Abstract
Purpose
To examine the effects of the occurrence and co-occurrence of comorbidities (COM), functional limitations (FL), and geriatric syndromes (GS) on treatment and outcomes in older cancer patients.
Materials and Methods
We used records from the Ohio Cancer Incidence Surveillance System linked with Medicare data, clinical assessment data from the home health care Outcomes and Assessment Information Set, and death certificate data. Our patient population included fee-for-service HHC Medicare beneficiaries diagnosed with incident loco-regional breast or colorectal cancer in years 1999–2001 (n=1236). We grouped patients according to the presence of multimorbidity: (0): none of COM, FL, or GS; (1): occurrence – but no co-occurrence – of COM, FL, or GS; (2): co-occurrence of any two of COM, FL, and GS; and (3): co-occurrence of all three of COM, FL, and GS. Our outcomes were receipt of standard treatment, as well as overall survival (OS) and disease-specific survival (DSS) through 2005. Multivariable regression models were developed to analyze the independent association between multimorbidity and the outcomes, before and after adjusting for age.
Results
The effect of multimorbidity on our outcomes was attenuated considerably by age. Adjusting for age and compared with no multimorbidity (0), high multimorbidity (3) remained significantly and negatively associated with receipt of standard treatment (adjusted odds ratio: 0.57, 95% Confidence Interval (CI): 0.33, 0.97). Furthermore, high multimorbidity (3) was associated with increased hazard for OS, but not for DSS (adjusted hazard ratio and 95% CI: 2.15 (1.58, 2.93) for three entities).
Conclusion
Multimorbidity is significantly and independently associated with cancer treatment and OS, but not DSS.
Keywords: Comorbidity, Functional Limitations, Geriatric Syndromes, Multimorbidity, Colorectal Cancer, Breast Cancer, Standard Treatment, Survival
Cancer is seldom the only condition with which older adults present1–3. The occurrence and co-occurrence of comorbidities, functional limitations, and geriatric syndromes (e.g., depression, dementia, and urinary incontinence) present a significant challenge to older cancer patients’ physiologic and functional reserves4, 5, as well as to clinicians managing their cancers. To date, studies on cancer outcomes in older patients have focused mostly on the effect of comorbidities relative to cancer treatment and survival outcomes. More recent studies have evaluated these outcomes in the presence of functional limitations or geriatric syndromes as well6, 7, and demonstrated that in colorectal cancer patients, functional limitations and geriatric syndromes are associated with treatment patterns and survival outcomes, independently of age and comorbidities. However, very little is known about the effect of co-occurrence of these conditions relative to these outcomes. The present study addresses this gap in the literature.
Research on the prevalence of multimorbidity8, 9 is emerging, and newly published studies are increasingly focusing on elucidating the often causal associations among comorbidities, functional limitations, and geriatric syndromes. For example, cognitive impairment, which is correlated with depression, has been shown to be associated with functional decline10. Indeed, low cognitive status is independently associated with functioning difficulties, even after controlling for a number of socio-demographic characteristics and chronic conditions11. Similarly, diabetes, depression, chronic obstructive pulmonary disease (COPD), arthritis, and poor lower-extremity physical performance are associated with urinary incontinence12, 13. COPD is linked to urinary incontinence through chronic cough, and arthritis may restrict an individual’s ability to disrobe quickly12, thus resulting in incontinence.
These clinical scenarios explain why these conditions seldom present in isolation, and why their co-occurrence is highly prevalent. In fact, an earlier study characterizing an older cancer population receiving home health care documented a prevalence of co-occurrence of 35% in breast cancer patients, 59% in prostate cancer patients, and 49% in colorectal cancer patients14.
Based on a framework proposed by Balducci and Extermann15, and consistent with prior studies by our group7, 14, we characterize patients’ clinical presentation by accounting for the presence of comorbidities, functional limitations, and/or geriatric syndromes. We further define multimorbidity as the co-occurrence of comorbidities, functional limitations, and/or geriatric syndromes. In this study, we aim to evaluate the effect of multimorbidity in older adults with incident loco-regional breast and colorectal cancer relative to receipt of standard treatment and survival, hypothesizing that multimorbidity is significantly associated with unfavorable treatment patterns and survival outcomes.
Methods
Data Sources
We used a database developed by linking records from the Ohio Cancer Incidence Surveillance system (OCISS) with Medicare enrollment and claims files, clinical assessment data from the Outcome and Assessment Information Set (OASIS), and Ohio death certificate files. As described in detail elsewhere16, the records were linked by using patient identifiers, including patient name, social security number, date of birth, and gender. This and related studies were approved by the Institutional Review Board, University Hospitals of Cleveland; the Ohio Department of Health, which administers the OCISS; and the Centers for Medicare & Medicaid Services, which supplied the Medicare and OASIS data.
The Ohio Cancer Incidence Surveillance System (OCISS)
Established in 1991, the OCISS is representative of over 90% of incident cancer cases diagnosed in residents of the state of Ohio. Exceptions are carcinoma in situ of the cervix, and non-melanoma cancers of the skin. The OCISS record carries patient identifiers, the date of cancer diagnosis, and tumor characteristics, including anatomic cancer site and cancer stage.
