Abstract
Objective
We previously reported an attenuation of both exercise hyperemia and measures of aerobic capacity in hypercholesterolemic mice. In this study we expanded upon the previous findings by examining the temporal and quantitative relationship of hypercholesterolemia to aerobic and anaerobic capacity and by exploring several potential mechanisms of dysfunction.
Methods
Eight-week old wild type (n=123) and apoE knockout (n=79) C57BL/6J mice were divided into groups with distinct cholesterol levels by feeding regular or high fat diets. At various ages the mice underwent treadmill ergospirometry. To explore mechanisms, aortic ring vasodilator function and nitrate (NOx) activity, urinary excretion of NOx, running muscle microvascular density and citrate synthase activity, as well as myocardial mass and histologic evidence of ischemia were measured.
Results
At 8 weeks of age, all mice had similar measures of exercise capacity. All indices of aerobic exercise capacity progressively declined at 12 and 20 weeks of age in the hypercholesterolemic mice as cholesterol levels increased while indices of anaerobic capacity remained unaffected. Across the 4 cholesterol groups, the degree of aerobic dysfunction was related to serum cholesterol levels; a relationship that was maintained after correcting for confounding factors. Associated with the deterioration in exercise capacity was a decline in measures of nitric oxide-mediated vascular function while there was no evidence of aberrations in functional or oxidative capacities or in other components of transport capacity.
Conclusion
Aerobic exercise dysfunction is observed in murine models of genetic and diet-induced hypercholesterolemia and is associated with a reduction in vascular nitric oxide production.
Keywords: Endothelial function, nitric oxide, oxygen consumption, cholesterol
Introduction
Hypercholesterolemia decreases the bioactivity and synthesis of endothelium-derived nitric oxide (EDNO) and impairs endothelial vasodilator function (1–5). However, notwithstanding the long-term consequences on the development of atherosclerosis, few immediate functional consequences of hypercholesterolemia have been described. We have reported that EDNO contributes to exercise hyperemia and is a determinant of aerobic exercise capacity in mice (6). In that study, the nitric oxide synthase (NOS) inhibitor, L-nitroarginine, attenuated post-exercise urinary nitrate (NOx) excretion (a measure of EDNO production during exercise), hindlimb exercise hyperemia and aerobic exercise capacity. In hypercholesterolemic Apo E deficient mice, we observed similar reductions in post-exercise urinary NOx, hindlimb blood flow and aerobic exercise capacity. The data suggest that conditions of reduced EDNO synthesis or activity cause inadequate exercise hyperemia that is rate limiting to oxygen transport and exercise capacity. This hypothesis is further supported by our observation that supplementation of E− mice with the EDNO precursor, L-arginine, restores aerobic capacity (7). The effect of L-arginine is associated with increased post-exercise urinary NOx, reflecting enhanced NO synthesis. These findings are consistent with the finding that Apo E deficient mice exhibit increased levels of the circulating NOS inhibitor ADMA (8). The present study was designed to determine the extent to which aerobic and anaerobic components of exercise capacity are affected, to establish the temporal and quantitative relationship of hypercholesterolemia with exercise dysfunction, and to determine which of the 3 major components of aerobic capacity: functional capacity, transport capacity and oxidative capacity are most affected by hypercholesterolemia.
Materials and Methods
Animals
Eight week old female wild type (E +, n = 123) and E− (9) (E−, n = 79) C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were entered into experimental protocols after a 1 week period of acclimation. All mice were inspected prior to the study by a veterinarian and monitored daily by technicians and investigators. Mice were housed 4 per cage, maintained on a 12 hour light/dark cycle, given unlimited access to food and water, handled daily and taught to run on a treadmill, but were otherwise confined to cages for the duration of the study. All experimental protocols were approved by the Administrative Panel on Laboratory Animal Care of Stanford University and conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Experimental protocol
In preliminary studies performed to determine serum cholesterol levels at various ages for the two strains while fed a chow diet, E+ and E− were sacrificed at 4, 6, 8, 10, 12 and 20 weeks for serum cholesterol measurements. From this data, 8, 12 and 20 weeks of age were selected to examine the indices and determinants of exercise capacity.
Eight-week-old E+ mice and E− mice that had been receiving a chow diet since weaning were randomized into 4 dietary groups. One group of E+ mice (E+, n = 49) was fed regular chow while a second group (E+chol, n = 22) received a high cholesterol diet (modified Thomas-Hartroft). One group of E− mice (E−, n = 24) received regular chow whereas the other E− group (E−chol, n = 10) received a high fat diet (10).
At 8 weeks (before initiating dietary intervention), 12 weeks and 20 weeks of age, randomly selected mice from each group underwent treadmill-testing and urinary NOx measurement. At 12 weeks of age, mice were randomly chosen from each group and sacrificed following treadmill testing by overdose of methoxyflurane (Pitman-Moore, Mundelein, IL) inhalation anesthesia. The aorta was harvested for studies of vascular reactivity and stimulated aortic NOx production. Blood was collected from the right atrium for measurement of serum total and HDL cholesterol levels. The heart was removed by transecting the major vessels at the base. After fat was removed, the heart was blotted dry and weighed then snap frozen and stored at −80°C for histologic examination. Gastrocnemius and vastus medialis muscles were collected for measurements of arteriolar and capillary density and citrate synthase activity.
Indices of Exercise Capacity and Treadmill Testing
The indices used to determine aerobic exercise capacity were maximal oxygen uptake (Vo2max), anaerobic threshold (AT), and aerobic work capacity (AWC). The distance run to exhaustion (DISTe) provides a good indicator of overall exercise capacity (aerobic as well as anaerobic). Respiratory quotient (RQ) was also determined to assess anaerobic work performance and to assess the form of substrate utilization. The definitions and methods of measurement of these indices, and methods of treadmill testing have been described previously (6). Briefly, mice were treadmill tested (Exer-4 Treadmill, Columbus Instruments, Columbus, OH) using shock-plate incentive at a constant 8° angle at an initial speed of 10 m/min which was incrementally increased 1 m/min every minute until the mouse reached exhaustion. Data on Vo2, Vco2, RQ, and DISTe were collected and stored on hard disk (Oxymax software, Columbus Instruments).
