Abstract
Background
Hip fracture affects more than 1.6 million persons worldwide and causes substantial changes in body composition, function, and strength. Usual care (UC) has not successfully restored function to most patients, and prior research has not identified an effective restorative program. Our objective was to determine whether a yearlong home-based exercise program initiated following UC could be administered to older patients with hip fracture and improve outcomes.
Methods
A randomized controlled trial of 180 community dwelling female patients with hip fracture, 65 years and older, randomly assigned to intervention (n=91) or UC (n=89). Patients were recruited within 15 days of fracture from 3 Baltimore-area hospitals from November 1998 through September 2004. Follow-up assessments were conducted at 2, 6, and 12 months after fracture. The Exercise Plus Program was administered by exercise trainers that included supervised and independently performed aerobic and resistive exercises with increasing intensity. Main outcome measures included bone mineral density of the contralateral femoral neck. Other outcomes included time spent and kilocalories expended in physical activity using the Yale Physical Activity Scale, muscle mass and strength, fat mass, activities of daily living, and physical and psychosocial functioning. The effect of intervention for each outcome was estimated by the difference in outcome trajectories 2 to 12 months after fracture.
Results
More than 80% of participants received trainer visits, with the majority receiving more than 3 quarters (79%) of protocol visits. The intervention group reported more time spent in exercise activity during follow-up (P<.05). Overall, small effect sizes of 0 to 0.2 standard deviations were seen for bone mineral density measures, and no significant patterns of time-specific between-group differences were observed for the remaining outcome measures.
Conclusion
Patients with hip fracture who participate in a yearlong, in-home exercise program will increase activity level compared with those in UC; however, no significant changes in other targeted outcomes were detected.
Hip fracture is a common problem with devastating consequences. At present, more Than 310 000 hip fractures occur annually in the United States,1 with an estimated cost of between $14 and $20 billion.2-7 By 2050, the number of hip fractures is expected to in crease to 700 000 in the United States and almost to 4 million worldwide.8 Between 16% and 32% of patients die with in a year.9-12 Among survivors, 50% need assistance to walk and 90% need assistance to climb stairs after 1 year.13 Furthermore, there are substantial changes in body composition;including loss of bone mineral density (BMD) of 4% to 7% per year, loss of lean body mass up to 6% within 2 months, and increase in fat mass of 3% to 4% in a year.13-16
On the basis of these findings under conditions of usual hip fracture care, it is important to identify novel interventions that older patients will comply with to ameliorate significant postfracture changes in order for gains to be realized beyond the 6-month recovery plateau observed for most functioning after hip fracture.17 Exercise is generally well tolerated by older adults after hip fracture with few serious adverse events18-20 and has the potential for increasing BMD and strength. Research has shown that weight-bearing, aerobic-type exercises alone or in combination with resistance exercises can slow or halt the rate of BMD loss in postmenopausal women.21-26 Unfortunately, the success of exercise in hip fracture rehabilitation, specifically, has varied. It is notable that few previous programs with this patient group lasted more than a few months,19,20,27 most began late in the recovery period,18,28 and with few exceptions19,27,29-31 most were gym or clinic based, rather than home based, which can limit access. Therefore, we designed a yearlong exercise program that could be delivered in patients’ homes in order to overcome the limitations of an extended center-based exercise program.
This study was designed to test the feasibility and identify preliminary indications of efficacy of the Exercise Plus Program, an aerobic and resistive exercise program administered after fracture by an exercise trainer in the home setting.32 We hypothesized that those randomized to the intervention, compared with those randomized to usual care (UC), would experience reduced losses in BMD, muscle mass, and strength; less increase in fat mass; greater increases in physical activity; improvements in ability to carry out physical and instrumental tasks of daily living; and increases in physical and psychosocial functioning.
METHODS
STUDY DESIGN
This randomized clinical trial (RCT) assigned patients with hip fracture to 1 of 2 groups: home exercise program continuing for 1 year after fracture (Exercise Plus Program) or UC.32 Women with hip fracture were recruited from 3 hospitals in the Baltimore Hip Studies (BHS) network between November 1998 and September 2004. Patients were screened for eligibility within 15 days of the fracture; those meeting entry criteria had dual energy x-ray absorptiometry (DXA) and additional measurements within 22 days of fracture. Participants completing at least 80% of the baseline survey were randomized. Follow-up data were collected at 2, 6, and 12 months after fracture. Measurement of outcomes was assessed at 2, 6, and 12 months after fracture to ensure comparability with other BHS studies on the natural history of recovery. Participants were called monthly to ascertain information on health care use and adverse events. Institutional review board approvals were obtained from the University of Maryland School of Medicine and the 3 participating hospitals; enrolled participants provided their own informed consent. A Data and Safety Monitoring Board met quarterly to review adverse events and safety reports.
PARTICIPANTS
A total of 180 community-dwelling women 65 years or older admitted within 72 hours of a nonpathological hip fracture receiving surgical repair were enrolled within 15 days of the fracture. Eligibility was determined through medical chart review, medical assessment, and cognitive screen. Exclusions included pathologic fracture, cardiovascular, neurologic, and respiratory diseases that could interfere with exercising independently at home, diseases of the bone (eg, Paget disease, osteomalacia), metastatic cancer, cirrhosis, end-stage renal disease, hardware in the contralateral hip, and conditions that increase risk of falling while exercising independently. Participants also had to be walking without human assistance prior to the fracture, cognitively intact (ie, score ≤20 on the Folstein Mini-Mental State Examination),33 and receive orthopedic surgeon clearance to participate. Patients enrolled were randomly assigned to receive a home exercise intervention or UC.
