Abstract
Treatment of human prostate carcinoma-derived LNCaP cells with androgen or oestradiol triggers simultaneous association of androgen receptor and oestradiol receptor β with Src, activates the Src/Raf-1/Erk-2 pathway and stimulates cell proliferation. Surprisingly, either androgen or oestradiol action on each of these steps is inhibited by both anti-androgens and anti-oestrogens. Similar findings for oestradiol receptor α were observed in MCF-7 or T47D cells stimulated by either oestradiol or androgens. Microinjection of LNCaP, MCF-7 and T47D cells with SrcK– abolishes steroid-stimulated S-phase entry. Data from transfected Cos cells confirm and extend the findings from these cells. Hormone-stimulated Src interaction with the androgen receptor and oestradiol receptor α or β is detected using glutathione S-transferase fusion constructs. Src SH2 interacts with phosphotyrosine 537 of oestradiol receptor α and the Src SH3 domain with a proline-rich stretch of the androgen receptor. The role of this phosphotyrosine is stressed by its requirement for association of oestradiol receptor α with Src and consequent activation of Src in intact Cos cells.
Keywords: androgen receptor/cross-talk/oestradiol receptor/Src association
Introduction
Steroid hormone receptors act as ligand-inducible transcriptional enhancer factors (Beato et al., 1995; Mangelsdorf et al., 1995; Parker and White, 1996; Evans, 1988; McKenna et al., 1999). Their transcriptional activity can even be activated in the absence of steroids through cross-talk with agonist-occupied membrane receptors (Power et al., 1991; Zhang et al., 1994; Pietras et al., 1995; Abreu-Martin et al., 1999; Yeh et al., 1999). In addition, steroid receptors regulate non-transcriptional events. This raises the question of the cellular localization and identity of the receptors responsible for these actions (Wehling, 1997; Revelli et al., 1998; McEwen and Alves, 1999). The classical model of steroid transcriptional action has not yet given exhaustive insight into steroid action on different events, including regulation of cell proliferation (Gorski, 1997). Different hypotheses have been proposed for cell proliferation. Steroids might act through interaction of their receptors with specific DNA sequences regulating the expression of genes required for cell multi plication (Weisz and Bresciani, 1993; Loose-Mitchell et al., 1988), production of polypeptide growth factors by the target cells (Dickson and Lippman, 1987) or other mechanisms. We recently observed that oestradiol, like peptide factors, activates the signal transducing Src/Ras/Erks pathway in human mammary cancer derived cell lines MCF-7 and T47D, as well as in the human colon cancer derived cell line Caco-2 (Migliaccio et al., 1993, 1996; Di Domenico et al., 1996). Progestins also activate the same pathway in T47D cells (Migliaccio et al., 1998). Activation of the pathway by either oestradiol or progestins triggers S-phase entry of the cells. This supports the view that the non-transcriptional activity of the two steroids is responsible for stimulation of cell growth (Castoria et al., 1999). These findings revealed for the first time that classical forms of oestrogen receptor α (ERα) and progesterone receptor B (PR-B) are coupled to cytosolic signal transducing proteins. These observations have been extended by other groups to different systems: a human neuroblastoma cell line (Watters et al., 1997); a rat osteoblast-like cell line (Endoh et al., 1997); rat cardiac fibroblasts (Lee and Eghbali-Webb, 1998); rat cerebral cortical explants (Singh et al., 1999); primary cortical neurons (Singer et al., 1999); and endothelial cells (Chen et al., 1999). In these systems and under different conditions this stimulation leads to different effects: induction of the c-fos gene (Watters et al., 1997), cell proliferation (Lee and Eghbali-Webb, 1998), neuroprotection (Singer et al., 1999) and vasorelaxation (Chen et al., 1999). We now report that the androgen R1881 stimulates the Src/Raf-1/Erks signal transducing pathway in LNCaP cells, which are derived from human prostatic adenocarcinoma. Furthermore, we observe that oestradiol has the same effects as R1881 on LNCaP cells. Interestingly, each of the two steroids induces assembly of a novel ternary complex constituted of the androgen receptor (AR), oestradiol receptor β (ERβ) and Src. The complex triggers activation of the pathway, S-phase entry and cell proliferation. An oestradiol antagonist (ICI 182,780) prevents complex assembly and pathway activation not only by oestradiol but also by androgen. Similarly, an androgen antagonist (Casodex) inhibits oestradiol action. The behaviour of human mammary cancer derived MCF-7 and T47D cells, once stimulated by oestradiol or R1881, is similar to that of LNCaP cells in terms of AR–ERα–Src complex assembly, Src activation, S-phase entry and cell stimulation. In these cells, as in LNCaP cells, Casodex inhibits oestradiol activity and ICI 182,780 prevents the androgen effects. Experiments in transfected Cos cells show that the single wild-type human AR or ERα or ERβ, once occupied by the cognate agonist, is also able to activate the signalling pathway. Nevertheless, in the same cells, assembly of the ternary complex leads to a stronger activation of Src. The association of Src with AR and ERα or ERβ was analysed using glutathione S-transferase (GST) fusion protein experiments. Our findings show that ERα or ERβ associates with the SH2 domain of Src. Phosphotyrosine 537 of ERα has been identified as the residue interacting with SH2. In turn, AR binds to SH3 of Src through a proline-rich stretch. We propose that simultaneous interaction of both SH2 and SH3 with the two receptors leads to a less constrained conformation of Src than that induced by the interaction of only one of the two domains with a single receptor. This conformation might be responsible for a stronger stimulation of Src activity.
Results
Antagonist cross-inhibition of LNCaP growth stimulated by androgen or oestradiol
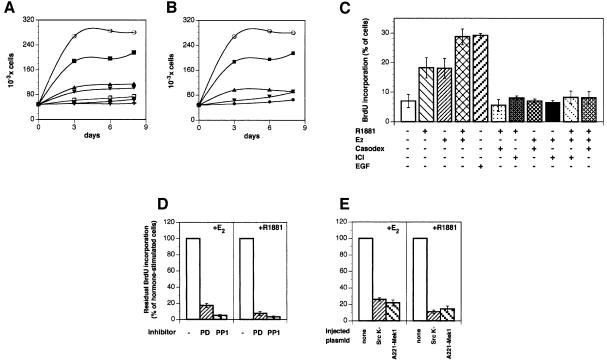
LNCaP cells were maintained for 4 days under conditions simulating hormone depletion. This was established using charcoal-treated serum and medium lacking phenol red, a substance with a weak oestrogen activity. Thereafter, different concentrations of R1881 (from 0.1 to 100 nM) were added to the medium and the growth rate was analysed. The androgen stimulated cell growth with the maximal effect at 10 nM (not shown). Stimulation of growth by 10 nM R1881 in the absence and presence of 10 µM Casodex and 10 nM oestradiol with and without 10 µM ICI 182,780 was evaluated (Figure 1A). As expected, the antagonist inhibited the agonist effect. However, when the antagonists were switched (Casodex was added together with oestradiol and ICI 182,780 with androgen), each of the two antagonists strongly reduced the steroid stimulatory effect (Figure 1B). LNCaP cells were also analysed for bromodeoxyuridine (BrdU) incorporation into newly synthesized DNA. The maximal incorporation after 24 h in the 0.001–100 nM range was detected at 10 nM R1881 (not shown). About 7% of cells synthesized DNA in the absence of steroids, whereas this fraction increased to 18% in the presence of 10 nM androgen or oestradiol (Figure 1C). When the two steroids were added to the medium simultaneously, this value was shifted to 28%. For comparison, the epidermal growth factor (EGF) effect was analysed. The growth factor stimulated BrdU incorporation to a similar extent as the two steroids added together. Like cell growth, DNA synthesis stimulated by androgen or oestradiol was inhibited not only by the cognate antagonists (Casodex or ICI 182,780, respectively) but also by the cross-antagonist (ICI 182,780 or Casodex, respectively) (Figure 1C). Either Casodex or ICI 182,780 abolished the stimulatory effect of the combined steroids.
