Summary
The thalamus has traditionally been thought to passively relay sensory information to the cortex. By showing that responses in visual thalamus are modulated by perceptual and cognitive tasks, recent fMRI and physiology studies have helped revise this view. The modulatory input to the visual thalamus derives from functionally distinct cortical and subcortical feedback pathways. These pathways enable the lateral geniculate nucleus and pulvinar to regulate the information transmitted to cortical areas according to cognitive requirements. Emerging evidence suggests that such regulation involves changing the degree of synchrony between neurons as well as changing the magnitude of thalamic activity. These findings support a role for the thalamus that extends as far as contributing to the control of visual attention and awareness.
Introduction
Cognitive processing was traditionally viewed as being confined to the cerebral cortex, relegating the thalamus to passively relay information to the cortex. Early reports of a lack of behavioral effects on the thalamus did little to change this view [1–3]. However, a role as a passive relay for the thalamus is at odds with the fact that it receives substantial modulatory input from brain areas involved in regulating attention and arousal [4]. Indeed, from an anatomical perspective, the visual thalamus is ideally positioned to regulate the transmission of information to the cortex and between cortical areas, a role proposed some time ago [4,5]. Here, we review the emerging evidence for thalamic control of visual attention, integration and awareness.
Visual thalamic physiology and thalamo-cortical circuitry
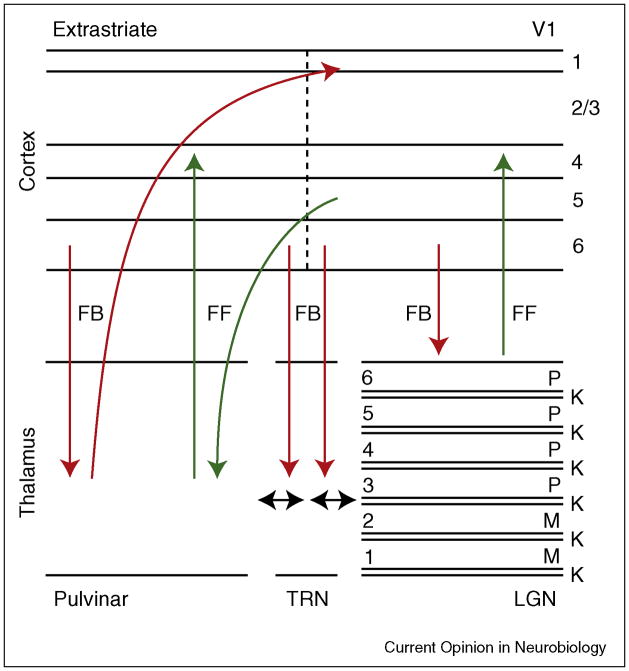
The visual thalamus consists of three main parts: the lateral geniculate nucleus (LGN), the pulvinar, and the thalamic reticular nucleus (TRN). These nuclei form thalamo-cortical circuits such as the cortico-LGN-cortical loop and the cortico-TRN-LGN-cortical loop. There are also loops incorporating the pulvinar, analogous to those for the LGN. The visual thalamo-cortical circuitry is schematically summarized in Figure 1.
Figure 1.
Thalamo-cortical circuitry. Parvocellular (P) neurons in layers 3–6, and magnocellular (M) neurons in layers 1–2 of the LGN, project to layer 4 of V1. Koniocellular (K) neurons in the thin layers of the LGN project to the superficial layers of the visual cortex (not shown). V1 provides feedback from layer 6 to the LGN. The pulvinar is thought to receive largely feedforward input from layer 5 of the cortex, i.e. information destined for more advanced levels of processing, and feedback input from layer 6. The pulvinar projection to the cortex terminates in layer 4 and in more superficial layers. Many different cortical areas are connected via the pulvinar; however, here we only show V1 and extrastriate cortex for clarity. FF = putative feedforward (green); FB = putative feedback (red).
