Abstract
γ-aminobutyric acid (GABA) acting on Cl−-permeable ionotropic type A (GABAA) receptors (GABAAR) is the major inhibitory neurotransmitter in the adult central nervous system of vertebrates. In immature brain structures, GABA exerts depolarizing effects mostly contributing to the expression of spontaneous activities that are instructive for the construction of neural networks but GABA also acts as a potent trophic factor. In the present paper, we concentrate on brainstem and spinal motoneurons that are largely targeted by GABAergic interneurons, and we bring together data on the switch from excitatory to inhibitory effects of GABA, on the maturation of the GABAergic system and GABAAR subunits. We finally discuss the role of GABA and its GABAAR in immature hypoglossal motoneurons of the spastic (SPA) mouse, a model of human hyperekplexic syndrome.
1. Introduction
γ-aminobutyric acid (GABA) is, with glycine, the major inhibitory neurotransmitter in the adult central nervous system (CNS) of vertebrates. GABA acts on Cl−-permeable ionotropic bicuculline-sensitive type A (GABAA) receptors (GABAAR) and metabotropic baclofen-sensitive GABABR, these latter being coupled through G-proteins to K+ and Ca2+ channels in neuronal membranes. More recently, it has been shown that GABA also activates Cl−-permeable bicuculline- and baclofen-insensitive GABACR, this receptor subtype being largely expressed in the retina and at lower level in other CNS area [1]. If all GABA receptors are present on the cell membrane, the common view is that GABABR are presynaptically located, whereas GABAAR and GABACR are postsynaptically. However, all GABA receptors seem to be located pre- and/or post-synaptically [2–5].
GABA is synthesized from the amino acid glutamate by the enzyme glutamic acid decarboxylase (GAD), this latter being present as two isoforms with different molecular weights of 65-kDa and 67-kDa [6]. The two GAD isoforms are product of two different genes. GAD65 gene (GAD2) is located on chromosome 10 (10p11.23) in human and on chromosome 2 (2 9.0 cM) in mouse, while GAD67 gene (GAD1) is located on chromosome 2 (2q31) in human and in chromosome 2 (2 43.0 cM) in mouse [7, 8]. In addition, during mouse and rat embryonic development, two alternatively splices forms are also synthesized from the GAD67 gene: the truncated 25-kDA leader (GAD25) and the enzymatically active protein GAD44 (for review, see [9]). GAD25 is a protein without GAD enzymatic activity. GAD25 and GAD44 are expressed during the development of the CNS, they are more abundant in proliferating progenitors [9–11], and they are downregulated during neuronal differentiation concomitant with an upregulation of GAD67 expression [12–14]. The 67-kDa GAD form is diffusely distributed in the cytoplasm of the cells, while the 65-kDa GAD form is mainly found attached to synaptic vesicles [15].
During CNS development, GABA exhibits a large panel of activity ranging from the control of cell proliferation to the formation of synapses (for review, see [16–19]). In immature brain structures, most studies described GABA as operating through GABAAR subclass [18, 20], and it was first proposed that the other GABAR subclasses were not functional at early stage of life [21]. However, this hypothesis was invalidated by the observation of a pre- and post-synaptic GABABR expression in the embryonic rat neocortex [22] and the modulation of cortical neuronal migration by GABABR activation [23–25]. GABABR activation triggers BDNF release and promotes the maturation of GABAergic synapses [26]. Finally, it has been shown that GABA can control the locomotor network in the rat neonatal spinal cord by acting on presynaptic GABABR as well as on postsynaptic GABAAR [27]. In the brainstem, it has been recently shown that the interaural time difference detection circuit is differentially controlled by GABABR during the second post-natal week [28]. An endogenous modulation of respiratory rhythm by GABABR that increases after birth has also been reported [29]. Finally, functional GABACR were detected in the spinal motoneurons (MNs) around birth, but a little is known about the function of these receptors in the immature spinal cord [1].
GABAAR-related effects on immature neuronal cells are opposed to those observed on mature neurons in the sense that GABA exerts depolarizing effects during development, while it induces hyperpolarizing effects in most mature CNS regions [30]. Such depolarizing GABA-mediated effects, coupled with conventional excitatory effect of glutamate and other classical neurotransmitters such as acetylcholine, lead to Ca2+ influx and generate spontaneous electrical activities that are the features of almost all immature structures of the CNS [31, 32]. Numerous studies have demonstrated the permissive role of depolarizing GABA in the maturation of neurite outgrowth [33], in promoting both excitatory and inhibitory synaptogenesis [34] and in controlling its switch from depolarizing to hyperpolarizing [35, 36].
Brainstem and spinal motoneurons that are largely targeted by GABAergic interneurons require an appropriate maturation of their GABA receptors and GABA innervations. In the present paper, we will describe the ontogeny of the GABAergic system in spinal MNs in parallel to the establishment of an inhibitory transmission, and then we will present data about the maturation of GABA receptors in hypoglossal motoneurons (HMs, motoneurons innervating the tongue) of the spastic (SPA) mouse, a model of human hyperekplexic syndrome in which the impaired glycinergic neurotransmission [37] may be compensated, in certain strain lines, by an increased aggregation of GABAAR [38, 39]. The hyperekplexic syndrome, as well as the amyotrophic lateral sclerosis (ALS) pathology, highlights the plasticity of the GABAergic system that may temporally compensate genetic alteration of other inhibitory systems [40, 41].
2. Maturation of Chloride-Mediated Inhibition in MNs
GABA, when binding to GABAAR, exerts effects that are mainly dependent upon the chloride equilibrium potential (E Cl). In mature neurons, the intracellular Cl− concentration [Cl−]i is lower than extracellular Cl− concentration [Cl−]o and the activation of the chloride permeable channels by GABA induces a chloride influx. However, in immature neurons that express a higher [Cl−]i compared to [Cl−]o, GABA acts as an excitatory neurotransmitter. Hence, during CNS development, a switch from excitatory to inhibitory effects of GABA occurs. In the mouse pre-Bötzinger complex (PBC), a brainstem respiratory structure that drives the rhythmic activity of the hypoglossal motoneurons, gramicidin perforated patch-clamp recordings that preserve the physiological [Cl−]i indicate that the reversal potential of GABAAR-mediated current (EGABAAR that corresponds to E Cl) switches from depolarizing to hyperpolarizing within the first postnatal (P) week (EGABAAR drops from −44 mV at P2 to −71 mV at P4) [42]. Because the resting membrane potential (rmp) for all PBC neurons was −56 mV, a switch from excitatory to inhibitory effects of GABA is evidenced between P2 and P4. Results obtained from gramicidin perforate-patched HMs are in good agreement with those collected in PBC neurons, because E Cl is measured as being −37 mV in neonates HMs (P2) and −73 mV in juveniles HMs (P15), but the exact time of the switch remains undetermined between P2 and P15 (rmp of HMs is −70 mV) [43]. However, two other studies [44, 45] reported that by birth, GABA induces a hyperpolarization of the membrane potential in respiratory medullary neurons and a suppression of respiratory frequency. These studies, which are based on gramicidin perforated-patch clamp recordings, rather indicate that the transition from excitatory to inhibitory effects occurs at approximately E19 but not during post-natal stages in respiratory networks. From a technical point of view, measures of the GABAAR-related driving force may be considered with caution because invasive recordings (including perforated patch-clamp) combined with large input resistances of immature neurons may lead to inexact resting membrane potential values, true resting membrane potential values being more hyperpolarized (see [46]).