The OCISS constituted the source file in this study, in that it was used to identify the patient population. All demographic and cancer-relevant variables originated from the OCISS.
The Medicare enrollment and claims files
The Medicare Denominator file includes one record per beneficiary. In addition to demographics, this file carries monthly variables indicating the individual’s participation in state buy-in or managed care programs.
The Medicare claims files included the Medicare Provider, Analysis and Review (MedPAR), carrying data pertaining to inpatient stays; the Outpatient Standard Analytic File (SAF); and the Physician Supplier or Carrier SAF. Each of these files carries diagnosis and procedure codes, which enabled us to identify treatment modalities in the −30 to +180 days relative to the date of cancer diagnosis. Records from the MedPAR carry up to 10 slots for each of the diagnosis and procedure codes, both in International Coding of Diseases, 9th Revision (ICD-9). The Outpatient and Carrier SAFs carry up to 4 slots for diagnosis codes, and procedure codes at the line item level in Current Procedural Terminology, 4th Edition (CPT-4) or in HealthCare Common Procedural Coding System (HCPCS).
The Outcome and Assessment Information Set (OASIS)
The OASIS is a repository of clinical assessment data for Medicare beneficiaries receiving home health care (HHC). The Medicare HHC is designed to address post-acute and other health care needs of patients who are homebound or confined to their residence. To qualify for HHC, Medicare beneficiaries must be under the care of a physician, and must require intermittent skilled nursing care, physical therapy, speech therapy, or occupational therapy17. The data are gathered by HHC providers upon admission, discharge, and every 60 days if the patient continues to receive HHC. Studies assessing the interrater reliability for a number of OASIS items reported Kappa scores of no less than 0.6 for a number of items that were evaluated in the study, but more than 0.7 for most items18, 19. As such, the reliability of these items in the OASIS has been deemed “sufficient for use in research.”18 Home health care agencies must submit the OASIS forms to the Centers for Medicare & Medicaid Services in order to be reimbursed by Medicare. This implies that patient assessment is available for nearly all Medicare beneficiaries receiving HHC.
In addition to patient identifiers, the OASIS record carries diagnosis codes, which we used to identify comorbid conditions, as well as variables on limitations in Activities of Daily Living (ADLs), cognitive status, depression, and urinary incontinence – among others. Most of these measures reflect the patient’s status as of the date of assessment, and their status in the 14 days prior. The latter variables were used as a proxy of their status at baseline.
The Ohio Death Certificate Data
Death certificate data are available for nearly all decedents of residents of the state of Ohio. In addition to demographics, the death certificate record carries the date of death and the cause of death coded in ICD-10.
Study population
We chose to study HHC patients in order to fully exploit the richness of clinical assessment data in the OASIS.
Our study population consists of residents from the state of Ohio, 65 years of age or older, diagnosed with incident loco-regional breast or colorectal cancer in the period August 1999 through November 2001, and admitted to HHC in the 30 days preceding or following their initial date of cancer diagnosis (n=1,371). The patients’ vital status was followed through December 31, 2005.
Of breast and colorectal patients receiving home health care (HHC), 15–20% had been receiving HHC one year or longer before cancer diagnosis; 10% had initiated HHC in the 30 to 365 days prior to cancer diagnosis; and 30–35% had initiated HHC in the 30-day window before or after cancer diagnosis16. The latter group constitutes our study population. The narrow window around the time of cancer diagnosis was to ensure that the assessment data reflected their clinical status at baseline.
To ensure completeness of claims data, we limited our study population to patients receiving their care through the traditional fee-for-service only, and excluded those enrolled in managed care programs (n=125). Furthermore, in order to minimize additional confounding from prior history of cancer, we excluded 10 patients diagnosed with another primary breast, colorectal, or prostate cancer during the period 1997–2001, leaving our study population at n=1,236.
Variables of interest
Outcome Variables
-
-Receipt of standard treatment, defined as follows20:
-
○Breast cancer:
-
▪Local stage: Mastectomy OR Lumpectomy + radiation therapy
-
▪Regional stage: Treatment for local stage + chemotherapy. Of note is that since we relied on Medicare data pre-dating the 2006 implementation of Plan D, the above category of chemotherapy does not include oral therapies such as tamoxifen or aromatase inhibitors.
-
▪
-
○Colorectal cancer:
-
▪Local stage: Colon resection
-
▪Regional stage: Treatment for local stage + chemotherapy
-
▪
-
○
The diagnosis and procedure codes pertaining to the above treatment modalities are presented in the Appendix.
-
-
Survival, defined as time elapsed between date of cancer diagnosis and date of death. Patients who survived until December 31, 2005 were censored. Using the cause of death, we accounted for overall survival, as well as disease-specific survival.
Independent Variables
The main independent variable, multimorbidity, describes the clinical presentation, based on the co-occurrence of clinical comorbidities, functional limitations, and geriatric syndromes in these patients. It is categorized as follows:
-
-
0, or absence of comorbidities, functional limitations, or geriatric syndromes;
-
-
1, or presence of comorbidities, functional limitations, or geriatric syndromes only.