The LGN contains three main classes of neurons: parvocellular, magnocellular and koniocellular. These neurons have different response characteristics, form different layers in the LGN, and project to different layers of the visual cortex. It is generally thought that parvocellular neurons, which are found in layers 3–6, are largely involved in form and color processing, whereas magnocellular neurons, which are found in layers 1 and 2, are largely involved in motion and depth processing. Less is known about koniocellular neurons, which are primarily located in a thin layer beneath each parvo- and magnocellular layer. The current evidence suggests that a subset of koniocellular neurons carry blue cone signals from the retina. Parvocellular and magnocellular neurons project to layer 4 of area V1, while koniocellular neurons project to superficial layers in V1 and to extrastriate cortex. Retinal afferents comprise only about 10% of the LGN’s input; the vast majority stems from various feedback sources including area V1 (originating from layer 6), the TRN and cholinergic projections from the parabrachial nucleus of the brainstem, which each provide roughly 30% of the input to the LGN [4]. The superior colliculus (SC) also provides input to the koniocellular layers of the LGN. The rich anatomical feedback architecture of the LGN provides the anatomical basis for the modulatory influences discussed below.
The pulvinar is traditionally subdivided into medial, lateral, inferior, and anterior nuclei, based on cytoarchitectonic criteria [6]. Broadly speaking, the dorsal pulvinar is connected with frontal, parietal and superior temporal cortical areas and the ventral pulvinar is connected with occipital and inferior temporal areas [6]. The superior colliculus also projects to the pulvinar, especially its ventral part [6]. Pulvinar neurons respond selectively to a number of visual stimulus features, including color, orientation, or motion [7]. This diversity presumably reflects the fact that the pulvinar receives input from (and provides output to) many cortical areas. As a general principle, if two cortical areas are directly connected, then they will be indirectly connected via the pulvinar as well [6]. In addition, regions of the pulvinar that are associated with different cortical areas are connected via long-range interneurons [8]. An intriguing characteristic of the overall connectivity pattern of the pulvinar is that it offers the possibility for the pulvinar to influence, and be influenced by, both feedforward and feedback processing in the visual cortex (Figure 1). The pulvinar receives input from layer 5 of the visual cortex, which is suggested to be feedforward, i.e. the transmitted information is destined for an area at a more advanced level within the processing hierarchy [4]. In contrast, cortical layer 6 provides feedback to the pulvinar, similar to the projection from V1 to the LGN. Pulvinar projections to the cortex terminate in cortical layers 1 through 4 [9].
Neurons in the visual sector of the thalamic reticular nucleus (TRN) have short latency responses to visual stimuli [10] and provide inhibitory input to the LGN or pulvinar [11]. These characteristics enable the TRN to modulate visual activity in the thalamus [10]. The TRN also has access to information regarding behavioral context from a diversity of sources, including the prefrontal cortex, extrastriate cortex, V1, LGN, pulvinar, and the SC [11,12]. After integrating these diverse inputs, the TRN is in a position to regulate information transmission in the LGN and pulvinar according to the behavioral context.
Attentional and perceptual modulation of LGN responses
The first compelling report of response modulation in the LGN during a cognitive task was provided by human fMRI studies. In a series of attention experiments [13,14], it was shown that selective attention affects visual processing in the LGN in at least three different ways, just as at the cortical level. First, neural responses to visual stimuli were greater when attended versus when ignored. Such attentional response enhancement was observed on both parvocellular and magnocellular LGN, but the effects tended to be larger on magnocellular layers [14]. Second, neural responses to ignored stimuli were attenuated depending on the load of attentional resources engaged elsewhere [13]. This response attenuation is consistent with results from a macaque study showing reduced metabolic activity in the magnocellular layers of the LGN in the region outside the representation of the attended stimulus [15]. Third, directing attention to a location in the absence of visual stimulation and in anticipation of stimulus onset increased neural baseline activity [13].
