SUMMARY
Although pregnancy confers unique susceptibility to infection, the pregnancy-associated immune defects that erode host defense remain largely undefined. Herein, we demonstrate that expansion of immune-suppressive Foxp3+ regulatory T cells (Tregs) which occurs physiologically during pregnancy or when experimentally induced in transgenic mice each caused enhanced susceptibility to prenatal pathogens including Listeria and Salmonella species. Reciprocally, infection susceptibility was uniformly reduced with Treg-ablation. Importantly however, the sustained expansion of maternal Tregs was essential for maintaining immune tolerance to the developing fetus because even partial transient ablation of Foxp3-expressing cells fractured maternal tolerance to fetal antigen and triggered fetal resorption. Interestingly, Foxp3 cell-intrinsic defects in the immune suppressive cytokine IL-10 alone were sufficient to override Treg-mediated infection susceptibility, while IL-10 was non-essential for sustaining pregnancy. Thus, maternal Treg expansion required for sustaining pregnancy creates naturally occurring holes in host defense that confers prenatal infection susceptibility.
INTRODUCTION
The pregnancy-associated susceptibility to intracellular pathogens has been classically attributed to a shift in helper T cell differentiation from a Th1 to Th2-dominated response required for maintaining pregnancy (Krishnan et al., 1996; Piccinni et al., 1998; Wegmann et al., 1993). However, the more recent identification other distinct CD4+ T cell lineages has blurred the dichotomy between Th2 responses that sustain pregnancy, and Th1 cells that promote rejection of the developing fetus by activating cellular immunity (Saito et al., 2010). These include Th17 cells that provide protection against extracellular pathogens by promoting inflammation, and immune-suppressive regulatory T cells (Tregs) that maintain peripheral tolerance by restraining the activation of self-reactive immune cells (Curtis and Way, 2009; Littman and Rudensky, 2010). In parallel with the identification of these additional T cell subsets, the requirement for expanded immune tolerance during pregnancy has become more specifically linked with the expansion of maternal Tregs. For example, circulating maternal Foxp3+ Tregs expand and peak mid-gestation to approximately 50% increased levels in human pregnancy (Santner-Nanan et al., 2009; Somerset et al., 2004). Reciprocally, defects in Treg expansion are associated with specific pregnancy complications such as preeclampsia or spontaneous abortion each related to maternal intolerance to the developing fetus (Prins et al., 2009; Santner-Nanan et al., 2009; Sasaki et al., 2004).
This association between maternal Tregs and pregnancy outcomes has also been experimentally explored in mouse models where CD25+CD4+ cells are required for maintaining healthy pregnancy (Aluvihare et al., 2004; Kahn and Baltimore, 2010). However, since previously employed approaches that cause the near complete ablation of these cells do not allow the importance of expanded Tregs from steady state pre-pregnancy levels in sustaining pregnancy to be addressed, and CD25 expression does not discriminate between bona fide Tregs and activated T cells, the actual requirement for expanded maternal Foxp3+ Tregs during pregnancy remains undefined. More importantly, given the fluid balance between immune suppression and stimulation controlled by Tregs, the expansion of Foxp3+ cells during pregnancy may also create holes in host defense that confers susceptibility to pathogens with a predilection for prenatal infection. In this regard, while the effects of Treg ablation on infection susceptibility have been investigated in numerous models of parasitic, bacterial, fungal, and viral infection (Belkaid, 2007; Suvas and Rouse, 2006), the impacts on host defense resulting from the more physiological expansion of these cells remain undefined.
Foxp3+ Tregs can use numerous molecules to mediate context specific immune suppression (Sakaguchi et al., 2009; Shevach, 2009; Vignali et al., 2008). For example, Tregs are enriched for CTLA-4 expression, and sustained CTLA-4 ablation in Foxp3+ cells causes non-specific T cell activation and systemic autoimmunity (Friedline et al., 2009; Wing et al., 2008). Interestingly, while CTLA-4 ablation in Foxp3+ cells reproduces some features of Treg deficiency, it does not recapitulate the more rapid onset of fatal systemic autoimmunity in mice with naturally-occurring or targeted defects in all Tregs due to Foxp3 deficiency (Fontenot et al., 2003; Khattri et al., 2003). Comparatively, ablation of IL-10 in Foxp3+ Tregs results in minimal systemic autoimmunity, but instead causes inflammation limited to sites with contact to the external environment (Rubtsov et al., 2008). This discordance in phenotype after sustained ablation of specific molecules in Foxp3-expressing cells illustrates unique, non-overlapping and specialized roles for individual Treg-expressed molecules. Accordingly, identifying how Foxp3+ cells mediate suppression in each context opens up the exciting possibility of dissociating the beneficial and harmful impacts of Tregs. Specifically, given the parallels between maternal Treg expansion and healthy uncomplicated pregnancy, together with the detrimental role Tregs play in host defense against infection, dissociating the Treg-associated molecules required for each may unlock therapeutic approaches that boost immunity against infection without compromising immune tolerance required for sustaining pregnancy.
To address these questions, we established a pregnancy model using mice with divergent MHC that recapitulates the natural variation between maternal and fetal antigen, and progressive expansion of maternal Foxp3+ Tregs during human pregnancy. Using this model, we demonstrate maternal Tregs impair host defense causing susceptibility to pathogens including Listeria and Salmonella species with a defined predilection for prenatal infection. In turn, the sustained expansion of maternal Foxp3+ Tregs was also essential for maintaining healthy pregnancy because even partial transient ablation to levels found in non-pregnant controls triggered sharply increased rates of fetal resorption and fractured tolerance to fetal antigen. Using mice containing Tregs with targeted defects in defined molecules implicated in suppression, we further demonstrate an essential role for Treg IL-10 in suppressing host defense against infection, while maternal IL-10 is non-essential for maintaining pregnancy. These results illustrate naturally occurring holes in host defense associated with physiological shifts in maternal Tregs, and identify Treg-associated molecules that dissociate the detrimental impacts of expanded Tregs on infection susceptibility from those required for sustaining pregnancy.