When does the switch from excitatory to inhibitory effects of GABA occur in spinal MNs? We have showed that there is a shift of EGABAAR toward negative values during the embryonic development of mouse lumbar spinal MNs [47]. Our data demonstrated that until E15.5, E Cl is above the spike threshold, whereas after E16.5, it drops significantly below spike threshold. During the course of the embryonic development, rmp of mouse spinal MNs remains below the E Cl. However, if GABAAR activation may trigger the firing of MNs until E15.5, after this embryonic developmental stage, such activation, although producing a depolarization, fails to trigger action potentials [47] (Figure 1). Our results indicate that GABA likely exerts a shunting action on mouse spinal MNs after E15.5, as demonstrated in the neonate rat spinal cord [48] and also described in current-clamp experiments by Hubner and collaborators in E18.5 mouse spinal MNs [49]. This shunting depolarizing GABA effect likely persists during postnatal stages even though our experimental measurements indicate that E Cl reaches MNs rmp at P0 [47]. A recent study based on conventional intracellular recordings clearly demonstrated that the shift from excitatory to inhibitory IPSPs occurs at P4-5 in rat spinal MNs [50]. This was in agreement with intracellular recordings performed by Wu and collaborators showing much smaller (but still) depolarizing effects of GABA at P0 compared to E16–E18 in rat spinal MNs [51]. Another study, based on gramicidin perforated patch-clamp recordings, indicates that EGABAAR shifts between P5 and P10 in mouse spinal MNs, that is, at a later developmental stage compared to the rat [52]. Hence, further experiments would be needed to precisely determine whether the switch from excitatory to inhibitory effects of GABA really occurs in mouse spinal MNs, and it would be interesting to determine whether an oxytocin-driven transient loss of chloride occurs at birth in spinal MNs as described in hippocampal neurons [53].
Figure 1.
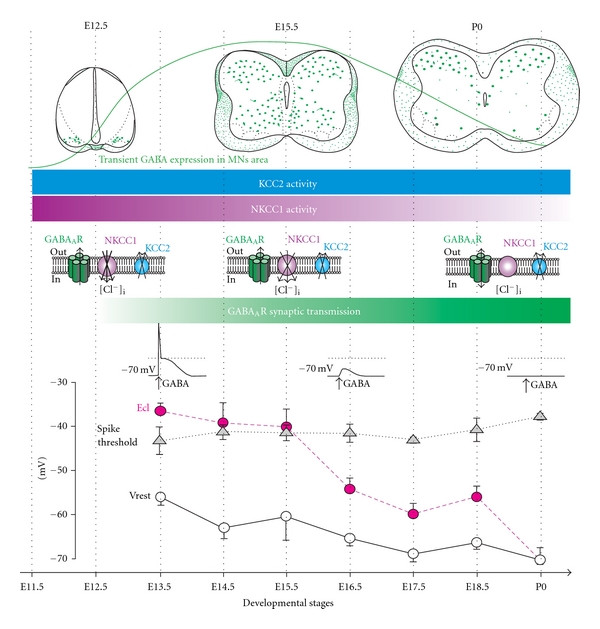
Development of the GABAAR-mediated inhibitory transmission in mouse lumbar spinal MNs. From top to bottom: schematic drawings (frontal views) depict the transient expression of GABA in spinal ventral interneurons (in green), while horizontal bars indicate the permanent KCC2 (in blue) and transient NKCC1 activity (in violet). The color intensity encodes the level of activity. NKCC1 inactivation combined to KCC2 activity leads to a significant decrease in [Cl−]i and a disappearance of GABAAR-mediated excitatory effects. In parallel to the maturation of the chloride cotransporters KCC2 and NKCC1, the spinal cord starts to convey first synaptic activity at E12.5 that is GABAergic (green horizontal bar). Bottom: maturation of the chloride equilibrium potential (E Cl), spike threshold and resting membrane potential (Vrest) across the embryonic stages of developmental. Note the drop of E Cl at E16.5 that accounts for the “shunting” GABAAR-mediated effect (modified from [47, 56, 66]).
3. Transient Expression of GABA in Motoneuronal Region during the Embryonic Life
Analyzing the maturation of GABA effects in MNs implies that an endogenous GABAergic innervation is present. GABA effects are indeed often tested using local application of exogenous GABA or GABAAR agonist (i.e., muscimol or isoguvacine) [42, 47]. It is thus essential to examine the ontogeny of GABA and GABA receptors. The detailed mapping of the GABAergic system has been extensively described in the adult brainstem by in situ hybridization, immunohistochemistry using antibodies directed against GABA or the GAD protein, specifically the 67-kDa isoform (GAD67) [54] or by taking advantage of the GAD67-GFP knock-in mouse [55]. However, to our knowledge, the ontogeny of the GABAergic innervation of brainstem MNs has not been precisely mapped.
We have described the process of embryonic maturation of GABA immunostaining in the mouse spinal cord [56]. Our study indicated that GABA-ir somata are first detected at embryonic day 11.5 (E11.5), exclusively at brachial level, in the ventral horn. By E13.5, the number of GABAergic neurons sharply increases throughout the extent of the ventral horn both at brachial and lumbar level. At E15.5, stained perikarya decrease in number in the ventral gray matter, while GABA-ir fibers are detected contacting MNs. Such a transient expression of GABA immunoreactivity in the spinal ventral horn was also described in the developing rat [57, 58] and chick [59].
4. GABAergic Synaptic Activity: A Predominant Neurotransmission in MNs at Early Developmental Stages
From a functional point of view, GABA effects differ according to the developmental stage. At early stages, excitatory GABA effects contribute, with cholinergic inputs, to the genesis of spontaneous network activity in the chick [60], mouse [61, 62] and rat [63, 64] spinal cord. At these early stages, MNs are still growing to their peripheral targets and the GABA-mediated spontaneous activity is required for correct motor axon guidance [65]. We have recently showed that first synaptic activity occurs at E12.5 in mouse spinal MNs [66] when the GABAergic phenotype starts to be largely expressed by interneurons located in the ventral gray matter [56]. GABAergic synaptic activity then increases in frequency and coexists with a glycinergic synaptic transmission [66]. In most immature CNS regions, GABA signaling is established before glutamatergic transmission, suggesting that GABA is the principal excitatory transmitter during early development [30]. In the spinal cord, pharmacological approaches performed while recording spontaneous activity showed as well that GABA generates, with acetylcholine [67], the earliest spontaneous motor activity and then glutamate interfere [64]. Our analysis also revealed that the glutamatergic synaptic transmission mainly develops in the embryonic spinal cord after the GABAergic one at around E14.5 (personal observation). Hence, GABA appears as a sort of automated expressed first ubiquitous signal, and then and only then does the adult behavior resumes. Interestingly, it has been shown that the glutamatergic transmission regulates the strength of GABAergic synapses [68].
If the synaptic transmission develops during the embryonic life in spinal MNs, it maturates during postnatal stages and a developmental shift from primarily long-duration GABAergic mIPSCs to short-duration glycinergic mIPSCs occurs after birth in rat MNs [69].
At E15.5 in the rat, commissural GABAergic connections mediate synchronous excitatory action on rhythm-generating networks in the ventral spinal cord, while at E18.5, these GABAergic commissural connections are responsible for reciprocal inhibition during left and right alternation [70]. Interestingly, at E20.5 in rat embryo, these inhibitory commissural inputs become mediated by glycine and not anymore by GABA [70]. These results that take over the primordial role of GABAAR for ensuring spontaneous activity and then reciprocal inhibition between left and right sides of the ventral spinal cord may explain why such a huge expression of GABA is detected in ventral spinal networks at E15.5, in the mouse [56]. At postnatal developmental stages, when commissural connections are mostly mediated by glycine [71–74], GABAergic inhibition has been shown to regulate the onset and duration of neurochemically induced locomotor activity [75].