There are a number of sources of input to the thalamus that are potentially able to modulate LGN activity based on attention allocations, including frontal and parietal cortex and the SC. The attentional selection of behaviorally relevant information is thought to be controlled by a widely distributed network of areas in frontal and parietal cortex. This higher-order attention network has a cortical pathway to the LGN via V1, and a subcortical pathway to the LGN via the TRN, e.g. the prefrontal cortico-TRN-LGN route [12,16]. The subcortical pathway offers a direct, and likely quicker, route to the thalamus. Other fast pathways potentially able to modulate LGN responses are derived from the superior colliculus, e.g. the retino-colliculo-TRN-LGN route [11]. Interestingly, the attention effects measured using fMRI tended to be larger in the LGN than in striate cortex [13], suggesting that attentional response modulation in the LGN is unlikely to be due solely to cortico-thalamic feedback from striate cortex, but may be further influenced by the subcortical sources of input such as the TRN. This view has been corroborated by a recent physiology study ([10]; but see Box 1) that demonstrated concurrent response modulation in parvo- and magnocellular LGN and the TRN. While the response amplitudes evoked by an attended stimulus increased relative to those evoked by unattended stimuli in the LGN, they decreased in the TRN and also preceded the LGN responses, suggesting a release of inhibitory control of the TRN over the LGN. These effects occurred in the first 100 ms after stimulus onset. A second effect of attention on the LGN manifested 200 ms after stimulus onset and was independent of the TRN. It is plausible that this late response modulation reflects cortical feedback via V1.
Box 1. Resolving discrepancies between human fMRI and monkey physiology studies of the LGN.
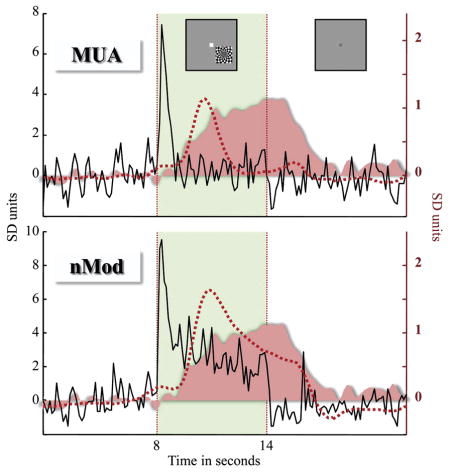
A number of fMRI studies have shown strong modulation of human LGN activity in attention [13,14] and rivalry tasks [18,19]. The size of attention effects on the LGN was greater than that on V1. In contrast, single-neuron recordings from awake, behaving monkeys have shown at most only moderate effects of attention on LGN activity [1–3,10], and the effect size appears to be on the order of that observed in macaque V1. The seeming discrepancy in the size of modulatory effects between the human neuroimaging and monkey physiology experiments may be due to the different nature of tasks involved, or possibly even differences between humans and monkeys. Alternatively, to help reconcile the fMRI and physiology data, it is useful to consider the neural basis of the BOLD signal measured with fMRI. The local field potentials that can be recorded in physiology experiments reflect subthreshold membrane potentials, including synaptic events, oscillatory activity and afterpotentials (that follow action potentials). BOLD responses have been shown to better correlate with local field potentials than action potentials [32]. An example from the work of Logothetis and colleagues [42] illustrates this point. Multi-unit spiking activity (action potentials from a number of neurons) and local field potentials (20–60 Hz) recorded from the same location in V1 are shown (in black) in the upper and lower panels, respectively (reproduction of Fig. 3B from [42], copyright (2008), with permission from Elsevier). There is a transient elevation in the multi-unit activity, and a sustained elevation in the 20–60 Hz component of the local field potential, in response to the stimulus (inset). When these neural signals are convolved with the theoretical hemodynamic response, the resulting regressor (red dashed line) for the local field potential better fits the sustained BOLD response (pink). In fact, in the absence of action potentials, local field potentials are strongly coupled to tissue oxygenation [34]. These results suggest that BOLD activity is more tightly linked to subthreshold membrane potentials than action potentials. To date, studies of the LGN in awake, behaving monkeys have generally involved measurements of action potential rate. Local field potentials and synchrony between neural responses, including the correlated firing of action potentials among multiple neurons, have not been recorded. It is likely that these neural measures, rather than action potential rate, will be more consistent with BOLD activity in the LGN.
