Abstract
In the context of a prevention of mother to child transmission of HIV program promoting exclusive breast-feeding (EBF) to 6 mo and offering HIV-PCR testing at ∼6 mo, we ascertained the feasibility of expressing and heat-treating (EHT) all breast milk fed to HIV-exposed, uninfected infants following 6 mo of EBF. Twenty mother-baby pairs were enrolled from a hospital in rural Zimbabwe. Research nurses provided lactation, EHT, and complementary feeding counseling through 21 home visits conducted over an 8-wk period and collected quantitative and qualitative data on the mothers' EHT experiences, children's diets, and anthropometric measurements. Mothers kept daily logs of EHT volumes and direct breast-feeding episodes. Mothers successfully initiated and sustained EHT for 4.5 mo (range, 1–11 mo), feeding 426 ± 227 mL/d (mean ± SD). By wk 2 of follow-up, children were receiving EHT and Nutributter-enriched complementary foods that satisfied 100% of their energy requirements. During the 8-wk follow-up period, no growth faltering was experienced [changes in weight-for-age, weight-for-length, and length-for-age Z scores = +0.03 ± 0.50; +0.77 ± 1.59; and +0.02 ± 0.85 (mean ± SD), respectively]. Stigma was not a major deterrent, likely due to a social marketing campaign for EBF that promoted EHT as a practice to sustain breast-feeding for all women. This study provides evidence that resource-poor rural women can initiate and sustain EHT given family and health systems support. EHT provides a strategy for improving the diets of HIV-exposed but uninfected children after direct breast-feeding has ceased.
Introduction
In developing countries, breast-feeding is the cornerstone of child survival, but it also causes about one-third of all pediatric HIV infections. Over the past 20 y, policy regarding feeding infants born to HIV-positive mothers has continually changed as new data have emerged quantifying these competing risks. The 2006 WHO technical consultation on HIV and Infant Feeding recommended exclusive breastfeeding to 6 mo, followed by continued breast-feeding along with complementary foods until an acceptable, feasible, affordable, sustainable, and safe (AFASS)9 replacement feeding option was available (1). Though continued breast-feeding after 6 mo is likely to provide the greatest chance of infection-free survival for the majority of infants living in resource-constrained settings, this policy proved difficult to implement (2). In our previous study of HIV-positive breast-feeding mothers in rural Zimbabwe, women whose children tested HIV-PCR negative at 6 mo overwhelmingly chose to stop breast-feeding, even though they did not meet AFASS criteria and provided grossly inadequate replacement diets (3).
In November 2009, WHO released “Rapid Advice” recommending that HIV-exposed infants in developing country settings be exclusively breast-fed to 6 mo, with continued breast-feeding to 12 mo along with complementary foods (4) while also providing either the mother with triple antiretroviral drug therapy or her infant with nevirapine prophylaxis (hereafter referred to as ARV) (5). These guidelines also listed expressing and heat-treating (EHT) breast milk as a possible interim strategy in 4 situations: 1) for low-birth weight or sick infants unable to suckle; 2) for mothers temporarily unable to breast-feed due to illness or mastitis; 3) to assist mothers to stop breast-feeding; and 4) in situations where ARV are temporarily not available. Though studies have demonstrated that EHT inactivates HIV (6) in breast milk while retaining most of its nutritional and immunological properties (7,8) and also extends its shelf life outside of refrigeration by lowering bacterial growth (9), the 2009 Guidance declined to recommend EHT more broadly until the acceptability, sustainability, and health system requirements for supporting EHT are better defined, and endorsed the need for continued research in this area.