RESULTS
Expanded maternal Foxp3+ Tregs dictate infection susceptibility during pregnancy
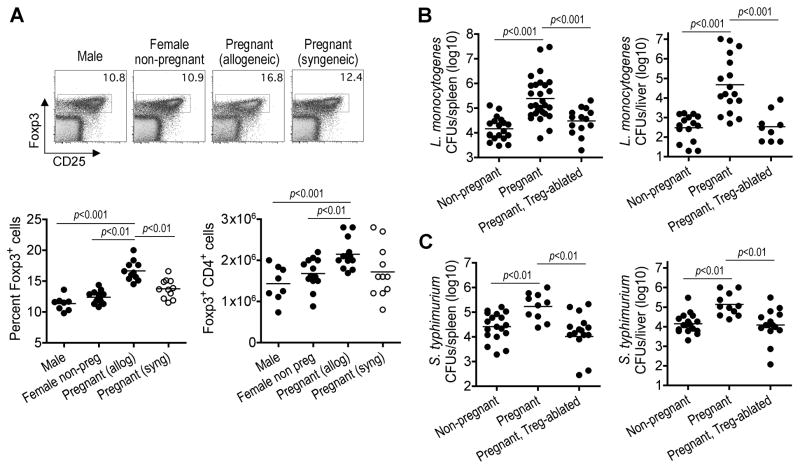
To reproduce the heterogeneity between maternal and fetal antigens during human pregnancy, we established synchronized matings between MHC mis-matched strains of inbred mice (Balb/c H-2d males with B6 H-2b females) that more fully recapitulates the expanded repertoire of non-self fetal antigen encountered by the maternal immune system. In this model, Foxp3+ Tregs expand in a synchronized fashion to ~50% increased levels by mid-gestation compared with non-pregnant controls or after syngeneic mating (Figure 1A). This magnitude of maternal Treg expansion is consistent with that reported in human and other models of mouse allogeneic pregnancy, and increased compared with syngeneic pregnancy where the only source of antigen-heterogeneity are those encoded by the Y-chromosome (Aluvihare et al., 2004; Santner-Nanan et al., 2009; Somerset et al., 2004). Interestingly, the expansion of maternal Tregs is not due to IFN-γ production against the allogeneic fetus because Foxp3+ cell expansion is also primed to a similar magnitude in IFN-γ receptor-deficient mice (12.2±0.3% Foxp3+ cells in non-pregnant compared with 17.0±0.6% at mid-gestation). These direct parallels between the magnitude of maternal Foxp3+ cell expansion and the degree of heterogeneity between maternal and fetal antigen suggest expanded Tregs play an important role in maintaining immune tolerance to the developing fetus during pregnancy.
Figure 1. Expanded maternal Foxp3+ Tregs dictate infection susceptibility during pregnancy.
(A) Percent and total number Foxp3+ among CD4+ splenocytes in male, virgin female, or pregnant B6 (H-2b) females at mid-gestation after allogeneic mating with Balb/c (H-2d) or syngeneic mating with B6 males. (B, C) Recoverable CFUs three days after infection in non-pregnant, pregnant Treg-sufficient, or pregnant Treg-abated Foxp3DTR/DTR females after allogeneic mating, and initiated on DT beginning mid-gestation and infected with Listeria monocytogenes (B) or Salmonella typhimurium (C) one day later.
Given the pivotal role Foxp3+ Tregs play in controlling the balance between immune suppression that maintains peripheral tolerance and immune activation required for optimal host defense against infection (Belkaid, 2007; Suvas and Rouse, 2006), we speculated the expansion of immune suppressive Tregs during pregnancy may also confer susceptibility to infection. In turn, this host defense defect would likely be exploited by pathogens that have a defined predisposition for infection during pregnancy. Among human pathogens, the intracellular bacterium Listeria monocytogenes (Lm) has a striking predilection for disseminated infection in pregnant women (Gellin et al., 1991; Mylonakis et al., 2002). Consistent with these epidemiological features, pregnant mice at mid-gestation compared with non-pregnant controls were markedly more susceptible to Lm infection (Figure 1B). To investigate the contribution of maternal Tregs on infection susceptibility, female Foxp3DTR mice on the B6 background were substituted for mating with Balb/c males. These transgenic mice co-express with Foxp3 the high-affinity human diphtheria toxin (DT) receptor allowing targeted ablation of maternal Foxp3-expressing cells with low-dose DT (Kim et al., 2007). We found the elimination of maternal Tregs beginning mid-gestation reversed the pregnancy-associated susceptibility to Lm infection (Figure 1B). This Treg-mediated defect in host defense was not limited only to Lm because susceptibility to Salmonella typhimurium (ST) during pregnancy was similarly reversed by the ablation of maternal Foxp3+ cells (Pejcic-Karapetrovic et al., 2007) (Figure 1C). Together, these results indicate maternal Foxp3+ Tregs contribute to pregnancy-associated infection susceptibility.
Foxp3+ Tregs impair host defense against infection
Given the expansion of fetal tissue that occurs in parallel with maternal Foxp3+ Tregs, infection susceptibility during pregnancy may also reflect more target tissue susceptible to direct invasion by Listeria and Salmonella species (Bakardjiev et al., 2006; Le Monnier et al., 2007; Pejcic-Karapetrovic et al., 2007). In agreement, we found Lm infection mid-gestation caused dose dependent increased rates of fetal invasion (Figure S1). Moreover, since the products of conception are not resorbed immediately after Foxp3+ cell-ablation, the precise impacts of expanded Tregs on infection susceptibility cannot be investigated exclusively using pregnant mice. Accordingly, to more definitively interrogate the impacts of Foxp3+ Tregs on host defense, we compared infection susceptibility of non-pregnant mice after Treg manipulation using complementary gain- and loss- of function approaches.