The feedback to the LGN from both the TRN and V1 is retinotopic. However, inputs from these different sources likely have different functions. Recent evidence suggests that there are three types of cortico-thalamic neurons, each type selectively targeting either magno-, parvo- or koniocellular LGN neurons [16]. In contrast, individual TRN neurons provide input to multiple LGN layers, hence the TRN input showing little specificity for magno-, parvo- or koniocellular neurons [17]. These connectivity patterns are consistent with the TRN modulating the transmission of information at particular locations in the visual field. In comparison, the cortico-thalamic feedback has the potential to be more specific, by additionally being able to selectively modulate information processing in magno-, parvo-, or koniocellular pathways to the cortex.
LGN activity can also be modulated under conditions of binocular rivalry. When dissimilar images such as gratings of orthogonal orientation are presented to the two eyes, they compete for perceptual dominance so that only one image is visible at a time while the other one is suppressed. FMRI activity in the human LGN has been shown to reflect the subjects’ reported percept and not necessarily the physical inputs from the two eyes. For example, while a grating of different contrast and orientation was presented to each eye, activity increased when the high-contrast horizontal grating was perceived and decreased when the low-contrast vertical grating was perceived [18,19]. Because LGN neurons are essentially monocular, the binocular rivalry effects most likely result from feedback to the LGN. These findings suggest that the thalamus contributes to the control of awareness of visual information as well as attention.
The pulvinar: an integrator of visual information?
The pulvinar has been much less studied than the LGN and consequently much less is known about its functions. Nevertheless, several lines of evidence suggest that the pulvinar should be considered as a subcortical component of the attention network in the brain. First, critical elements of the attention network, such as the prefrontal cortex, posterior parietal cortex, and SC, are connected with the pulvinar [6]. Second, spatial attention can increase (by more than 25%) or attenuate the action potential rate of pulvinar neurons in awake, behaving monkeys [1,7]. Third, in addition to change in the magnitude of pulvinar responses, modulation of action potential timing and variability has been reported ([7]; see Box 2 for the effect of response timing on gain control). Fourth, selective attention has been shown to increase BOLD responses [20,21] and glucose uptake [22] in the human pulvinar. Finally, pulvinar lesions are commonly associated with attention deficits, including neglect [23], altered salience signaling [24], impaired spatial and temporal processing [25] and poor filtering of distracters [26,27].
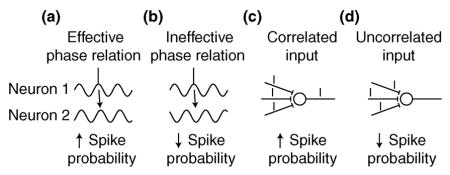
Box 2. Gain control mechanisms.