Vascular Reactivity
A 7 mm segment (ring) of thoracic aorta was dissected free of connective tissue and immediately placed in cold physiologic saline solution (PSS). Aortic segments were quickly mounted on wire stirrups, hung from force transducers and submerged in oxygenated PSS at 37°C. Over the course of 60 minutes, the segments were progressively stretched to the optimum point of their length-tension relationship (determined previously to be 3 g). Subsequently, the concentration of norepinephrine inducing half-maximal response (EC50NE) was determined by exposing the segments to increasing concentrations of norepinephrine (in half-log increments from 10−9 to 10−4 M). Once a maximal response was obtained, the segments were washed repeatedly with fresh PSS for 60 minutes until the tension returned to the previous baseline value. Responses to the vasodilating agents, nitroglycerine (NTG; in ½ log incremental doses from 1×10−10 to 5 × 10−5) and acetylcholine (ACh; in ½ log incremental doses from 1×10−10 to 1 × 10−5), were studied after precontracting the segments with EC50NE. After a stable contraction was obtained, the segments were exposed to increasing doses of vasodilator.
Measurement of Aortic NOx
A 7 mm segment from the abdominal aorta was removed and placed in ice-cold PSS. After the removal of connective tissue, the segment was bisected longitudinally and incubated in 300µl HBSS medium (Irvine Scientific, Santa Ana, CA) containing calcium ionophore A23187 which stimulates endothelial NO release (final concentration of 10−6 M) and L-arginine (100µM/L) at 37°C. After 120 min, the medium was centrifuged at 15,000 rpm for 5 min and the supernatant was stored at −80°C. NOx was measured with a commercially available chemiluminescence apparatus (model 2108, Dasibi Corp., Glendale, CA) as previously described (11). The samples were injected (50µl) into boiling acidic vanadium (III) chloride. Signals from the detector were analyzed by computerized integration of curve areas.
Measurement of Urinary NOx
At 8 and 12 weeks of age, mice were placed in metabolic chambers for 2 hours for basal and post-exercise urinary NOx collection. Previous work has demonstrated that urinary NOx excretion correlates with cGMP excretion and is a reflection of microvascular EDNO production (11, 12). For the basal state, mice were confined to cages for greater than 24 hours and for the post-exercise state, mice were treadmill exercised over 22 minutes to a final treadmill speed of 32 m/min. Metabolic chambers were constructed from 250ml Plexiglas utility boxes. Each metabolic chamber drained into a test tube containing 100µl of isopropyl alcohol cooled by ice water for the duration of the 5-hour urine collection. Urine was centrifuged at 4,000rpm for 5 min and the supernatant was collected and stored at −80°C for measurement of NOx and creatinine. Urine NOx was measured in an identical fashion to that of aortic NOx except that samples were diluted 1:9 in deionized, distilled water. Urine creatinine was measured using a kit from Sigma-Aldrich (12).
Hematology, Biochemistry and Histology
Blood samples were collected in serum separator tubes at the time of sacrifice and centrifuged at 3,000rpm for 15 minutes. The serum was separated and stored at −80°C until analysis. Total serum and HDL cholesterol were analyzed using the enzymatic method of Allain et al (13).
To measure muscle oxidative capacity, gastrocnemius and vastus medialis muscles were removed, frozen in liquid nitrogen and stored at −80°C until assayed. Maximal citrate synthase activity was assayed on muscle homogenates by the method of Srere (14). Values were expressed as an average of both muscles.
Hearts were fixed in 10% formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin. Sections (16 per animal; n=6 in each group) were examined by microscopy for evidence of necrosis, fibrosis, contraction bands or other changes representing myocardial ischemia by an experienced cardiac pathologist blinded to the experimental groups.
To determine microvessel density, gastrocnemius muscles were fixed in 10% formalin. The specimens were then sectioned once transversely and the two pieces were embedded in paraffin such that each section would contain a transverse and longitudinal cut. For arteriolar density determination, sections (5µm) were stained by Avallone’s modification of the Jones silver methenamine method for staining basement membranes. For capillary density, sections (2µm) were stained using toludine blue. Arteriolar and capillary density measurements were determined separately using stereologic analysis.
Diets & Drugs
Three diets were used in these experiments; the regular chow diet (0.022% cholesterol, 11% total fat by weight, Purina, Richmond, IN), the modified Thomas-Hartroft diet (1.3% cholesterol, 15% fat from cocoa butter, Dyets, Bethlehem, PA (10)), and the Western-type diet (0.15% cholesterol, 21% fat from butterfat, Dyets (15,16)).
Physiologic saline solution was composed of NaCl, 118(mM); KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2, NaHCO3, 25; Na2EDTA, 0.026; dextrose, 11.1; L-arginine, 0.1. All solutions were prepared in distilled water except for oxaloacetic acid and 5,5'-dithiobis-(2-nitrobenzoate) (DTNB) which were prepared in 0.1M and 1M Tris-HCl, respectively. Acetyl Coenzyme A, DTNB, oxaloacetic acid, norepinephrine bitartrate, ACh, calcium ionophore A23187, L-arginine were purchased from Sigma Chemical Co. (St. Louis, MO). NTG was obtained from DuPont Chemicals (Wilmington, DE).
Data Analysis
Data are expressed as mean ± standard error of the mean (SE). Comparisons of means from multiple populations were made by one and two factor analysis of variance (ANOVA) followed by Fisher's Protected Least Significant Difference. Multivariate regression analysis was used for simultaneous comparisons of relationships. Comparisons of multiple means from repeat-measures experiments were made by multivariate analysis of variance (MANOVA). Comparisons of multiple covariate-adjusted means were made by one and two factor analysis of covariance (ANCOVA). A p-value less than 0.05 was accepted as statistically significant.