5. Ontogeny of KCC2 and NKCC1 Immunoreactivity
The switch from excitatory to inhibitory GABAAR-related effects is closely related to the lowering of [Cl−]i during the course of the development. This latter mainly relies on the differential ontogenic expression of the Na+/K+/2Cl− cotransporter isoform 1 (NKCC1), which uptakes chloride ions [76–78], and the neuronal K+/Cl−cotransporter type 2 (KCC2) [79], which extrudes chloride ions [49, 80]. However, other exchangers can control the chloride gradient as the anion (Cl−–HCO3 −) exchangers, either Na+- independent (AE) or Na+-driven (NDCBE also called NDAE) [81] (NCBE) [82]. AE mediates influx of Cl− while exporting HCO3 −, these exchanges being triggered by intracellular alkalinisation. NDCBE, known as an acid extruder (extrudes H+), moves Cl− out in exchange of HCO3 −, driven by the Na+ gradient [83, 84]. NCBE also lowers [Cl−]i (and [H+]i) while importing Na+ and HCO3 − [82, 85].
It is generally accepted that early in development, NKCC1 is predominant and, therefore, maintains a high [Cl−]i, while at later stages, NKCC1 vanishes, and KCC2 develops, lowering intracellular chloride levels [86–88]. In spinal cord MNs, it was shown that the expression of KCC2 transcripts parallels neuronal differentiation during the embryonic life and preceded the decline of the GABAAR reversal potential (EGABAAR) [52]. Thus, the relationship between KCC2, NKCC1, and EGABAAR during the course of the embryonic development remained an open question. We addressed this question in a previous study [47] and found that KCC2 immunoreactivity (KCC2-ir) can be detected in MNs area as early as E11.5, confirming the Stein's study [52], when NKCC1 is also largely expressed. At E14.5, KCC2 is largely present in the ventral gray matter and at later stages this protein keeps stable. At E11.5, a dense NKCC1 labelling is detected throughout the ventral grey matter. Thus, our data indicated that the main drop of E Cl occurring at E16.5 is likely dependant on a reduction of the NKCC1 efficacy rather than a later expression of KCC2. In the rat, Stil and co-workers investigated the expression of KCC2 and NKCC1 in the ventral horn of the spinal cord from E17 to P20 and found that the expression of KCC2 increases significantly, while the expression of NKCC1 decreases during postnatal life when the shift from depolarizing to hyperpolarizing IPSPs occurs (at P4-P5) [50].
It must be mentioned that analyzing the shift from depolarizing to hyperpolarizing effects of GABA in spinal MNs by taking into account only KCC2 and NKCC1 may be simplistic, because the anion exchangers AE has been clearly demonstrated as accumulating chloride in immature chick MNs [89]. Hence, the expression of inhibitory GABA effects likely also relies on the reduction of AE in addition to NKCC1. Also, NCBE that is expressed as early as E14.5 in the mouse SC [90] may play an important role in lowering [Cl−]i.
On the whole, even though likely oversimplified, Figure 1, that is based on our data, illustrates the ontogeny of the GABAergic inhibitory synaptic transmission in parallel to the activity of the two main cotransporters KCC2 and NKCC1. It must be noted that the transient maximum expression of GABA in ventral motor network precedes the drop of E Cl.
6. Ontogenic Changes of the GABAergic Receptors in MNs
GABAAR and GABACR as glycine, nicotinic acetylcholine, and 5-hydroxytryptamine type 3 receptors belong to the cystein-loop receptor family. They are both pentameric assemblies of subunits, each subunits being characterized by extracellular N and C terminals and by four transmembrane domains (TM1–TM4), the domain TM2 forming the anionic channel pore [91]. GABAARs are composed of a large variety of different subunits, sixteen GABAARs subunits being cloned so far (α1–6, β1–3, γ1–3, δ, ε, θ, and π) and three (ρ1–3) for GABACR [92, 93]. The number of GABAAR subunits is also theoretically increased by alternative splicing but only a dozen of subunit combinations have been detected so far [93]. The agonist binding site is carried mainly by α subunits, while γ subunits are responsible for linking GABAARs to the postsynaptic cytoskeleton. The most abundantly expressed GABAAR in the adult CNS has a stoichiometry of 2α, 2β, and 1γ2 subunit. In addition GABAAR subunit combination varies according to the synaptic and extrasynaptic location of this receptor. For example, GABAARs containing the δ subunit or the α5 subunit cannot accumulate at postsynaptic site, likely because they cannot anchor to postsynaptic scaffold protein complex [93–95]. Remarkably, the extrasynaptic GABAARs containing the δ subunit (αβδ GABAAR) have a higher apparent affinity for GABA and desensitize more slowly and less extensively than postsynaptic GABAARs containing the β and/or the γ subunits [96], while GABAARs containing the α5 subunit display a reduction in their desensitization kinetics when compared with receptors containing other α subunits [97].
In the adult lumbar rat spinal cord, only α2, α3, β3, and γ2 mRNAs are expressed at significant levels, the α3, β3 and γ2 transcripts being present in many neurons throughout the Rexed laminae, whereas the α2 mRNA is restricted to motor neurons and adjacent cells [98]. A high expression level of the α1 and the α2 subunits is detected using immunohistochemistry in the adult rat oculomotor trochlear nuclei, the hypoglossal nucleus, and the dorsal nucleus of the vagus [99]. Interestingly, the motor trigeminal nucleus mainly expresses the α2 subunits, while α5 and β2/3 are poorly present in these CNS areas and the δ subunit is undetectable [99]. A recent immunohistochemical study, performed in human brainstem and cervical spinal cord, shows roughly similar results [100]. In this study, Waldvogel et al. did not analyze the expression of α4–α6 subunits and δ subunits, but they showed that α1, α2, α3, β2/3, and γ2 GABAAR subunits are largely detected in the brainstem motoneuron nuclei and in the lamina IX as well as, in less extend, in the lamina X of the cervical spinal cord [100]. However, their data, collected from human brain, differ from Fritschy's group results obtained from rat tissue. Indeed, Waldvogel et al. find a high expression of α1, α2, α3, and β2/3 subunits in the motor trigeminal nucleus, while the γ2 subunit was poorly expressed [100]. This could reflect differences in GABAAR subunit expression between species. However, because these two studies are based on a semi quantitative analysis of immunostaining, at a macroscopic level, discrepancies must be taken with caution. Effectively, it is well known that immunostaining, particularly for GABAAR subunits, can strongly vary depending on the fixation procedure [101, 102].
From a developmental point of view, little is known about changes in GABAAR subunit expression during spinal cord MNs development. In the rat cervical spinal cord, the α6 and δ subunits mRNAs are not detectable at all ages tested (from E12 to adult). During the ontogeny, as demonstrated for GABA [56, 57], subunits mRNA expression emerges along a ventrodorsal gradient. In fact, α2, α3, α5, β2, β3, γ2, and γ3 subunits emerge in presumptive MNs at E12–E13 and then can be detected in more dorsal regions [103]. A synchronized peak of α2, α3, β2, β3, γ2, and γ3 subunits mRNAs is detected at neonatal stages. In the adult rat cervical spinal cord, GABAAR α1, α4, α5, β1-2, γ1, and γ3 subunit mRNAs are found only in relatively few cells scattered in the gray matter, whereas mature MNs exhibit α2β3γ2 transcripts [103]. Thus, contrary to that observed for glycine receptors [104], there is no obvious switch in GABA subunit expression during prenatal and postnatal development of MNs. Interestingly, the α3 mRNA level observed at early developmental stage in the lateral motor column decreases around birth and was no longer detected in the adult [103]. In the hypoglossal nuclei, indirect proofs based on immunochemistry favor a switch from α1 to α2 subunits, during prenatal development [105]. As mentioned above, the α1 and α2 GABAAR subunits, together with the γ2 GABAAR subunit, are the main GABAAR subunits expressed in the hypoglossal nucleus of the adult rat [99]. Assuming that γ2 GABAAR clusters that do not colocalize with α1 GABAAR clusters reflect the presence of GABAAR containing α2 subunits, Muller and collaborators concluded for an increase in the proportion of GABAAR containing α2 GABAAR subunits [105]. However, this is in apparent contradiction to other studies showing that the α2 GABAAR subunits are expressed early in development and are progressively replaced by α1 GABAAR subunit in many brain areas [106]. A further quantitative immunohistochemical analysis of the developmental changes in the proportion of α2 and α1 GABAAR subunits in the hypoglossal nucleus is thus required in order to verify that developmental maturation processes of GABAARs can vary between CNS areas.