In Zimbabwe, expressing breast milk is promoted by the Baby-Friendly Hospital Initiative as a method of sustaining breast-feeding during periods of separation for all mother-infant pairs. EHT is promoted by the national prevention of mother to child transmission of HIV (PMTCT) program as a strategy to reduce postnatal transmission for HIV-positive mothers and their infants (10) and formative research has indicated it could be an acceptable PMTCT strategy (11). The PMTCT program is also rolling out Early Infant Diagnosis (EID). In addition, district-wide campaigns are being conducted in rural Zimbabwe to encourage exclusive breast-feeding (EBF) from birth to 6 mo for all infants (i.e. regardless of maternal HIV status; A. Jenkins, N. Tavengwa, B. Chasekwa, K. Chatora, N. Taruberekera, W. Mushayi, M. Mbuya, R. Madzima, unpublished data). EHT is promoted in these campaigns as a means to sustain breast-feeding for both HIV-positive and HIV-negative mothers during times when they must be away from their infants for several hours. Within this context, we designed this study to determine whether resource-constrained, HIV-positive rural mothers can: 1) initiate EHT; 2) consistently and correctly perform EHT to ensure milk safety; and 3) express, heat-treat, and feed sufficient volumes of EHT milk and complementary foods to provide a nutritionally adequate diet that supports normal infant growth. Because the study was implemented prior to the 2009 guidance, it focused on implementation of EHT following 6 mo of EBF by HIV-positive mothers who had received PCR-negative test results for their infants at 5–6 mo of age through EID. Qualitative methods were incorporated to identify maternal and infant health, socioeconomic, cultural, psychological, and health system factors that facilitated or deterred women in practicing EHT.
Materials and Methods
Study setting.
The study was conducted from March 2008 to June 2009 among women seeking postpartum and baby care at St. Theresa's hospital, located 250 km south of Harare in a predominantly subsistence farming rural district. At the time of the study, PMTCT services included antenatal HIV counseling and testing and maternal/neonatal single-dose nevirapine prophylaxis during the perinatal period. EBF from birth to 6 mo was promoted for all mother-infant pairs by St. Theresa's staff (except for the rare HIV-positive mother in this region who met AFASS criteria for replacement feeding) and by the EBF campaign. Postpartum, HIV-positive mothers were encouraged to return monthly for clinical monitoring, infant feeding counseling, and infant cotrimoxazole prophylaxis.
Infant HIV diagnosis was offered at 5–6 mo through the EID program. Dry blood spot samples were transported to a DNA PCR laboratory in Harare for HIV testing with results usually made available at St. Theresa's within 1 mo, along with post-test counseling. This session included counseling to inform context-appropriate infant feeding decisions. Mothers of PCR-positive infants were referred to the Opportunistic Infections/High Activity Antiretroviral Therapy clinic, provided continuing cotrimoxazole, and encouraged to continue breast-feeding while initiating complementary feeding. Mothers of PCR-negative infants were assessed to determine whether they met AFASS criteria for replacement feeding and encouraged to stop breast-feeding if they did, but they were encouraged to continue breast-feeding along with beginning complementary foods if they did not. Cotrimoxazole prophylaxis was provided until the mother stopped breast-feeding.
Recruitment.
Between March and October 2008, 39 HIV-positive mothers requested HIV diagnosis for their infant at ∼5 mo of age from St. Theresa's; 32 of these infants tested HIV-PCR-negative and their mothers were invited to participate in the study. Ten mothers declined due to reluctance to disclose their HIV status (disclosure was not an enrollment criterion) or discomfort with either practicing EHT or participating in a study of HIV-exposed children, and 2 of the remaining mothers were excluded because they met AFASS criteria for replacement feeding. The 20 remaining mothers provided written informed consent and were enrolled.
EHT support and data collection.