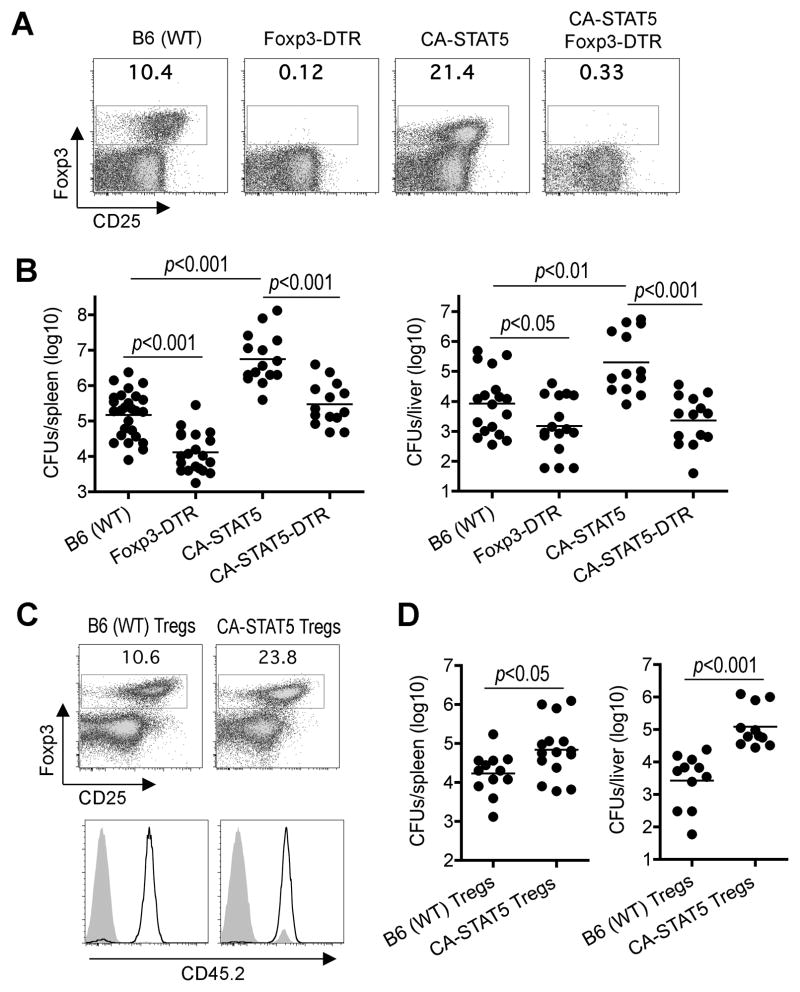
Foxp3 expression in T cells is stimulated by STAT5b promoter binding, and mice containing a constitutively active isoform of STAT5b (CA-STAT5b) have expanded Foxp3+ Tregs (Burchill et al., 2003) (Figure 2A, S2). Consistent with the notion that expanded Tregs promote infection susceptibility, significantly more recoverable CFUs were found in CA-STAT5b compared with B6 control mice after Lm infection (Figure 2B). Since aberrations in other immune cells (e.g. CD8+ T and CD19+ B cells) in CA-STAT5b mice may also contribute to infection susceptibility, related experiments more specifically investigated the contribution of expanded Foxp3+ Tregs in these mice. First, CA-STAT5b mice were intercrossed with Foxp3DTR mice allowing for the ablation of expanded Foxp3+ Tregs with DT. We found DT treatment in CA-STAT5b Foxp3DTR mice efficiently ablated expanded Foxp3+ cells and restored resistance to levels comparable to Treg un-manipulated controls (Figure 2A, 2B). In turn, DT treatment in Foxp3DTR compared with Foxp3WT mice also caused significant reductions in recoverable Lm CFUs congruent with the effects of Treg-ablation on infection susceptibility for pregnant mice. As a complementary approach, we exploited the efficiency whereby adoptively transferred Tregs not susceptible to DT repopulates this ablated cell compartment by using Tregs from CA-STAT5b or control mice to reconstitute Treg-ablated Foxp3DTR mice sustained on low-dose DT. We found Treg-ablated mice reconstituted with CA-STAT5b compared with WT Tregs contained ~2-fold increased Foxp3+ cells (23% compared with 11%) due to cell-intrinsic STAT5b stimulation that drives Foxp3+ cell expansion (Figure 2C). Foxp3+ CD4+ cells in mice reconstituted with CA-STAT5b or WT Tregs were each > 99% donor derived, while < 1% Foxp3− cells were donor derived based on expression of the CD45.2 congenic marker. After Lm infection, Treg-ablated Foxp3DTR mice reconstituted with expanded CA-STAT5b compared with WT Tregs contained significantly more recoverable CFUs that paralleled the increased susceptibility of CA-STAT5b mice (Figure 2D). Thus, expanded Foxp3+ Tregs impair host defense and confer susceptibility to disseminated Lm infection.
Figure 2. Expanded Foxp3+ Tregs impair host defense against Lm infection.
(A) Percent Foxp3+ among CD4+ splenocytes after DT treatment in B6 (WT), Foxp3DTR, CA-STAT5b, or CA-STAT5b Foxp3DTR mice. (B) Recoverable CFUs three days after Lm infection for each group of mice initiated on DT treatment one day prior to infection. (C) Percent Foxp3+ Tregs day 14 after the initiation of sustained DT in Foxp3DTR CD45.1+ mice reconstituted with donor Tregs (CD45.2+) from WT or CA-STAT5b mice (top). CD45.2 expression by Foxp3+ (line) or Foxp3− (shaded) CD4+ T cells (bottom). (D) Recoverable CFUs three days after Lm infection for the mice described in (C).
Sustained expansion of maternal Foxp3+ Tregs is required for maintaining pregnancy
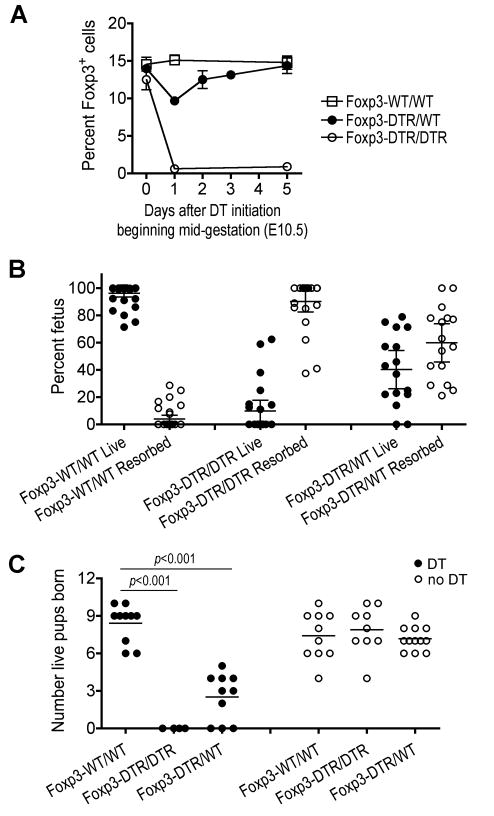
The infection susceptibility associated with the physiological expansion of maternal Foxp3+ cells during allogeneic pregnancy suggests the proposed role these cells play in facilitating antigenic diversity is more important for species survival outweighing transient defects in host defense against infection. In this regard, although the importance of maternal Tregs during pregnancy has been described using strategies that cause the near complete ablation of these cells based on CD25 expression (Aluvihare et al., 2004; Kahn and Baltimore, 2010), the specific requirement for maternal Foxp3+ cells expanded from steady state levels found in non-pregnant controls in maintaining pregnancy remains undefined. To investigate this question, we exploited the X-linked inheritance of foxp3 where random chromosome inactivation in Foxp3DTR/WT heterozygous females results in distinct Foxp3DTR and Foxp3WT Treg subsets. We found the initiation of DT beginning mid-gestation in Foxp3DTR/WT mice caused more modest (~35%) reductions in Foxp3+ Tregs, compared with the expected 50%, that closely approximates the level found in non-pregnant controls (Figure 3A and 1A). Even with sustained DT, these reductions were transient as Foxp3+ cells rapidly rebounded to levels found in pregnant Foxp3WT/WT controls. These findings are each consistent with the continuous refilling of this partially depleted compartment with Foxp3WT Tregs that are not susceptible to ablation in Foxp3DTR/WT mice (Kim et al., 2007). Interestingly, these partial transient reductions in maternal Tregs achieved with DT in Foxp3DTR/WT mice were nevertheless sufficient to trigger sharply increased rates of fetal resorption (10-fold, p < 0.001) with reciprocal reductions in the number of live pups born (70% reduction, p < 0.001) each compared with Treg-sufficient pregnancies (Figure 3B, 3C). By extension, DT treatment in pregnant Foxp3DTR/DTR mice caused the sustained near-complete ablation of Tregs, and more profound rates of fetal resorption with reciprocal elimination of live pups born (Figure 3A–3C).