Results
Aerobic Capacity in Relation to Onset of Hypercholesterolemia
At 8 weeks of age, all 5 measures of exercise capacity were similar (Table 1). However, the E− mice demonstrated a decline in the 4 indices of exercise capacity that have aerobic capacity as component (Vo2max, AT, DISTe and AWC) as they aged from 8 to 12 and 20 weeks (Table 1; Figure 1). The decline in aerobic capacity was associated with an increase in cholesterol over time in the E− animals (Figure 2). By comparison, E+ mice maintained their aerobic capacity with a slight downward trend in Vo2max, AT and AWC but an upward trend in DISTe. By contrast, the RQ of all mice at all three ages were similar suggesting little impact on anaerobic capacity.
Table 1.
Physical, Biochemical and Exercise Characteristics of E+ and E− Mice at 8 (n = 25 & 25), 12 (n = 32 & 15) and 20 (n= 17 & 9) Weeks of Age
| Measurement | Age | E+ | E− |
|---|---|---|---|
| Body Weight (g) | 8 Week | 20.8 ± 0.5 | 20.2 ± 0.5 |
| 12 Week | 22.4 ± 0.5 | 22.9 ± 0.8 | |
| 20 Week | 23.1 ± 0.4 | 30 ± 1** | |
| Total Cholesterol (mg/dl) | 8 Week § | 131 ± 19 | 422 ± 53 |
| 12 Week | 153 ± 21 | 1175 ± 107** | |
| 20 Week | 157 ± 2 | 807 ± 57** | |
| Basal Urinary NOx (PM NO/mg creatinine) | 8 Week | 181± 21 | 284 ± 43 |
| 12 Week | 279 ± 65 | 187 ± 26 | |
| Post-Exercise Urinary NOx (PM NO/mg creatinine) | 8 Week | 202 ± 43 | 346 ± 52 ** |
| 12 Week | 429 ± 105 | 165 ± 20 ** | |
| Vo2max (mlO2/min/kg) | 8 Week | 122 ± 3 | 123 ± 4 |
| 12 Week | 117 ± 2 | 102 ± 4** | |
| 20 Week | 110 ± 2 | 97 ± 2* | |
| Anaerobic Threshold (mlO2/min/kg) | 8 Week | 100 ± 4 | 96 ± 3 |
| 12 Week | 93 ± 2 | 78 ± 4 ** | |
| 20 Week | 77 ± 2 | 71 ± 3 | |
| DISTe (m) | 8 Week | 456 ± 23 | 468 ± 23 |
| 12 Week | 476 ± 17 | 453 ± 42 | |
| 20 Week | 524 ± 24 | 306 ± 32 ** | |
| AWC (J/g) | 8 Week | 12 ±1 | 13 ± 1 |
| 12 Week | 10.9 ± 0.6 | 7.0 ± 1** | |
| 20 Week | 8.0 ± 0.7 | 4.8 ± 0.7** | |
| RQe | 8 Week | 1.07 ± .03 | 1.02 ± .01 |
| 12 Week | 1.00 ± .01 | 1.00 ± .02 | |
| 20 Week | 1.03 ± .02 | 1.02 ± .01 | |
Values are mean ± SE.
based on smaller n
p < .05,
p < .01 vs. E+
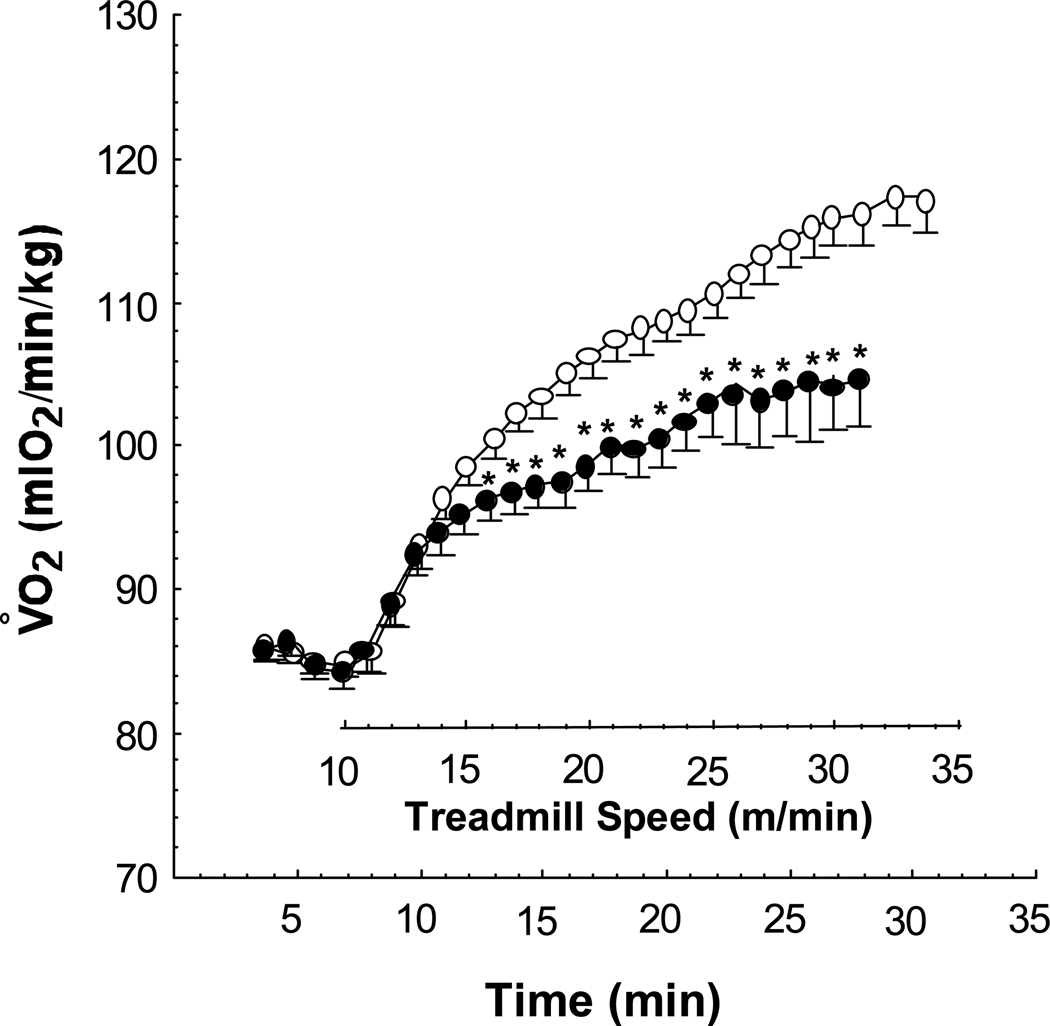
Figure 1.