Fig. 1. Effect of androgen and oestradiol on LNCaP cell growth and S-phase entry under different conditions. (A) Quiescent LNCaP cells were left untreated (filled circles) or treated for the indicated times with either 10 nM oestradiol in the absence (open circles) or presence of 10 µM ICI 182,780 (upward filled triangles) or with 10 nM R1881 in the absence (filled squares) or presence (downward filled triangles) of 10 µM Casodex. Control cells were also treated with 10 µM ICI 182,780 (open squares) or 10 µM Casodex (crosses) alone. (B) Cells were left untreated (filled circles) or treated for the indicated times with either 10 nM oestradiol in the absence (open circles) or presence (downward filled triangles) of 10 µM Casodex or with 10 nM R1881 in the absence (filled squares) or presence (upward filled triangles) of 10 µM ICI 182,780. (C) Quiescent LNCaP cells were left unstimulated or stimulated with the indicated compounds for 24 h. BrdU was added and the cells were fixed and permeabilized. DNA synthesis was followed as described in Materials and methods. Several coverslips were analysed and BrdU incorporation calculated according to the formula: percentage BrdU-positive cells = (BrdU-positive cells/total cells) × 100. Results from different experiments were pooled. The means ± SEM are shown. (D) Quiescent LNCaP cells were stimulated for 24 h with either 10 nM oestradiol (right) or 10 nM R1881 (left) in the absence or presence of the indicated inhibitors. PD98059 was utilized at 50 µM and PP1 at 10 µM. BrdU was included in the cell medium and DNA synthesis analysed as described in Materials and methods. Data from different coverslips were pooled and residual BrdU incorporation was expressed as a percentage of steroid-stimulated incorporating cells, which was 28 and 25% for oestradiol- and androgen-stimulated cells, respectively. The basal BrdU incorporation (<5%) was evaluated and subtracted in each case. (E) Quiescent LNCaP cells were either not injected or injected with the indicated constructs (either SrcK– or A221 MEK-1). Either 10 nM oestradiol (left) or 10 nM R1881 (right) was added to the cells for 24 h. BrdU incorporation into DNA was analysed as in (D). Steroid-stimulated BrdU incorporation was 26 and 27% for oestradiol- and androgen-stimulated cells, respectively. The basal BrdU incorporation (<4%) was evaluated and subtracted. For each plasmid, data are derived from at least 150 scored cells. The means ± SEM are shown. A construct expressing the pSG5 empty plasmid was also microinjected into nuclei of quiescent LNCaP cells together with plasmid pEGFP, as an injection marker. The cells injected with pSG5 were left unstimulated or stimulated with either oestradiol or R1881. A total of 23 and 24% of the GFP-expressing LNCaP cells entered S-phase upon stimulation with oestradiol and R1881, respectively. BrdU incorporation of unstimulated cells was <3.5%.
Since oestradiol stimulates S-phase entry of MCF-7 cells through activation of the Src/Ras/Erks pathway (Castoria et al., 1999), the effect of cell-permeable inhibitors of the pathway on steroid-stimulated BrdU incorporation was evaluated. Figure 1D shows that addition of either the MEK-1 inhibitor PD98059 (Alessi et al., 1995) or the Src inhibitor PP1 (Hanke et al., 1996) strongly reduced androgen- or oestradiol-dependent S-phase entry of LNCaP cells. Inhibitors alone did not interfere with the basal BrdU incorporation (not shown). Furthermore, microinjection of LNCaP cells with dominant-negative Src or MEK-1 (SrcK– and A221-Mek 1, respectively) strongly reduced the number of oestradiol- or androgen-stimulated cells incorporating BrdU into DNA (Figure 1E). These data show that steroid activation of Src and MEK-1 is required for cell cycle progression of prostate cancer cells.
Antagonist cross-inhibition of signalling pathway activation by androgen or oestradiol in LNCaP cells
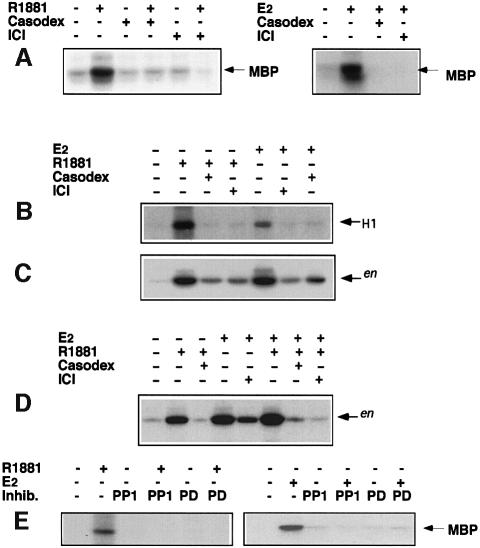
Based on the effects of cell-permeable inhibitors on S-phase entry, the action of the androgen R1881 on Erk-2 activity in LNCaP cells was evaluated. Cell lysates were immunoprecipitated with anti-Erk-2 antibody. Control samples were immunoprecipitated with excess C14 peptide against which the antibody was raised. The myelin basic protein (MBP) phosphorylating activity of the immunoprecipitates was assayed. A 10 nM R1881 treatment gave strong and rapid stimulation of Erk-2 activity, which reached maximal values after 5 min and returned to the basal level after 90 min. In the control samples no phosphorylation of MBP was detected (not shown). The anti-androgen Casodex (10 µM) abolished the 5 min androgen stimulation without affecting basal Erk activity (Figure 2A, left). Interestingly, the pure anti-oestrogen ICI 182,780 (10 µM) was as effective as Casodex in preventing the 5 min stimulatory effect of R1881 treatment (Figure 2A, left). A 10 nM oestradiol treatment of LNCaP cells for 5 min also stimulated Erk-2 activity. Not only ICI 182,780 but also Casodex abolished this stimulation (Figure 2A, right).
Fig. 2. Effect of androgen and 17β-oestradiol on Erk-2, Raf-1 and Src activities in LNCaP cells. (A) (Left) Cells were treated for 5 min with 10 nM R1881 alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780, or with 10 µM Casodex alone or 10 µM ICI 182,780 alone. (Right) Cells were treated for 5 min with 10 nM oestradiol alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780. Cell lysates were immunoprecipitated with anti-Erk-2 antibodies. Erk-2 kinase activity was assayed using MBP as a substrate The arrows indicate the MBP position. (B) Cells were left untreated or treated for 2 min with either 10 nM oestradiol or 10 nM R1881 alone or in the presence of 10 µM Casodex or ICI 182,780. Cell lysates were immunoprecipitated with anti-Raf antibodies and Raf-1 kinase activity was assayed in the immunoprecipitates using histone H1 as substrate. (C and D) Cells were left untreated or treated for 2 min with the indicated compounds. Cell lysates were immunoprecipitated with anti-Src antibodies and immunoprecipitates assayed for Src kinase activity using acidified enolase as substrate. The arrows indicate the enolase position (en). (E) Cells were left unstimulated or stimulated for 5 min with 10 nM R1881 (left) or 10 nM oestradiol (right) in the absence or presence of 50 µM PD98059 or 10 µM PP1. Control cells were treated with inhibitors alone (not shown).
We next analysed the effect of steroids on steps of the signal transduction pathway upstream of MAP kinases. Raf-1 was immunoprecipitated with anti-Raf-1 antibodies and the ability of the immunoprecipitates to phosphorylate H1 histone was assayed. A 2 min R1881 treatment stimulated this activity and stimulation was abolished by either Casodex or ICI 182,780. In turn, oestradiol stimulated Raf-1 activity and ICI 182,780 or Casodex prevented stimuation (Figure 2B).
Treatment of LNCaP cells with 10 nM R1881 for different times also enhanced Src kinase activity measured using enolase as substrate in cell lysates immunoprecipitated with monoclonal 327 anti-Src antibody. This activity was stimulated 1 min after hormone addition, reached a peak after 2 min and decreased towards basal levels after 15 min (not shown). Casodex reduced the stimulation of Src induced by a 2 min R1881 treatment. A similar reduction was caused by ICI 182,780. A 2 min oestradiol treatment also stimulated Src activity and this effect was not only antagonized by ICI 182,780, but also by Casodex (Figure 2C).
When LNCaP cells were simultaneously treated for 2 min with R1881 and oestradiol, Src stimulation was stronger than with the single agonists (Figure 2D). Either ICI 182,780 or Casodex prevented the combined action of both agonists. Stimulation by the combined agonists over the single agonists of Src activity was similar to that observed on S-phase entry (1.7- and 1.9-fold, respectively) (Figure 1C), reinforcing the view of a linkage between Src and DNA synthesis stimulation by steroids.
Steroid-stimulated Erk-2 activity inhibition by PP1 (Figure 2E) indicates that Src is upstream of Erk-2.