Figure 3. Sustained expansion of maternal Foxp3+ Tregs is required for maintaining pregnancy.
(A) Percent Foxp3+ among CD4+ splenocytes during allogeneic pregnancy in Foxp3WT/WT, Foxp3DTR/WT, or Foxp3DTR/DTR mice after the initiation of DT treatment beginning mid-gestation (E10.5). Bar, one standard error. (B) Percent live or resorbed fetuses among total placental-fetus in individual pregnancies for each group of mice day 5 after the initiation of DT beginning mid-gestation. Bar, mean and 95% confidence interval. (C) Number of live pups born after allogeneic mating for each group of female mice either treated with DT for four consecutive days beginning mid-gestation (filled) or without DT treatment (open).
Importantly, these detrimental pregnancy outcomes were not due to non-specific effects related to DT because each group of mice received the same dosing of this reagent beginning mid-gestation; nor were they caused by inherent defects in Foxp3DTR/DTR or Foxp3DTR/WT mice because without DT, the number of live pups born for each was indistinguishable from Treg-sufficient controls (Figure 3C). Furthermore, given the potential for DT-mediated ablation of fetal Foxp3+ cells in Foxp3DTR pregnancies, we examined Foxp3 expression in E10.5 to E16.5 embryos using both anti-Foxp3 antibody and Foxp3GFP reporter mice (Fontenot et al., 2005b). In each method, no detectable Foxp3 expression in utero above background levels was observed (Figure S3). The absence of in utero Foxp3 expression is consistent with the birth of males hemizygous for defects in Foxp3 at the expected Mendelian ratios (Brunkow et al., 2001), the ability of donor Tregs adoptively transferred after birth to rescue Foxp3-deficient mice from systemic autoimmunity (Fontenot et al., 2003), and the very minuscule levels of Foxp3 expression in one day old mice (Fontenot et al., 2005a). Moreover, the observed rates of in utero fetal resorption triggered by DT in Foxp3DTR/WT (57%) and Foxp3DTR/DTR (90%) pregnancies significantly exceed the expected percentage of Foxp3DTR pups after allogeneic mating with Foxp3WT/WT males. Accordingly, the effects of DT treatment in Foxp3DTR/WT and Foxp3DTR/DTR pregnancies most likely reflect the manipulation of maternal and not fetal Tregs. Since the majority of expanded Foxp3+ Tregs during pregnancy are also CD25+ (Figure 1A), these results are consistent with the importance of CD25+ CD4+ T cells in maintaining pregnancy (Aluvihare et al., 2004; Kahn and Baltimore, 2010). However, our use of reagents that manipulate Tregs based on Foxp3-expression and cause the partial ablation of these cells to pre-pregnancy levels more specifically establishes the importance of sustained maternal Treg expansion during pregnancy.
Expanded maternal Tregs sustain tolerance to fetal antigen
The requirement for sustained expansion of Foxp3+ cells during pregnancy suggests these cells provide uninterrupted active immune tolerance to the developing fetus. To investigate this hypothesis, the impacts of Treg ablation on the priming and activation of maternal T cells with specificity to the fetus were enumerated. To more precisely track the response to fetal antigen, we substituted mice where ovalbumin (OVA) is expressed in all cells on the Balb/c background for mating with non OVA-expressing females on the B6 background (Ehst et al., 2003). This strategy allows T cells among maternal immune cells with specificity to defined peptides within the surrogate fetal-(OVA) antigen to be tracked using well-characterized immunological tools (Erlebacher et al., 2007). We found Treg-ablation beginning mid-gestation triggered similar increased rates of fetal resorption in Foxp3DTR/WT (57%) and Foxp3DTR/DTR (96%) pregnancies that was associated with robust expansion of OVA-specific CD4+ and CD8+ T cells compared with Foxp3WT/WT pregnancies (Figure 4A). Importantly, the expansion of these OVA-specific T cells was specific to the developing fetus and not caused by non-specific effects related to Foxp3+ cell manipulation because only background levels were found in Treg-ablated females impregnated with non-OVA expressing males (Figure S4). Furthermore, maternal Tregs also suppress the activation of fetal-(OVA)-specific T cells because CD8+ T cells from pregnancies with partial (Foxp3DTR/WT) or near complete (Foxp3DTR/DTR) Treg ablation produce significantly more IFN-γ after OVA257-264 peptide stimulation, and eliminated adoptively transferred OVA257-264 peptide-coated target cells more efficiently each compared with Treg-sufficient (Foxp3WT/WT) pregnancies (Figure 4B, 4C). Thus, fetal resorption and fractured tolerance to fetal antigen triggered by even partial reductions in maternal Foxp3+ Tregs in mice recapitulates human pregnancy complications related to fetal intolerance (e.g. spontaneous abortion, preeclampsia) each associated with blunted expansion of maternal Tregs (Prins et al., 2009; Santner-Nanan et al., 2009; Sasaki et al., 2004).
Figure 4. Expanded maternal Tregs sustain tolerance to fetal antigen.
(A) Percent and number of adoptively transferred CD90.1+ fetal (OVA323-339)-specific CD4+ (top) or (OVA257-264)-specific CD8+ (bottom) cells among splenocytes in Foxp3WT/WT, Foxp3DTR/DTR, or Foxp3DTR/WT females impregnated by Actin-OVA transgenic males five days after the initiation of DT beginning mid-gestation (E10.5). (B) Percent and number of IFN-γ-producing CD90.1+ CD8+ T cells after in vitro OVA257-264 peptide stimulation (line) or un-stimulated controls (shaded). (C) Percent OVA257-264 peptide-pulsed (CFSElo) relative to untreated control cells (CFSEhi) pre-transfer, or after adoptive transfer into each group of females impregnated by Actin-OVA males initiated on DT treatment beginning mid-gestation.