Changing the number of action potentials generated by pre-synaptic neurons can obviously affect the action potential output of the post-synaptic neuron. However, action potential timing, particularly the degree of synchrony between neurons, in addition to action potential rate, is important for gain control [43–45]. Gain control refers to changing the relationship between the input and the output of a neuron. A relevant example is the increased responsiveness of a neuron representing an attended stimulus. A number of mechanisms for gain control have been proposed, and here we will briefly discuss two of them. First, neurons undergo oscillations of membrane potential, which are below the threshold for action potentials. Inputs arriving at a depolarizing phase of a neuron are more likely to generate action potentials; conversely, inputs arriving at a hyperpolarizing phase of a neuron are less likely to generate action potentials. Thus, the output of a neuron may be modulated by changing the relative timing or synchrony between pre- and post-synaptic neurons ([43,45]; e.g., neuron 2 is more likely to fire an action potential in panel (a) than (b)). Second, synchronized inputs generally have a larger effect on neurons. Therefore, the output of a neuron can also be modulated by changing the synchrony of pre-synaptic neurons ([44]; e.g., the post-synaptic neuron is more likely to fire in panel (c) than (d)). Neural synchrony may manifest between subthreshold oscillatory processes of various frequencies, e.g. in the gamma frequency band (25–90 Hz), and is modulated within [46] and between [43,47] brain areas by cognitive activities such as selective attention. Just how these oscillatory processes are regulated, in particular the relationship between processes, is fundamental to a better understanding of the neural mechanisms of gain control. However, our knowledge regarding the regulation of oscillatory processes during cognition is limited.
Thalamo-cortical circuits are able to support oscillations [48]. Inhibitory neurons are involved in generating fast oscillations, and the TRN has been shown to modulate oscillations through inhibitory inputs to other thalamic neurons [49]. Recently, it has been shown that attention changes the responses of TRN neurons [10]. This provides a means for attention to regulate thalamo-cortical oscillations. There are also intrinsic cell properties that give rise to oscillatory behavior [50]. Oscillations may originate in the cortex or thalamus, and subthreshold oscillatory patterns appear to shape action potential output, especially action potential timing [44]. Changing thalamo-cortical oscillatory patterns (frequency, amplitude or phase) is likely to change the gain of thalamic and cortical neurons.
It is useful here to consider pulvinar connectivity in more detail, before discussing how the pulvinar contributes to attentional selection at a mechanistic level. Cortico-pulvino-cortical pathways travel between fronto-parietal areas and between occipito-temporal areas. Fronto-parietal areas also connect to occipito-temporal areas via a cortico-colliculo-ventral pulvino-cortical pathway and possibly a minor cortico-dorsal pulvino-cortical pathway [6]. This cortico-pulvino-cortical circuitry suggests at least two ways for the pulvinar to be involved in attentional selection: one at the level of the thalamus, and the other at the cortical level. At the level of the thalamus, the pulvinar could filter visual information from different inputs and only transmit information relevant to behavior. Alternatively, at the level of the cortex, pulvino-cortical neurons could modulate the efficacy of direct inputs from one cortical area to another.
The pulvinar may serve a broader role than one limited to visual attention. For example, physiology and fMRI studies have shown that the pulvinar responds to global motion in both random dot patterns [21] and in plaid patterns [28,29], suggesting that the pulvinar may integrate the different visual components for the global percepts [28]. Such integration is made possible by the interconnections between the pulvinar and multiple visual cortical areas, e.g. those involved in pattern-motion processing such as MT+ and V3. In support of this idea, pulvinar lesions in humans can disturb the binding of visual features [25,30]. These results are consistent with the idea that the pulvinar integrates visual information according to the behavioral context.
Thalamic regulation of cortical responses
Thus far, we have described perceptual and cognitive conditions during which the responsiveness, or gain, of thalamic neurons changes. Importantly, changing thalamic gain influences cortical responses in turn. For example, increasing the number of action potentials that are output by thalamic neurons is likely to increase the response of cortical target neurons. However, modulating the overall spike rate is only one way to influence the weighting of information transmitted to cortical areas. Alternatively, cortical gain may be regulated by adjusting the degree of synchrony between neurons, which may not even be reflected in the magnitude of thalamic responses (Box 2). That is, even if the action potential rate of thalamic neurons does not change, synchronizing action potential timing across thalamic neurons may increase the impact of thalamic output on the cortex. In addition, synchronizing oscillations of membrane potentials (below the threshold for action potentials) of thalamic and cortical neurons may increase the effectiveness of thalamo-cortical transmission.