Within 2 d of recruitment, a research nurse visited the mother in her home. Demographic and socioeconomic characteristics, infant illness during the previous week, and breast-feeding frequency were ascertained by questionnaire. Infant weight (Salter scale) and length (length board) were measured. An EHT kit was provided to the mother that included a cooking pot, glass jars (6/20 mothers experienced breakages; therefore, extra jars were provided), soap, and a lidded plastic storage container. The nurse demonstrated and instructed the mother in the 8-point EHT protocol: 1) wash hands with soap prior to expressing; 2) clean jar and feeding cup with soap; 3) sterilize jar and feeding cup by boiling; 4) express milk into the glass jar; 5) express from both breasts; 6) place the jar in a pot with sufficient water to come 2 finger-widths above milk level and heat until water reaches a rolling boil; 7) remove pot as soon as water boils; 8) immediately remove jar from pot and cover. The mother was instructed to feed EHT milk by cup within 8 h, the shelf life at room temperature (9). The research nurse stayed in the mother's home for up to 5 h to observe at least 1 EHT episode. During the first episode, the nurse measured and recorded peak milk temperature and observed and recorded the mother's adherence to the EHT protocol using a questionnaire checklist. The nurse also recorded milk volumes expressed, heat-treated, and fed, and time taken for each of these steps, and instructed the mother to record these data daily on EHT log forms. Following the initial visit, the research nurse visited the mother daily for the next 6 d, each time staying long enough to observe and record protocol adherence, temperature, volumes, and time taken during at least 2 EHT episodes. On d 3 of the protocol, after EHT was established, the nurse began instructing the mother to prepare and feed locally available foods for complementary feeding and to record type and amount of all foods consumed by the infant daily on diet log forms. During this home visit, the nurse also introduced Nutributter (Nutriset), a peanut-based, fortified spread previously pilot-tested in the study area (12), and instructed the mother to feed 20 g/d, an amount containing 1 Recommended Dietary Allowance (RDA) of all micronutrients and 440 kJ, mixed into other foods. After the first week of daily visits, the nurse visited the mother twice weekly from wk 2 to 8 and collected the same outcomes as during wk 1, staying long enough to observe at least 1 EHT episode. During this time, the nurse also conducted a 24-h diet history and collected and verified EHT and diet log forms. Additionally, through semistructured interviews, the nurse discussed the mother's experience with EHT focusing on time taken, stigma and HIV status disclosure, breast pain and other issues the mother felt constrained her ability or willingness to express and heat-treat. Thus, a total of 21 home visits to each mother were conducted over this 8-wk support period. After this 8-wk period, mothers were encouraged to continue using EHT for as long as possible, although home-based support was no longer provided. A final follow-up visit was conducted when each child was between 12 and 18 mo of age to ascertain how long EHT had been sustained beyond the 8-wk period of support and to offer a second HIV-PCR test.
This study was conducted during a time of food shortages; a local nongovernmental organization provided corn-soy blend rations to vulnerable children, including HIV-exposed children, within the study area.
Data analysis.
All data were entered and managed in dbase Plus version 2.01 (dataBased Intelligence). Quantitative data were analyzed using Stata version 10.0 (Stata Corp LP). The nutrient composition of the infants' diets was analyzed using Nutrisurvey (WHO). A Nutrisurvey function was used to estimate nutrient retention of cooked foods. For some indigenous foods, the Nutrisurvey nutrient composition database was supplemented with data from a local unpublished food composition table (13), PROTA (14), and published articles (15). The energy adequacy of diets was determined by comparing nutrient intakes with daily energy requirements (16,17). Weight-for-age (WAZ), weight-for-length (WLZ), and length-for-age (LAZ) Z scores were calculated using the 2006 WHO International Standards (18). A percent adherence score was calculated for each home observation as the sum of EHT practices completed divided by a maximum of 8 practices multiplied by 100. Using the chi-square test and the t test for categorical and continuous variables, respectively, differences between maternal, infant, and household characteristics and infant diet among children who experienced more positive and more negative ΔWAZ and ΔLAZ between recruitment and the 8-wk visit and between recruitment and the 12–18 mo final visit were ascertained. For all tests, P < 0.05 was considered significant. Values in the text are means ± SD unless indicated otherwise.
Qualitative data were coded and analyzed using Atlas.ti software version 6.0 (ATLAS.ti GmbH).
Interviews were structured based on themes that were determined a priori based on the findings from the study of the acceptability of EHT (11) and common constraints in child feeding (19). These themes were evaluated and appropriate quotes selected to emphasize pertinent points.
Ethical approval.
Ethical approval to conduct this study was granted by the Medical Research Council of Zimbabwe and the Institutional Review Boards of Johns Hopkins University Bloomberg School of Public Health and the Research Institute of McGill University Health Centers. The study pediatrician and an external safety-monitoring pediatrician were responsible for attending to and reviewing adverse events to assess the likelihood that they resulted directly or indirectly from the study interventions.