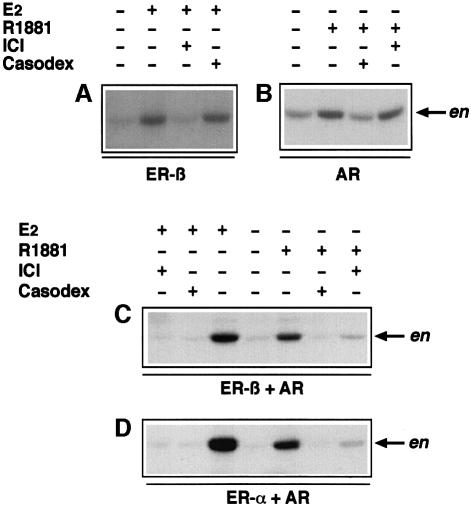
R1881 or oestradiol induces simultaneous association of AR, ERβ and Src in LNCaP cells
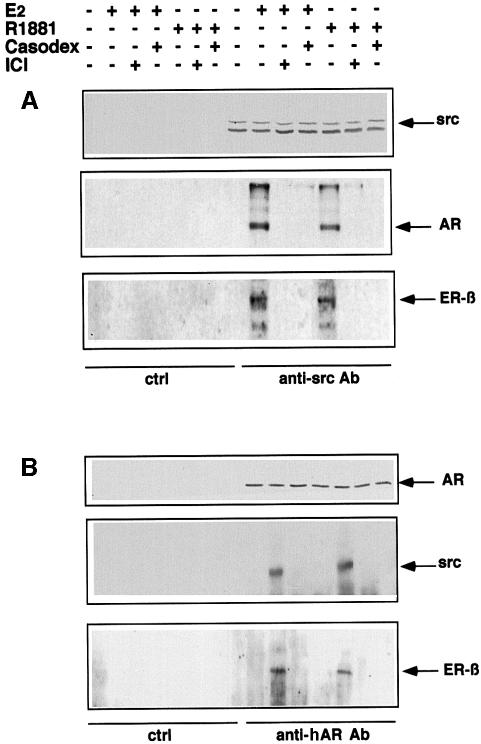
Cross-inhibited stimulation of Src, Raf-1 and Erk-2 in LNCaP cells treated with oestradiol or the R1881 androgen suggests a reciprocal cross-talk between the two receptors. This possibility was investigated by immunoprecipitating proteins from LNCaP cell lysates using antibodies raised against Src. Cells were either unstimulated or stimulated by androgen or oestradiol (10 nM each) for 2 min in the absence or presence of either the cognate antagonist or the cross-antagonist (10 µM each). Cell lysate proteins were immunoprecipitated with anti-Src antibody and submitted to SDS–PAGE, then blotted with antibodies directed against AR, Src or ERβ. Similar amounts of Src were immunoprecipitated. Under basal conditions, neither AR nor ERβ co-immunoprecipitated with Src. Such co-immunoprecipitation was detected after steroid-induced cell stimulation and disappeared in the presence of each of the two antagonists (Figure 3A). In a different experiment, lysate proteins were immunoprecipitated by anti-AR antibody. Again, Src was associated with AR and ERβ only in stimulated cells (Figure 3B). Whatever the agonist used, assembly of the steroid-induced complex was prevented by each of the two antagonists. In conclusion, stimulation of LNCaP cells by either R1881 or oestradiol induces association of the two receptors with Src.
Fig. 3. Androgen and oestradiol trigger ERβ–AR–Src association in LNCaP cells. Quiescent LNCaP cells were left untreated or treated for 2 min with either 10 nM oestradiol alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780, or with 10 nM R1881 in the absence or presence of 10 µM ICI 182,780 or 10 µM Casodex. (A) Cell lysates were immunoprecipitated with anti-Src antibodies. Parallel samples were precipitated with control antibodies. Immunoprecipitated proteins were resolved by SDS–PAGE, transferred onto nitrocellulose filters, then probed with anti-Src, anti-hAR or anti-hERβ antibodies. (B) Cell lysates were immunoprecipitated with anti-hAR or control antibodies. Immunoprecipitates were resolved by SDS–PAGE, transferred to nitrocellulose filters and probed with anti-hAR, anti-Src or anti-ERβ antibodies. The arrows indicate the expected position of the indicated proteins.
Antagonist cross-inhibition in MCF-7 and T47D cells of androgen or oestradiol action
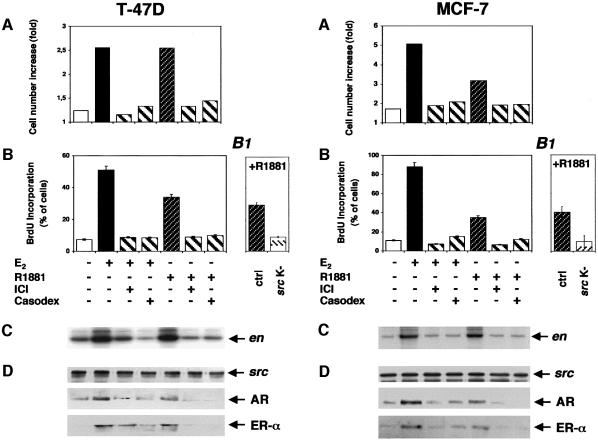
To answer the question of whether the cross-inhibition observed in LNCaP cells can be observed in other cells, we analysed the behaviour of two well-known human mammary cancer derived cell lines: MCF-7 and T47D. Both of them express AR in addition to ER. Both 10 nM oestradiol and 10 nM R1881 stimulated cell growth and S-phase entry, although the androgen was less active than oestradiol (Figure 4A and B). As in LNCaP cells, an excess of Casodex or ICI 182,780 cross-inhibited hormone-stimulated activity. It has been previously reported that oestradiol and progestins activate the Src/Ras/Erks pathway of these cell lines (Migliaccio et al., 1996, 1998). We now observe that, in addition to oestradiol, androgen stimulates Src activity in both cell lines (Figure 4C). Antagonists cross-inhibited Src activated by either oestradiol or androgen. Association of ERα and AR with Src was investigated by analysing proteins immunoprecipitated by Src antibodies. In T47D and MCF-7 cells, androgen or oestradiol treatment stimulated ERα–AR–Src ternary complex assembly (Figure 4D). Assembly was inhibited when cells were incubated with hormone in the presence of either antagonist or cross-antagonist. Microinjection of T47D and MCF-7 cells with SrcK– abolished the S-phase entry stimulated by R1881 (Figure 4B1). Together these findings show that cross-talk between the oestradiol and androgen receptors in cell lines derived from mammary cancers is similar to that of cells derived from prostate cancer. In MCF-7 and T47D cells, this cross-talk also leads to Src activation and cell proliferation.
Fig. 4. Effects of androgen and oestradiol in T47D and MCF-7 cells. (Left) Quiescent T47D cells were left unstimulated or stimulated with the indicated compounds. The cells were then analysed for growth rate (A), which was expressed as increase in cell number and BrdU incorporation (B), which was calculated as in Figure 1C. (B1) Quiescent T47D cells, either uninjected or injected with a SrcK–-expressing plasmid, were stimulated with R1881 (10 nM). BrdU was included in the cell medium and DNA synthesis analysed. It was calculated as in Figure 1C. Basal incorporation of BrdU (<5%) was evaluated and subtracted. Data derived from at least 150 scored cells were pooled. The means ± SEM are shown. Lysates from quiescent T47D cells were immunoprecipitated with anti-Src antibody. (C) Src kinase activity was assayed using enolase as substrate. (D) Immunocomplexes were probed with either anti-Src (upper), anti-AR (middle) or anti-ERα (lower) antibodies. (Right) Quiescent MCF-7 cells untreated or treated with the indicated compounds were analysed for growth rate (A) and BrdU incorporation (B). (B1) MCF-7 cells, either uninjected or injected with SrcK–-expressing plasmid, were stimulated with R1881 (10 nM). BrdU was included in the cell medium and DNA synthesis analysed. Basal incorporation of BrdU (<3%) was evaluated and subtracted. Data derived from at least 150 scored cells were pooled. The means ± SEM are shown. Lysates from quiescent MCF-7 cells were immunoprecipitated with anti-Src antibody. (C) Src kinase activity was assayed. (D) Immunocomplexes were probed with either anti-Src (upper), anti-AR (middle) or anti-ERα (lower) antibodies.
Src activation by androgen or oestradiol in Cos cells transfected with AR and ERα or ERβ
To investigate further the role of cross-talk between the androgen and oestrogen receptors in hormone-driven signal transduction pathway activation, plasmids encoding wild-type human AR or human ERβ were separately transfected or co-transfected into Cos-7 cells. In the experiments shown in Figure 5A and B, Cos cells were transfected with the cDNAs of ERβ or AR, respectively. In ERβ-transfected cells, stimulation of Src kinase activity by a 2 min 10 nM oestradiol treatment was observed (Figure 5A). It was abolished by ICI 182,780. R1881 (10 nM) stimulated Src activity in Cos cells transfected with AR, and Casodex inhibited this stimulation (Figure 5B). Neither inhibition of oestrogen action by 10 µM anti-androgen (Figure 5A) nor inhibition of the androgen effect by 10 µM anti-oestrogen was detected (Figure 5B). These findings demonstrate that each of the two receptors, once occupied by the agonist, activates Src in the absence of the other receptor. Only the cognate antagonist prevents this activation. Finally, Cos-7 cells were co-transfected with AR cDNA together with either the β or α form of human ER. When cells were co-transfected with AR and ERβ (Figure 5C), either oestradiol or R1881 induced stimulation of Src activity. However, in contrast to Cos cells expressing a single steroid receptor and similar to LNCaP cells, either Casodex or ICI 182,780 prevented stimulation by each of the two steroids. Similar findings were obtained when cells were co-transfected with AR and the α form of ER (Figure 5D), suggesting that ERα, like ERβ, is able to cross-talk with AR. These experiments show that cross-inhibition of oestrogen and androgen action on signal transduction by antagonists requires both receptors.
Fig. 5. Effect of androgen and oestradiol on Src activity in Cos cells transiently transfected with ER and AR cDNAs. (A) Cos cells were transfected with pSG5-ERβ vector encoding human ER (β form) and treated for 2 min with 10 nM oestradiol alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex. (B) Cells were transfected with pSG5-AR vector encoding human AR and treated for 2 min with 10 nM R1881 alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780. (C) Cells were co-transfected with pSG5-ERβ and pSG5-AR. They were either untreated or treated for 2 min with 10 nM oestradiol alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780, or with 10 nM R1881 alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780. (D) Cells were co-transfected with pSG5-ERα and pSG5-AR. They were treated for 2 min either with 10 nM oestradiol alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex, or with 10 nM R1881 alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex. Lysates were immunoprecipitated with anti-Src antibody and immunoprecipitates assayed for Src kinase activity using acidified enolase as substrate. The arrows (en) indicate the enolase position.