STUDY GROUPS
Participants in both groups were provided information on bone health and management of osteoporosis. Their baseline DXA scan was sent to their primary care provider along with information on interpreting results and the National Osteoporosis Foundation recommendations for treatment of osteoporosis.34,35 Participants were encouraged to discuss results with their physicians.
In-Home Exercise Intervention
The Exercise Plus Program consisted of an exercise component and a self-efficacy based motivational component to help optimize program adherence throughout the 12-month intervention.36 The Exercise Plus Program, as described elsewhere,32 was initiated at completion of skilled rehabilitation services by exercise trainers in participant’s postacute discharge location with more frequent supervised sessions early in the intervention to ensure safety and build independence. Participants started the program receiving 3 trainer-supervised exercise sessions per week during the first 2 months and then received 2 supervised sessions per week for 2 months. The frequency dropped to once a week and then once every other week for a maximum of 56 supervised sessions by the end of the protocol. The trainer supplemented the decrease in supervised sessions with telephone calls to remind participants to exercise and address any questions or concerns.
The exercise sessions combined aerobic exercise using a Stairstep,32 a comprehensive strengthening program that covered the main muscle groups relevant to hip fracture recovery, and stretching exercises (20- to 30-minute warm-up and cool down periods). Participants were expected to perform aerobic activity at least 3 days per week and strength training 2 days per week for 30 minutes. Each participant started at her own individual level with regard to time spent in aerobic activities and amount of repetitions and resistance used in the strengthening program but was advanced to a higher level according to a standard protocol.32 Participants were assessed at the initial visit to identify an appropriate exercise prescription tailored to the individual’s medical status and level of conditioning.
Strength training included a series of 11 exercises for the upper and lower extremities using Thera-Band products (The Hygenic Corporation, Akron, Ohio) and/or ankle and wrist cuff weights. The duration of each exercise was increased until the participant could do 3 sets of 10 repetitions on both sides. Intensity was then augmented by increasing the resistance of exercise bands and/or adding ankle and wrist cuff weights.32 Duration of aerobic stepping was increased with the goal of completing 30 minutes of continued stepping. Once participants could perform 20 minutes, light ankle weights were added to increase exercise intensity.
Usual Care
The UC group received the physician-prescribed postfracture standard of care for patients with hip fracture in the greater Baltimore, Maryland, region at the time of study, which included relatively short hospital stays and approximately 2 to 4 weeks of rehabilitation.
TREATMENT ASSIGNMENT
PC-PLAN software37 was used to randomize participants within blocks of 2 or 10 patients for each study hospital, depending on estimated number of admissions, to eliminate between-hospital differences and ensure that each hospital is equally represented in the same frequency across the 2 study groups. Randomization occurred a mean (standard deviation [SD]) of 11 (4.5) days following fracture, which was prior to discharge to the community for almost all participants. The study coordinator then contacted participants by telephone to explain their group assignment. Those in the exercise group were told that an exercise trainer would be calling within 2 days to schedule the first home visit. Study staff conducting assessments were blind to group assignment.
MEASURES
Outcome Measures
Outcomes were assessed at 2, 6, and 12 months after hip fracture; most were also obtained prior to randomization with reference to the current, in-hospital, or prefracture period. Measures were collected on-site at the time of BMD testing at participating hospitals; when this was not possible or additional sessions were needed, participants were measured in their own residence.
Primary Outcome—BMD
Bone mineral density at the contralateral femur was measured using DXA (models QDR-1000 W, QDR-1500; Hologic, Waltham, Massachusetts). Each participant’s follow-up DXA was obtained on the same machine as her prerandomization assessment; daily calibration of DXA machines prevented significant drift in measurements over time.
Secondary Outcomes
Whole-body DXA was performed to measure total lean body mass and fat mass. Physical activity was assessed using the Yale Physical Activity Scale.38 Both hours spent exercising and kilocalories (participation in hours per week multiplied by an intensity code [kilocalories per hour]) expended in a week were calculated. The 6-minute walk test assessed maximal distance walked in 6 minutes on an indoor course.39,40 Lower extremity performance was measured with the Lower Extremity Gain Scale that evaluated performance of 9 lower-extremity tasks considered important for patients with hip fracture and their care providers.41 A single chair rise time and a timed walk were excerpted from the Lower Extremity Gain Scale and analyzed separately. A measure of gait and global balance, developed for older patients with hip fracture was used.42,43 Grip strength was measured with the JAMAR hand-held dynamometer (JAMAR Technologies Inc, Hatfield, Pennsylvania).44,45 Lower Extremity Physical Activities of Daily Living were assessed using a modified form of the Functional Status Index,46 revised to specifically address functional issues relevant to patients after hip fracture.17 Instrumental Activities of Daily Living were obtained using a modified version of the Older Americans Resources and Services Instrument,47 which asks about performance of 7 tasks. The Geriatric Depression Scale was used to assess participants for depressive symptoms,48-50 and health-related quality of life was assessed using the 36-Item Short Form Health Survey (SF-36).51
Demographic information and medical history, fracture type, and course of hospitalization were obtained by abstracting medical charts. Monthly telephone calls were made to participants to ascertain falls and resultant injuries, outpatient and emergency department visits, hospitalizations, and deaths.