Results
Baseline characteristics and retention
All study mothers were literate and the majority had attended secondary school (Table 1). Most mothers were married or cohabiting. Seventeen (85%) had access to a protected water source, but only 8 (40%) had a latrine in their household.
TABLE 1.
General characteristics of the children and their mothers1
| Characteristics | |
|---|---|
| Maternal factors | |
| Education level, % (n) | |
| Primary | 30 (6) |
| Secondary | 70 (14) |
| Marital status, % (n) | |
| Married/cohabiting | 70 (14) |
| Separated/divorced | 15 (3) |
| Never married | 5 (1) |
| Widowed | 10 (2) |
| Children cared for, n | 2.8 ± 1.42 |
| Household factors, % (n) | |
| Main source of water is protected | 85 (17) |
| Latrine in household | 40 (8) |
| Child factors | |
| Sex, male, % (n) | 60 (12) |
| Low birth weight (<2500 g), % (n) | 25 (5) |
| Age at PCR test, mo | 5.5 ± 0.63 |
| Age at recruitment, mo | 6.3 ± 0.84 |
| Ill during the week prior to recruitment, % (n) | 20 (4) |
| Breast-feeding episodes at recruitment, n/d | 3.5 ± 2.44 |
Values are means ± SD, n = 20 or % (n).
Blood samples had been collected from study infants at age 5.5 ± 0.6 mo for PCR testing and mothers were given these results and recruited into the study 19 d later; age at recruitment was 6.3 ± 0.8 mo. The turnaround time for PCR testing was variable (range 7–70 d) with 4 (20%) mothers receiving test results >30 d after blood collection. Three delays were responsible for this protracted interval: 1) delays in transporting samples to the laboratory due to political/security issues prevailing during the study period; 2) delays in processing some samples due to stock-outs of PCR kits in the laboratory; and 3) delays in both sending results and the mother coming to the hospital to receive the test results. The first 2 delays accounted for a mean of 12 d (range: 5–55 d) and the 3rd delay accounted for 8 d (range: 0–29 d).
Feasibility of heat-treating expressed breast milk
Mothers' proficiency and impact of EHT on daily activities/routines.
Mothers achieved a 96% mean adherence to the implementation protocol computed based on the 8 measures of compliance (Table 2). Adherence remained high throughout follow-up, staying above 94% on average, while time spent expressing, heat-treating, and cup feeding the baby decreased over time. In semistructured interviews, mothers attributed these decreases in time to increasing proficiency: “…the time it takes to do EHT activities is like preparing any other meals for the baby. It's even getting shorter with experience.”
TABLE 2.
Peak milk temperature during heat-treating, mothers' adherence to the EHT protocol, and time taken to express, heat-treat and feed breast milk over the 8-wk follow up period1
| Process indicators | All Visits | Visits 1–7 | Visits 8–14 | Visits 15–21 |
|---|---|---|---|---|
| Peak temperature, °C | 80.5 ± 1.7 | — | — | — |
| Overall adherence to protocol,2% | 95.5 ± 4.3 | 94.4 ± 6.3 | 96.0 ± 4.3 | 96.8 ± 4.2 |
| Time taken to express, min | 14.7 ± 5.7 | 18.1 ± 7.4 | 13.1 ± 5.5 | 12.7 ± 7.0 |
| Time taken to heat-treat, min | 17.5 ± 3.5 | 20.4 ± 6.0 | 16.7 ± 4.9 | 15.5 ± 5.1 |
| Time taken to feed baby, min | 5.7 ± 2.0 | 6.6 ± 1.9 | 6.5 ± 3.7 | 3.9 ± 1.6 |
Values are means ± SD, n = 20.
Scored as a percent of 8 measures of adherence to the protocol, assessed as: 1) washing hands with soap and water before expressing, 2) cleaning utensils with soap, 3) sterilizing jar and feeding cup by boiling, 4) expressing into heating jar, 5) expressing from both breasts, 6) correct amount of water in the pot (2 finger widths above breast milk), 7) removal of pot from flame as soon as water boiled, and 8) immediate removal of jar from pot.