Src activation by oestradiol in Cos cells transfected with ERβ alone (Figure 5A) or together with AR (Figure 5C) and Src activation by androgens in cells expressing AR alone (Figure 5B) or together with either ERβ (Figure 5C) or ERα (Figure 5D) show that co-transfected cells respond to each of the two steroids with a 3-fold stronger stimulation than cells transfected with only one receptor. This value has been averaged from three different experiments, including that represented in Figure 5.
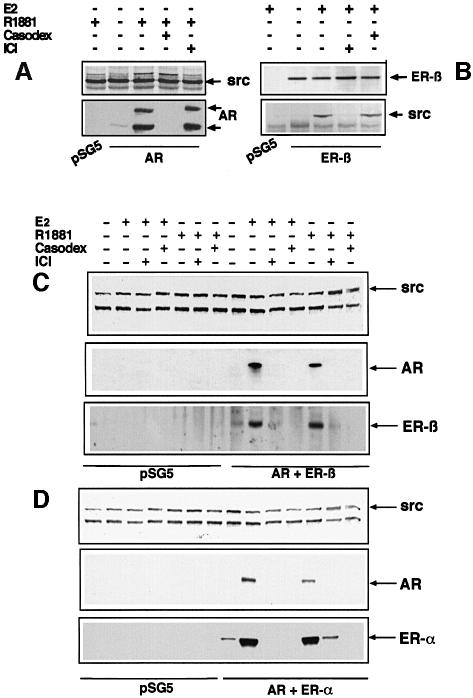
R1881 and oestradiol induce AR, ERα or ERβ and Src association in transfected Cos cells
Cos cells were transiently transfected with either AR or ERβ plasmids alone (Figure 6A and B) or co-transfected with plasmids containing the cDNAs of hAR and either ERβ or ERα (Figure 6C and D). Cells were stimulated by oestradiol or androgen alone or in the presence of antagonists. Cell lysates were immunoprecipitated by antibodies against Src and blotted with antibodies against Src, AR or ER. Cell lysate proteins were immunoprecipitated with antibodies against ERβ only in the experiment presented in Figure 6B. In cells transfected with only one receptor (Figure 6A and B), the cognate agonist induced Src–receptor association. Like Src activation (Figure 5A and B), this association was prevented by the corresponding antagonist and was unaffected by the cross-antagonist. As in LNCaP cells (Figure 3), in Cos cells transfected with two receptors, AR and ERβ co-precipitated with Src when treated with oestradiol or R1881 and each of the two antagonists inhibited these associations (Figure 6C). The same experiment with Cos cells expressing ERα instead of ERβ gave similar results (Figure 6D). Taken together, our data support the view that, regardless of the agonist used, R1881 or oestradiol induced the ternary complex in cells expressing AR and ER, and this complex stimulated Src activity. Prevention of this assembly by antagonists also prevented Src activation.
Fig. 6. Androgen- and oestradiol-induced association of ER–AR–Src in transiently transfected Cos cells. (A) Cos cells were transfected with void pSG5 or pSG5-AR vectors, then treated for 2 min with 10 nM R1881 alone or in the presence of 10 µM Casodex or 10 µM ICI 182,780. Cell lysates were immunoprecipitated with anti-Src antibody and immunoprecipitates subjected to SDS–PAGE. (B) Cos cells were transfected with pSG5 or pSG5-ERβ and treated for 2 min with 10 nM oestradiol alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex. Lysates were immunoprecipitated with anti-ERβ antibody and immunoprecipitates subjected to SDS–PAGE. (C) Cos cells were transfected with void pSG5 vector or with both pSG5-AR vector and pSG5-ERβ. They were treated for 2 min with 10 nM oestradiol alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex, or with 10 nM R1881 alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex. (D) Cos cells were transfected with pSG5 or with both pSG5-hAR and pSG5-HEG0 encoding human ERα. They were treated for 2 min with 10 nM oestradiol alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex, or with 10 nM R1881 alone or in the presence of 10 µM ICI 182,780 or 10 µM Casodex. Cell lysates were immunoprecipitated with anti-Src antibody and immunoprecipitates subjected to SDS–PAGE. (C and D) Proteins transferred onto nitrocellulose filters were probed with anti-hAR, anti-ERβ, anti-Src or H222 anti-ERα antibodies. The arrows indicate the expected position of the indicated proteins.
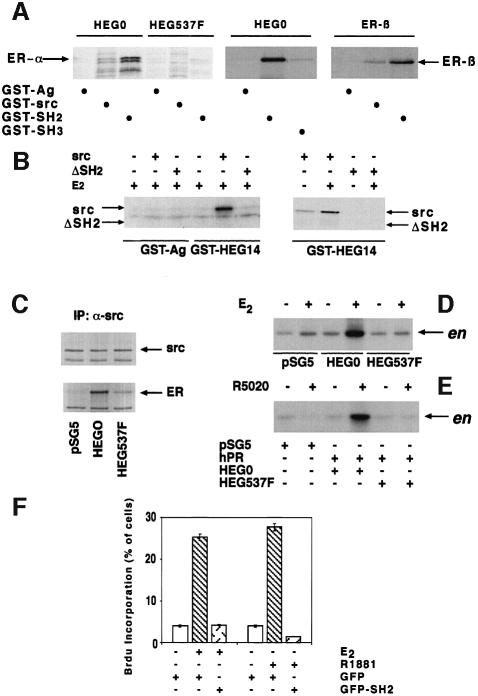
Definition of domains involved in Src–oestradiol receptor interaction
Association between Src and steroid receptors was investigated in vitro by pull-down experiments with GST fusion protein constructs. Human ERα (HEG0) or its mutant substituted at Tyr537 with Phe (HEG537F) was incubated with either GST–Src or GST–agarose, the latter used as a control. Both receptors were synthesized in reticulocyte lysate and labelled with [35S]methionine. Although HEG0 in the presence of oestradiol interacted with GST–Src, no interaction was detectable when HEG537F was used, indicating a role of phosphotyrosine 537 (Figure 7A, left). A candidate for the interaction with the phosphotyrosine of ERα is the SH2 domain of Src (SH2). This was confirmed by the strong association between GST–SH2 and HEG0, whereas HEG537F was unable to interact (Figure 7A, left). In addition, HEG0 weakly, probably non-specifically, interacted with the SH3 domain of Src (GST–SH3), while the same receptor interacted strongly with GST–SH2 (Figure 7A, middle). Like ERα, human ERβ also interacted with GST–Src and GST–SH2 (Figure 7A, right). The chimera GST–HEG14 (HEG14 is the C-terminal half of HEG0 including the hormone-binding domain and Tyr537) was employed in similar experiments together with [35S]Src or [35S]Src lacking SH2 (ΔSH2) (Figure 7B, left). GST–HEG14 interacted with Src in the presence of oestradiol. No interaction was observed with ΔSH2, further indicating the role of the SH2 domain in the association of Src with HEG0 (Figure 7B, right). Parallel experiments performed with GST–agarose instead of GST–HEG14 showed weak, non-specific interaction with Src. Interestingly, oestradiol stimulated the association of GST–HEG14 with Src (Figure 7B, right).
Fig. 7. Role of the Src SH2 domain in hER–Src association and signalling activation by steroids. (A) (Left) GST–agarose (GST-Ag), GST–Src and GST–SH2 were incubated in the presence of 10 nM oestradiol with either HEG0 or HEG537F labelled with [35S]methionine during their synthesis in reticulocyte lysate. (Middle) GST–Ag, GST–SH2 and GST–SH3 were incubated with [35S]HEG0 in the presence of 10 nM oestradiol. (Right) GST–Ag, GST–Src and GST–SH2 were incubated with [35S]hERβ in the presence of 10 nM oestradiol. Proteins were eluted with SDS, subjected to SDS–PAGE and revealed by autoradiography. (B) (Left) GST–Ag and GST–HEG14 (HEG14 is the C-terminal half of HEG0) were incubated in the presence of 10 nM oestradiol alone or together with either [35S]Src or [35S]Src lacking the SH2 domain (ΔSH2). (Right) GST–HEG14 was incubated in the absence or presence of 10 nM oestradiol with either Src or ΔSH2. Proteins were eluted with SDS, subjected to SDS–PAGE and revealed by fluorography. (C) Cos cells, transfected with either empty pSG5 vector or vector pSG5-HEG0 or pSG5-HEG537F, were treated with 10 nM oestradiol for 2 min. Cell lysates were immunoprecipitated with anti-Src antibody. The immunoprecipitates were blotted either with H222 anti-ER (lower) or anti-Src (upper) antibody. (D) Cells transfected as in (C) were incubated in the absence or presence of 10 nM oestradiol for 2 min. Immunoprecipitates by anti-Src antibody were assayed for Src activity using enolase (en) as substrate. (E) Cos cells were transfected with the empty pSG5 vector or PSG5-PR-B together with either PSG5-HEG0 or pSG5-HEG537F. Cells were stimulated for 2 min with 10 nM R5020 progestin. Src activity of the immunoprecipitates from cell lysates was assayed. (F) Quiescent LNCaP cells were injected with either GFP- or GFP-SH2-expressing plasmids. Cells were then left unstimulated or stimulated with either 10 nM oestradiol or R1881. BrdU was included in the cell medium and after 24 h DNA synthesis was analysed. It was calculated as in Figure 1C. Data from several coverslips were pooled and evaluated statistically. The means ± SEM are also shown.