STATISTICAL ANALYSIS
The intention-to-treat principle was followed in analyses to assess the effect of intervention on outcome (ie, all randomized participants were included using available data). The completed trial had from 50 to 70 participants per arm with usable data at 12-month follow-up, depending on the outcome measurement. For tests on the between-arm change from baseline at 12 months after fracture, effect sizes ranging from 0.37 to 0.43 SDs were detectable with 80% power at the 5% significance level, 2-tailed (within-subject correlation was on the order of 0.7 for most measurements). Thus, the detectable effect sizes are considered smaller than medium effect sizes according to Cohen.52
Generalized estimating equations53 were used to perform repeated measures analyses. Model terms included an indicator variable for the intervention and indicator variables for 2-, 6-, and 12-month time points with the prerandomization baseline serving as the reference. For outcomes without baseline measurement, models were revised to provide estimates for the 3 follow-up time points only. Time-by-treatment interaction terms were included in the longitudinal model. This model was used to estimate the mean and standard error of the outcome measure at each time point for each of the 2 groups. Robust standard error estimates were obtained using a technique described by Huber.54
The effect of intervention for each outcome was estimated by the difference in mean changes from baseline during 2 to 12 months after fracture. This approach corrected for between-group differences at baseline. A global P value for the differences in postran-domization changes between groups was obtained from a test of the null hypothesis that all the treatment by time interaction co-efficients were simultaneously zero. Time-specific differences were transformed to standardized effect sizes by dividing them by the square roots of the deviance dispersion (a within-group SD) obtained from fitting the generalized estimating equation model. P<.05 was considered statistically significant.
To investigate potential bias due to missing data, sensitivity analyses were performed using weighted estimating equations.55 Weights were the inverse estimated probabilities of being observed at each visit derived from a logistic regression of observed status (yes/no) on time, group, their interaction, and selected baseline factors. Results from generalized estimating equations are only unbiased when data are missing completely at random in the sense of Robins et al55 and Rubin.56
RESULTS
A total of 1276 female patients with hip fracture were screened during the study period (Figure 1). Of these, 243 (19%) were eligible and 180 (74%) were randomized: 91 to intervention and 89 to UC group. The 4 most common reasons for ineligibility were prefracture nursing home residency (24%), prefracture dementia or scoring below 20 on the Mini-Mental State Examination within 15 days after fracture (13%), chronic atrial fibrillation or other cardiac arrhythmia (12%), and having hardware in the contralateral hip (10%).
Figure 1.
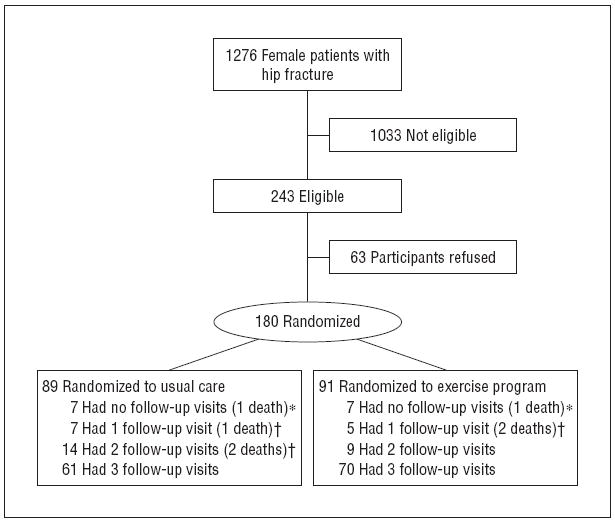
Follow-up data available over 12 months by group. *Patient died before any follow-up visits. †Patient died after providing follow-up data.
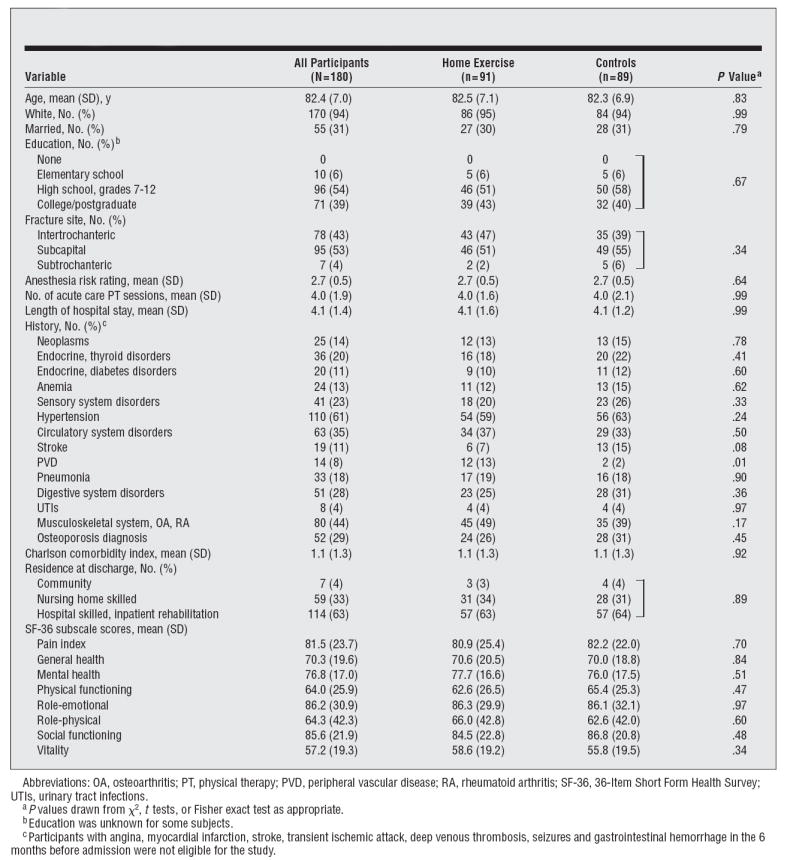
The mean (SD) age was 82.4 (7.0) years (Table1). More than 94% of the participants were white, 31% were married, and 73% had at least a high school education. Participants had a mean (SD) length of stay in acute care of 4.1 (1.4) days and had a mean (SD) of 1.1 (1.3) comorbidities. Prior to the fracture, the lowest scores on the SF-36 were reported for the vitality (57.2), physical functioning (64.0), and role-physical (64.3) subscales, while reported scores were relatively high on general health (70.3) and mental health (76.8) subscales. Seven participants in each group (7.7%) provided no follow-up data; the remaining participants provided data for at least 1 follow-up visit.