Treg cell-intrinsic IL-10 confers infection susceptibility
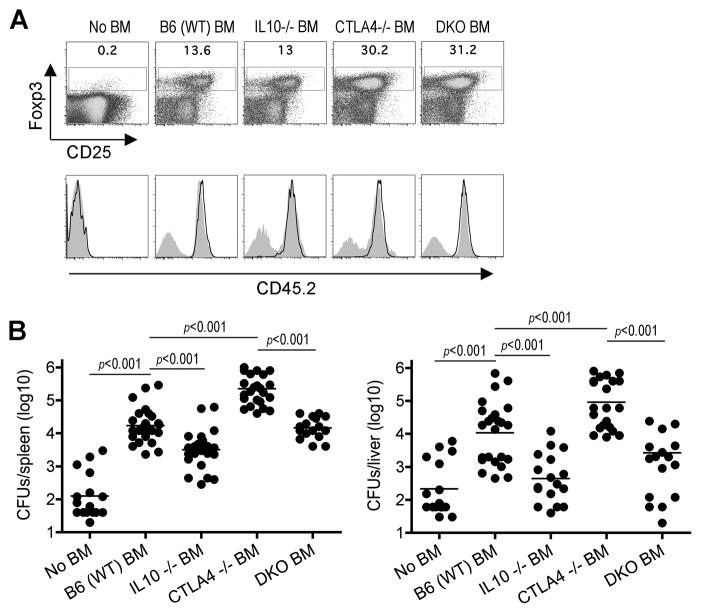
Given the divergent roles for expanded maternal Tregs that are on one hand required for sustaining pregnancy, but also impair host defense that confers infection susceptibility, we explored if targeted ablation of individual Treg-associated molecules would allow these protective and harmful effects to be dissociated. In this regard, although numerous Treg-associated molecules that can each mediate immune suppression in vitro have been identified (Sakaguchi et al., 2009; Shevach, 2009; Vignali et al., 2008), the relative importance for each in dictating the multi-faceted role Foxp3+ cells play in vivo remain incompletely defined. We focused initially on CTLA-4 and IL-10 because each play dominant roles for Treg suppression in other in vivo contexts (Asseman et al., 1999; Belkaid et al., 2002; Friedline et al., 2009; Rubtsov et al., 2008; Wing et al., 2008). To evaluate the requirement for Treg expression of each molecule, bone marrow cells from CTLA-4-deficient, IL-10-deficient or WT mice were adoptively transferred into sub-lethally irradiated Foxp3DTR CD45.1+ recipient mice. After reconstitution and before DT treatment, these mixed chimera mice contain Tregs with targeted defects in either CTLA-4 or IL-10 each derived from donor bone marrow cells and WT Tregs from Foxp3DTR recipient mice. However, after DT treatment, Tregs derived from recipient Foxp3DTR mice are eliminated leaving behind only those derived from donor CD45.2+ bone marrow cells that rapidly expand to refill this partially ablated compartment (Figure 5A). Using this approach that allows the more synchronized ablation of each Treg-associated molecule, mice containing only CTLA-4-deficient compared with WT Tregs were found to have expanded Foxp3+ cells consistent with the role of CTLA-4 in limiting Treg proliferation (Kolar et al., 2009; Tang et al., 2008). In turn, mice with expanded CTLA-4 deficient compared with WT Tregs were also more susceptible to Lm infection (Figure 5B). Comparatively, while IL-10-deficient and WT Tregs each expand to a similar extent in irradiated Foxp3DTR mice, those containing only IL-10-deficient Tregs compared with mice containing either WT or CTLA-4-deficient Tregs were markedly more resistant to Lm infection (Figure 5A, 5B).
Figure 5. Treg-mediated infection susceptibility requires cell-intrinsic IL-10.
(A) Percent Foxp3+ among CD4+ splenocytes day 10 after the initiation of sustained DT in irradiated Foxp3DTR CD45.1+ mice without donor bone marrow, or reconstituted with bone marrow from WT, IL-10-deficient, CTLA-4-deficient mice, or mice with combined defects in both IL-10 and CTLA-4 (DKO) (top). CD45.2 expression by Foxp3+ (line) or Foxp3− (shaded) CD4+ cells (bottom). (B) Recoverable CFUs three days after Lm infection for the mice described in (A).
To further investigate the requirement for IL-10 and CTLA-4 expression by Tregs in host defense against infection, mixed chimera mice containing Foxp3+ cells with targeted defects in both molecules were generated by using bone marrow cells from mice with combined defects in both IL-10 and CTLA-4 (DKO) for reconstituting sub-lethally irradiated Foxp3DTR mice. Although the rate of recombination between these two genes separated by 39.8 centi-Morgan on chromosome 1 was somewhat lower than expected (Figure S5A), mice with defects in both IL-10 and CTLA-4 could be generated, and began to appear runty with a “scurfy” phenotype beginning 23 to 27 days of life that was indistinguishable from CTLA-4-deficient mice (Tivol et al., 1995; Waterhouse et al., 1995). After adoptive transfer into irradiated Foxp3DTR mice, bone marrow cells from these DKO mice led to expanded levels of Foxp3+ Tregs comparable to reconstitution with bone marrow from CTLA-4-deficient mice (Figure 5A). However, despite the expansion of Foxp3+ Tregs due to cell-intrinsic defects in CTLA-4, these mice were significantly more resistant to Lm infection with significantly reduced CFUs compared with mice containing CTLA-4-deficient (IL-10-sufficient) Tregs (Figure 5B). Importantly, these impacts on infection susceptibility are not explained by potential differences in non-specific immune activation because donor bone marrow derived Tregs from each group of mice compared with Treg-ablated mice without donor bone marrow suppressed activation (percent CD44hiCD62Llo) among bulk T cells to the same extent (Figure S5B). Together, these results indicate that while CTLA-4 controls host defense against Lm indirectly by limiting Treg-expansion, ablation of IL-10 overrides the infection susceptibility associated with both expanded CTLA-4-deficient and normal levels of WT Tregs.
Related experiments sought to investigate whether resistance against Lm infection in mice containing exclusively IL-10-deficient Tregs could be attributed to the mixed population of WT and IL-10 deficient non-Treg cells derived from donor bone marrow in irradiated Foxp3DTR mice. Donor Tregs from IL-10-deficient or B6 control mice were used to repopulate non-irradiated Foxp3-cell ablated Foxp3DTR mice; and with sustained DT for seven to ten days, >99% of Foxp3+ Tregs and <1% non-Tregs were donor derived based on CD45.2 expression (Figure 6A). After Lm infection, mice reconstituted with IL-10-deficient compared with WT Tregs were more resistant containing significantly reduced numbers of recoverable CFUs (Figure 6B). Similar to reconstituted Tregs in irradiated mice, adoptively transferred IL-10 and WT Tregs each suppressed non-specific T cell activation to a similar extent compared with Treg-ablated mice without donor cells (Figure S6). Taken together, these results demonstrate a critical role for Foxp3+ Tregs and IL-10 production by these cells in suppressing host defense against Lm infection.
Figure 6. Treg IL-10 impairs resistance against Lm infection.