It has been shown that oscillations of subthreshold membrane potentials help synchronize action potentials [31]. Interestingly, there is growing evidence that BOLD responses measured with fMRI are more closely related to subthreshold membrane potentials rather than action potentials (Box 1). BOLD responses have been reported to strongly correlate with subthreshold oscillatory activity, especially in the gamma frequency band [32–34]. Therefore, it is possible that the modulatory effects on the human LGN discussed above may involve changes in oscillatory processes and consequently changes in action potential timing. Physiology studies in awake, behaving animals have primarily measured response magnitude (e.g., [1,3,10]), and for this reason there is limited data on the temporal structure of LGN activity during cognitive manipulations. However, there is already some support for the idea that task demands modulate the timing of LGN responses. For example, visual attention has been shown to increase (beta) oscillatory activity in the cat LGN and V1 [35,36] and cortico-thalamic feedback appears to potentiate the LGN responses [35]. Using in vitro and anaesthetized animal preparations, it has been shown that cortico-thalamic feedback regulates oscillations and action potential timing, thereby helping to synchronize activity in the LGN and between the LGN and visual cortex [37]. Importantly, correlated firing of LGN cells is more effective in eliciting V1 responses than uncorrelated inputs [38]. Thus, oscillations and neuronal synchrony are able to modify the efficacy of retino-cortical transmission.
Similarly, the cortico-pulvino-cortical connectivity appears suitable for adjusting the relationship between oscillatory processes in different cortical areas, in order to control cortical gain. To this end, connections between the TRN and the pulvinar [11] are suitable for modulating oscillations, and pulvinar projections to superficial cortical layers may be able to entrain networks of neurons [9]. A few physiology studies in the cat provide initial evidence in support of the hypothesis that the pulvinar adjusts cortical oscillations and thereby controls cortical gain. There is synchrony between the visual cortex and the lateral posterior-pulvinar complex in the beta frequency band during attentional selection [39] and in the gamma frequency band elicited by visual stimulation in anaesthetized preparations [40,41]. Deactivating the pulvinar has been shown to reduce the synchrony and oscillation strength in the visual cortex [40,41]. These studies of the cat pulvinar suggest that disturbed oscillatory mechanisms may contribute to the attention deficits experienced by human subjects with pulvinar lesions [23–25,27].
Conclusions
There is growing evidence for modulation of neural responses in the visual thalamus by perception and cognition (Box 3). Changes in the action potential rate or synchrony of LGN neurons are likely to change the gain of cortical neurons. Due to its rich feedback connectivity the LGN is well positioned to regulate retino-cortical transmission according to attentional or other cognitive demands. Analogously, the pulvinar is well positioned to regulate cortico-cortical transmission, which may mediate the integration of visual information flexibly in the ever-changing behavioral context. Such a role may involve setting oscillatory patterns in different cortical areas and/or manipulating their phase relationships in order to control gain.
Box 3. Future directions.
To better understand top-down modulation of the visual thalamus, future experiments need to characterize the temporal structure of LGN and pulvinar activity in monkeys performing cognitive tasks. As for the LGN, there is currently no published data on koniocellular neurons, the third major LGN cell type, in awake, behaving monkeys, and because of the different inputs (e.g. SC and extrastriate feedback) and outputs (e.g. superficial layers of V1 and extrastriate cortex) of koniocellular neurons, their behavioral modulation is bound to differ from that of magno- and parvocellular neurons. As for the pulvinar, it has recently proved amenable to fMRI investigation, permitting clarification of pulvinar functions. In addition, physiological experiments aimed at resolving functions of pulvinar subdivisions are much needed. From a systems perspective, thalamic modulation of cortical gain during cognition would ideally be studied using simultaneous neural recordings from the visual thalamus and cortex in awake, behaving monkeys.