Our previous work showed that the interaction of ERα with Src is crucial for Src activation, not only by oestradiol but also by progestin (Migliaccio et al., 1998). In addition, our in vitro findings demonstrate a reduction in or lack of this interaction when HEG537F was used in place of the wild-type receptor. On this basis it is expected that HEG537F should be unable to activate Src in whole cells stimulated with either steroid. Cos cells were transfected with the empty plasmid (pSG5) or plasmids expressing HEG0 or its mutant HEG537F. In cells stimulated for 2 min by 10 nM oestradiol, association of HEG0 with Src was clearly observed, whereas association of HEG537F was weak (Figure 7C). These findings confirm the in vitro experiments. Furthermore, in contrast to cells transfected with HEG0, no activation of Src was detected upon oestradiol stimulation of cells transfected with HEG537F (Figure 7D). When Cos cells were co-transfected with PR-B and HEG0, then treated with 10 nM progestin R5020, Src activity was stimulated, as expected (Migliaccio et al., 1998). In cells co-transfected with PR-B and HEG537F, the mutant was unable to transmit the progestin signal to Src (Figure 7E). From these findings it appears that phosphotyrosine 537 is crucial for ER association with Src and Src activation by oestradiol. Phosphotyrosine 537 is also required for the progestin-induced cross-talk between PR-B and ERα leading to Src activation by progestin. This is in agreement with the observation that such cross-talk requires ERα–Src association (Migliaccio et al., 1998). LNCaP cells microinjected with a GFP–Src SH2 construct were unresponsive to oestradiol or R1881 in terms of S-phase entry (Figure 7F). This is compatible with excess Src SH2 interfering with the Src–ER–AR association that is required for the oestradiol and androgen effects.
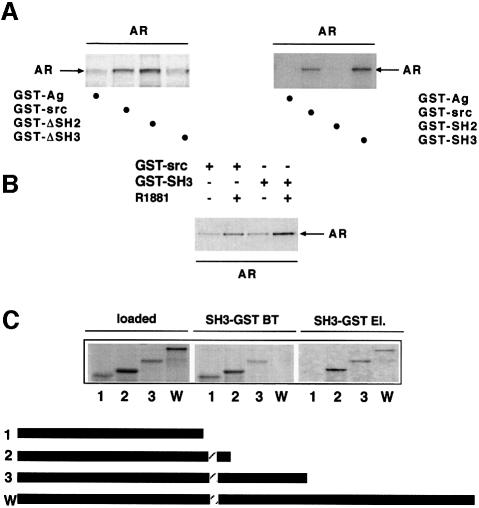
Definition of domains involved in Src–androgen receptor interaction
The interaction between human AR and Src was then analysed. In the presence of androgen, [35S]AR interacted with GST–Src and even more strongly with GST–ΔSH2. An interaction with GST–ΔSH3 (Src lacking SH3) was barely detectable, suggesting that SH3 of Src is the domain responsible for the interaction of Src with AR (Figure 8A, left). This is supported by the experiment in Figure 8A (right), showing that AR interacted with GST–Src and GST–SH3 but not with GST–SH2. Figure 8B shows androgen stimulation of the association of [35S]AR with both GST–Src and GST–SH3. Analysis of the interaction of [35S]AR mutants with GST–SH3 showed that deletion of amino acids 371–422 abolished this interaction (Figure 8C). This sequence contains a stretch of prolines, which are candidates for the association of AR with Src SH3.
Fig. 8. Steroid-stimulated in vitro association of hAR with Src via its SH3 domain. (A) (Left) GST–Ag, GST–Src, GST–ΔSH2 and GST–ΔSH3 (ΔSH3 is Src with the SH3 domain deleted) were incubated in the presence of 10 nM R1881 with [35S]AR synthesized in reticulocyte lysate. (Right) GST–Ag, GST–Src, GST–SH2 and GST–SH3 were incubated with [35S]AR in the presence of 10 nM R1881. (B) GST–Src or GST–SH3 was incubated with [35S]AR in the absence or presence of R1881. Proteins were eluted with SDS, subjected to SDS–PAGE and revealed by fluorography. (C) Different 35S-labelled AR deletion mutants were incubated with GST–SH3 in the presence of 10 nM R1881. Equivalent aliquots from samples incubated with GST–SH3 (loaded), break-through (BT) and SDS-eluted (El.) proteins were subjected to SDS–PAGE and revealed by fluorography. The bars below the figure are a diagrammatic representation of AR deletion mutants used in the experiment. Mutants 1–3 include amino acids 1–370, 1–422 and 1–564 of hAR, respectively. W indicates the wild-type receptor. The dashed bar corresponds to the 372–379 proline stretch of AR.
In conclusion, our experiments show that Src undergoes association with ERα via interaction of its SH2 domain with phosphotyrosine 537 of ERα and with AR via binding of its SH3 domain to proline residues present in this receptor. These associations are stimulated by the presence of the agonists. Src also associates with ERβ via its SH2 domain.
Discussion
LNCaP cells have largely been used as a model of androgen-responsive growth (Horoszewics et al., 1983). These cells contain a point mutation in the ligand-binding domain of the AR, leading to a decrease in steroid binding specificity (Veldscholte et al., 1990). In addition, they express ERβ, as shown by the present findings. The presence of mRNA for ERβ in the prostate (Kuiper et al., 1997) implies a physiological role for the receptor in this organ. LNCaP cells respond to oestrogens by increasing growth (Sonnenschein et al., 1989; Schuurmans et al., 1991; Castagnetta et al., 1995). Prostatic hyperplasia and carcinoma share this behaviour (Wilson, 1980; Wilding, 1992), further supporting the view that LNCaP cells represent a model for human prostatic carcinoma, the second leading cause of cancer death and the most prevalent cancer in men (Carter and Coffey, 1990; Scardino et al., 1992). Like breast cancer, it is generally felt that for prostate cancer there is a need for innovative therapies. Our scant knowledge of the mechanisms responsible for androgen- and oestradiol-dependent growth has hindered new treatments. The present paper has filled some of this gap.
We now observe that the synthetic androgen R1881 induces S-phase entry of LNCaP cells through stimulation of the entire Src/Raf/Erks pathway. In fact, Src and MEK-1 small molecule inhibitors as well as their dominant-negative forms block S-phase entry of LNCaP cells. Similar findings are observed when LNCaP cells are stimulated by oestradiol. In our previous reports on MCF-7 cells, oestradiol also activated this pathway (Migliaccio et al., 1996; Castoria et al., 1999). In MCF-7 cells, oestradiol action is mediated by ERα, in LNCaP cells ERβ is involved in the hormonal action. Erk stimulation by androgens in different whole cells has recently been observed (Peterziel et al., 1999). However, this stimulation was not linked to upstream Src and Raf activation, and its role was not investigated.
In cells expressing AR and ER, like LNCaP cells, stimulation by androgen or oestradiol couples classical steroid receptors to the cytosolic signalling pathway and cell proliferation through induction of an ER–AR–Src ternary complex. Similar events are also detectable in cells derived from human mammary cancer and expressing ERα. This supports a general role of these events. Similar results have been obtained in Cos cells co-transfected with wild-type AR, Erβ or ERα. This observation makes it unlikely that the oestradiol-induced complex in LNCaP cells is due to binding of the hormone to point-mutated AR. This ternary complex is strictly required to activate Src; hormonal antagonists prevent both complex assembly and pathway activation. Under basal conditions, the catalytic domain of the Src family kinases is constrained in an inactive state through two distinct intramolecular interactions. Binding of the SH2 domain to the C-terminal phosphorylated tyrosine locks the molecule in an inhibited conformation (Matsuda et al., 1990). Binding of the SH3 domain to the SH2 kinase linker is also important for Src inactivation (Superti-Furga et al., 1993). Full catalytic activity requires release of these restraints. In fact, the kinase activity of Src can be enhanced by binding of the SH2 domain to phosphotyrosine-containing sequences, binding of the SH3 domain to proline-rich sequences, dephosphorylation of the C-terminal phosphotyrosine (Hubbard et al., 1998; Xu et al., 1999; Schlessinger, 2000). Our data strongly support the view that direct protein–protein interactions are responsible for Src activation by steroid hormones. We observe that Src interacts with ERα through its SH2 domain. Phosphotyrosine 537 of HEG0, initially identified in our laboratory (Castoria et al., 1993), plays a major role in the interaction of ERα with the Src SH2 domain. This residue has also been reported to be the only phosphotyrosine in ERα in MCF-7 cells (Arnold et al., 1995) and is in a key position (White et al., 1997). The ER–Src association is required not only for oestradiol, but also for progestin signal activation (Migliaccio et al., 1998). In fact, from our present experiments it appears that phosphotyrosine 537 is required for Src activation by progestin. We also observe that Src interacts with AR through its SH3 domain. AR contains proline-rich sequences; our findings show that one of them is responsible for the observed interaction. Interestingly, we observe that in Cos cells co-transfected with ERα and AR, and stimulated by either androgen or oestradiol, Src is more efficiently activated than in Cos cells transfected with only one of these receptors. This should be the consequence of the unrestrained conformation of the kinase when Src, through the SH2 and SH3 domains, interacts with the two steroid receptors. A model of the ternary complex is presented in Figure 9.