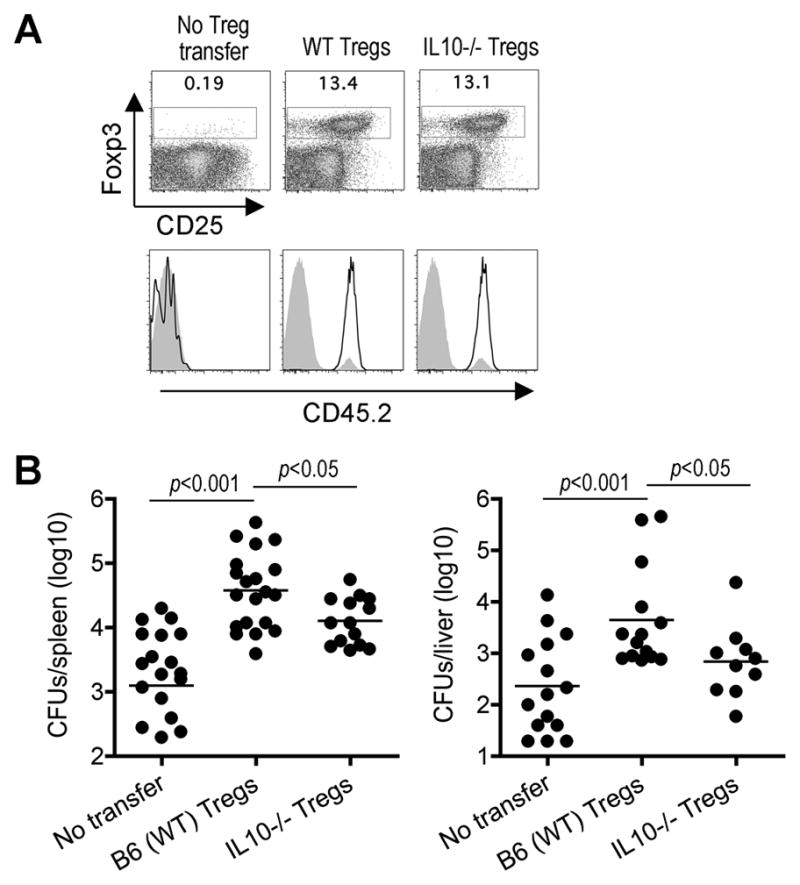
(A) Percent Foxp3+ among CD4+ splenocytes day 12 after the initiation of sustained DT in non-irradiated Foxp3DTR CD45.1+ mice without donor Tregs, or reconstituted with donor Tregs from WT or IL-10-deficient mice (top). CD45.2 expression by Foxp3+ (line) or Foxp3− (shaded) CD4+ cells (bottom). (B) Recoverable CFUs three days after Lm infection for the mice described in (A).
IL-10 is dispensable for sustaining pregnancy, but impairs host defense against prenatal infection
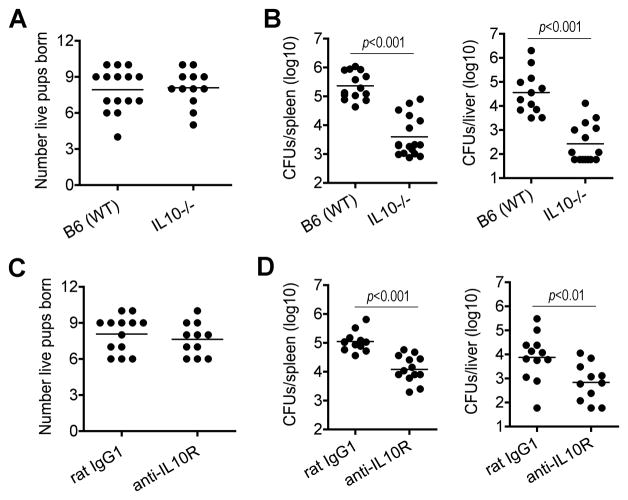
Given the requirement for maternal Foxp3+ Tregs in sustaining pregnancy, we examined whether parallel requirements exist for maternal IL-10 by enumerating pregnancy outcomes in IL-10-deficient compared with B6 control mice after allogeneic mating with Balb/c males. In sharp contrast to pregnancy loss that occurs with even partial transient Treg ablation, IL-10-deficient compared with IL-10-sufficient pregnancies had indistinguishable normal numbers of live pups born at term (Figure 7A). The non-essential role for maternal IL-10 in sustaining allogeneic pregnancy together with the importance of Treg IL-10 in promoting susceptibility to Lm infection suggest IL-10 neutralization may be used for boosting immunity against infection without compromising pregnancy outcomes. Consistent with this hypothesis, we found pregnant IL-10-deficient compared with pregnant B6 controls were significantly less susceptible to Lm infection (Figure 7B). These results are in agreement with the increased resistance against Lm for IL-10-deficient non-pregnant mice (Dai et al., 1997), and extend the importance of this cytokine to infection susceptibility during pregnancy. As a complementary approach, we enumerated the impacts of IL-10 receptor neutralization on pregnancy outcomes and resistance to Lm infection. Similar to findings using IL-10-deficient mice, anti-IL-10 receptor compared with isotype control antibody treatment at mid-gestation had no significant impacts on the number of live births after allogeneic pregnancy, but caused sharp reductions in the number of recoverable Lm CFUs after infection (Figure 7C, 7D). Together, these results establish that although IL-10 is dispensable for sustaining pregnancy, it impairs host defense against prenatal Lm infection.
Figure 7. Maternal IL-10 is dispensable for sustaining pregnancy, but impairs host defense against prenatal Lm infection.
(A) Number of live pups born for IL-10-deficient or B6 (WT) mice after allogeneic mating with Balb/c males. (B) Recoverable CFUs three days after Lm infection at mid-gestation (E10.5) for IL-10-deficient or B6 (WT) pregnant mice. (C) Number of live pups born for B6 mice after allogeneic mating with Balb/c males treated with anti-IL-10 receptor or isotype control (rat IgG1) antibody beginning mid-gestation. (D) Recoverable CFUs in pregnant B6 mice three days after Lm infection and treatment with anti-IL-10 receptor or isotype control antibody at mid-gestation.
DISCUSSION
Regulatory T cells control the fluid balance between immune suppression that maintains peripheral tolerance and immune stimulation required for optimal host defense against infection. Herein, we demonstrate pregnancy triggers the physiological expansion of Foxp3+ Tregs that shifts this balance allowing maternal tolerance to the developing fetus to be maintained. Using tools that manipulate Tregs based on Foxp3 expression and cause the partial ablation of these cells, the blunted expansion of maternal Tregs observed in human pregnancy complications such as spontaneous abortion or preeclampsia where these cells are reduced to pre-pregnancy levels, but not eliminated is more closely recapitulated (Prins et al., 2009; Santner-Nanan et al., 2009; Sasaki et al., 2004). We find the sustained expansion of Foxp3+ Tregs was imperative because even transient partial ablation to levels found in non-pregnant mice was sufficient to cause sharply increased rates of fetal resorption, reductions in the number of live pups born, and the expansion and activation of maternal T cells with specificity to the fetus. Given the parallel requirements for tryptophan catabolism through indoleamine 2,3-dioxygenase (IDO) in sustaining pregnancy, and the potency whereby Tregs stimulate tryptophan catabolism (Fallarino et al., 2003; Munn et al., 1998), blunted expansion or transient ablation of maternal Tregs may complicate pregnancy by disrupting this pathway. This is consistent with the up-regulation of IDO during uncomplicated human pregnancy compared with spontaneous abortion cases (Miwa et al., 2005). Our ongoing studies are aimed at dissecting the molecular basis for how maternal Tregs sustain pregnancy, and the pregnancy-associated signals that drive the expansion of maternal Tregs. In this regard, while maternal Tregs suppress IFN-γ production by fetal-specific T cells and IFN-γ is a potent inducer of IDO expression (Taylor and Feng, 1991), IFN-γ responsiveness was non-essential for Treg expansion illustrating other unidentified signals stimulate shifts in maternal Foxp3+ cells during allogeneic pregnancy.