Table 1.
Demographic and Medical Characteristics of the Sample
|
FEASIBILITY: DELIVERY OF PROGRAM
Of the 91 participants randomized to receive the exercise program, 75 (82%) were followed up by an exercise trainer; 16 (18%) refused to participate after being assigned to the intervention group. Only 1 person refused to participate after receiving a trainer visit. Of the intervention participants, 51 (56%) started exercising before their 2-month assessment. The mean time to initiate exercise was 67.8 days after fracture (range, 25-203 days). The mean number of visits for participants randomized to exercise was 36.2; however, those who agreed to trainer visits received a mean of 44 of 56 visits (79%) over the postfracture year.
Table 2 gives the proportion of participants affected by adverse events by group over the year. There were no statistically significant differences between groups in the adverse events monitored. There was only 1 treatment-related serious adverse event experienced while exercising in the intervention group; a participant fractured her ulna while doing a chest stretch during the warm-up exercise.
Table 2.
Participants Experiencing Adverse Events by Treatment Group
| Event | Exercise Group, No. (% With ≥1 Event) (n=91) | Control Group, No. (% With ≥1 Event) (n=89) | P Valuea |
|---|---|---|---|
| Falls | 31 (34) | 31 (35) | .91 |
| Outpatient visits | 81 (89) | 74 (83) | .26 |
| Hospital admissions | 21 (23) | 24 (27) | .55 |
| ED visits | 17 (19) | 12 (13) | .34 |
| Deaths | 3 (3) | 4 (4) | .72 |
Abbreviation: ED, emergency department.
P values drawn from χ2 tests.
Figure 2 shows the mean reported time spent engaged in exercise behavior per week, and the calculated number of kilocalories expended per week as an indication of participation in the exercise program. The intervention group reported more time spent exercising (Figure 2A): this amounted to 0.5 9 hours (95% confidence interval [CI], 0.15-1.33 hours) at 2 months after fracture; 0.77 hours (95% CI, 0.03-1.50 hours) at 6 months; and 0.68 hours (95% CI, 0.05-1.41 hours) at 12 months after fracture. The mean number of kilocalories expended while engaged in exercise behavior was greater in the intervention group at all follow-uptime points (Figure 2B): 184.5 kcal more (95% CI, 15.7-353.4 kcal) at 2 months; 249.1 kcal (95% CI, 54.5-443.6 kcal) at 6 months; and 169.9 kcal (95% CI, 31.9-371.6 kcal) at 12 months (global P=.03). Trends for total time and kilocalories spent in all physical activities were similar to the results for exercise behavior reported above, but the differences were smaller and not statistically significant (Figure 3).
Figure 2.
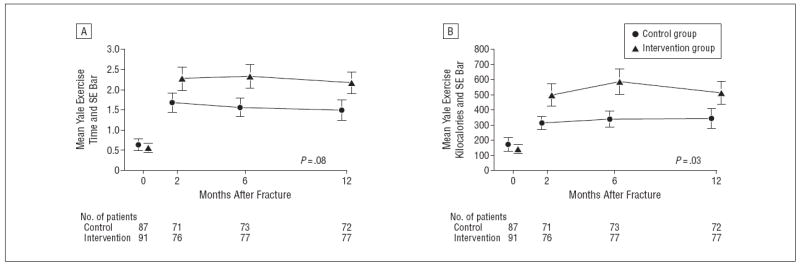
Yale exercise time in hours (A) and Yale exercise kilocalories (B) expended over time in the intervention and control groups. The P value indicated on the graph pertains to a test on the null hypothesis of no between-group difference in the 2- to 12-month trajectories.
Figure 3.
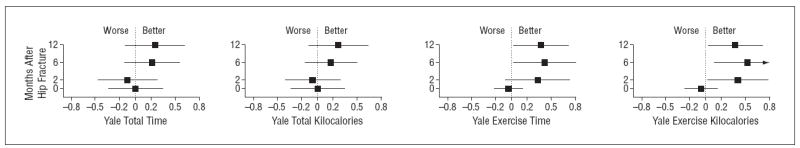
Effect of intervention on Yale physical activity measures. Data are given as standard effect size for exercise relative to control, with 95% confidence intervals.