-
-2, or simultaneous presence of:
-
○Comorbidities and functional limitations
-
○Comorbidities and geriatric syndromes
-
○Functional limitations and geriatric syndromes
-
○
-
-
3, or simultaneous presence of comorbidities, functional limitations, AND geriatric syndromes.
Respectively, the terms comorbidities, functional limitations, and geriatric syndromes refer to the presence of at least one comorbid condition, according to the listing of comorbidities by the National Institute on Aging/National Cancer Institute21; limitations in at least one Activity of Daily Living; and presence of at least one geriatric syndrome (dementia, depression, delirium, urinary incontinence, falls, malnutrition). The definitions used herein were consistent with a framework proposed earlier by Balducci and Extermann15, as well as previously published studies7, 14.
Other independent variables consisted of demographics: age (grouped in 5-year increments); race (African American vs. all others); sex; and marital status (married vs. all Other); and cancer-related variables, including cancer site (female breast vs. colorectal), and cancer stage (local vs. regional). We note that while the tumor size, number of lymph nodes, and metastasis were available through the OCISS, a high proportion of missing data in these variables precluded us from being able to use staging data according to the American Joint Commission on Cancer (AJCC). Instead, we relied on the Surveillance, Epidemiology, and End Results (SEER) summary stage.
Analysis
We examined the distribution of the study population by the variables of interest, and compared receipt of standard treatment by our independent variables in bivariate analyses. We developed logistic regression models to evaluate the association between multimorbidity and receipt of standard treatment after adjusting for patient demographics and tumor site/stage. We then developed Kaplan-Meier curves and Cox Proportional Hazard models to analyze disease-specific and overall survival. In the latter models, receipt of standard treatment was used as an independent variable as well.
Results of the bivariate analysis indicated a strong and statistically significant association between age and multimorbidity, as shown below. To address potential confounding, and to gain a better understanding of the association between age, multimorbidity, and the outcomes of interest, we developed regression models with and without the age variable. In our interpretation of the findings, we rely on the results obtained by the models that included the age variable. Nonetheless, we chose to also present the model without the age variable in order to show the extent to which the inclusion of the age variable in the model attenuated the effects of multimorbidity.
To obtain adequate statistical power, we combined data for both cancer sites. To ensure that this approach did not mask any site-specific differences in the outcomes of interest, we tested the interaction between multimorbidity and cancer site, and conducted stratified analysis by cancer site. The interaction term was not statistically significant (p=0.5321), and the stratified analysis yielded results comparable to the model using data for both sites, albeit at lower levels of statistical power. Consequently, we present our results from the models using combined data for both cancer sites.
We used SAS version 9.1 (Cary, NC) in all our analyses.
Results
Our study population included 601 breast cancer patients and 635 colorectal cancer patients, distributed almost equally between local- and regional-stage cancer (Table 1). Fourteen percent were 85 years of age or older, and 8% were African American. The majority were women (78%) and 39% were married.
Table 1.
Distribution of multimorbidity (composite co-morbidity, functional limitation and geriatric syndromes) and other variables by standard treatment
| Variable of interest | Total Population n (% of total) |
Standard treatment | ||
|---|---|---|---|---|
| Yes n (row %) |
No n (row %) |
P-value | ||
| Demographics: | ||||
| Age: | ||||
| 65–69 | 174 (14.1) | 142 (81.6) | 32 (18.4) | |
| 70–74 | 265 (21.4) | 222 (83.8) | 43 (16.2) | |
| 75–79 | 339 (27.4) | 255 (75.2) | 84 (24.8) | |
| 80–84 | 281 (22.7) | 171 (60.9) | 110 (39.2) | |
| 85+ | 177 (14.3) | 86 (48.6) | 91 (51.4) | <0.0001 |
| Race: | ||||
| African-American | 97 (7.9) | 67 (69.1) | 30 (30.9) | |
| All others | 1139 (92.2) | 809 (71.0) | 330 (29.0) | 0.6841 |
| Sex: | ||||
| Men | 276 (22.3) | 184 (66.7) | 92 (33.3) | |
| Women | 960 (77.7) | 692 (72.1) | 268 (27.9) | 0.0809 |
| Marital status | ||||
| Married | 478(38.7) | 358 (74.9) | 120 (25.1) | |
| other | 758 (61.3) | 518 (68.3) | 240 (31.7) | 0.0135 |
| Cancer Stage: | ||||
| Local | 635 (51.4) | 584 (92.0) | 51 (8.0) | |
| Regional | 601 (48.6) | 292 (48.6) | 309 (51.4) | <0.0001 |
| Cancer Site: | ||||
| Breast | 601 (48.6) | 470 (78.2) | 131 (21.8) | |
| Colon | 635 (51.4) | 406 (63.9) | 229 (36.1) | <0.0001 |
| Multimorbidity: | ||||
| 0 | 262 (21.2) | 205 (78.2) | 57 (21.8) | |
| 1 | 448 (36.3) | 339 (75.7) | 109 (24.3) | |
| 2 | 371 (30.0) | 240 (64.7) | 131 (35.3) | |
| 3 | 155(12.5) | 92(59.4) | 63(40.7) | <0.0001 |
| Total | 1236 (100.0) | 876 (100.0) | 360 (100.0) | |
Receipt of standard treatment varied significantly by age (49% among the oldest, and 82% among those in the 65–69 age group); by marital status (75% among married patients vs. 68% in others); anatomic cancer site (64% among colorectal cancer patients, and 78% among breast cancer patients); and by cancer stage (49% among those diagnosed with regional-stage cancer compared to 92% among their counterparts diagnosed with local-stage cancer). With regard to multimorbidity, the proportion of patients receiving standard treatment decreased from 78% among those with no multimorbidity to 59% among those presenting with high multimorbidity (3).