Mean minute Vo2 of 12 week old E+ (n = 32, open circles) and E− (n = 22, solid circles) over the course of treadmill testing. After 7 minutes of basal measurements, the treadmill is started at 10 m/min and advanced 1 m/min every minute until exhaustion. ** P < .05 vs. E+ by MANOVA.
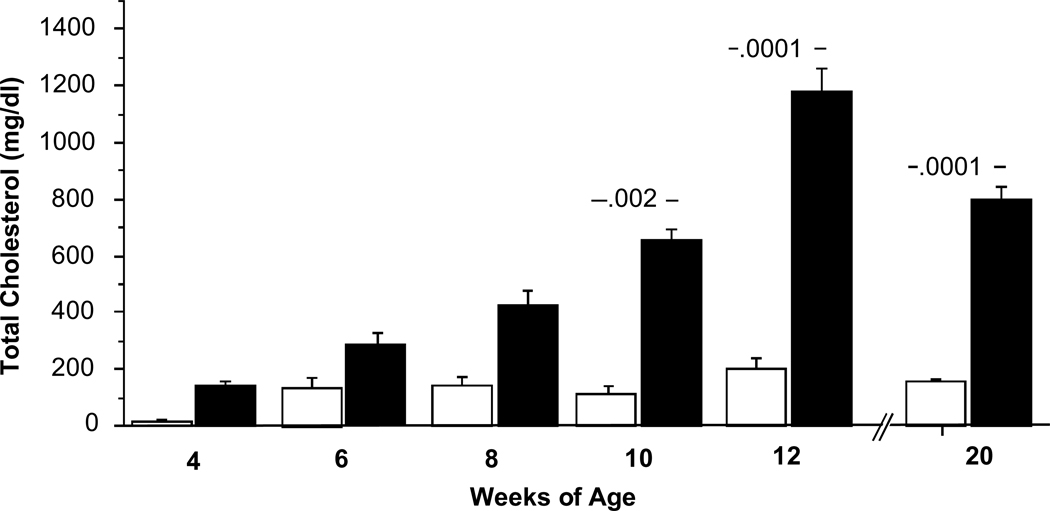
Figure 2.
Bar graph of total serum cholesterol over time. Cholesterol means ± SE of E+ (n = 10, 5, 5, 5, 59 and 16 respectively, open bars) and E− (n = 5, 4, 5, 4, 30 and 20, solid bars) at 4, 6, 8, 10, 12 and 20 weeks. Numbers over bars = p value vs. E+ by ANOVA.
Aerobic Capacity in Relation to Degree and Origin of Hypercholesterolemia
The combinations of 2 diets and 2 genetic strains resulted in 4 groups with significantly different total serum cholesterol levels (p < .0001) that increased in the order of E+ < E+chol < E− < E−chol and ranged from 153 ± 21 mg/dl to 2154 ± 223 mg/dl. The HDL cholesterol levels of each of the groups were not significantly different from each other.
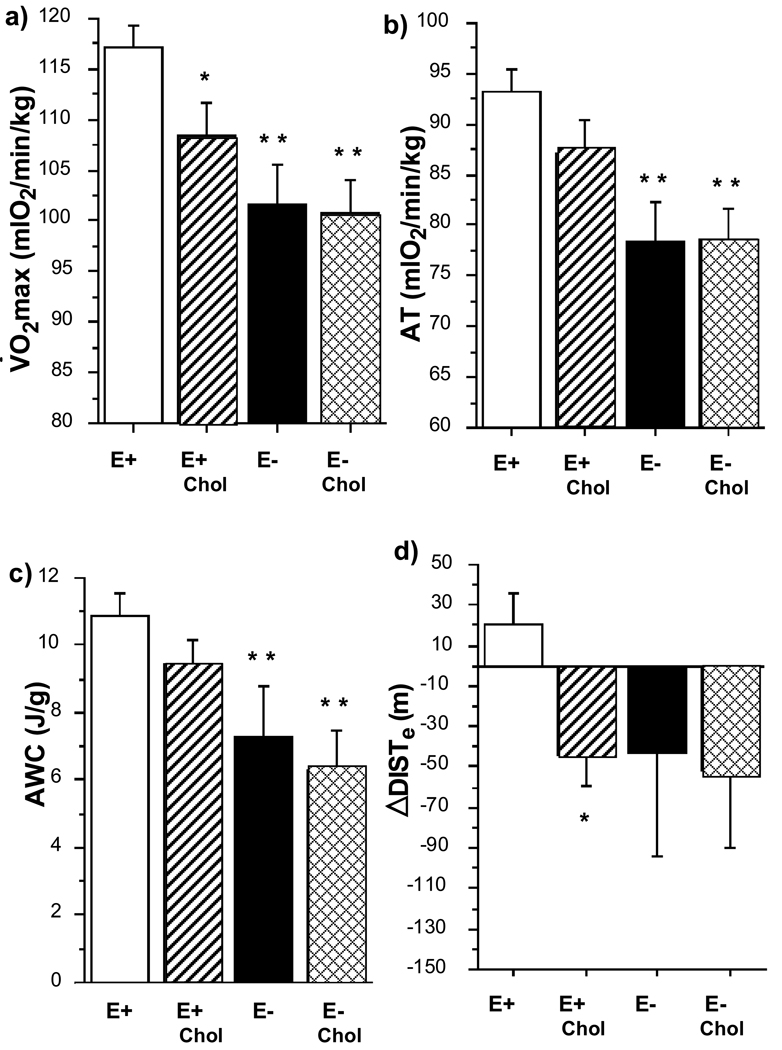
At 12 weeks, the Vo2max of the 3 hypercholesterolemic groups was significantly less than that of E+ (p < .001) and decreased across groups with increasing cholesterol levels (Figure 3). Similar inverse trends across groups were observed with AT and AWC with the values of the E− groups being significantly lower than that of E+ (p < .005 and p < .01 respectively). As the mice aged from 8 weeks to 12 weeks, the E+ group improved in running distance (ΔDISTe) while the other three groups demonstrated a progressive decline in running distance with age.