If it is now clearly demonstrated that GABAAR subunits may evolve during development and vary according to brain areas, few data are available concerning the cellular location of these subunits on a single MNs. Using immunocytochemistry and confocal microscopy, Lorenzo et al. compared the subcellular patterns of expression of the main GABAAR subunits (GABAAR α1, α2, α3, and α5) in the somatic versus dendritic compartments of rat abducens MNs [107] and revealed a differential organization of GABAAR subunits. They found that MNs somata contain only GABAAR α1, while both GABAAR α1 and GABAAR α3 are detected on dendrites [107].
7. Maturation of the GABAergic System on Motoneuron in Normal and Pathological Conditions: Mixed GABA/glycine Synapses and Mismatch between Pre- and Postsynaptic Elements
During the first 3 weeks of rodent postnatal development, inhibitory synaptic transmission changes in multiple ways that differ depending on brain areas. Electrophysiology and immunocytochemistry suggest that the respective contribution of the glycinergic and GABAergic transmission to the overall inhibitory message received by postsynaptic neurons may vary during the developmental period. For example, a developmental switch from a predominant GABAergic to main glycinergic neurotransmission occurs in the lumbar spinal cord [69] and in the lateral superior olive of young rodents [108, 109], while GABAergic neurotransmission dominates in developing collicular neurons [110] (Figure 2(a)).
Figure 2.
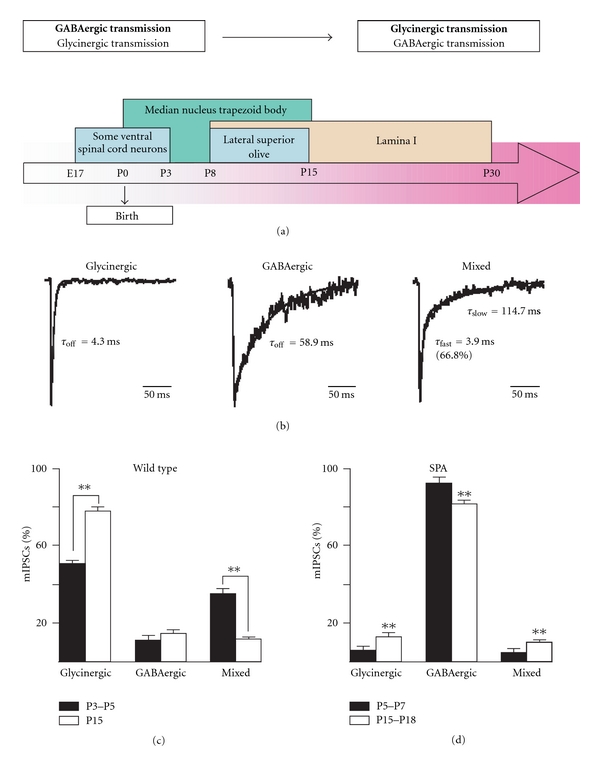
(a) Developmental changes in the proportions of GABAergic and glycinergic synaptic activity in various areas of the central nervous system. (b) Examples of individual glycinergic (left) GABAergic (middle) and mixed (right) miniature inhibitory postsynaptic currents (mIPSCs) recorded in a hypoglossal motoneuron at P15, in the presence of tetrodotoxin (a blocker of voltage-gated sodium channels). Note the slower decay phase of the GABAergic mIPSC compared to the glycinergic mIPSC. Decay phase of GABAergic and glycinergic events is better fitted with a single exponential function, while a double exponential function is required to fit the decay phase of mixed events. (c) Relative proportions of glycinergic, GABAergic and mixed mIPSCs at P3–P5 (black bars) and at P15 (white bars) in wild-type mice. (d) Relative proportions of glycinergic, GABAergic and mixed miniature postsynaptic events at P5–P7 (black bars) and at P15–P18 (white bars) in SPA mice. (Adapted from [118, 126]).
As first demonstrated in neonatal spinal MNs, glycine and GABA can be coreleased from the same presynaptic vesicle resulting in a mixed glycinergic/GABAergic synaptic event [111]. Mixed inhibitory synapses have also been functionally identified in MNs of the hypoglossal nucleus [112, 113], but mixed synapses are not particular to inhibitory input on MNs, because they are also described on spinal interneurons [114, 115]. If mixed inhibitory synapses appear to reflect an intermediate stage of maturation of glycinergic synapses, it must be noted that although the proportion of mixed synapses decreases during development in Renshaw cells and other spinal cord interneurons [116], mixed inhibitory synapses remain functional in the adult [114, 116]. This is also the case in abducens MNs during rat postnatal development: before birth, only GABAergic axon terminals develop, whereas mixed GABA/glycine axon terminals appear at birth, and their number increases during the first postnatal week [117].
Functional mixed inhibitory synapses have also been described in rat HMs [112, 113]. However, a complete morphofunctional study of the development of inhibitory synapse on the mouse HMs, between P3–P5 and P15, revealed that the developmental shift from glycinergic/GABAergic to pure glycinergic neurotransmission depends mainly on the maturation of the presynaptic elements, while postsynaptic GlyRs and GABAARs remain associated at the same postsynaptic density at all age tested. Effectively, although miniature inhibitory postsynaptic currents (mIPSCs) are mainly glycinergic and mixed glycinergic/GABAergic at P3–P5 and then predominantly glycinergic at P15 (Figures 2(b) and 2(c)), postsynaptic GlyRs and GABAARs remain associated at the same postsynaptic density at all age tested [118]. In addition, because many GABAergic synapses are unlikely to contain postsynaptic GABAARs yet, it was supposed that they represent newly formed ‘‘nonfunctional” GABAergic synaptic contacts, as previously observed in the cerebellum [119, 120]. It is, however, unclear whether such a discrepancy between the pre- and the postsynaptic element also occurs in other CNS area during development, but it must be noted that a similar maturation process of the inhibitory presynaptic terminals was also observed in neurons of the rat lateral superior olive [109]. Moreover, postsynaptic GABAARs facing presynaptic terminals that do not release GABA have also been reported in the spinal cord and brain neuropil in culture [121–125]. Such a mismatch between the pre- and the postsynaptic element of inhibitory synapses was also observed in the adult Renshaw cells of the rat spinal cord [114]. In that case, it was proposed that GABAergic presynaptic terminals could face postsynaptic GlyR clusters [114]. Altogether, these data suggest that the maturation of inhibitory synapses rather results from a differential regulation of the GlyT2 and GAD65 expression at the level of a single synaptic terminal but not from a redistribution of GlyRs and GABAARs at postsynaptic site.