EFFECTS OF INTERVENTION ON OUTCOMES
Postfracture differences in mean changes were examined for all study outcomes in the same manner as for time spent exercising; none of the differences comparing the exercise intervention and UC groups were statistically significant. The weighted effects estimation analyses (weighted estimating equation) provided no evidence for a systematic difference between patients responding (or measured) and those lost to follow-up. Time-specific between-group differences, expressed as standardized effect sizes for select longitudinal analyses, are shown in Figure 4.
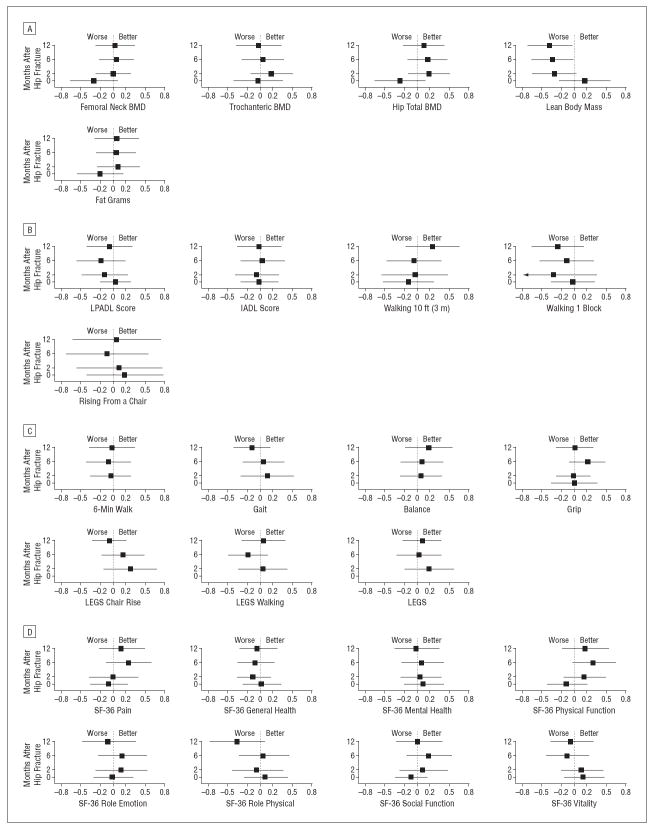
Figure 4.
Effect of intervention on bone and muscle (A), activities of daily living (B), performance measures (C), and 36-Item Short Form Health Survey (SF-36) subscales (D). Data are given as standard effect size for exercise relative to control, with 95% confidence intervals. BMD indicates bone mineral density; IADL, Instrumental Activities of Daily Living; LEGS, Lower Extremity Gain Scale; and LPADL, Lower Extremity Physical Activities of Daily Living.
Overall, small effect sizes of 0 to 0.2 SDs were seen for body composition measures including femoral neck, trochanteric and total hip BMD, and fat mass (Figure 4A). No pattern of time-specific between-group differences were observed for physical performance outcomes (Figure 4B), Lower Extremity Physical Activities of Daily Living or Instrumental Activities of Daily Living summary scores (Figure 4C), SF-36 subscales (Figure 4D), or Geriatric Depression Scale (P>.05).
PER-PROTOCOL ANALYSIS
Additional analyses were performed to assess any dose-response relationships comparing participants in the intervention group who received more than 43 visits from a trainer (the median number of visits received) vs those receiving 43 or fewer visits. No statistically significant differences were observed in patient characteristics or study outcomes (data not shown).
COMMENT
This study demonstrates the feasibility of delivering a year-long, home-based exercise intervention to older women with hip fractures. The data showed that more than 80% of participants allowed a trainer to come into their home to provide the intervention over a 12-month period and that the majority of participants received more than three-quarters (77%) of protocol visits. The limited number of treatment-related adverse events suggests that the intervention was safe and well-tolerated. Those assigned to the intervention reported more physical activity and exercise behavior than those in UC, and a greater amount of energy expended in these activities, particularly within the first 6 months. This is consistent with participants receiving approximately 85% of supervised sessions by 6 months after fracture according to the schedule of visits; however, there is evidence of a sustained effect of the remaining 15% of trainer visits between 6 and 12 months, with greater time in exercise reported at 12 months compared with 2 months.