Most mothers felt that the time required was comparable to the demands of preparing meals for her children. All mothers, except 1 who lapsed to direct breast-feeding for a few days and opted to reduce study contact consequent to her poor health, felt that EHT activities had been well integrated into their daily routines. The mother who opted to reduce study contacts continued to receive and feed Nutributter and receive complementary feeding counseling. She also permitted research staff to continue to make some support visits during which the infant was monitored.
Family support.
All 20 mothers reported that immediate family members (particularly their husbands, mothers, mothers-in-law, and older children) had supported them to EHT through encouragement, assisting with child-care, or taking on some of her household chores. Most mothers reported this family support began at the beginning of EHT and continued throughout the entire period they practiced it: “My husband prepares family food and takes care of the baby while I am doing EHT activities…same with my in-laws.”
For 6/20 mothers, however, developing this supportive environment was a more gradual process as illustrated by the following example where health system intervention facilitated the process: “...my husband… did not take it lightly that I had taken the baby off the breast without his knowledge...When we went for family planning counseling, I asked the counselor to explain to my husband about EHT... He then consented for me to continue EHT for the sake of a healthy baby.”
Stigma and disclosure.
None of the women reported feeling stigmatized as a result of EHT. The stigma of being HIV-positive and the associated rejection by some neighbors and family members did occur among this study population, but it was not exacerbated by EHT. All participants reported feeling able to disclose their EHT practice without needing to disclose their HIV status as well. This was likely due to support received through the PMTCT program at St. Theresa's and the EBF campaign that promoted EHT for all women to sustain breast-feeding during times of maternal-infant separation: “When I told my neighbors that I had joined a program on expressing and heat treating, which some of them had seen and heard about during the EBF campaigns in our area, they said they had heard that EHT and EBF can be practiced by any mother with a child, whether HIV-negative or positive....”
The experiences of other mothers also helped normalize EHT in the community. The following example highlights the role that success stories played in creating readiness to accept EHT among some families: “The family knew about EHT before I started. They had seen another woman in the village expressing and heat treating and had seen how her baby was growing healthy. This enabled them to accept EHT….”
Indeed, as EHT visits continued, all 20 mothers reported having disclosed their HIV status to more people, both within (20/20) and outside (11/20) their households. There is no evidence that this increasing disclosure was a consequence of others observing her expressing and/or heat-treating breast milk or being visited by the research nurse: “I have long back disclosed my doing EHT to most relatives and neighbors but not about my HIV status. It's only my mother who knows about it.”
Duration of EHT and reasons for stopping.
At the final visit conducted at infant age of 16.4 ± 2.40 mo, all mothers had stopped EHT. By history, they had continued EHT for a duration of 4.5 ± 2.7 mo, range: 5 wk–11.1 mo (Table 3). Nineteen of the 20 mothers said they stopped EHT because of inadequate milk production. The remaining mother was falsely advised by a friend, a mother of a child of similar age, that she “should stop expressing and heat-treating when her child has reached 18 mo of age.” Food insecurity was the most frequently mentioned cause, with mothers citing the direct effects of hunger on their milk production: “I could not get enough food for myself…I was expressing 20 mL/d, which was not enough for (the) baby.” For 3 mothers, their family circumstances also contributed toward cessation of EHT. Two were advised to feed solid foods and other liquids (exclusively, instead of with EHT milk) by their families: “…my brother... said that I had lost weight and he thought it was better for me to stop EHT then he would provide the baby with food...I agreed with his decision because I was surely losing weight. My baby could eat other foods without problems.”
TABLE 3.