Figure 3.
Bar graphs of exercise indices of 4 cholesterol groups at 12 weeks. a) Vo2max, b) AT, c) ΔDISTe and d) AWC of E+ (n = 32, open bars), E+chol (n = 22, striped bars) and E− (n = 14, solid bars) and E−chol (n = 9, cross-hatched bars). Expressed in mean ± SE. * p < .05 and ** p < .01 vs. E+. Numbers over bars = p value vs. E+ by ANOVA.
Independence of Association of Hypercholesterolemia with Aerobic Capacity
We examined several known physical and biochemical determinants of aerobic capacity in comparison to total serum cholesterol of the mice at 12 weeks of age. These determinants included body weight, cardiac mass, and oxidative capacity (Table 2). We also examined the confounding variables of genetic strain and diet. Only the E−chol group demonstrated a significantly higher average body weight than the other groups at 12 weeks of age (p < .001). Similarly, only the E− demonstrated significantly greater cardiac mass when indexed to body weight (p < .005). There were no differences between groups with respect to citrate synthase activity. By multivariate analysis, both total serum cholesterol and body weight demonstrated inverse relationships with the indices of exercise capacity. Weak correlations were observed with strain, cardiac mass and diet. However, after adjusting for serum cholesterol level, differences in indices of exercise capacity between groups disappeared indicating that, of these variables, total serum cholesterol level was the strongest predictor of aerobic exercise capacity.
Table 2.
Physical, & Biochemical Characteristics of 12 Week Old Mice
| Measurement | E+ (n = 32) |
E+chol (n = 22) |
E− (n = 15) |
E−chol (n = 10) |
|---|---|---|---|---|
| Body Weight (g) | 22.4 ± 0.5 | 21.9 ± 0.5 | 22.9 ± 0.8 | 26.5 ± 0.6** |
| Total Cholesterol (mg/dl) | 153 ± 21 | 306 ± 40 | 1175 ± 107** | 2154 ± 223** |
| HDL Cholesterol § | 60 ± 21 | 80 ± 19 | 17 ± 9 | 38 ± 17 |
| Cardiac Mass Index (mg heart/g body) | 5.3 ± .2 | 5.0 ±.2 | 6.3 ± .3 ** | 5.5 ± .1 |
| Arteriolar density (#/mm2) | 102 ± 3 | 99 ± 10 | ||
| Capillary density (#/mm2) | 690 ± 114 | 661 ± 122 | ||
| Citrate Synthase (µg/min/g muscle) | 34 ± 1 | 34 ± 1 | 32 ± 1 | 34 ± 2 |
| Basal Urinary NOx (pM/mg creatinine) | 279± 65 | 289± 51 | 187 ± 26 | 200 ± 40 |
| Post-Exercise Urinary NOx (pM/mg creatinine) | 429 ± 105 | 354 ± 40 | 165 ± 20 ** | 140 ± 40 ** |
| Aortic NOxActivity (pM/mm aorta) | 1139 ± 253 | 937 ± 104 | 419 ± 106 | 532 ± 84 |
| RQe | 1.00 ± .01 | 0.99 ± .02 | 1.00 ± .02 | 0.99 ± .01 |
Values are mean ± SE.
p < .05,
p < .01 vs. E+,
based on smaller n
Mechanisms of Aerobic Exercise Impairment by Hypercholesterolemia
Effect on endothelial function
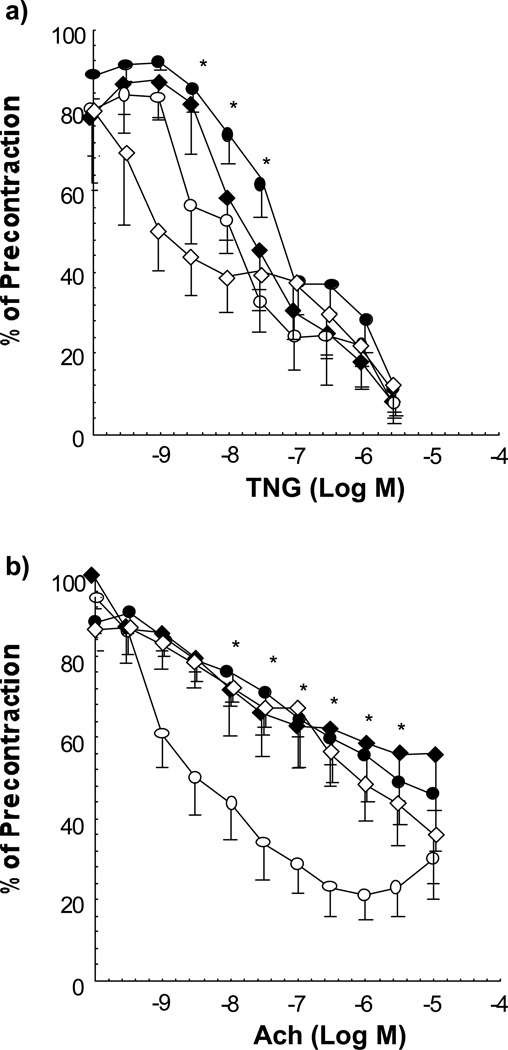
1) Vascular reactivity: The response to endothelium-independent vasorelaxation to NTG of aortic rings was similar in all groups at 12 weeks of age except for an attenuation in E− at three intermediate doses of NTG (p = 0.01 by MANOVA, Figure 4). In contrast, endothelium-dependent vasodilation to ACh was significantly impaired in all three of the hypercholesterolemic groups compared with E+ (p < 0.0001 by MANOVA). 2) EDNO activity: There was an inverse trend between aortic NOx activity and cholesterol level (r = −.32, p = .06) as well as a direct correlation of aortic NOx activity with measures of aerobic capacity; ΔDISTe (r= .55, p < .01) and AT (r = .43, p < .05). Likewise, there was a trend toward a decline across all 4 groups in basal urinary NOx excretion which became more pronounced in the post-exercise measures.