Multimorbidity was strongly associated with older age (Table 2). The proportion of patients with no multimorbidity was 30.5% in the 65–69 age group, and 14.7% in patients 85 years of age or older. Conversely, the proportion of patients with multimorbidity (3) was 7.5% in the youngest age group and 22.0% in the oldest age group (p < 0.001).
Table 2.
Distribution of multimorbidity by age
| MULTIMORBIDITY | Total N (Row %) |
||||
|---|---|---|---|---|---|
| Age group | 0 N (Row %) |
1 N (Row %) |
2 N (Row %) |
3 N (Row %) |
|
| 65–69 | 53 (30.5) | 69 (39.7) | 39 (22.4) | 13 (7.5) | 174 (100.0) |
| 70–74 | 66 (24.9) | 105 (39.6) | 69 (26.0) | 25 (9.4) | 265 (100.0) |
| 75–79 | 73 (21.5) | 131 (38.6) | 102 (30.1) | 33 (9.7) | 339 (100.0) |
| 80–84 | 44 (15.7) | 95 (33.8) | 97 (34.5) | 45 (16.0) | 281 (100.0) |
| 85+ | 26 (14.7) | 48 (27.1) | 64 (36.2) | 39 (22.0) | 177 (100.0) |
p < 0.001
Table 3 reports the results from the logistic regression models predicting receipt of standard treatment, before and after including the age variable in the model. Model 1 includes multimorbidity, as well as all other covariates, except age. Results from this model indicated that patients with multimorbidity levels (2) and (3) were significantly less likely than those with multimorbidity (0) to receive standard treatment (adjusted odds ratio (AOR): 0.64, 95% confidence interval (CI; 0.42, 0.97), and 0.42 (0.25, 0.71), respectively).
Table 3.
Logistic models to analyze the association between receipt of standard treatment and multimorbidity, before and after adjusting for age.
| Variable | Model 1* Adjusted odds ratio (95% Confidence Interval) |
Model 2** Adjusted odds ratio (95% Confidence Interval) |
|---|---|---|
| Multimorbidity: | ||
| 0 | Reference | Reference |
| 1 | 0.99 (0.65, 1.50) | 1.04 (0.68, 1.61) |
| 2 | 0.64 (0.42, 0.97) a | 0.79 (0.51, 1.22) |
| 3 | 0.42 (0.25, 0.71) b | 0.57 (0.33, 0.97) a |
| Race: | ||
| All others | Reference | Reference |
| African-American | 1.21 (0.72, 2.04) | 0.92 (0.53, 1.60) |
| Sex: | ||
| Women | Reference | Reference |
| Men | 0.90 (0.62, 1.33) | 0.89 (0.59, 1.33) |
| Marital status | ||
| Other | Reference | Reference |
| Married | 1.85 (1.36, 2.52) c | 1.43 (1.03, 1.99) a |
| Cancer Stage: | ||
| Local | Reference | Reference |
| Regional | 0.08 (0.06, 0.11) c | 0.07 (0.05, 0.09) c |
| Cancer Site: | ||
| Colon | Reference | Reference |
| Breast | 1.11 (0.79, 1.56) | 0.961 (0.67, 1.37) |
| Age | 0.91 (0.89, 0.93) c | |
| (continuous) | ||
Model 1 includes multimorbidity, and all other covariates, except age
Model 2 includes multimorbidity, all other covariates, including age
0.05 ≤ p < 0.01;
0.01 ≤ p < 0.001;
p < 0.001; all other statistics not significant at p < 0.05
These odds ratios changed considerably when the age variable was entered in the model. Results from Model 2 indicated that multimorbidity (3) remained significantly associated with lower likelihood to receive standard treatment (AOR: 0.57, 95% CI: 0.33, 0.97). In this model, multimorbidity (2) was no longer associated with receipt of standard treatment. We also note that age was also independently and significantly associated with lower likelihood to undergo standard treatment (AOR: 0.91, 95% CI: 0.89, 0.93), implying that an increase in age by one year is associated with 9% lower likelihood to undergo standard treatment.