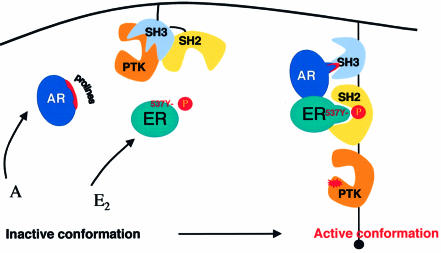
Fig. 9. Model of Src activation by androgen or oestradiol. The kinase domain of Src is held in a restrained, inactive conformation by intramolecular interactions: binding of SH2 with PTyr527 and binding of SH3 with the SH2 kinase linker. Each steroid (A or E2) in the presence of both AR and ERα is able to enhance kinase activity by inducing binding of a proline-rich sequence of AR to SH3 of Src and phosphotyrosine 537 of ERα to SH2, thus changing the inactive conformation of Src (left) to a ‘fully’ active conformation (right). In the presence of either AR or ER alone, androgen or oestradiol leads to a binary AR/Src or ER/Src complex. In this complex, Src might assume an intermediary conformation corresponding to ‘partial’ activation. ERβ behaves like ERα.
The role of ERβ in oestradiol activation of Erk-2 and DNA synthesis has recently been observed in transfected Chinese hamster ovary (CHO) cells (Razandi et al., 1999). In the present experiments, ERβ behaves like ERα. It forms a binary complex with Src in Cos cells transfected with ERβ cDNA alone and a ternary complex with AR and Src in LNCaP cells or Cos cells co-transfected with ERβ and AR cDNAs. Like ERα, ERβ undergoes association with the SH2 domain of Src. Since hERβ is highly homologous to hERα in the hormone-binding domain (HBD) (Kuiper et al., 1998) and the residue corresponding to Tyr537 of hERα is conserved at position 443 (Enmark et al., 1997), we expect that phosphorylation of this residue is involved in the interaction with the SH2 domain of Src.
Cross-talk between two steroid receptors (PR-B and ERα) has been described previously (Migliaccio et al., 1998). In T47D cells and in transiently transfected Cos cells, progestin binds PR-B and induces activation of Src through association of Src with ERα. Nevertheless, molecular and functional differences exist between the cross-talk between PR-B and ERα and that described in this paper. PR-B is associated with ERα in the absence of agonist, whereas AR and ERβ or ERα undergo association in the presence of agonists. PR-B does not associate with Src, whereas AR and ER do. No ternary complex between PR-R, ERα and Src can be detected (Migliaccio et al., 1998). Furthermore, the biological relevance of these differences is strengthened by the finding that anti-oestrogens block progestin stimulation of T47D cell growth (Migliaccio et al., 1998), whereas anti-progestins do not inhibit the stimulatory effect of oestradiol on the same cells (unpublished). In contrast, in LNCaP and transfected Cos cells, the oestradiol effects are inhibited by anti-androgen and those of androgen are prevented by anti-oestrogen. On the basis of these findings, we propose the existence of a functional one-way cross-talk between PR-B and ER, whereas a reciprocal cross-talk occurs between AR and ER. The first is required for progestin activation of Src, the latter to fully activate Src.
Our studies show that cross-talk between steroid receptors takes place in vivo, thus regulating the non-genomic proliferative pathways of different steroids. Activation of these pathways represents an important mechanism by which progestins stimulate proliferation of mammary cancer cells (Migliaccio et al., 1998; Castoria et al., 1999), and oestradiol and androgen stimulate S-phase entry of prostate and mammary cancer cells. Identification of mutant receptors, which have lost the ability to cooperate in signalling pathway activation, might contribute to a better understanding of the role of this cross-talk. Furthermore, the finding of steroid receptor mutants that constitutively activate the cell cycle machinery, either through the Src/Ras/Erks pathway or using alternative pathways, will be a powerful tool to study the transition from hormone-dependent to -independent cell proliferation.
Materials and methods
Constructs
The cDNAs encoding hAR, the wild-type (HEG0) form of hERα and hERβ were cloned into expression vector pSG5 as reported (Chang et al., 1988; Tora et al., 1989; Kuiper and Gustafsson, 1997). cDNA encoding the kinase-inactive form of Src (Lys259→Met) was cloned into pSG5 as described (Barone and Courtneidge, 1995). cDNA encoding kinase-inactive MEK-1 (Ser221→Ala, A221-MEK1) was kindly provided by J.Downward. It was subcloned into pEXV3 as reported (Cowley et al., 1994). Chicken full-length Src cDNA as well as the ΔSH2 and ΔSH3 deletion mutants were subcloned into plasmid vector pGEX-2TK (Pharmacia) from pSGTSrc, pRSP-ΔSH2 and pSGT-1ΔSH3. The Src SH2 domain was amplified by PCR from the pSGT-1Src vector using oligos (GENSET) from the N- and C-termini of the SH2 domain with the added restriction sites BamHI (5′-CTGAGGATCCATCCAGGCTGA AGAGTGG-3′) and EcoRI (5′-GACTGAATTCGGACGTGGGGCAGA-3′). The Src SH3 domain was amplified by PCR from the pSGT-1Src vector using oligos from the N- and C-termini of the SH3 domain with the added restriction sites BamHI (5′-GCACAGGATCCGCTGGCGGCG TCACCACTTTCGTG-3′) and EcoRI (5′-CTGAGAATTCAGCCT GGATGGAGTCTGAGGGCGCGAC-3′). The resulting PCR fragments were digested with BamHI and EcoRI, gel purified and ligated into pGEX-2TK. The pGEX-Src constructs were used to generate GST–Src fusion proteins. The pEGFP-SH2 construct was obtained from the pSGT-1Src vector by PCR amplification of the Src SH2 domain, using oligos (GENSET) from the N- and C-termini of the SH2 domain with the added restriction sites EcoRI (5′-CTGAGAATTCTCAGACTCCA TCCAGGCTGAAGAGTGG-3′) and BamHI (5′-CTGAGGATCCGG ACGTGGGGCAGACGTTGGTCAG-3′). The resulting PCR fragment was digested with EcoRI and BamHI, gel purified and ligated into the plasmid vector pEGFP (C2 from Clontech). All PCR products as well as junctions were verified by sequencing. HEG537 cDNA encoding the point-mutated hER was obtained from wild-type HEG0 by substituting the 500 bp BglII fragment with the same from HE537F (Castoria et al., 1993).
Cell culture, transfection and microinjection techniques
Human prostate cancer LNCaP-FGC cells (passage 7; American Type Culture Collection) were grown at 37°C in 5% CO2 in air in RPMI-1640 medium supplemented with phenol red, l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 U/ml), gentamicin (50 µg/ml) and 10% fetal calf serum. For cell counting, LNCaP cells were plated at 40% confluence in multiwell tissue culture plates and maintained in phenol red-free RPMI-1640 containing insulin (Humulin I, 20 U/l) and charcoal-stripped calf serum (CSCS) for 4 days. The cells were then left unstimulated or stimulated for the indicated times with either R1881 (Zeneca, Italy) or oestradiol (Sigma). The effect of either Casodex or ICI 182,780 (both from Zeneca, Italy) on cell number was monitored using a 1000-fold excess of each antagonist. Cells were finally counted in a haemocytometer in quadruplicate as described (Auricchio et al., 1995). For S-phase entry, LNCaP cells were seeded onto gelatine-precoated coverslips at 40–50% confluence, then maintained for 3 days in phenol red-free RPMI-1640 medium containing insulin (Humulin I, 20 U/l) and CSCS. The cells were then left unstimulated or stimulated for 24 h with either the androgen R1881, oestradiol or EGF (recombinant EGF; Boehringer). When indicated, cells were stimulated with R1881 plus oestradiol. The effect of either Casodex or ICI 182,780 on S-phase entry was monitored using a 1000-fold excess of antagonist. The effect of inhibitors on steroid-stimulated S-phase entry was analysed using the indicated concentrations of PP1 and PD98059 (Calbiochem, CA). MCF-7 and T47D cells were grown as described (Castoria et al., 1999). For cell counting, they were plated at 40% confluence in multi-well tissue culture plates, then made quiescent according to the same report. The cells were then left unstimulated or stimulated for the indicated times with either R1881 or oestradiol. The effect of either Casodex or ICI 182,780 on cell number was monitored using a 1000-fold excess of each antagonist. Cells were finally counted in a haemocytometer in quadruplicate as described (Auricchio et al., 1995). For S-phase entry, the cells were seeded onto gelatine-precoated coverslips at 40–50% confluence, then made quiescent. The cells on coverslips were left unstimulated or stimulated for 24 h with the indicated steroids, in the absence or presence of a 1000-fold excess of antagonist. Cos-7 cells were cultured and transfected as previously reported (Migliaccio et al., 1998) using 2.5 µg of plasmid pSG5 encoding either hAR, hERβ, hERα or hPR-B. Empty plasmid pSG5 (at 2.5 µg) was in each case transfected as a control. Microinjection experiments were performed in quiescent cells as described (Castoria et al., 1999). Empty plasmid pSG5 (at 25 ng/µl) was injected into cell nuclei together with 25 ng/µl of GFP-expressing plasmid (pEGFP; Clontech, CA), as an injection marker. A221-MEK-1 was injected at 40 ng/µl together with 10 ng/µl GFP-expressing plasmid, to help in identification of injected cells. All other plasmids were injected at 50 ng/µl. The indicated steroids were added 4–6 h after injection of cDNAs and the cells incubated at 37°C for another 24 h.