The physiological expansion of Tregs during pregnancy and non-antigen-specific fashion whereby these cells mediate suppression suggest other detrimental or potentially protective immune responses may be suppressed as well. For example for patients with rheumatoid arthritis or autoimmune hepatitis, reduction in disease severity or disease remission occurs during pregnancy (Buchel et al., 2002; Ostensen and Villiger, 2007). On the other hand, given the intricately regulated balance between immune stimulation and suppression, sustained expansion of immune tolerance during pregnancy may also cause defects in host defense especially against pathogens such as Listeria monocytogenes and Salmonella typhimurium with a predilection for prenatal infection (Gellin et al., 1991; Mylonakis et al., 2002; Pejcic-Karapetrovic et al., 2007). Accordingly, we investigated the impacts of expanded Tregs on infection susceptibility in complementary in vivo models that include allogeneic pregnancy, CA-STAT5b transgenic mice, and mice with Treg-intrinsic defects in CTLA-4. In each condition where Tregs are expanded, increased infection susceptibility was uniformly identified. Furthermore, in each model of expanded Tregs where host defense against infection is impaired, secondary defects that include the ablation of expanded Tregs or specific Treg-intrinsic molecules were used to confirm the overall importance of Tregs, or to identify Treg-associated molecules that mediate infection susceptibility. Although our experiments were not designed to evaluate the additional incremental impacts whereby direct invasion into the products of conception cause susceptibility to disseminated Lm infection (Bakardjiev et al., 2006; Le Monnier et al., 2007), the direct links between Tregs manipulated using these complementary gain- and loss-of function approaches in pregnant and non-pregnant mice on susceptibility to Lm infection establish that expanded Tregs required for sustaining pregnancy also impair host defense against this important prenatal pathogen. These findings suggest the expansion of maternal Tregs that facilitates increased genetic diversity between maternal and paternal antigen is more important for species survival outweighing transient defects in host defense.
The deleterious impacts of expanded Tregs on host defense against Lm is consistent with the accelerated pathogen eradication caused by ablation of CD25+ or Foxp3+ Tregs during infection with an increasingly wide assortment of other pathogens (Belkaid, 2007; Johanns et al., 2010; Suvas and Rouse, 2006). However, while prior studies have primarily used loss-of-function approaches for investigating the role of Tregs in host defense, this study uses complementary approaches that mimic the physiological prenatal expansion of these cells to demonstrate how increased Foxp3+ Tregs dictates infection susceptibility during pregnancy. An important area for future investigation is to explore if similar impacts on host defense are found after infection with other pathogens using gain-of-function approaches for Foxp3+ Tregs. In this regard, although the number of pathogens where Tregs have been implicated to suppress host defense far exceeds the limited number with defined predilection for prenatal infection, the results of these studies will likely have boarder implications for understanding infection susceptibility in aging and other contexts where the physiological expansion of Tregs also occurs (Gregg et al., 2005).
Numerous Foxp3+ cell-associated molecules have been shown to mediate the suppressive properties of Tregs (Sakaguchi et al., 2009; Shevach, 2009; Vignali et al., 2008). Recently, intercrossing mice where the Cre-recombinase and foxp3 are co-expressed with other mice where each Treg-associated molecule of interest is flanked by loxP sites have revealed unique and discordant roles for defined Treg-associated molecules in controlling specific aspects of peripheral tolerance (Rubtsov et al., 2008; Wing et al., 2008). Although ideal for identifying Treg-associated molecules that with sustained ablation cause autoimmunity, this strategy that results in the ablation of each molecule in Foxp3+ cells throughout development is not suitable for interrogating their role in host defense against infection where the synchronized ablation of each molecule in Tregs is required. Accordingly, for interrogating the relative importance of Treg-associated molecules in controlling host defense against Lm infection, we exploited the efficiency whereby donor Tregs from mice with targeted defects in each Treg-associated suppression molecule expands to fill the partially ablated cellular compartment in mixed chimera mice, or completely empty compartment in Treg-ablated mice. These approaches that allow more synchronized ablation of specific Treg-associated molecules in Foxp3+ cells identified important roles for Treg IL-10 in suppressing host defense against Lm infection. Interestingly however, elimination of Treg IL-10 in neither non-irradiated Treg-reconstituted mice nor irradiated mice containing expanded CTLA-4-deficient Tregs restored resistance to levels found in Treg-ablated mice. These findings indicate other Treg-associated molecules likely play functionally redundant roles and/or act synergistically with IL-10 in impairing host defense against Lm infection.
The importance of IL-10 in suppressing immunity against Lm infection during pregnancy is in agreement with the previously reported importance of this cytokine in promoting susceptibility to other pathogens (Belkaid et al., 2002; Brooks et al., 2006; Ejrnaes et al., 2006). Together with controlling inflammation at mucosal surfaces (Asseman et al., 1999; Rubtsov et al., 2008), our results extend the context specific role of Treg IL-10 to include suppressing host defense components after acute pathogenic infection during pregnancy. Given the non-essential roles for maternal IL-10 in maintaining pregnancy even after allogeneic mating, these results demonstrate IL-10 can dissociate the detrimental impacts of expanded Tregs on infection susceptibility from the beneficial effects required for sustaining pregnancy. Accordingly, it is tantalizing to consider that the benefits of transiently neutralizing IL-10 for boosting host defense during complicated prenatal infections would not be harmful for pregnancy, and may therefore outweigh the potentially detrimental impacts on peripheral tolerance.
EXPERIMENTAL PROCEDURES
Mice
B6, Balb/c, SJL CD45.1+, IL-10-deficient (Kuhn et al., 1993), and IFN-γ receptor-deficient (Huang et al., 1993) mice were each purchased from The Jackson Laboratory. Foxp3DTR and Foxp3GFP mice were generously provided by Dr. Alexander Rudensky (Fontenot et al., 2005b; Kim et al., 2007). Foxp3DTR mice were backcrossed > 15 generations to B6 mice, and intercrossed with CD45.1+ mice. Actin-OVA mice (Ehst et al., 2003) were backcrossed > 10 generations to Balb/c mice. OT-1 and OT-II TCR transgenic mice were maintained on a Rag1-deficient CD90.1 (B6) background. CA-STAT5b mice (Burchill et al., 2003) were intercrossed with Foxp3DTR mice to generate CA-STAT5b Foxp3DTR mice. CTLA-4-deficient mice have been described (Tivol et al., 1995; Waterhouse et al., 1995). Mice with combined defects in IL-10 and CTLA-4 were generated by intercrossing mice with individual defects (Figure S5). For Treg ablation, mice were treated with an initial dose of DT (25 μg/kg) followed by subsequent daily doses of 5 μg/kg. All experiments were performed using University of Minnesota IACUC approved protocols.