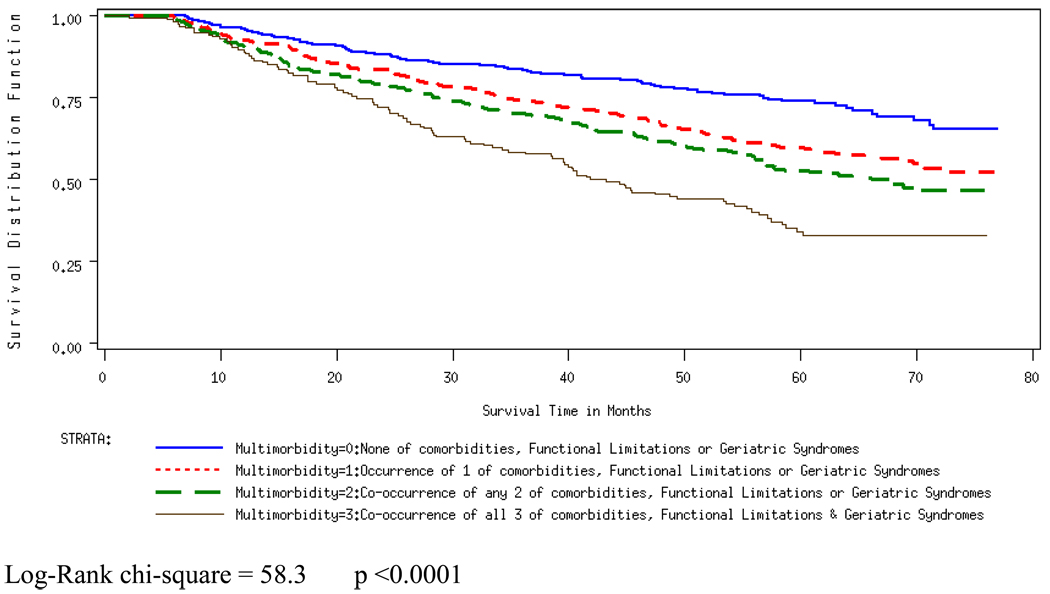
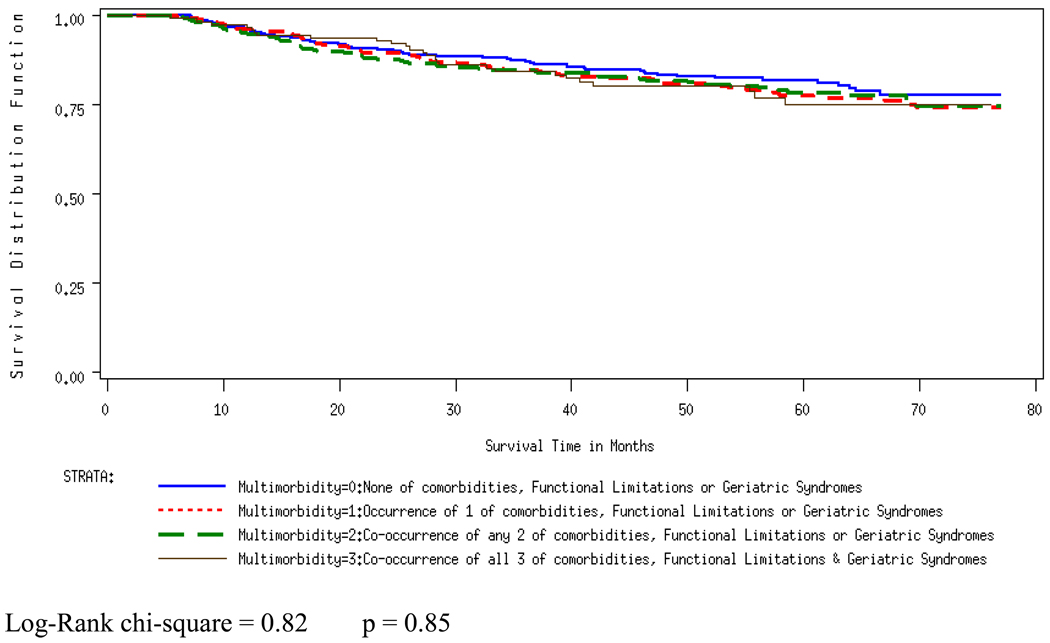
Figures 1 and 2 present the Kaplan-Meier curves, and Table 4 presents the results from the overall survival and disease-specific survival models. In the overall survival model the occurrence and co-occurrence of clinical entities were significantly associated with increased hazard, even after entering the age variable in the model (Adjusted Hazard Ratio (AHR): 2.15, 95% CI: 1.58, 2.93 for multimorbidity (3), compared to multimorbidity (0)). Multimorbidity was not associated with disease-specific survival, however, and this did not change with the inclusion of the age variable in the model.
Figure 1.
Overall survival by Multimorbidity
Figure 2.
Disease-specific survival by Multimorbidity
Table 4.