Acknowledgments
We thank Marius Peelen and Ryan Mruczek for comments on the manuscript. Supported by grants NEI RO1 EY017699 and NIMH RO1 MH64043.
Footnotes
Conflicts of interest
We declare that we have no conflict of interest.
Contributor Information
Yuri B. Saalmann, Green Hall, Princeton Neuroscience Institute, Princeton University, Princeton, NJ 08540 USA, Telephone: +1-609-258-8318, saalmann@princeton.edu
Sabine Kastner, Green Hall, Psychology Department, Princeton University, Princeton, NJ 08540 USA, Telephone: +1-609-258-0479, Fax: +1-609-258-1113, skastner@princeton.edu.
References
- 1.Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol. 2001;85:219–234. doi: 10.1152/jn.2001.85.1.219. [DOI] [PubMed] [Google Scholar]
- 2.Lehky SR, Maunsell JH. No binocular rivalry in the LGN of alert macaque monkeys. Vision Res. 1996;36:1225–1234. doi: 10.1016/0042-6989(95)00232-4. [DOI] [PubMed] [Google Scholar]
- 3.Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I: distribution and timing of effects across visual areas. Cereb Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2. Cambridge, Mass.: MIT Press; 2006. [Google Scholar]
- 5.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- 8.Imura K, Rockland KS. Long-range interneurons within the medial pulvinar nucleus of macaque monkeys. JComp Neurol. 2006;498:649–666. doi: 10.1002/cne.21085. [DOI] [PubMed] [Google Scholar]
- 9.Jones EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci. 2002;357:1659–1673. doi: 10.1098/rstb.2002.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. Using single-neuron recordings from awake, behaving macaques, the authors showed that attention moderately but significantly increased the action potential rate of LGN neurons as well as reduced the activity of TRN neurons. In light of the fact that the TRN provides an inhibitory input to the LGN, these results suggest that the TRN contributes to the gating of LGN responses by attention. This study is the first to show attentional modulation of single LGN neurons, consistent with the effects of attention on the LGN measured using fMRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 12.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 14••.Schneider KA, Kastner S. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J Neurosci. 2009;29:1784–1795. doi: 10.1523/JNEUROSCI.4452-08.2009. Using high resolution fMRI, the authors measured activity in the LGN and SC during sustained spatial attention. Spatial attention enhanced activity in both the LGN and SC, although the effect was significantly stronger in the SC. Weak suppression was also observed in some regions of the LGN. These results suggest that visual information can be filtered at the first processing stage after the retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanduffel W, Tootell RB, Orban GA. Attention-dependent suppression of metabolic activity in the early stages of the macaque visual system. Cereb Cortex. 2000;10:109–126. doi: 10.1093/cercor/10.2.109. [DOI] [PubMed] [Google Scholar]
- 16.Briggs F, Usrey WM. Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron. 2009;62:135–146. doi: 10.1016/j.neuron.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlrich DJ, Manning KA, Feig SL. Laminar and cellular targets of individual thalamic reticular nucleus axons in the lateral geniculate nucleus in the prosimian primate Galago. J Comp Neurol. 2003;458:128–143. doi: 10.1002/cne.10568. [DOI] [PubMed] [Google Scholar]
- 18.Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunderlich K, Schneider KA, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat Neurosci. 2005;8:1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastner S, O’Connor DH, Fukui MM, Fehd HM, Herwig U, Pinsk MA. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol. 2004;91:438–448. doi: 10.1152/jn.00553.2003. [DOI] [PubMed] [Google Scholar]
- 21.Smith AT, Cotton PL, Bruno A, Moutsiana C. Dissociating vision and visual attention in the human pulvinar. J Neurophysiol. 2009;101:917–925. doi: 10.1152/jn.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- 24.Michael GA, Buron V. The human pulvinar and stimulus-driven attentional control. Behav Neurosci. 2005;119:1353–1367. doi: 10.1037/0735-7044.119.5.1353. [DOI] [PubMed] [Google Scholar]
- 25.Arend I, Rafal R, Ward R. Spatial and temporal deficits are regionally dissociable in patients with pulvinar lesions. Brain. 2008;131:2140–2152. doi: 10.1093/brain/awn135. [DOI] [PubMed] [Google Scholar]