Immunofluorescence and DNA synthesis analysis
DNA synthesis was assayed by a 6 h pulse with 100 µM BrdU (Boehringer). Cells on coverslips were fixed, permeabilized and stained for Src as described (Castoria et al., 1999). Quantification from co-injection experiments with dominant-negative MEK-1 was obtained by immunostaining of MEK-1 with diluted [1:50 in phosphate-buffered saline (PBS)] anti-MEK-1 antibody (Ab C-18; Santa Cruz Biotechnology) followed by incubation with diluted (1:100) Texas red-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories). It showed that >90% of cells expressing GFP were positive for MEK-1. BrdU incorporation was assessed as reported (Castoria et al., 1999). Cell nuclei were stained with Hoechst 33258 (Sigma) and the coverslips were finally inverted and mounted in Moviol (Calbiochem). Slides were analysed using an Axiophot fluorescent microscope (Zeiss).
Production of recombinant proteins
The GST–HEG14 fusion protein was produced and purified as reported (Abbondanza et al., 1998). All other GST fusion protein constructs, along with the parental pGEX-2T carrying GST alone, were transformed into Escherichia coli JM109 cells. Fusion proteins were extracted as reported (Kaelin et al., 1991), with minor modifications. Briefly, the cells were resuspended in a one-hundredth volume and extract obtained by three cycles of freezing and thawing in lysis buffer [PBS, 1% Triton X-100 pH 7.4, containing the protease inhibitors leupeptin, antipain and pepstatin (LAP) at 10 µg/ml and 1 mM phenylmethylsulfonyl fluoride (PMSF)]. Lysozyme at 5 mg/ml was added to the lysis buffer and lysates clarified at 13 000 r.p.m. Fusion proteins were then purified on glutathione–agarose beads (Fluka) by incubating 1 ml of supernatant with 40 µl of beads (1:1 in PBS containing 1% Triton X-100, 10 µg/ml LAP, 1 mM PMSF) for 1 h at 4°C. After three washings with PBS (containing 0.2% Triton X-100, 10 µg/ml LAP, 1 mM PMSF), the matrix with adsorbed fusion proteins was utilized for protein–protein interaction assay.
Protein–protein interaction assay
Coupled in vitro transcription/translation reactions were used to produce 35S-labelled human oestrogen receptors α (HEG0 and HEG537F) and β (hERβ), human androgen receptor (hAR) and chicken Src and Src derivatives (SH2, SH3, ΔSH2 and ΔSH3) in rabbit reticulocyte lysate (Promega). For protein–protein interaction assay, the matrix with adsorbed fusion proteins was incubated with radiolabelled proteins for 1 h at room temperature by gentle shaking in PBS (containing 0.2% Triton X-100, 10 µg/ml LAP, 1 mM PMSF) in the absence or presence of hormone. Beads were washed three times in the same buffer and proteins eluted with Laemmli sample buffer. They were finally resolved by SDS–PAGE and protein bands revealed by fluorography.
Immunoprecipitation and kinase assays
Immunoprecipitation of cell lysates with either anti-Src, anti-Raf-1 or anti-Erk-2 antibodies was performed as reported (Migliaccio et al., 1998; Castoria et al., 1999). Src, Raf-1 and Erk-2 kinase activities were assayed according to the same reports. Immunoprecipitation of AR from LNCaP cells was performed at 4°C for 18 h using polyclonal rabbit anti-hAR antibody (1 µg/2 mg of cellular protein; clone PA1-110; ABR, Golden, CO). Immunoprecipitation of ERβ from either LNCaP or transfected Cos cells was performed at 4°C for 18 h using polyclonal rabbit anti-hERβ antibody (1 µg/2 mg of cellular protein; UBI, Lake Placid, NY). Protein concentrations were measured with a Bio-Rad protein assay kit (Bio-Rad, CA). Immunocomplexes were precipitated using 30 µl of protein A–Sepharose CL4B (Pharmacia BioTech) pre-equilibrated with lysis buffer. Protein A–Sepharose beads were washed three times with 1 ml of lysis buffer and immunocomplexes reduced using 60 µl of Laemmli sample buffer.
Electrophoresis and immunoblotting
All procedures were performed as described (Migliaccio et al., 1996, 1998; Castoria et al., 1999). Human AR from either LNCaP or transfected Cos-7 cells was detected using polyclonal rabbit anti-AR antibody (clone PA1-110; ABR). Human ERβ was revealed using goat polyclonal anti-ERβ antibodies (clone N19; Santa Cruz). Human ERα was detected using H222 monoclonal antibody as described (Auricchio et al., 1995). Immunoreactive proteins were revealed using an ECL detection system (Amersham, UK).
Acknowledgments
Acknowledgements
We are grateful to G.Santelli and A.Mineo for allowing access to equipment in their laboratory. We thank J.Downward for the A221-MEK-expressing plasmid, P.Chambon for the HEG0-expressing plasmid, J.A.Gustafsson for the hERβ-expressing plasmid, S.Gonfloni for pRSP-ΔSH2, S.A.Courtneidge for pSGT-1Src and pSGT-1ΔSH3, and M.Capra for LNCaP-FGC. The H222 anti-ERα monoclonal antibodies were kindly provided by Abbott Laboratories (Abbott Park, IL) and Zeneca (Italy) provided the anti-oestrogen ICI 182,780 and the anti-androgen Casodex. The editorial assistance of Gian Michele La Placa as well as the technical assistance of Flavia Vitale, Domenico Piccolo and Leandra Sepe are also acknowledged. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro and Ministero dell’Università e della Ricerca Scientifica (Cofinanziamento MURST 1997 e 1998, Fondi 40 e 60%).