Despite the effectiveness of the delivery system and the reported improvements in exercise and physical activity, the exercise intervention did not result in clinically important or statistically significant changes in targeted outcomes, including BMD, compared with controls. There are very few RCTs testing the impact of exercise interventions on BMD among older adults and even fewer among people after a hip fracture. A recent meta-analysis57 of 10 RCTs with postmenopausal women (n=595) reported a trend toward an increase in femoral neck BMD for exercising subjects compared with controls. However, studies with participants more similar to patients with hip fracture have shown no or only moderate improvements in BMD,58,59 even with higher intensity exercise.60,61 Studies that reported significant improvements in BMD included lower extremity exercises that would be contraindicated during fracture healing.62-64
Other studies of postfracture exercise for patients with hip fracture have shown that regular exercise (resistive and/or aerobic) can improve physical performance (eg, gait, strength),65 mobility,27,66 walking speed, and quadriceps strength.18 In a study of a center-based intervention initiated 6 months after a hip fracture, benefits in muscle strength, walking speed, balance, and physical performance were found.20 However, a Cochrane Review of 13 trials of 1065 patients comparing exercise interventions concluded that there was insufficient evidence to determine if physical intervention affected outcome after hip fracture.55 In a more recent study, participation in physical activity, particularly in the early postfracture period, was predictive of functional recovery.67
One of the challenges in comparing outcomes of prior studies to those in the present report is that studies have varied greatly with regard to program content and type of exercises (aerobic, resistance training, and functional training); delivery site (gym, clinic, or patient’s home); time of initiation after fracture; intensity and duration of program; level of supervision; background of trainer or therapist; subject selection; and frailty status of participants. Furthermore, the dose of the exercises tested in our program may have lacked intensity to improve functional tasks65 and lacked the specificity needed to translate into improvements in outcomes such as balance and rising from a chair. It is possible that our program may have proved efficacious if tested with a lower functioning group of patients. Likewise, it is possible that a more intense program that provided more weight-bearing exercises with increased loads, a higher level of resistive training for strengthening, and longer aerobic training beyond 6 months would have affected the primary and some of the secondary outcomes of interest in this higher-functioning group. There are preliminary data that indicate patients with hip fracture can tolerate more intensive exercise programs and that such a program can affect aspects of functioning.31
Strengths of the present program include that the intervention was delivered in participants’ homes and that they were visited by certified trainers early in the intervention period; this may have led to the high level of acceptance and the greater level of activity in the intervention group. Adherence to the program was high, despite the long duration and the total number of visits received by participants, suggesting that this program can increase independent exercise and enhance sustained adherence.
This study was restricted to a select group of female participants recruited from a representative population of women with hip fractures who had the potential to exercise safely and independently in the home setting. Participants were healthier than the average hip fracture patient, as reflected in their high 1-year survival rate (96%) compared with that of patients with hip fracture overall (68%-84%),9-12 and had greater physical functioning17 and smaller relative decline in BMD14-16 as shown in prior studies. Participants included in this study may have been functioning at too high a level to benefit from the program being tested.
The significant losses and impairments that remain after hip fracture are remarkable and warrant further attention. One of the challenges clinicians will face in the coming decades is finding ways to deliver rehabilitative and other services to frail older adults over a long period to maximize recovery from acute events such as hip fracture. Our low-intensity, home-based exercise program is a feasible delivery strategy and can be used as a model for developing more home-based services that can enhance adherence and promote independence after completion of services; however, further research is needed to assess the overall cost-effectiveness of the program. Additional studies of postfracture exercise and rehabilitation programs are needed particularly in combination with nutritional and pharmacologic interventions designed to have an impact on study outcomes.
Acknowledgments
Funding/Support: This research was supported by the National Institute on Aging, Bethesda, Maryland (grants R37 AG09901, R01 AG18668, R01 AG17082, T32 AG00262), and the Claude D. Pepper Older Americans Independence Center (grants P60 AG12583 and P30 AG028747).
Footnotes
Author Contributions: Dr Orwig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Orwig, Hochberg, Yu-Yahiro, Resnick, Hawkes, Hebel, Zimmerman, and Magaziner. Acquisition of data: Orwig, Yu-Yahiro, Resnick, Hebel, Golden, and Magaziner. Analysis and interpretation of data: Orwig, Hochberg, Hawkes, Shardell, Hebel, Colvin, Miller, and Magaziner. Drafting of the manuscript: Orwig, Hawkes, Hebel, and Magaziner. Critical revision of the manuscript for important intellectual content: Orwig, Hochberg, Yu-Yahiro, Resnick, Hawkes, Shardell, Hebel, Colvin, Miller, Golden, Zimmerman, and Magaziner. Statistical analysis: Hawkes, Shardell, Hebel, Miller, and Golden. Obtained funding: Hebel and Magaziner. Administrative, technical, and material support: Orwig and Zimmerman. Study supervision: Orwig, Hochberg, Yu-Yahiro, Resnick, Colvin, and Magaziner.
Additional Contributions: We thank the facilities, orthopedic surgeons, and hospital personnel; the Baltimore Hip Studies research staff; members of the data and safety monitoring board; and the patients with hip fracture and their families for volunteering their time and information for this work. Thera-Band Academy generously contributed the Thera-Band resistive bands used by study participants. Lynn Lewis provided editorial assistance.
Financial Disclosure: None reported.
References
- 1.National Center for Health Statistics Centers for Disease Control and Prevention. National Nursing Home Survey (NNHS) Public-Use Data Files. [June 2007]; http://www.cdc.gov/nchs/products/elec_prods/subject/nnhs.htm.