Cox proportional hazards models to analyze the association between multimorbidity and each of the overall and disease-specific survival, before and after adjusting for age.
| OVERALL SURVIVAL | DISEASE-SPECIFIC SURVIVAL | |||
|---|---|---|---|---|
| Variable | Model1* Adjusted hazard ratio (95% Confidence Interval) |
Model2** Adjusted hazard ratio (95% Confidence Interval) |
Model1* Adjusted hazard ratio (95% Confidence Interval) |
Model2** Adjusted hazard ratio (95% Confidence Interval) |
| Multimorbidity: | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.47 (1.13, 1.92) b | 1.43 (1.10, 1.87) b | 0.98 (0.70, 1.39) | 0.98 (0.69, 1.38) |
| 2 | 1.57 (1.19, 2.06) b | 1.47 (1.12, 1.94) b | 0.84 (0.58, 1.21) | 0.83 (0.53, 1.38) |
| 3 | 2.39 (1.76, 3.24) c | 2.15 (1.58, 2.93) c | 0.88 (0.54, 1.41) | 1.01 (0.99, 1.03) |
| Standard treatment | ||||
| No | Reference | Reference | Reference | Reference |
| yes | 0.67 (0.55, 0.83) c | 0.77 (0.63, 0.96) a | 0.97 (0.73, 1.28) | 1.00 (0.74, 1.34) |
| Race | ||||
| All others | Reference | Reference | Reference | Reference |
| African-American | 0.97 (0.71, 1.32) | 1.07 (0.78, 1.46) | 1.12 (0.72, 1.74) | 1.14 (0.73, 1.79) |
| Sex | ||||
| Women | Reference | Reference | Reference | Reference |
| Men | 1.28 (1.03, 1.60) a | 1.31 (1.05, 1.64) a | 1.11 (0.80, 1.53) | 1.11 (0.80, 1.53) |
| Marital status | ||||
| Other | Reference | Reference | Reference | Reference |
| Married | 0.83 (0.69, 1.00) a | 0.91 (0.75, 1.10) | 0.87 (0.66, 1.14) | 0.88 (0.67, 1.17) |
| Cancer Stage | ||||
| Local | Reference | Reference | Reference | Reference |
| Regional | 1.58 (1.28, 1.94) c | 1.67 (1.36, 2.06) a | 4.51 (3.20, 6.37) c | 4.58 (3.24, 6.49) c |
| Cancer Site: | ||||
| Colon | Reference | Reference | Reference | Reference |
| Breast | 0.73 (0.59, 0.90) b | 0.77 (0.63, 0.95) c | 0.645 (0.47, 0.88) b | 0.65 (0.48, 0.90) b |
| Age | 1.03 (1.02, 1.05) c | 1.01 (0.99, 1.03) | ||
| (continuous) | ||||
Model 1 includes multimorbidity, and all other covariates, except age
Model 2 includes multimorbidity, all other covariates, including age
0.05 ≤ p < 0.01;
0.01 ≤ p < 0.001;
p < 0.001; all other statistics not significant at p < 0.05
Sensitivity analysis
We used the OASIS, rather than claims data to identify comorbid conditions. However, as we have reported in a recent study by our group22, the OASIS may have under-reported conditions that may bear little or no relevance to care management during the home health care episode. We repeated the analysis based on the Charlson comorbid conditions identified from the claims data, rather than the OASIS data. The comparison of the findings obtained from using these different data sources and approaches did not yield any substantial differences in the results. Of note, however, was the confidence interval for the adjusted odds ratio for multimorbidity (3) in association with receipt of standard treatment. Using the OASIS comorbid conditions, we obtained an AOR 0.57 (95% confidence interval: 0.33, 0.97; please refer to Table 3, Model 2), while the model based on claims-based Charlson comorbid conditions yielded an AOR 0.52 (0.30, 0.89). Similarly, we note that in the model analyzing overall survival, multimorbidity (1) lost statistical significance when age was entered in the model: from Adjusted Hazard Ratio (AHR) 1.43 (1.10, 1.87) as reported in Table 4, Model 2, to AHR 1.12 (0.90, 1.40) when relying on claims-based Charlson comorbid conditions.
Discussion
Multimorbidity is highly prevalent in older patients with cancer; yet its effects relative to cancer treatment and survival outcomes had not been evaluated until the present study. In this study, we examined the effects of occurrence and co-occurrence of comorbidities, functional limitations, and geriatric syndromes on treatment and survival outcomes in older patients with loco-regional breast or colorectal cancer. Our findings indicated that multimorbidity was significantly and independently associated with cancer treatment and overall survival, but not with disease-specific survival. In addition, the effect of multimorbidity on our outcomes was attenuated considerably by age.
These findings carry methodological, as well as clinical implications. From a methodological standpoint, we highlight the significance of accounting for clinical factors that reflect the complexity of clinical presentation in older adults with cancer in a more nuanced fashion. Unfortunately however, most population-based databases lack these pertinent variables, precluding their use in cancer-related outcomes studies of large scale6, 16. From a clinical perspective, we note that the clinical conditions used in constructing the multimorbidity variable are integral components of the Comprehensive Geriatric Assessment (CGA)23–26, and that our findings point to the importance of incorporating them in disease management and prognostication. Indeed, the results from the present study indicate that, regardless of age, older adults with high multimorbidity constitute the most vulnerable subgroup of our study population, as they are less likely than those with no multimorbidity to receive definitive treatment, and more likely to experience unfavorable survival outcomes. In turn, these findings strongly support the benefits of relying on the CGA or other instruments that correctly identify vulnerable older adults. For this subgroup of patients, clinicians can follow the non-cancer conditions closely, and intervene in a timely fashion to correct the course of treatment in case the patient shows signs of decline.