Our data from the hypoglossal nucleus also suggest that pre- and postsynaptic elements mature independently [118]. However, a more recent study performed on spastic (SPA) mice, a model for hyperekplexia, argues against this hypothesis [126]. SPA mice display an insertion of an LINE-1 transposable element into the gene coding for the GlyR β subunit, which results in a truncated protein that impairs accumulation of GlyRs at postsynaptic sites and leads to a strong dysfunction of glycinergic synaptic transmission [127, 128]. In C57BL/6J strain, SPA mice which express a lower amount of GlyR β subunits die 2-3 weeks after birth [129], suggesting that GABAergic compensation does not necessarily take place. It was first hypothesized that the progressive postnatal developmental lost of GABAergic presynaptic terminals that normally occurs in wild-type mice due to a switch to glycinergic terminals [118] could explain the progressive impairment of inhibitory synaptic activity and thus the lethality of this mutation. But surprisingly, in opposition to our observations made in wild-type animal, the inhibitory synaptic activity is mainly GABAergic in SPA mice (Figure 2(d)): a developmental decrease in glycinergic presynaptic terminals occurs, while the density of GABAergic presynaptic terminals increases [126]. In addition, the proportion of inhibitory presynaptic terminals facing GABAARs significantly increases during postnatal development in HMs of SPA mice. It must, however, be noted that many GABAergic synaptic boutons face diffuse GABAARs staining, which contrasts to the situation observed in wild-type animal which most of the presynaptic terminals face aggregated GABAARs. It is, thus, likely that GABAergic synapses are less efficient in SPA mice than in wild type [126]. Also, because SPA mice cannot survive, these results indicate that GABAergic neurotransmission does not compensate for defects in GlyR postsynaptic aggregation in this hyperekplexia model. They also suggest, contrary to that previously hypothesized [118], that a crosstalk exists between postsynaptic and presynaptic elements, leading to the developmental regulation of the presynaptic terminal neurotransmitter content that could be related to a downregulation of GlyT2 expression and an up-regulation of GAD65 expression at inhibitory presynaptic terminals depending on the level of postsynaptic GlyR aggregation.
Alteration of GABAAR and GlyR expression was also analyzed in MNs vulnerable and resistant to amyotrophic lateral sclerosis (ALS) [41]. Because a reduced level of expression of the GABAAR α1 subunit mRNA has been shown in neurons of the motor cortex of patients with ALS [130], Lorenzo et al. investigated, using a quantitative immunohistochemical study, the possibility that GABAAR and GlyR might be expressed differentially in ALS-vulnerable and ALS-resistant brainstem MNs in an ALS rat model [41]. Indeed, MNs controlling eye movements and bladder contraction are surprisingly unaffected (they are ALS-resistant) during terminal stages of ALS, while other MNs underlie an invariably fatal degeneration (they are ALS-vulnerable) [131]. Their main hypothesis was a reduction in GABAAR and GlyR expression in vulnerable MNs, which could account for an alteration of the inhibition and hence for an amplification of the glutamatergic synaptic activity onto these MNs, an excessive excitatory transmission being known to be detrimental. Interestingly, Lorenzo et al. showed a differential expression of GABAAR (and GlyR) in brainstem ALS-resistant oculomotor (III), trochlear (IV), abducens (VI) versus ALS-vulnerable MNs trigeminal (V), facial (VII), hypoglossal (XII) [41]. They demonstrated that GABAAR in ALS-vulnerable MNs mostly express α2 subunits while GABAAR in ALS-resistant MNs are α1 subunits enriched. They also showed that ALS-resistant MNs contain a larger proportion of extrasynaptic GABAAR clusters than ALS-vulnerable MNs. Because extrasynaptic GABAAR are activated by GABA spillover from synapses [132–134] and mediate a tonic inhibition that plays a crucial role in regulating neuronal excitability [135], the authors hypothesized that the presence of extrasynaptic GABAAR in ALS-resistant MNs could protect these neurons from excessive depolarization by abnormal glutamate release. Their data demonstrated that the rate of occurrence of extrasynaptic GABAAR clusters was approximately twice as high in ALS-resistant as in ALS-vulnerable MNs, but more experiments are necessary to determine to what extend this difference accounts for the vulnerability of MNs, as for example by manipulating extrasynaptic GABAAR expression in specific MNs. On the contrary, recent reports show that glycinergic innervation but not GABAergic innervation of spinal MNs is deficient in the ALS mouse model expressing the mutant form of human superoxide dismutase-1 with G93A substitution (SOD1 G93A ) [136, 137]. The authors examined, using whole-cell patch-clamp recordings, GlyR-mediated currents in cultured spinal MNs from this ALS mouse model. They found that glycine-evoked current density was significantly smaller in the SOD1 MNs compared to control. However, they did not find any change in GABAergic synaptic activity. This alteration in glycinergic synaptic activity is likely to be due to a lower GlyRα1 subunit mRNA expression in SOD1G93A MNs [137]. These results suggest that a selective alteration in GlyR expression can partly account for an alteration of inhibitory synapse efficacy in MNs early in the disease process of ALS, with SOD1G93A substitution at least. But these data obtained from GlyR expression in this ALS mouse model do not demonstrate, as data regarding GABAAR expression, that a reduction of receptor subunit expression can effectively account for MNs vulnerability in ALS. Again, more experiment is necessary to resolve this issue.
Finally, these results on GABAAR or GlyRs expression in ALS could be complementary rather than contradictory if one supposes that the expression of the different GlyR and GABAAR subunits can be region specific. For example, GABAAR α1 subunit is poorly expressed in the spinal cord compared to more central region [103], and it is important to note that glycinergic and GABAergic synapses control MNs development in a region-specific manner during programmed cell death as exemplified by data obtained in gephyrin-deficient mice that lack all postsynaptic GlyRs and some GABAAR clusters [138]. In these gephyrin-deficient mice, there is a reduced respiratory MN survival and decreased innervation of the diaphragm, whereas limb-innervating MNs show increased survival and increased innervation of their target muscles [138].
8. Concluding Remarks
If GABAergic interneurons constitute only 17%–20% of the neurons in the brain [139], their primordial role in the maintenance of a good balance in neuronal connections is obvious. GABAAR activation is likely to play an important role on spinal cord and brainstem MNs development as well as during pathological conditions, but it is unclear to what extend such a diversity leading to functionally different GABAARs is important for a proper development of functional locomotor networks and to what extend a defect in a subunit expression can impact neuronal survival during development and in pathological condition as in ALS. For example, it will be of interest to determine to what extend the expression of α2 GABAARs instead of α1 is important for neuronal development. This can be done using genetic tools as the knock in technique by substituting α2 expression by α1. Another unknown mechanism that must be determined is the communication pathway between GABAergic/glycinergic pre-synaptic neurons and post-synaptic receptors. Thus, it would be worthy to examine changes in the pre-synaptic GABAergic and/or glycinergic phenotype, during development or in pathological conditions, when a post-synaptic receptor type is missing or altered.
Acknowledgment
The authors are grateful to Dr. Evelyne Sernagor (Newcastle University Medical School, UK) for helping to prepare the paper.