Antibodies and flow cytometry
Fluorophore-conjugated antibodies and other reagents for cell surface, intracellular, and intranuclear staining were purchased from eBioscience or BD-Biosciences. Intracellular cytokine staining was performed for bulk ex vivo splenocytes (containing OVA-specific CD8+ T cells and antigen presenting cells) after stimulation with OVA257-264 peptide or no stimulation control in media containing GolgiPlug (BD Biosciences). Antibodies for IL-10 receptor neutralization (1B1.3A) (Brooks et al., 2006; Ejrnaes et al., 2006) and rat IgG1 isotype control were purchased form BioXcell, and inoculated intraperitoneally (1 mg/mouse) into pregnant mice at mid-gestation.
Cell transfers
Purified CD8+ T cells (105) from OT-I or CD4+ T cells (106) from OT-II TCR transgenic mice were adoptively transferred into Foxp3WT/WT, Foxp3DTR/DTR, or Foxp3DTR/WT females impregnated with Actin-OVA or non-OVA-expressing Balb/c males at mid-gestation (E10.5) with the initiation of DT. To measure cytotoxicity, splenocytes from congenic (CD45.2+) mice were stained with either high (1 μM) or low (50 nM) CFSE, pulsed with OVA257-264 peptide (50 nM CFSE) or no peptide control (1 μM CFSE), mixed at a 1:1 ratio, adoptively transferred into pregnant mice four days after the initiation of DT at mid-gestation, and harvested 8 hours after transfer.
Infections
Lm strain 10403s (Portnoy et al., 1988) and ST strain SL1344 (Johanns et al., 2010) were each grown to early log phase (OD600 0.1) in brain heart infusion media at 37°C, washed and diluted with saline to 200 μl, and injected intravenously (5 × 103 CFUs for Lm, and 1 × 102 CFUs for ST). For enumerating susceptibility, the number of recoverable bacteria in the spleen or liver three days after infection were quantified by organ homogenization, and plating serial dilutions onto agar plates as described (Rowe et al., 2008). For enumerating fetal invasion, each placental-fetal unit was individually dissected, homogenized in saline, and cultured on agar plates.
Bone marrow chimera and Treg reconstitutions
Bone marrow cells were harvested from the tibias and femurs from donor mice, intravenously transferred into sub-lethally irradiated (725 rads) Foxp3DTR mice, and allowed to reconstitute for 8–10 weeks. Thereafter, mice were treated with DT for 10 days, then infected with Lm to evaluate infection susceptibility. For reconstituting Foxp3+ cells in non-irradiated Foxp3DTR mice, donor Tregs from B6, CA-STAT5b, or IL-10-deficient mice were adoptively transferred into Foxp3DTR mice (5 × 105 donor Foxp3+ CD4+ cells per mouse) with the initiation of daily DT, and infected with Lm 15 days later.
Statistical analysis
The number and percent live pups, resorbed concepti, cell numbers, and recoverable log10 bacterial CFUs were first analyzed and found to be normally distributed. Thereafter, differences between each group were analyzed using the unpaired Student’s t-test (Prism, GraphPad) with p < 0.05 taken as statistical significance.
Supplementary Material
HIGHLIGHTS.
Expanded maternal Foxp3+ Tregs confer infection susceptibility during pregnancy
Sustained maternal Treg expansion maintains immune tolerate to fetal antigen
Treg IL-10 is dispensable for sustaining pregnancy, but impairs prenatal host defense
Acknowledgments
We thank Dr. Alexander Rudensky for providing Foxp3DTR and Foxp3GFP mice, Dr. Marc Jenkins for providing Actin-OVA mice, Dr. Daniel Mueller for providing CTLA-4-deficient mice, Drs. Michael Bevan, Bryce Binstadt, Kristen Hogquist, Stephen McSorley, Matthew Mescher, Joseph Sun, and Viava Vezys for helpful discussions, and Sandra Horn and the UMN Mouse Genetics Laboratory for assistance in synchronized mouse breeding. This research was supported by NIH grants F30-DK084674 (JHR) and R01-AI087830 (SSW).
Footnotes
This manuscript contains six supplementary figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nature immunology. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. The Journal of experimental medicine. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakardjiev AI, Theriot JA, Portnoy DA. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS pathogens. 2006;2:e66. doi: 10.1371/journal.ppat.0020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nature reviews. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature medicine. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature genetics. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Buchel E, Van Steenbergen W, Nevens F, Fevery J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. The American journal of gastroenterology. 2002;97:3160–3165. doi: 10.1111/j.1572-0241.2002.07124.x. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, Bensinger SJ, Turka LA, Brennan P, Jameson SC, Farrar MA. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. The Journal of experimental medicine. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. The Journal of clinical investigation. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nature immunology. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. The Journal of experimental medicine. 2005a;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005b;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. The Journal of experimental medicine. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellin BG, Broome CV, Bibb WF, Weaver RE, Gaventa S, Mascola L. The epidemiology of listeriosis in the United States--1986. Listeriosis Study Group. American journal of epidemiology. 1991;133:392–401. doi: 10.1093/oxfordjournals.aje.a115893. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25 high regulatory T cells increases with age. Clinical and experimental immunology. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science (New York, NY. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS pathogens. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kolar P, Knieke K, Hegel JK, Quandt D, Burmester GR, Hoff H, Brunner-Weinzierl MC. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis and rheumatism. 2009;60:123–132. doi: 10.1002/art.24181. [DOI] [PubMed] [Google Scholar]
- Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Le Monnier A, Autret N, Join-Lambert OF, Jaubert F, Charbit A, Berche P, Kayal S. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infection and immunity. 2007;75:950–957. doi: 10.1128/IAI.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Miwa N, Hayakawa S, Miyazaki S, Myojo S, Sasaki Y, Sakai M, Takikawa O, Saito S. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Molecular human reproduction. 2005;11:865–870. doi: 10.1093/molehr/gah246. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science (New York, NY. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine. 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Seminars in immunopathology. 2007;29:185–191. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- Pejcic-Karapetrovic B, Gurnani K, Russell MS, Finlay BB, Sad S, Krishnan L. Pregnancy impairs the innate immune resistance to Salmonella typhimurium leading to rapid fatal infection. J Immunol. 2007;179:6088–6096. doi: 10.4049/jimmunol.179.9.6088. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nature medicine. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. The Journal of experimental medicine. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Johanns TM, Ertelt JM, Way SS. PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J Immunol. 2008;180:7553–7557. doi: 10.4049/jimmunol.180.11.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? International immunology. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Molecular human reproduction. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Rouse BT. Treg control of antimicrobial T cell responses. Current opinion in immunology. 2006;18:344–348. doi: 10.1016/j.coi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Tang AL, Teijaro JR, Njau MN, Chandran SS, Azimzadeh A, Nadler SG, Rothstein DM, Farber DL. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806–1813. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science (New York, NY. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunology today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science (New York, NY. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.