Our findings indicating that receipt of standard treatment is not associated with disease specific survival are puzzling. One explanation may be that older adults face competing causes of death, and patients may be dying of other causes before they can benefit from cancer treatment.
Absent data infrastructure to evaluate cancer outcomes in older adults and our use of the unique database, built by linking records from multiple population-based databases, constitutes a great strength of this study. As well, we exploited the richness of clinical assessment data in the OASIS to identify multimorbidity.
Our study also bears limitations, however:
First, we note the inclusion criteria for our patient population, consisting of older adults residing in Ohio and admitted to home health care in the 30-day window before or after cancer diagnosis. On the one hand, limiting our study to this patient population may compromise the generalizability of our findings, especially because being eligible for home health care implies being homebound and with greater complexity of care needs than those not admitted to home health care27. On the other hand, we note that because of the 30-day window around the time of cancer diagnosis, we believe that our patient population may be representative of the community-dwelling elderly population with cancer, not receiving home health care. We speculate that these patients may have experienced functional decline following receipt of cancer treatment, and then admitted to home health care. As well, provider and psycho-social characteristics may have influenced their likelihood to be admitted to HHC following receipt of cancer treatment. A comparison of HHC with non-HHC patients on demographics and cancer stage (all stages combined) revealed the breast cancer/HHC patients were slightly older than their non-HHC counterparts, with a greater representation of patients aged 75–79 years of age among the HHC group than in the non-HHC group. As well, we note a greater proportion of African Americans in the HHC than in the non-HHC group for both breast and colorectal cancer patients (10.4% vs. 7.1% and 9.4% vs. 7.9% in breast and colorectal cancer patients, respectively). Finally, we note a greater representation of patients with regional-stage cancer in the HHC group compared with their non-HHC counterparts, but a considerably larger proportion of unknown stage/unstaged cases in the non-HHC than in the HHC group14.
Second, given that our data originated from administrative sources, the accuracy and completeness of clinical data may be compromised, at least to some extent: a) as noted above, we were bound to use the SEER-summary cancer stage rather than the more detailed AJCC staging, and this may have resulted in a less refined categorization of the variable on standard treatment; b) comorbidities may be under-reported in the OASIS, as noted above; and c) inaccuracies in the OASIS may also have affected other variables used in deriving our measures of functional limitations and geriatric syndromes.
Because of the above limitations, we believe that these findings should be replicated in larger studies that would make it possible to fully evaluate the effects of multimorbidity relative to cancer treatment and outcomes in older adults. Furthermore, future assessments of outcomes should encompass changes in health and functional status, in addition to survival. However, such research endeavors require a suitable data infrastructure that captures a broad spectrum of carefully designed measures, preferably collected prospectively, that can be used to answer various research questions in cancer and aging.
The absence of such a data infrastructure at present warrants us to make best use of existing secondary data to study pertinent questions. Indeed, while building on a framework proposed by Balducci and Extermann, we were not only limited in defining our study cohort, but also by the variables available through the OASIS, especially when defining the conditions under the rubric of geriatric syndromes. Unfortunately, existing secondary databases present serious limitations – ranging from total absence of data on components of the CGA, as with the SEER-Medicare data, to the difficulties in adapting the available measures to our analytic needs, as we have done in the present study.
In closing, we note the importance of accounting for the co-occurrence of functional limitations and geriatric syndromes along with comorbid conditions in studying receipt of cancer treatment and outcomes. While our findings do not provide a basis to recommend more or less standard therapy among patients with multimorbidity, we believe that the results presented herein inform the discussion on future directions of research in cancer and aging. Before treatment recommendations can be made, it is imperative that studies on multimorbidity be repeated in larger and representative groups of older adults with cancer.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Georgette Haydu of the Ohio Department of Health, which maintains the Ohio Cancer Incidence Surveillance System for her careful review of the manuscript.
Research Support:
This study was supported in part by a Career Development Grant from the National Cancer Institute (K07 CA096705 to Dr. Koroukian), and a NIH Cancer-Aging Research Development Grant (P20 CA103736 to Dr. Nathan Berger, Case School of Medicine; Dr. Koroukian, pilot project investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
Cancer incidence data were obtained from the Ohio Cancer Incidence Surveillance System (OCISS), Ohio Department of Health. Use of these data does not imply that the Ohio Department of Health either agrees or disagrees with any presentation, analyses, interpretations, or conclusions. Information about the OCISS may be obtained at odh.state.oh.us/ODHPrograms/CI_SURV/ci_surv1.htm".
These results were presented in part at the annual meeting of the Societé Internationale d’Oncologie Geriatrique, Berlin, Germany, October 2009.