References
- 1.Rozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor ρ1 and ρ2 subunits in developing rat brain and spinal cord. European Journal of Neuroscience. 2002;15(11):1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- 2.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Progress in Neurobiology. 1995;46(4):423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- 3.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Progress in Biophysics and Molecular Biology. 2005;87(1):33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirischuk S, Akyeli J, Iosub R, Grantyn R. Pre- and postsynaptic contribution of GABAC receptors to GABAergic synaptic transmission in rat collicular slices and cultures. European Journal of Neuroscience. 2003;18(4):752–758. doi: 10.1046/j.1460-9568.2003.02805.x. [DOI] [PubMed] [Google Scholar]
- 5.Clark SE, Garret M, Platt B. Postnatal alterations of GABA receptor profiles in the rat superior colliculus. Neuroscience. 2001;104(2):441–454. doi: 10.1016/s0306-4522(01)00087-2. [DOI] [PubMed] [Google Scholar]
- 6.Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 7.Bu DF, Erlander MG, Hitz BC, et al. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(6):2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brilliant MH, Szabo G, Katarova Z, et al. Sequences homologous to glutamic acid decarboxylase cDNA are present on mouse chromosomes 2 and 10. Genomics. 1990;6(1):115–122. doi: 10.1016/0888-7543(90)90455-4. [DOI] [PubMed] [Google Scholar]
- 9.Varju P, Katarova Z, Madarasz E, Szabo G. GABA signalling during development: new data and old questions. Cell and Tissue Research. 2001;305(2):239–246. doi: 10.1007/s004410100356. [DOI] [PubMed] [Google Scholar]
- 10.Szabo G, Katarova Z, Greenspan R. Distinct protein forms are produced from alternatively spliced bicistronic glutamic acid decarboxylase mRNAs during development. Molecular and Cellular Biology. 1994;14(11):7535–7545. doi: 10.1128/mcb.14.11.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varju P, Katarova Z, Madarász E, Szabo G. Sequential induction of embryonic and adult forms of glutamic acid decarboxylase during in vitro-induced neurogenesis in cloned neuroectodermal cell-line, NE-7C2. Journal of Neurochemistry. 2002;80(4):605–615. doi: 10.1046/j.0022-3042.2001.00733.x. [DOI] [PubMed] [Google Scholar]
- 12.Behar T, Ma W, Hudson L, Barker JL. Analysis of the anatomical distribution of GAD mRNA encoding truncated glutamic acid decarboxylase proteins in the embryonic rat brain. Developmental Brain Research. 1994;77(1):77–87. doi: 10.1016/0165-3806(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 13.Katarova Z, Sekerkova G, Prodan S, Mugnaini E, Szabo G. Domain-restricted expression of two glutamic acid decarboxylase genes in midgestation mouse embryos. Journal of Comparative Neurology. 2000;424(4):607–627. doi: 10.1002/1096-9861(20000904)424:4<607::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Ma W, Behar T, Chang L, Barker JL. Transient increase in expression of GAD65 and GAD67 mRNAs during postnatal development of rat spinal cord. Journal of Comparative Neurology. 1994;346(1):151–160. doi: 10.1002/cne.903460111. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CC, Davis KM, Jin H, et al. Association of L-glutamic acid decarboxylase to the 70-kDa heat shock protein as a potential anchoring mechanism to synaptic vesicles. Journal of Biological Chemistry. 2000;275(27):20822–20828. doi: 10.1074/jbc.M001403200. [DOI] [PubMed] [Google Scholar]
- 16.Lauder JM, Liu J, Devaud L, Morrow AL. GABA as a trophic factor for developing monoamine neurons. Perspectives on Developmental Neurobiology. 1998;5(2-3):247–259. [PubMed] [Google Scholar]
- 17.Ziskind-Conhaim L. Physiological functions of GABA-induced depolarizations in the developing rat spinal cord. Perspectives on Developmental Neurobiology. 1998;5(2-3):279–287. [PubMed] [Google Scholar]
- 18.Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Frontiers in Cellular Neuroscience. 2010;4(11):11 pages. doi: 10.3389/fncel.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130(3):567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Dave KA, Bordey A. GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: implications for proliferation. IUBMB Life. 2009;61(5):496–503. doi: 10.1002/iub.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leinekugel X, Khalilov I, McLean H, et al. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Advances in Neurology. 1999;79:189–201. [PubMed] [Google Scholar]
- 22.Lopez-Bendito G, Shigemoto R, Kulik A, Paulsen O, Fairen A, Lujan R. Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. European Journal of Neuroscience. 2002;15(11):1766–1778. doi: 10.1046/j.1460-9568.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- 23.Behar TN, Schaffner AE, Colton CA, et al. GABA-induced chemokinesis and NGF-induced chemotaxis of embryonic spinal cord neurons. Journal of Neuroscience. 1994;14(1):29–38. doi: 10.1523/JNEUROSCI.14-01-00029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behar TN, Li Y, Tran HT, et al. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. Journal of Neuroscience. 1996;16(5):1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Bendito G, Lujan R, Shigemoto R, Ganter P, Paulsen O, Molnar Z. Blockade of GABAB receptors alters the tangential migration of cortical neurons. Cerebral Cortex. 2003;13(9):932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- 26.Fiorentino H, Kuczewski N, Diabira D, et al. GABAB receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. Journal of Neuroscience. 2009;29(37):11650–11661. doi: 10.1523/JNEUROSCI.3587-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cazalets JR, Bertrand S, Sqalli-Houssaini Y, Clarac F. GABAergic control of spinal locomotor networks in the neonatal rat. Annals of the New York Academy of Sciences. 1998;860:168–180. doi: 10.1111/j.1749-6632.1998.tb09047.x. [DOI] [PubMed] [Google Scholar]
- 28.Hassfurth B, Grothe B, Koch U. The mammalian interaural time difference detection circuit is differentially controlled by GABAB receptors during development. Journal of Neuroscience. 2010;30(29):9715–9727. doi: 10.1523/JNEUROSCI.1552-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer A, Zhang W. Postnatal development of GABAB-receptor-mediated modulation of potassium currents in brainstem respiratory network of mouse. Respiratory Physiology and Neurobiology. 2007;158(1):22–29. doi: 10.1016/j.resp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiological Reviews. 2007;87(4):1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 31.O’Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Current Opinion in Neurobiology. 1999;9(1):94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 32.Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nature Reviews Neuroscience. 2009;11(1):18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends in Neurosciences. 2005;28(6):278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends in Neurosciences. 2007;30(8):382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Ganguly K, Schinder AF, Wong ST, Poo MM. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 36.Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. Journal of Neuroscience. 2003;23(20):7621–7629. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White WF, Heller AH. Glycine receptor alteration in the mutant mouse spastic. Nature. 1982;298(5875):655–657. doi: 10.1038/298655a0. [DOI] [PubMed] [Google Scholar]
- 38.Biscoe TJ, Fry JP. Some pharmacological studies on the spastic mouse. British Journal of Pharmacology. 1982;75(1):23–35. doi: 10.1111/j.1476-5381.1982.tb08754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham BA, Schofield PR, Sah P, Callister RJ. Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. Journal of Physiology. 2003;551(3):905–916. doi: 10.1113/jphysiol.2003.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller E, Le Corronc H, Scain AL, Triller A, Legendre P. Despite GABAergic neurotransmission, GABAergic innervation does not compensate for the defect in glycine receptor postsynaptic aggregation in spastic mice. European Journal of Neuroscience. 2008;27(10):2529–2541. doi: 10.1111/j.1460-9568.2008.06217.x. [DOI] [PubMed] [Google Scholar]
- 41.Lorenzo LE, Barbe A, Portalier P, Fritschy JM, Bras H. Differential expression of GABAA and glycine receptors in ALS-resistant vs. ALS-vulnerable motoneurons: possible implications for selective vulnerability of motoneurons. European Journal of Neuroscience. 2006;23(12):3161–3170. doi: 10.1111/j.1460-9568.2006.04863.x. [DOI] [PubMed] [Google Scholar]
