS One. 2008;3(5):e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pernot M, Aubry JF, Tanter M, et al. In vivo transcranial brain surgery with an ultrasonic time reversal mirror. J Neurosurg. 2007;106(6):1061–1066. doi: 10.3171/jns.2007.106.6.1061. [DOI] [PubMed] [Google Scholar]
- 133.Gateau J, Marsac L, Pernot M, Aubry JF, Tanter M, Fink M. Transcranial ultrasonic therapy based on time reversal of acoustically induced cavitation bubble signature. IEEE Trans Biomed Eng. 57(1):134–144. doi: 10.1109/TBME.2009.2031816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chopra R, Curiel L, Staruch R, Morrison L, Hynynen K. An MRI-compatible system for focused ultrasound experiments in small animal models. Med Phys. 2009;36(5):1867–1874. doi: 10.1118/1.3115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu HL, Wai YY, Chen WS, et al. Hemorrhage detection during focused-ultrasound induced blood–brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med Biol. 2008;34(4):598–606. doi: 10.1016/j.ultrasmedbio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 136.Liu HL, Hsu PH, Chu PC, et al. Magnetic resonance imaging enhanced by superparamagnetic iron oxide particles: usefulness for distinguishing between focused ultrasound-induced blood–brain barrier disruption and brain hemorrhage. J Magn Reson Imaging. 2009;29(1):31–38. doi: 10.1002/jmri.21599. [DOI] [PubMed] [Google Scholar]
- 137▪.Lin KJ, Liu HL, Hsu PH, et al. Quantitative micro-spect/ct for detecting focused ultrasound-induced blood–brain barrier opening in the rat. Nucl Med Biol. 2009;36(7):853–861. doi: 10.1016/j.nucmedbio.2009.04.011. Results from this study demonstrated the feasibility of using micro-SPECT/CT for detection US induced blood–brain barrier opening. [DOI] [PubMed] [Google Scholar]
- 138.Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007;32(2):51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 139▪.O’Brien WD., Jr Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007;93(1–3):212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. Detailed review of the physical and biophysical principles and phenomena produced by ultrasound interaction with biological systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112(5 Pt 1):2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 141.Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: A new era in ultrasound. BMJ. 2001;322(7296):1222–1225. doi: 10.1136/bmj.322.7296.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borden MA, Zhang H, Gillies RJ, Dayton PA, Ferrara KW. A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials. 2008;29(5):597–606. doi: 10.1016/j.biomaterials.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koo YE, Reddy GR, Bhojani M, et al. Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev. 2006;58(14):1556–1577. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]