Figure 4.
Vascular reactivity studies represented by concentration-effect curves of rings of aortae from 12 week old mice. a) endothelium-independent vasorelaxation in response to NTG of segments of aorta from E+ (n = 6, open circles), E+chol (n = 6, open diamonds), E− (n = 6, filled circles) and E−chol (n = 9, solid diamonds). The E− curve demonstrates significant attenuation of vasorelaxation at the middle three doses. This effect was not observed in the other two high cholesterol groups. b) Concentration-effect curves demonstrating endothelium-dependent vasorelaxation in response to ACh. The curves of the three high cholesterol groups demonstrate significant attenuation of relaxation by MANOVA. * P < 0.05 vs. E+ at the same dose.
Effect on cardiac function, angiogenesis and oxidative capacity
To exclude myocardial injury as a mechanism for reduced aerobic capacity, myocardial sections were examined by light microscopy. No histologic evidence for myocardial ischemia, injury or necrosis was found in either the E+ or E− groups at 20 weeks of age. We also considered an effect of hypercholesterolemia on developmental angiogenesis and oxidative capacity of skeletal muscles. There were no differences in arteriolar or capillary density or in citrate synthase levels however.
Discussion
The salient findings of this study are;
Aerobic dysfunction is observed in murine models of genetic and diet induced hypercholesterolemia
The aerobic dysfunction is associated with a disturbance of endothelium-derived nitric oxide activity and vasodilatory function whereas no evidence of disturbances in other determinants of aerobic capacity was found.
This study confirms our previous observation that lipid-induced reduction in exercise capacity is a true aerobic dysfunction. Indeed, 4 indices of exercise capacity which have an aerobic component; oxygen uptake, anaerobic threshold, overall work performance and running distance, all declined in proportion to the degree of hypercholesterolemia. This occurred whether the elevation in serum cholesterol was genetically determined or diet-induced.
To examine if factors other than serum cholesterol might be contributing determinants of impaired aerobic capacity, we examined the contribution of several known physical and biochemical determinants of exercise capacity with our measures of aerobic capacity. Body weight, cardiac mass and heart volume have all been shown to correlate with aerobic capacity (17–19). In our model, only the E−chol group demonstrated a significantly higher average body weight and cardiac mass (when indexed to body weight) than the other groups. However, an increase in cardiac mass would be expected to augment cardiac performance and aerobic capacity rather than reduce it as occurred in the E−chol group. Oxidative enzyme activity in running muscle is also a known determinant of aerobic capacity. Oxidative enzyme capacity is commonly measured by assessing mitochondrial citrate synthase activity. Mitochondrial citrate synthase is a highly inducible enzyme that is upregulated with other mitochondrial enzymes in trained skeletal muscle mitochondria (20). However, in our study there were no significant differences in running muscle citrate synthase activity amongst any of the groups. By multivariate analysis, effects of genetic strain, diet, body weight and cardiac mass were not significant determinants of aerobic capacity when total serum cholesterol was included in the analysis. Taken together, these data suggest that total serum cholesterol level is the strongest determinant of impaired aerobic capacity in our model. However, one limitation of this study is that two different diets are typically employed to induce hypercholesterolemia in wild-type and apoE deficient mice. Accordingly, it is possible that other components of the two diets may have contributed to the observed impairments in aerobic capacity and endothelial function in the hypercholesterolemic mice.
We investigated potential mechanisms by which hypercholesterolemia might adversely affect exercise capacity. Hypercholesterolemia can potentially affect any or all of the three major components of aerobic capacity (functional capacity, transport capacity and oxidative capacity (19)). One of these components, oxygen transport capacity, is perhaps most limited by vascular conduction and distribution of blood flow (21,22). Our finding of reduced EDNO activity as measured by aortic and urinary NOx production, and attenuated endothelium-dependent vasodilation coupled with our previous findings of attenuated exercise hyperemia in hypercholesterolemic mice supports a disturbance in this component (7). One limitation of our approach was that aortic vascular reactivity and aortic NO release were measured in different segments of the aorta, the thoracic and abdominal aorta specifically, regions of the aorta that are known to have differences in vascular response.
Oxygen transport capacity can also be limited by blood vessel density. Vessel density might be altered in development by a depression in angiogenesis, which is modulated by EDNO activity (23). Indeed, E− mice have been shown to have an age-dependent impairment in angiogenesis in response to surgically-induced ischemia (24). Indeed, we have shown that the impaired angiogenesis in response to hindlimb ischemia is a function of lipid-induced elevations in plasma levels of ADMA, the endogenous NOS inhibitor (8). Hypercholesterolemia could act by inhibiting angiogenesis in response to training. However, at a time when there was significant impairment of aerobic capacity (12 weeks), there was no significant reduction in microvascular density in the hypercholesterolemic mice. It is of course possible that such a deficiency develops at a later time course.
Hypercholesterolemia may also affect the functional capacity of the cardiovascular system by causing cardiac ischemia and dysfunction, particularly during intense exercise. However, other investigators have demonstrated that hemodynamically significant disease in the coronary arteries of E− mice is not observed at this age (16). Indeed, we observed no histological evidence for ischemic insults to the myocardium.
Finally, hypercholesterolemia could alter oxidative capacity by effecting nitric oxide activity within the skeletal myocyte. Nitric oxide modulates mitochondrial respiration via the inhibition of cytochrome c oxidase, the terminal component, and rate-limiting step of the electron transport chain (25). However, a reduction in skeletal muscle nitric oxide activity would increase oxygen utilization as observed ex vivo (26) or down-regulate oxidative enzymes such as citrate synthase. Our data does not support a lipid-induced disinhibition of mitochondrial respiration as the hypercholesterolemic mice had reduced VO2max.