- 42.Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. European Journal of Neuroscience. 2000;12(8):2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- 43.Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. Journal of Neurophysiology. 1998;80(5):2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- 44.Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. Journal of Neuroscience. 2006;26(14):3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. European Journal of Neuroscience. 1998;10(12):3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 46.Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. Journal of Neurophysiology. 2003;90(5):2964–2972. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- 47.Delpy A, Allain AE, Meyrand P, Branchereau P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. Journal of Physiology. 2008;586(4):1059–1075. doi: 10.1113/jphysiol.2007.146993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jean-Xavier C, Mentis GZ, O’Donovan MJ, Cattaert D, Vinay L. Dual personality of GABA/glycine-mediated depolarizations in immature spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11477–11482. doi: 10.1073/pnas.0704832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30(2):515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 50.Stil A, Liabeuf S, Jean-Xavier C, Brocard C, Viemari JC, Vinay L. Developmental up-regulation of the potassium-chloride cotransporter type 2 in the rat lumbar spinal cord. Neuroscience. 2009;164(2):809–821. doi: 10.1016/j.neuroscience.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Wu WL, Ziskind-Conhaim L, Sweet MA. Early development of glycine- and GABA-mediated synapses in rat spinal cord. Journal of Neuroscience. 1992;12(10):3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. Journal of Comparative Neurology. 2004;468(1):57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- 53.Tyzio R, Cossart R, Khalilov I, et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314(5806):1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 54.Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. Journal of Comparative Neurology. 2005;493(2):274–290. doi: 10.1002/cne.20758. [DOI] [PubMed] [Google Scholar]
- 55.Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki JI, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. Journal of Comparative Neurology. 2003;467(1):60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 56.Allain AE, Bairi A, Meyrand P, Branchereau P. Ontogenic changes of the GABAergic system in the embryonic mouse spinal cord. Brain Research. 2004;1000(1-2):134–147. doi: 10.1016/j.brainres.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 57.Ma W, Behar T, Barker JL. Transient expression of GABA immunoreactivity in the developing rat spinal cord. Journal of Comparative Neurology. 1992;325(2):271–290. doi: 10.1002/cne.903250210. [DOI] [PubMed] [Google Scholar]
- 58.Tran TS, Alijani A, Phelps PE. Unique developmental patterns of GABAergic neurons in rat spinal cord. Journal of Comparative Neurology. 2003;456(2):112–126. doi: 10.1002/cne.10511. [DOI] [PubMed] [Google Scholar]
- 59.Antal M, Berki AC, Horvath L, O’Donovan MJ. Developmental changes in the distribution of gamma-aminobutyric acid- immunoreactive neurons in the embryonic chick lumbosacral spinal cord. Journal of Comparative Neurology. 1994;343(2):228–236. doi: 10.1002/cne.903430204. [DOI] [PubMed] [Google Scholar]
- 60.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. Journal of Neuroscience. 1999;19(8):3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Branchereau P, Chapron J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. Journal of Neuroscience. 2002;22(7):2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. Journal of Neuroscience. 2003;23(2):587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. Journal of Neurophysiology. 2003;89(3):1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- 64.Nishimaru H, Iizuka M, Ozaki S, Kudo N. Spontaneous motoneuronal activity mediated by glycine and GABA in the spinal cord of rat fetuses in vitro. Journal of Physiology. 1996;497(1):131–143. doi: 10.1113/jphysiol.1996.sp021755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43(5):687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 66.Scain AL, Le Corronc H, Allain AE, et al. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. Journal of Neuroscience. 2010;30(1):390–403. doi: 10.1523/JNEUROSCI.2115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers CP, Lewcock JW, Hanson MG, et al. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46(1):37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 68.Rosato-Siri M, Grandolfo M, Ballerini L. Activity-dependent modulation of GABAergic synapses in developing rat spinal networks in vitro. European Journal of Neuroscience. 2002;16(11):2123–2135. doi: 10.1046/j.1460-9568.2002.02291.x. [DOI] [PubMed] [Google Scholar]
- 69.Gao BX, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. Journal of Neurophysiology. 2001;86(1):492–502. doi: 10.1152/jn.2001.86.1.492. [DOI] [PubMed] [Google Scholar]
- 70.Nakayama K, Nishimaru H, Kudo N. Basis of changes in left-right coordination of rhythmic motor activity during development in the rat spinal cord. Journal of Neuroscience. 2002;22(23):10388–10398. doi: 10.1523/JNEUROSCI.22-23-10388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iizuka M, Nishimaru H, Kudo N. Development of the spatial pattern of 5-HT-induced locomotor rhythm in the lumbar spinal cord of rat fetuses in vitro. Neuroscience Research. 1998;31(2):107–111. doi: 10.1016/s0168-0102(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 72.Fukuroda T, Ozaki S, Ihara M, et al. Necessity of dual blockade of endothelin ET and ET receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. British Journal of Pharmacology. 1996;117(6):995–999. doi: 10.1111/j.1476-5381.1996.tb16688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kudo N, Nishimaru H, Nakayama K. Developmental changes in rhythmic spinal neuronal activity in the rat fetus. Progress in Brain Research. 2004;143:49–55. doi: 10.1016/s0079-6123(03)43005-7. [DOI] [PubMed] [Google Scholar]
- 74.Branchereau P, Morin D, Bonnot A, Ballion B, Chapron J, Viala D. Development of lumbar rhythmic networks: from embryonic to neonate locomotor-like patterns in the mouse. Brain Research Bulletin. 2000;53(5):711–718. doi: 10.1016/s0361-9230(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 75.Hinckley C, Seebach B, Ziskind-Conhaim L. Distinct roles of glycinergic and GABAergic inhibition in coordinating locomotor-like rhythms in the neonatal mouse spinal cord. Neuroscience. 2005;131(3):745–758. doi: 10.1016/j.neuroscience.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 76.Russell JM. Sodium-potassium-chloride cotransport. Physiological Reviews. 2000;80(1):211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 77.Rohrbough J, Spitzer NC. Regulation of intracellular Cl− levels by Na+-dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. Journal of Neuroscience. 1996;16(1):82–91. doi: 10.1523/JNEUROSCI.16-01-00082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. Journal of Physiology. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain: a neuronal-specific isoform. Journal of Biological Chemistry. 1996;271(27):16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 80.Rivera C, Voipio J, Payne JA, et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 81.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Progress in Brain Research. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 82.Wang CZ, Yano H, Nagashima K, Seino S. The Na-driven Cl/HCO/ exchanger: cloning, tissue distribution, and functional characterization. Journal of Biological Chemistry. 2000;275(45):35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- 83.Chesler M. Regulation and modulation of pH in the brain. Physiological Reviews. 2003;83(4):1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 84.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO transporters. Pflugers Archiv European Journal of Physiology. 2004;447(5):495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 85.Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. European Journal of Neuroscience. 2003;18(11):2935–2945. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- 86.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 87.Ben-Ari Y. Developing networks play a similar melody. Trends in Neurosciences. 2001;24(6):353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 88.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nature Reviews Neuroscience. 2002;3(9):715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Islas C, Chub N, Wenner P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. Journal of Neurophysiology. 2009;101(2):507–518. doi: 10.1152/jn.90986.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hübner CA, Hentschke M, Jacobs S, Hermans-Borgmeyer I. Expression of the sodium-driven chloride bicarbonate exchanger NCBE during prenatal mouse development. Gene Expression Patterns. 2004;5(2):219–223. doi: 10.1016/j.modgep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiological Reviews. 2004;84(4):1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 92.Macdonald RL, Olsen RW. GABAA receptor channels. Annual Review of Neuroscience. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 93.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Reviews Neuroscience. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 94.Brünig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. Journal of Comparative Neurology. 2002;443(1):43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- 95.Crestani F, Keist R, Fritschy JM, et al. Trace fear conditioning involves hippocampal α 5GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. Journal of Pharmacology and Experimental Therapeutics. 2006;316(3):1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- 97.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure-function relations and synaptic transmission. Journal of Physiology. 1995;489(2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Molecular Brain Research. 1991;10(2):179–183. doi: 10.1016/0169-328x(91)90109-b. [DOI] [PubMed] [Google Scholar]