- 2.Schneider EL, Guralnik JM. The aging of America: impact on health care costs. JAMA. 1990;263(17):2335–2340. [PubMed] [Google Scholar]
- 3.Tosteson A, Solomon D, King A, Dawson-Hughes B, Burge R, Wong J. Projections of osteoporosis fractures and costs by skeletal site in the USA [abstract] J Bone Miner Res. 2005;20(suppl 1):S21. [Google Scholar]
- 4.Praemer A, Furner S, Rice D. Musculoskeletal Conditions in the United States: Surgeon General’s Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1992. [Google Scholar]
- 5.Brainsky A, Glick H, Lydick E, et al. The economic cost of hip fractures in community-dwelling older adults: a prospective study. J Am Geriatr Soc. 1997;45(3):281–287. doi: 10.1111/j.1532-5415.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 6.Ray NF, Chan JK, Thamer M, Melton LJ., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Office of Technology Assessment, Congress of the United States. Hip Fracture Outcomes in People Aged 50 and Over: Mortality, Service Use, Expenditures, and Long-term Functional Impairment. Washington, DC: US Government Printing Office; 1994. [Google Scholar]
- 8.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 9.Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007;17(7):514–519. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Deakin DE, Boulton C, Moran CG. Mortality and causes of death among patients with isolated limb and pelvic fractures. Injury. 2007;38(3):312–317. doi: 10.1016/j.injury.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence VA, Hilsenbeck SG, Noveck H, Poses RM, Carson JL. Medical complications and outcomes after hip fracture repair. Arch Intern Med. 2002;162(18):2053–2057. doi: 10.1001/archinte.162.18.2053. [DOI] [PubMed] [Google Scholar]
- 12.Jiang HX, Majumdar SR, Dick DA, et al. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res. 2005;20(3):494–500. doi: 10.1359/JBMR.041133. [DOI] [PubMed] [Google Scholar]
- 13.Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023–1031. doi: 10.1093/aje/kwg081. [DOI] [PubMed] [Google Scholar]
- 14.Fox KM, Magaziner J, Hawkes WG, et al. Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000;11(1):31–35. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson M, Nilsson JA, Sernbo I, Redlund-Johnell I, Johnell O, Obrant KJ. Changes of bone mineral mass and soft tissue composition after hip fracture. Bone. 1996;18(1):19–22. doi: 10.1016/8756-3282(95)00422-x. [DOI] [PubMed] [Google Scholar]
- 16.Dirschl DR, Piedrahita L, Henderson RC. Bone mineral density 6 years after a hip fracture: a prospective, longitudinal study. Bone. 2000;26(1):95–98. doi: 10.1016/s8756-3282(99)00234-3. [DOI] [PubMed] [Google Scholar]
- 17.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498–M507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- 18.Hauer K, Specht N, Schuler M, Bärtsch P, Oster P. Intensive physical training in geriatric patients after severe falls and hip surgery. Age Ageing. 2002;31(1):49–57. doi: 10.1093/ageing/31.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Tinetti ME, Baker DI, Gottschalk M, et al. Systematic home-based physical and functional therapy for older persons after hip fracture. Arch Phys Med Rehabil. 1997;78(11):1237–1247. doi: 10.1016/s0003-9993(97)90338-5. [DOI] [PubMed] [Google Scholar]
- 20.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292(7):837–846. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- 21.Bérard A, Bravo G, Gauthier P. Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporos Int. 1997;7(4):331–337. doi: 10.1007/BF01623773. [DOI] [PubMed] [Google Scholar]
- 22.Caplan GA, Ward JA, Lord SR. The benefits of exercise in postmenopausal women. Aust J Public Health. 1993;17(1):23–26. doi: 10.1111/j.1753-6405.1993.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 23.Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ., Jr Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med. 1988;108(6):824–828. doi: 10.7326/0003-4819-108-6-824. [DOI] [PubMed] [Google Scholar]
- 24.Sinaki M, McPhee MC, Hodgson SF, Merritt JM, Offord KP. Relationship between bone mineral density of spine and strength of back extensors in healthy postmenopausal women. Mayo Clin Proc. 1986;61(2):116–122. doi: 10.1016/s0025-6196(12)65197-0. [DOI] [PubMed] [Google Scholar]
- 25.Sinaki M, Offord KP. Physical activity in postmenopausal women: effect on back muscle strength and bone mineral density of the spine. Arch Phys Med Rehabil. 1988;69(4):277–280. [PubMed] [Google Scholar]
- 26.Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R. Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res. 1990;5(6):589–595. doi: 10.1002/jbmr.5650050608. [DOI] [PubMed] [Google Scholar]
- 27.Sherrington C, Lord SR, Herbert RD. A randomized controlled trial of weight-bearing versus non-weight-bearing exercise for improving physical ability after usual care for hip fracture. Arch Phys Med Rehabil. 2004;85(5):710–716. doi: 10.1016/s0003-9993(03)00620-8. [DOI] [PubMed] [Google Scholar]
- 28.Lauridsen UB, de la Cour BB, Gottschalck L, Svensson BH. Intensive physical therapy after hip fracture: a randomised clinical trial. Dan Med Bull. 2002;49(1):70–72. [PubMed] [Google Scholar]
- 29.Sherrington C, Lord SR. Home exercise to improve strength and walking velocity after hip fracture: a randomized controlled trial. Arch Phys Med Rehabil. 1997;78(2):208–212. doi: 10.1016/s0003-9993(97)90265-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsauo JY, Leu WS, Chen YT, Yang RS. Effects on function and quality of life of postoperative home-based physical therapy for patients with hip fracture. Arch Phys Med Rehabil. 2005;86(10):1953–1957. doi: 10.1016/j.apmr.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Mangione KK, Craik RL, Tomlinson SS, Palombaro KM. Can elderly patients who have had a hip fracture perform moderate- to high-intensity exercise at home? Phys Ther. 2005;85(8):727–739. [PubMed] [Google Scholar]
- 32.Yu-Yahiro JA, Resnick B, Orwig D, Hicks GE, Magaziner J. Design and implementation of a home-based exercise program post-hip fracture: the Baltimore hip studies experience. PM R. 2009;1(4):308–318. doi: 10.1016/j.pmrj.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. p. 843. [PubMed] [Google Scholar]