References
- Abbondanza C. et al. (1998) Interaction of vault particles with estrogen receptor in the MCF-7 breast cancer cell. J. Cell Biol., 141, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu-Martin M.T., Chari,A., Palladino,A.A., Craft,N.A. and Sawyers,C.L. (1999) Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol. Cell. Biol., 19, 5143–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi D.R., Cuenda,A., Cohen,P., Dudley,D.T. and Saltiel,A.R. (1995) PD098059 is a specific inhibitor of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem., 270, 27489–27494. [DOI] [PubMed] [Google Scholar]
- Arnold S.F., Obourn,J.D., Jaffe,H. and Notides,A.C. (1995) Phosphoryl ation of the human estrogen receptor on tyrosine 537 in vivo and by Src family tyrosine kinases in vitro. Mol. Endocrinol., 9, 24–33. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Di Domenico,M., Migliaccio,A., Castoria,G. and Bilancio,A. (1995) The role of estradiol receptor in the proliferative activity of vanadate in MCF-7 cells. Cell Growth Differ., 6, 105–113. [PubMed] [Google Scholar]
- Barone M.V. and Courtneidge,S.A. (1995) Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature, 378, 509–512. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich,P. and Schutz,G. (1995) Steroid-hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- Carter H.B. and Coffey,D.S. (1990) The prostate: an increasing medical problem. Prostate, 16, 39–48. [DOI] [PubMed] [Google Scholar]
- Castagnetta L.A., Miceli,M.D., Sorci,C.M.G., Pfeffer,U., Farruggio,R., Oliveri,G., Calabrò,M. and Carruba,G. (1995) Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology, 136, 2309–2319. [DOI] [PubMed] [Google Scholar]
- Castoria G., Migliaccio,A., Green,S., Di Domenico,M., Chambon,P. and Auricchio,F. (1993) Properties of a purified estradiol-dependent calf uterus tyrosine kinase. Biochemistry, 32, 1740–1750. [DOI] [PubMed] [Google Scholar]
- Castoria G., Barone,M.V., Di Domenico,M., Bilancio,A., Ametrano,D., Migliaccio,A. and Auricchio,F. (1999) Non-transcriptional action of estrogen and progestin triggers DNA synthesis. EMBO J., 18, 2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.S., Kokontis,J. and Liao,S.T. (1988) Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science, 240, 324–326. [DOI] [PubMed] [Google Scholar]
- Chen Z., Yuhanna,I.S., Galcheva-Garcova,Z., Karas,R.H., Mendelsohn,M.E. and Shaul,P.W. (1999) Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Invest., 103, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S., Paterson,H., Kemp P. and Marshall,C.J. (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH3T3 cells. Cell, 77, 841–852. [DOI] [PubMed] [Google Scholar]
- Di Domenico M., Castoria,G., Bilancio,A., Migliaccio,A. and Auricchio,F. (1996) Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res., 56, 4516–4521. [PubMed] [Google Scholar]
- Dickson R.B. and Lippman,M.E. (1987) Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocr. Rev., 8, 29–43. [DOI] [PubMed] [Google Scholar]
- Endoh H., Sasaki,H., Maruyama,K., Takeyama,K., Waga,I., Shimizu,T., Kato,S. and Kawashima,H. (1997) Rapid activation of MAP kinase by estrogen in the bone cell line. Biochem. Biophys. Res. Commun., 235, 99–102. [DOI] [PubMed] [Google Scholar]
- Enmark E., Pelto-Huikko M., Grandien,K., Lagerkrantz,J., Fried G., Nordenskjold,M. and Gustafsson,J.A. (1997) Human estrogen receptor β—gene structure, chromosomal localization and expression pattern. J. Clin. Endocrinol. Metab., 82, 4258–4265. [DOI] [PubMed] [Google Scholar]
- Evans R.M. (1988) The steroid and thyroid hormone receptor superfamily. Science, 240, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. (1997) Do we need new paradigms? Endocr. News, 22, 4-12. [Google Scholar]
- Hanke J.H., Gardner,J.P., Dow,R.L., Changeliau,P.S., Brisselte,W.H., Weringer,E.J., Pollok,B.A. and Connelly,P.A. (1996) Discovery of a novel, potent and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem., 271, 695–701. [DOI] [PubMed] [Google Scholar]
- Horoszewics J.S., Leong,S.S., Kawinski,E., Karr,J.P., Rosenthal,H., Chu,T.M., Mirand,E.A. and Murphy,G.P. (1983) LNCaP model of human prostatic carcinoma. Cancer Res., 43, 1809–1818. [PubMed] [Google Scholar]
- Hubbard S.R., Mohammadi,M. and Schlessinger,J. (1998) Auto regulatory mechanisms in protein-tyrosine kinases. J. Biol. Chem., 273, 11987–11990. [DOI] [PubMed] [Google Scholar]
- Kaelin W.G., Pallas,D.C., De Caprio,J.A., Kaye,F.J. and Livingston,D.M. (1991) Identification of cellular proteins that can interact with the T/E1A-binding region of the retinoblastoma product. Cell, 64, 521–532. [DOI] [PubMed] [Google Scholar]
- Kuiper G.G.J.M. and Gustafsson,J.A. (1997) The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett., 410, 87–90. [DOI] [PubMed] [Google Scholar]
- Kuiper G.G.J.M., Carlsson,B., Grandien,K., Enmark,E., Häggblad,J., Nilsson,S. and Gustafsson,J.A. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology, 138, 863–870. [DOI] [PubMed] [Google Scholar]
- Kuiper G.G.J.M., Lemmen,J.G., Carlsson,B., Corton,J.C., Safe,S.H., van der Saag,P.T., van der Burg,B. and Gustafsson,J.A. (1998) Interaction of estrogenic chemicals and phytestrogens with estrogen receptor β. Endocrinology, 139, 4252–4263. [DOI] [PubMed] [Google Scholar]
- Lee H.W. and Eghbali-Webb,M., (1998) Estrogen enhances proliferative capacity of cardiac fibroblasts by estrogen receptor- and mitogen-activated protein kinase-dependent pathway. J. Mol. Cell. Cardiol., 30, 1359–1368. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell D.S., Chiappetta,C. and Stancel,G.M. (1988) Estrogen regulation of c-fos messenger ribonucleic acid. Mol. Endocrinol., 2, 946–951. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Mayer,B.J., Fukui,Y. and Hanafusa,H. (1990) Binding of transforming protein, P47gag-crk to a broad range of phosphotyrosine-containing proteins. Science, 248, 1537–1539. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. and Alves,S.E. (1999) Estrogen actions in the central nervous system. Endocr. Rev., 20, 279–307. [DOI] [PubMed] [Google Scholar]
- McKenna N.J., Lanz,R.B. and O’Malley,B.W. (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev., 20, 321–344. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Pagano,M. and Auricchio,F. (1993) Immediate and transient stimulation of protein tyrosine phosphorylation by estradiol in MCF-7. Oncogene, 8, 2183–2191. [PubMed] [Google Scholar]
- Migliaccio A., Di Domenico,M., Castoria,G., de Falco,A., Bontempo,P., Nola,E. and Auricchio,F. (1996) Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol–receptor complex in MCF-7 cells. EMBO J., 15, 1292–1300. [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Piccolo,D., Castoria,G., Di Domenico,M., Bilancio,A., Lombardi,M., Gong,W., Beato,M. and Auricchio,F. (1998) Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen. EMBO J., 17, 2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M.G. and White,R. (1996) Nuclear receptors spring into action. Nature Struct. Biol., 3, 113–115. [DOI] [PubMed] [Google Scholar]
- Peterziel H., Mink,S., Schonert,A., Becker,M., Kocler,H. and Cato,A.C.B. (1999) Rapid signalling by androgen receptor in prostate cancer cells. Oncogene, 18, 6322–6329. [DOI] [PubMed] [Google Scholar]
- Pietras R.J. et al. (1995) HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene, 10, 2435–2446. [PubMed] [Google Scholar]
- Power R.F., Mani,S.K., Codina,J., Conneely,O.M. and O’Malley,B.W. (1991) Dopaminergic and ligand-independent activation of steroid hormone receptor. Science, 254, 1636–1639. [DOI] [PubMed] [Google Scholar]
- Razandi M., Pedram,A., Greene,G.L. and Levin,E.R. (1999) Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol., 13, 307–319. [DOI] [PubMed] [Google Scholar]
- Revelli A., Massobrio,M. and Tesarik,J. (1998) Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev., 19, 3–17. [DOI] [PubMed] [Google Scholar]
- Scardino P.T., Weaver,R. and Hudson,M.A. (1992) Early detection of prostate cancer. Hum. Pathol., 23, 211–222. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. (2000) New roles for Src kinases in control of cell survival and angiogenesis. Cell, 100, 293–296. [DOI] [PubMed] [Google Scholar]
- Schuurmans A.L., Bolt,J., Velscholte,J. and Mulder,E. (1991) Regulation of growth of LNCaP human prostate tumor cells by growth factors and steroid hormones. J. Steroid Biochem. Mol. Biol., 40, 193–197. [DOI] [PubMed] [Google Scholar]
- Singer C.A., Figueroa-Masot,X.A., Batchelor,R.H. and Dorsa,D.M. (1999) The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci., 19, 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Setáló,G., Guan,X., Warren,M. and Toran-Allerand,C.D. (1999) Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J. Neurosci., 19, 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenschein C., Olea,N., Pasanen,M.E. and Soto,A.M. (1989) Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res., 49, 3474–3481. [PubMed] [Google Scholar]
- Superti-Furga G., Fumagalli,S., Koegl,M., Courtneidge,S.A. and Draetta,G. (1993) Csk inhibition of c-Src activity requires both the SH2 and SH3 domains of Src. EMBO J., 12, 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., Mullick,A., Metzger,D., Ponglikitmongkol,M., Park,I. and Chambon,P. (1989) The cloned human estrogen receptor contains a mutation which alters its hormone binding properties. EMBO J., 8, 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldscholte J. et al. (1990) A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun., 173, 534–540. [DOI] [PubMed] [Google Scholar]
- Watters J.J., Campbell,J.S., Cunningham,M.J., Krebs,E.G. and Dorsa,D.M. (1997) Rapid membrane effects of steroids in neuroblastoma cells: effect of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology, 138, 4030–4033. [DOI] [PubMed] [Google Scholar]
- Wehling M. (1997) Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol., 59, 365–393. [DOI] [PubMed] [Google Scholar]
- Weisz A. and Bresciani,F. (1993) Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit. Rev. Oncog., 4, 361–388. [PubMed] [Google Scholar]
- White R., Sjöberg,M., Kalkhoven,E. and Parker,G.M. (1997) Ligand independent activation of oestrogen receptor by mutation of a conserved tyrosine. EMBO J., 16, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding G. (1992) The importance of steroid hormones in prostate cancer. Cancer Surv., 14, 113–130. [PubMed] [Google Scholar]
- Wilson J.D. (1980) The pathogenesis of benign prostatic hyperplasia. Am. J. Med., 68, 745–747. [DOI] [PubMed] [Google Scholar]
- Xu W., Doshi,A., Lei,M. Eck,M.J. and Harrison,S.C. (1999) Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell, 3, 629–638. [DOI] [PubMed] [Google Scholar]
- Yeh S., Lin,H.-K., Kang,H.-Y., Thin,T.H., Lin,M.-F. and Chang,C. (1999) From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc. Natl Acad. Sci. USA, 96, 5458–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bai,W., Allgood,V.E. and Weigel,N.L. (1994) Multiple signalling pathways activate chicken progesterone receptor. Mol. Endocrinol., 8, 577–584. [DOI] [PubMed] [Google Scholar]