REFERENCES
- 1.Satariano WA, Silliman RA. Comorbidity: implications for research and practice in geriatric oncology. Crit Rev Oncol Hematol. 2003 Nov;48(2):239–248. doi: 10.1016/j.critrevonc.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Yates JW. Comorbidity considerations in geriatric oncology research. CA Cancer J Clin. 2001 Nov-Dec;51(6):329–336. doi: 10.3322/canjclin.51.6.329. [DOI] [PubMed] [Google Scholar]
- 3.Berger DA, Megwalu II, Vlahiotis A, et al. Impact of comorbidity on overall survival in patients surgically treated for renal cell carcinoma. Urology. 2008 Aug;72(2):359–363. doi: 10.1016/j.urology.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balducci L. Aging, frailty, and chemotherapy. Cancer Control. 2007 Jan;14(1):7–12. doi: 10.1177/107327480701400102. [DOI] [PubMed] [Google Scholar]
- 5.Given B, Given CW. Older adults and cancer treatment. Cancer. 2008 Dec 15;113(12 Suppl):3505–3511. doi: 10.1002/cncr.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koroukian SM. Assessment and interpretation of comorbidity burden in older adults with cancer. J Am Geriatr Soc. 2009;57(s2):s275–s278. doi: 10.1111/j.1532-5415.2009.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koroukian SM, Xu F, Bakaki PM, Diaz-Insua M, Towe TP, Owusu C. Comorbidities, functional limitations, and geriatric syndromes in relation to treatment and survival patterns among elders with colorectal cancer. J Gerontol A Biol Sci Med Sci. 2010 Mar;65(3):322–329. doi: 10.1093/gerona/glp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med. 2009 Feb;265(2):288–295. doi: 10.1111/j.1365-2796.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- 9.Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008 Jul;98(7):1198–1200. doi: 10.2105/AJPH.2007.121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002 Jun;50(6):1045–1050. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaum CS, Ofstedal MB, Liang J. Low cognitive performance, comorbid disease, and task-specific disability: findings from a nationally representative survey. J Gerontol A Biol Sci Med Sci. 2002 Aug;57(8):M523–M521. doi: 10.1093/gerona/57.8.m523. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RA, Vittinghoff E, Kanaya AM, et al. Urinary incontinence in elderly women: findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004 Aug;104(2):301–307. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 13.Brown JS, Vittinghoff E, Lin F, Nyberg LM, Kusek JW, Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES) 2001–2002. Diabetes Care. 2006 Jun;29(6):1307–1312. doi: 10.2337/dc05-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006 May 20;24(15):2304–2310. doi: 10.1200/JCO.2005.03.1567. [DOI] [PubMed] [Google Scholar]
- 15.Balducci L, Extermann M. Management of the frail person with advanced cancer. Crit Rev Oncol Hematol. 2000 Feb;33(2):143–148. doi: 10.1016/s1040-8428(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 16.Koroukian SM. Linking the Ohio Cancer Incidence Surveillance System with Medicare, Medicaid, and clinical data from home health care and long term care assessment instruments: Paving the way for new research endeavors in geriatric oncology. J Registry Manag. 2008;35:156–165. [PMC free article] [PubMed] [Google Scholar]
- 17.United States General Accounting Office. Report to Congressional Committees. Medicare Home Health Care Payments to Home Health Agencies are Considerably Higher than Costs. GAO-02-663. 2002 May
- 18.Madigan EA, Fortinsky RH. Interrater reliability of the outcomes and assessment information set: results from the field. Gerontologist. 2004 Oct;44(5):689–692. doi: 10.1093/geront/44.5.689. [DOI] [PubMed] [Google Scholar]
- 19.Hittle DF, Shaughnessy PW, Crisler KS, et al. A study of reliability and burden of home health assessment using OASIS. Home Health Care Serv Q. 2003;22(4):43–63. [PubMed] [Google Scholar]
- 20.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002 Mar 6;94(5):334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 21.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998 Jun 1;82(11):2123–2134. [PubMed] [Google Scholar]
- 22.Koroukian SM, Scharpf T, Bakaki PM, Madigan EA. Identifying comorbidities in home health care patients: Does the Outcome Assessment Information Set have incremental value to Medicare claims data? Home Health Serv Quarterly. doi: 10.1080/01621424.2011.545726. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 23.Rao AV, Seo PH, Cohen HJ. Geriatric assessment and comorbidity. Semin Oncol. 2004 Apr;31(2):149–159. doi: 10.1053/j.seminoncol.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol. 2000 Sep;35(3):147–154. doi: 10.1016/s1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Lachs MS, Cohen HJ, Muss HB, Kornblith AB. Geriatric assessment for oncologists: rationale and future directions. Crit Rev Oncol Hematol. 2006 Sep;59(3):211–217. doi: 10.1016/j.critrevonc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005 Sep;55(3):241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Koroukian SM, Xu F, Beaird H, Diaz M, Murray P, Rose JH. Complexity of care needs and unstaged cancer in elders: a population-based study. Cancer Detect Prev. 2007;31(3):199–206. doi: 10.1016/j.cdp.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.