Based on these findings as well as those from our previous report, we conclude that hypercholesterolemia adversely affects aerobic capacity by impairing EDNO synthesis resulting in depressed vascular conductance and distribution (i.e. oxygen transport capacity). It is well documented that hypercholesterolemia impairs endothelial vasodilator function in the microvasculature as well as the conduit vessels (27–30). These alterations could disrupt the normal mechanisms that redistribute blood flow to exercising muscle (31). That depressed EDNO activity attenuates exercise-induced hyperemia is controversial. In two studies, administration of NOS inhibitors did not affect exercise-induced hyperemia in the human forearm, nor hyperemia in response to electrically stimulated contractions in dogs (32,33). However, others have reported a diminution of exercise hyperemia in the human forearm and in certain rat hindlimb muscles with infusion of NOS inhibitor (34,35). Still other studies suggest that EDNO is involved at low-intensity but not at high intensity exercise (36) or only partly responsible for exercise hyperemia (37).
An interdependence of physical performance, cholesterol fractions and coronary heart disease risk has been observed in multiple clinical studies. Notable is the direct relationship of Vo2max and exercise endurance with HDL cholesterol (38,39), ApoA-1 (40) and HDL/LDL ratio (41) and the inverse relationship with total cholesterol (38) and plasma triglyceride levels (42). In all of these studies, the investigators assumed that Vo2max or exercise endurance reflected the subjects’ level of physical activity and conditioning and that serum cholesterol was a dependent variable in this relationship. They concluded that, as a consequence of greater energy expenditure, physically active individuals have lower cholesterol levels. Whereas this may be true in part, our studies suggest that the causality of the relationship may be ambiguous. Indeed, elevated levels of serum cholesterol appear to reduce Vo2max. In this scenario, Vo2max is not only determined by physical activity habits, but also by serum cholesterol and its effects upon endothelial function.
Our observations take on greater clinical relevance with the recent finding that treatment with HMG coA-reductase inhibitors can improve treadmill exercise time in patients with peripheral arterial disease (43,44). In these trials, the improvement in treadmill exercise time was associated with a reduction in total and LDL-cholesterol. The beneficial effect of statins on walking distance in PAD is not likely due to a hemodynamic effect related to the regression of lesions. The effect of statins on conduit vessel luminal area is quite modest (45). Furthermore, the improvement in walking time occurred in the absence of any increase in ankle-brachial index in the treated patients (44). Accordingly, it seems plausible that the reduction of cholesterol in these studies, combined with lipid-independent effects of statins on the NO synthase pathway (46), may have improved vascular reactivity of the conduit, collateral and/or arteriolar vessels sufficiently to improve limb blood flow and oxygen transport capacity.
To conclude, hypercholesterolemia is associated with an aerobic dysfunction in mice as assessed by several indices of exercise performance. A lipid-induced defect in the NOS pathway may impair exercise capacity by reducing oxygen transport capacity. The impairment of exercise capacity is observed in diet-induced as well as genetically determined hypercholesterolemia. These studies may provide insight into recent clinical trials showing a benefit of statins on exercise capacity in patients with peripheral arterial disease.
Acknowledgments
This study was supported by grants to Dr. Cooke from the National Heart, Lung and Blood Institute (1RO1-HL-58638, R01 HL-63685, R01 AT/HL00204 and P01 AG18784), and was done during the tenure of a Grant-in-Aid Award from the American Heart Association and Sanofi Winthrop. Dr. Maxwell was a recipient of a Bugher Foundation Fellowship of the American Heart Association. Dr. Niebauer was a recipient of a stipend award from the Deuche Forschungsgemeinschaft, Bonn, Germany (Ni 456/1-1). The authors thank Dr. Margaret Billingham for her review of the pathologic specimens and appreciate the advice of Dr. Edward Rubin of University of California at Berkeley.
Abbreviations
- E+
wild type mice on a regular chow diet
- E+chol
wild type mice on a Thomas-Hartroft high cholesterol diet
- E−
apoE deficient mice on a regular chow diet
- E−chol
apoE deficient mice on a high fat diet
- Vo2max
Maximal oxygen uptake
- Vco2
Rate of carbon dioxide exhaled
- AT
Anaerobic threshold
- DISTe
Distance run to exhaustion
- ΔDISTe
Change in distance run to exhaustion from 8 weeks
- AWC
Aerobic work capacity
- RQ
Respiratory quotient
- NTG
Nitroglycerin
- PSS
Physiologic saline solution
- NOx
Nitrogen oxide (NO2 and NO3)
- EDNO
Endothelium-derived nitric oxide
Contributor Information
Andrew J. Maxwell, Program in Vascular Medicine and Biology, Division of Cardiovascular Medicine, Stanford University.
Josef Niebauer, Program in Vascular Medicine and Biology, Division of Cardiovascular Medicine, Stanford University
Patrick S. Lin, Program in Vascular Medicine and Biology, Division of Cardiovascular Medicine, Stanford University
Philip S. Tsao, Program in Vascular Medicine and Biology, Division of Cardiovascular Medicine, Stanford University
Daniel Bernstein, Program in Vascular Medicine and Biology, Division of Pediatric Cardiology, Stanford University.
John P. Cooke, Program in Vascular Medicine and Biology, Division of Cardiovascular Medicine, Stanford University.