- 99.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. Journal of Comparative Neurology. 1995;359(1):154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 100.Waldvogel HJ, Baer K, Eady E, et al. Differential localization of gamma-aminobutyric acid type A and glycine receptor subunits and gephyrin in the human pons, medulla oblongata and uppermost cervical segment of the spinal cord: an immunohistochemical study. The Journal of Comparative Neurology. 2010;518(3):305–328. doi: 10.1002/cne.22212. [DOI] [PubMed] [Google Scholar]
- 101.Saper CB. A guide to the perplexed on the specificity of antibodies. Journal of Histochemistry and Cytochemistry. 2009;57(1):1–5. doi: 10.1369/jhc.2008.952770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schneider Gasser EM, Straub CJ, Panzanelli P, Weinmann O, Sassoè-Pognetto M, Fritschy JM. Immunofluorescence in brain sections: simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nature Protocols. 2006;1(4):1887–1897. doi: 10.1038/nprot.2006.265. [DOI] [PubMed] [Google Scholar]
- 103.Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. Journal of Comparative Neurology. 1993;338(3):337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- 104.Legendre P. The glycinergic inhibitory synapse. Cellular and Molecular Life Sciences. 2001;58(5-6):760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muller E, Triller A, Legendre P. Glycine receptors and GABA receptor alpha 1 and gamma 2 subunits during the development of mouse hypoglossal nucleus. European Journal of Neuroscience. 2004;20(12):3286–3300. doi: 10.1111/j.1460-9568.2004.03785.x. [DOI] [PubMed] [Google Scholar]
- 106.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. Journal of Neuroscience. 1994;14(9):5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorenzo LE, Russier M, Barbe A, Fritschy JM, Bras H. Differential organization of gamma-aminobutyric acid type A and glycine receptors in the somatic and dendritic compartments of rat abducens motoneurons. The Journal of Comparative Neurology. 2007;504(2):112–126. doi: 10.1002/cne.21442. [DOI] [PubMed] [Google Scholar]
- 108.Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. Journal of Neuroscience. 1998;18(12):4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nabekura J, Katsurabayashi S, Kakazu Y, et al. Developmental switch from GABA to glycine release in single central synaptic terminals. Nature Neuroscience. 2004;7(1):17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- 110.Meier J, Juttner R, Kirischuk S, Grantyn R. Synaptic anchoring of glycine receptors in developing collicular neurons under control of metabotropic glutamate receptor activity. Molecular and Cellular Neuroscience. 2002;21(2):324–340. doi: 10.1006/mcne.2002.1161. [DOI] [PubMed] [Google Scholar]
- 111.Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281(5375):419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 112.O’Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. Journal of Neurophysiology. 1999;82(3):1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- 113.O’Brien JA, Berger AJ. The nonuniform distribution of the GABAA receptor α1 subunit influences inhibitory synaptic transmission to motoneurons within a motor nucleus. Journal of Neuroscience. 2001;21(21):8482–8494. doi: 10.1523/JNEUROSCI.21-21-08482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geiman EJ, Zheng W, Fritschy JM, Alvarez FJ. Glycine and GABAA receptor subunits on renshaw cells: relationship with presynaptic neurotransmitters and postsynaptic gephyrin clusters. Journal of Comparative Neurology. 2002;444(3):275–289. doi: 10.1002/cne.10148. [DOI] [PubMed] [Google Scholar]
- 115.Keller AF, Coull JAM, Chéry N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. Journal of Neuroscience. 2001;21(20):7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonzalez-Forero D, Alvarez FJ. Differential postnatal maturation of GABAA, glycine receptor, and mixed synaptic currents in Renshaw cells and ventral spinal interneurons. Journal of Neuroscience. 2005;25(8):2010–2023. doi: 10.1523/JNEUROSCI.2383-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dufour A, Tell F, Baude A. Perinatal development of inhibitory synapses in the nucleus tractus solitarii of the rat. European Journal of Neuroscience. 2010;32(4):538–549. doi: 10.1111/j.1460-9568.2010.07309.x. [DOI] [PubMed] [Google Scholar]
- 118.Muller E, Le Corronc H, Triller A, Legendre P. Developmental dissociation of presynaptic inhibitory neurotransmitter and postsynaptic receptor clustering in the hypoglossal nucleus. Molecular and Cellular Neuroscience. 2006;32(3):254–273. doi: 10.1016/j.mcn.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Dumoulin A, Triller A, Dieudonné S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. Journal of Neuroscience. 2001;21(16):6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takayama C, Inoue Y. Morphological development and maturation of the GABAergic synapses in the mouse cerebellar granular layer. Developmental Brain Research. 2004;150(2):177–190. doi: 10.1016/j.devbrainres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 121.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18(5):1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. Journal of Neuroscience. 1996;16(3):974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kannenberg K, Sieghart W, Reuter H. Clusters of GABAA receptors on cultured hippocampal cells correlate only partially with functional synapses. European Journal of Neuroscience. 1999;11(4):1256–1264. doi: 10.1046/j.1460-9568.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 125.Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. Journal of Neuroscience. 2000;20(22):8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muller E, Le-Corronc H, Legendre P. Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Frontiers in Molecular Neuroscience. 2008;1(3) doi: 10.3389/neuro.02.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kingsmore SF, Giros B, Suh D, Bieniarz M, Caron MG, Seldin MF. Glycine receptor β-subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nature Genetics. 1994;7(2):136–142. doi: 10.1038/ng0694-136. [DOI] [PubMed] [Google Scholar]
- 128.Mulhardt C, Fischer M, Gass P, et al. The spastic mouse: aberrant splicing of glycine receptor β subunit mRNA caused by intronic insertion of L1 element. Neuron. 1994;13(4):1003–1015. doi: 10.1016/0896-6273(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 129.Becker CM, Hermans-Borgmeyer I, Schmitt B, Betz H. The glycine receptor deficiency of the mutant mouse spastic: evidence for normal glycine receptor structure and localization. Journal of Neuroscience. 1986;6(5):1358–1364. doi: 10.1523/JNEUROSCI.06-05-01358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Petri S, Krampfl K, Hashemi F, et al. Distribution of GABAA receptor mRNA in the motor cortex of ALS patients. Journal of Neuropathology and Experimental Neurology. 2003;62(10):1041–1051. doi: 10.1093/jnen/62.10.1041. [DOI] [PubMed] [Google Scholar]
- 131.Reiner A, Medina L, Figueredo-Cardenas G, Anfinson S. Brainstem motoneuron pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS. Experimental Neurology. 1995;131(2):239–250. doi: 10.1016/0014-4886(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 132.Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25(3):673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 133.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends in Neurosciences. 2004;27(9):569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 134.Syková E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience. 2004;129(4):861–876. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 135.Petrini EM, Marchionni I, Zacchi P, Sieghart W, Cherubini E. Clustering of extrasynaptic GABAA receptors modulates tonic inhibition in cultured hippocampal neurons. Journal of Biological Chemistry. 2004;279(44):45833–45843. doi: 10.1074/jbc.M407229200. [DOI] [PubMed] [Google Scholar]
- 136.Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. American Journal of Pathology. 2009;174(2):574–585. doi: 10.2353/ajpath.2009.080557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang Q, Martin LJ. Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis. Journal of Neuroscience. 2011;31(8):2815–2827. doi: 10.1523/JNEUROSCI.2475-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Banks GB, Kanjhan R, Wiese S, et al. Glycinergic and GABAergic synaptic activity differentially regulate motoneuron survival and skeletal muscle innervation. Journal of Neuroscience. 2005;25(5):1249–1259. doi: 10.1523/JNEUROSCI.1786-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Research Reviews. 1998;26(2-3):113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]