- 26.Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harb Symp Quant Biol. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- 27••.Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci U S A. 2009;106:4054–4059. doi: 10.1073/pnas.0810086106. The authors tested the performance of patients with pulvinar lesions in an orientation discrimination task in which the oriented target was flanked by distracters. Irrelevant but physically salient distracters impaired patients’ performance. However, increasing the salience (contrast) of the target relative to the distracters improved performance. This study suggests that the pulvinar plays a significant role in selective attention, and in particular in filtering distracter information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merabet L, Desautels A, Minville K, Casanova C. Motion integration in a thalamic visual nucleus. Nature. 1998;396:265–268. doi: 10.1038/24382. [DOI] [PubMed] [Google Scholar]
- 29.Villeneuve MY, Kupers R, Gjedde A, Ptito M, Casanova C. Pattern-motion selectivity in the human pulvinar. Neuroimage. 2005;28:474–480. doi: 10.1016/j.neuroimage.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Ward R, Danziger S, Owen V, Rafal R. Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nat Neurosci. 2002;5:99–100. doi: 10.1038/nn794. [DOI] [PubMed] [Google Scholar]
- 31.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 32.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 33.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 34.Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- 35.Bekisz M, Wrobel A. 20 Hz rhythm of activity in visual system of perceiving cat. Acta Neurobiol Exp (Wars) 1993;53:175–182. [PubMed] [Google Scholar]
- 36.Wrobel A, Bekisz M, Kublik E, Waleszczyk W. 20 Hz bursting beta activity in the cortico-thalamic system of visually attending cats. Acta Neurobiol Exp (Wars) 1994;54:95–107. [PubMed] [Google Scholar]
- 37.Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci. 1998;18:6395–6410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- 39••.Wrobel A, Ghazaryan A, Bekisz M, Bogdan W, Kaminski J. Two streams of attention-dependent beta activity in the striate recipient zone of cat’s lateral posterior-pulvinar complex. J Neurosci. 2007;27:2230–2240. doi: 10.1523/JNEUROSCI.4004-06.2007. The authors simultaneously recorded local field potentials from the lateral posterior-pulvinar complex and visual cortex of cats performing either a visual or an auditory version of a spatial discrimination task. Amplitudes of oscillations in the beta frequency band (12–25Hz) increased in the lateral posterior-pulvinar complex and visual cortex when the cat anticipated a visual target but not when anticipating an auditory target. Results of a phase-correlation analysis of the local field potentials suggest synchronization between the beta-band oscillations in the thalamus and cortex during the anticipatory period. These results are consistent with a contribution of the pulvinar to cortical oscillations, which are important for gain control during attentional processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molotchnikoff S, Shumikhina S. The lateral posterior-pulvinar complex modulation of stimulus-dependent oscillations in the cat visual cortex. Vision Res. 1996;36:2037–2046. doi: 10.1016/0042-6989(95)00311-8. [DOI] [PubMed] [Google Scholar]
- 41.Shumikhina S, Molotchnikoff S. Pulvinar participates in synchronizing neural assemblies in the visual cortex, in cats. Neurosci Lett. 1999;272:135–139. doi: 10.1016/s0304-3940(99)00497-8. [DOI] [PubMed] [Google Scholar]
- 42.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 43.Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- 44.Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J Neurosci. 2006;26:1673–1676. doi: 10.1523/JNEUROSCI.3737-05d.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 46.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 47.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 48.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Steriade M, Contreras D, Amzica F, Timofeev I. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llinas RR, Choi S, Urbano FJ, Shin HS. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci U S A. 2007;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]