References
- 1.Jayakody RL, Senaratne MP, Thomson AB, Kappagoda CT. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can J Physiol Pharmacol. 1985;63:1206–1209. doi: 10.1139/y85-199. [DOI] [PubMed] [Google Scholar]
- 2.Guerra R, Jr, Brotherton AF, Goodwin PJ, Clark CR, Armstrong ML, Harrison DG. Mechanisms of abnormal endothelium-dependent vascular relaxation in atherosclerosis: implications for altered autocrine and paracrine functions of EDRF. Blood Vessels. 1989;26:300–314. doi: 10.1159/000158779. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Bossaller C, Cartwright J, Jr, Henry PD. Videomicroscopic demonstration of defective cholinergic arteriolar vasodilation in atherosclerotic rabbit. J Clin Invest. 1988;81:1752–1758. doi: 10.1172/JCI113516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RA, Zitnay KM, Haudenschild CC, Cunningham LD. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DG, Ohara Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: implications for impaired vasomotion. Am J Cardiol. 1995;75:75B–81B. doi: 10.1016/0002-9149(95)80018-n. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell AJ, Ho HV, Le CQ, Lin PS, Bernstein D, Cooke JP. L-arginine enhances aerobic exercise capacity in association with augmented nitric oxide production. J Appl Physiol. 2001;90:933–938. doi: 10.1152/jappl.2001.90.3.933. [DOI] [PubMed] [Google Scholar]
- 8.Jang JJ, Ho HK, Kwan HH, Fajardo LF, Cooke JP. Angiogenesis is impaired by hypercholesterolemia: role of asymmetric dimethylarginine. Circulation. 2000;102:1414–1419. doi: 10.1161/01.cir.102.12.1414. [DOI] [PubMed] [Google Scholar]
- 9.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990;10:316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- 11.Tsao PS, McEvoy LM, Drexler H, Butcher EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation. 1994;89:2176–2182. doi: 10.1161/01.cir.89.5.2176. [DOI] [PubMed] [Google Scholar]
- 12.Heinegard D, Tiderstrom G. Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta. 1973;43:305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 13.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 14.Srere PA. Citrate Synthase. Methods Enzymology. 1969;13:3–5. [Google Scholar]
- 15.el-Rimawi AS, James NT. Quantitative morphometric analyses of the exercised extensor digitorum longus muscle of the mouse. Acta Anat. 1989;134:191–198. doi: 10.1159/000146686. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 17.Bergh U, Sjodin B, Forsberg A, Svedenhag J. The relationship between body mass and oxygen uptake during running in humans. Med Sci Sports Exerc. 1991;23:205–211. [PubMed] [Google Scholar]
- 18.Astrand PO, Rodahl K. Textbook of Work Physiology. 2nd ed. New York: McGraw-Hill; 1977. [Google Scholar]
- 19.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 20.Chi MM, Hintz CS, Coyle EF, et al. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983;244:C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 21.Barclay JK, Stainsby WN. The role of blood flow in limiting maximal metabolic rate in muscle. Med Sci Sports. 1975;7:116–119. [PubMed] [Google Scholar]
- 22.di Prampero PE. An analysis of the factors limiting maximal oxygen consumption in healthy subjects. Chest. 1992;101:188S–191S. doi: 10.1378/chest.101.5_supplement.188s. [DOI] [PubMed] [Google Scholar]
- 23.Murohara T, Asahara T, Silver M, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 25.Brown G. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Letters. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 26.King CE, Melinyshyn MJ, Mewburn JD, et al. Canine hindlimb blood flow and O2 uptake after inhibition of EDRF/NO synthesis. J Appl Physiol. 1994;76:1166–1171. doi: 10.1152/jappl.1994.76.3.1166. [DOI] [PubMed] [Google Scholar]
- 27.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohara Y, Petersen TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. Journal of Clinical Investigation. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 30.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 31.Line PD, Kvernebo K, Helgerud J, Ingjer F. Aerobic endurance, anatomical factors and time properties of laser Doppler recorded skin postocclusive hyperaemia. Eur J Appl Physiol. 1992;64:508–512. doi: 10.1007/BF00843759. [DOI] [PubMed] [Google Scholar]
- 32.Persson MG, Gustafsson LE, Wiklund NP, Hedqvist P, Moncada S. Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. Br J Pharmacol. 1990;100:463–466. doi: 10.1111/j.1476-5381.1990.tb15829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson JR, Kapoor S. Contribution of endothelium-derived relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol. 1993;75:2740–2744. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]
- 34.Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol (Lond) 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- 36.O'Leary DS, Dunlap RC, Glover KW. Role of endothelium-derived relaxing factor in hindlimb reactive and active hyperemia in conscious dogs. Am J Physiol. 1994;266:R1213–R1219. doi: 10.1152/ajpregu.1994.266.4.R1213. [DOI] [PubMed] [Google Scholar]
- 37.Hussain SN, Stewart DJ, Ludemann JP, Magder S. Role of endothelium-derived relaxing factor in active hyperemia of the canine diaphragm. J Appl Physiol. 1992;72:2393–2401. doi: 10.1152/jappl.1992.72.6.2393. [DOI] [PubMed] [Google Scholar]
- 38.Abbott RD, Levy D, Kannel WB, et al. Cardiovascular risk factors and graded treadmill exercise endurance in healthy adults: The Framingham Offspring Study. Am J Cardiol. 1989;63:342–346. doi: 10.1016/0002-9149(89)90343-3. [DOI] [PubMed] [Google Scholar]
- 39.Berg A, Keul J, Ringwald G, Deus B, Wybitul K. Physical performance and serum cholesterol fractions in healthy young men. Clinica Chimica Acta. 1980;106:325–330. doi: 10.1016/0009-8981(80)90317-4. [DOI] [PubMed] [Google Scholar]
- 40.Haskell WL. Exercise-induced changes in plasma lipids and lipoproteins. Prev Med. 1984;13:23–36. doi: 10.1016/0091-7435(84)90038-0. [DOI] [PubMed] [Google Scholar]
- 41.Schnabel A, Kindermann W. Effect of maximal oxygen uptake and different forms of physical training on serum lipoproteins. Eur J Appl Physiol. 1982;48:263–277. doi: 10.1007/BF00422987. [DOI] [PubMed] [Google Scholar]
- 42.Andersen LB, Haraldsdottir J. Tracking of cardiovascular disease risk factors including maximal oxygen uptake and physical activity from late teenage to adulthood. An 8-year follow-up study. J Intern Med. 1993;234:309–315. doi: 10.1111/j.1365-2796.1993.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 43.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–1486. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 44.Mondillo S, Ballo P, Barbati R, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–364. doi: 10.1016/s0002-9343(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 45.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781–1791. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- 46.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]