- 35.Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older US adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 36.Resnick B, Magaziner J, Orwig D, Zimmerman S. Evaluating the components of the Exercise Plus Program: rationale, theory and implementation. Health Educ Res. 2002;17(5):648–658. doi: 10.1093/her/17.5.648. [DOI] [PubMed] [Google Scholar]
- 37.Dallal G. PC-PLAN Randomization Plans. Malden, MA: StatTools; 1986. [Google Scholar]
- 38.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25(5):628–642. [PubMed] [Google Scholar]
- 39.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5, pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman S, Hawkes WG, Hebel JR, Fox KM, Lydick E, Magaziner J. The Lower Extremity Gain Scale: a performance-based measure to assess recovery after hip fracture. Arch Phys Med Rehabil. 2006;87(3):430–436. doi: 10.1016/j.apmr.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Fox KM, Felsenthal G, Hebel JR, Zimmerman SI, Magaziner J. A portable neu-romuscular function assessment for studying recovery from hip fracture. Arch Phys Med Rehabil. 1996;77(2):171–176. doi: 10.1016/s0003-9993(96)90163-x. [DOI] [PubMed] [Google Scholar]
- 43.Fox KM, Hawkes WG, Hebel JR, et al. Mobility after hip fracture predicts health outcomes. J Am Geriatr Soc. 1998;46(2):169–173. doi: 10.1111/j.1532-5415.1998.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 44.Resnick B, Simpson M, Galik E, et al. Making a difference: nursing assistants’ perspectives of restorative care nursing. Rehabil Nurs. 2006;31(2):78–86. doi: 10.1002/j.2048-7940.2006.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 45.Alpert PT, Miller SK, Wallmann H, et al. The effect of modified jazz dance on balance, cognition, and mood in older adults. J Am Acad Nurse Pract. 2009;21(2):108–115. doi: 10.1111/j.1745-7599.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 46.Jette AM, Harris BA, Cleary PD, Campion EW. Functional recovery after hip fracture. Arch Phys Med Rehabil. 1987;68(10):735–740. [PubMed] [Google Scholar]
- 47.Fillenbaum GG. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services procedures. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 48.Sheikh JI, Yesavage JA, Brooks JO, III, et al. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3(1):23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- 49.Friedman B, Heisel M, Delavan R. Validity of the SF-36 five-item Mental Health Index for major depression in functionally impaired, community-dwelling elderly patients. J Am Geriatr Soc. 2005;53(11):1978–1985. doi: 10.1111/j.1532-5415.2005.00469.x. [DOI] [PubMed] [Google Scholar]
- 50.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 51.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 52.Cohen J. Statistical Power for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 53.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 54.Huber PJ. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Vol. 1. Berkeley, CA: University of California Press; 1967. The behavior of maximum likelihood estimates under non-standard conditions; pp. 221–233. [Google Scholar]
- 55.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in presence of missing data. J Am Stat Assoc. 1995;90(429):106–121. [Google Scholar]
- 56.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. [Google Scholar]
- 57.Kelley GA, Kelley KS. Exercise and bone mineral density at the femoral neck in postmenopausal women: a meta-analysis of controlled clinical trials with individual patient data. Am J Obstet Gynecol. 2006;194(3):760–767. doi: 10.1016/j.ajog.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Korpelainen R, Keinänen-Kiukaanniemi S, Heikkinen J, Väänänen K, Korpelainen J. Effect of impact exercise on bone mineral density in elderly women with low BMD: a population-based randomized controlled 30-month intervention. Osteoporos Int. 2006;17(1):109–118. doi: 10.1007/s00198-005-1924-2. [DOI] [PubMed] [Google Scholar]
- 59.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12(6):913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 60.Bemben DA, Fetters NL, Bemben MG, Nabavi N, Koh ET. Musculoskeletal responses to high- and low-intensity resistance training in early postmenopausal women. Med Sci Sports Exerc. 2000;32(11):1949–1957. doi: 10.1097/00005768-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 61.Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y. Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Metab. 2004;22(5):500–508. doi: 10.1007/s00774-004-0514-2. [DOI] [PubMed] [Google Scholar]
- 62.Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res. 1996;11(2):218–225. doi: 10.1002/jbmr.5650110211. [DOI] [PubMed] [Google Scholar]
- 63.Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc. 2002;34(1):17–23. doi: 10.1097/00005768-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Judge JO, Kleppinger A, Kenny A, Smith JA, Biskup B, Marcella G. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos Int. 2005;16(9):1096–1108. doi: 10.1007/s00198-004-1816-x. [DOI] [PubMed] [Google Scholar]
- 65.Resnick B, Hicks G, Orwig D, Yu-Yahiro J, Magaziner J. Review of the impact of exercise interventions on function post hip fracture and recommendations for future interventions. [January 4, 2011];Int J Disability Community Rehabil. 2010 9(1) http://www.ijdcr.ca/09_01/articles/resnick.shtml.
- 66.Tinetti ME, Baker DI, Gottschalk M, et al. Home-based multicomponent rehabilitation program for older persons after hip fracture: a randomized trial. Arch Phys Med Rehabil. 1999;80(8):916–922. doi: 10.1016/s0003-9993(99)90083-7. [DOI] [PubMed] [Google Scholar]
- 67.Talkowski JB, Lenze EJ, Munin MC, Harrison C, Brach JS. Patient participation and physical activity during rehabilitation and future functional outcomes in patients after hip fracture. Arch Phys Med Rehabil. 2009;90(4):618–622. doi: 10.1016/j.apmr.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]