Time from EHT initiation to no direct breast feeding, EHT frequency and duration, and breast milk volumes expressed, heat-treated, and fed to children over the 8-wk follow-up period1
| EHT initiation to cessation, mo | 4.5 ± 2.7 (1.1–11.1) |
| EHT initiation to no direct breastfeeding, d | 6.6 ± 6.2 (1–23) |
| EHT initiation to no direct breastfeeding, %(n) | |
| 0 – 2 d | 35 (7) |
| 3 – 7 d | 25 (5) |
| 8 – 14 d | 30 (6) |
| >14 d | 10 (2) |
| Total | 100 (20) |
| EHT episodes, n/d | 3.9 ± 1.4 (1.8–7.8) |
| Breast milk expressed and heat-treated, mL/d | 427 ± 172 (182–799) |
| EHT breast milk fed, mL/d | 418 ± 170 (182–795) |
Values are means ± SD (range), n = 20 or % (n).
EHT and complementary feeding.
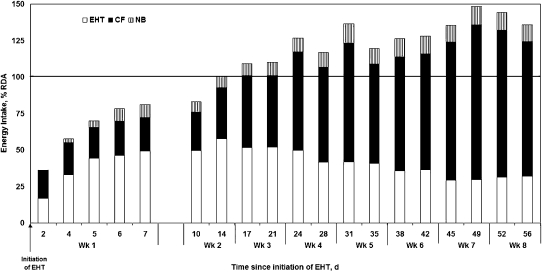
All 20 mothers initiated EHT, transitioning from EBF to exclusive EHT by 6.6 ± 6.2 d (Table 3). The duration of the transition period, during which mothers were concurrently feeding directly from the breast and EHT milk from a cup, varied from zero (no overlap) to >2 wk. By the end of wk 1, mothers were expressing, heat-treating, and feeding 363 ± 179 mL/d (providing 49% of their infants' energy requirement) while also feeding sufficient complementary food to provide 81 ± 31.4% of their total energy requirement of the infants' energy RDA (Fig. 1). EHT milk consumption was 479 ± 203 mL/d (providing 49.1% of their infants' energy requirement) from wk 2 to 4 and then declined to 390 ± 186 mL/d (providing 33.6% of their infants' energy requirement) from wk 4 to 8. Concomitantly, intake of Nutributter-enriched complementary food increased such that from the end of wk 2, mean total energy intake met or exceeded 100% of the infants' energy RDA. The Nutributter provided 13.9 and 8.6% of the complementary food and total energy intakes, respectively, and ensured intake of 100% of the RDA of all micronutrients. Without the Nutributter, the 2 most limiting nutrients were iron and calcium; EHT milk and locally available complementary foods met only 45.8% of the RDA for iron and 81.2% of the Reference Nutrient Intake (RNI) for calcium but provided 100% of the infants' requirements for energy, protein, and all other micronutrients.
FIGURE 1 .
Dietary energy consumption from EHT milk, complementary foods (CF), and Nutributter (NB) as the percent of the RDA by 6- to 9-mo-old infants over the 8-wk follow-up period. Values are means, n = 20.
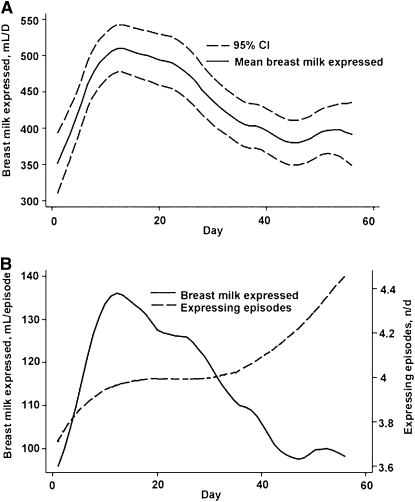
Although mean volume of milk expressed daily decreased over time after wk 2 (Fig. 2A), it is unlikely that this was attributable to the frequency of expressing episodes, because this increased over time (Fig. 2B).
FIGURE 2 .
Volume of breast milk expressed daily (A) and during each expressing episode compared with the number of expressing episodes (B) over the 8-wk follow-up period. Values are means and 95% CI (A), n = 20.
One mother stopped EHT 5 wk after initiating it, reporting that she was expressing inadequate volumes of milk. Prior to stopping, she had been providing 175 mL/d EHT milk and feeding a total (EHT milk plus Nutributter-enriched complementary foods) of 2730 kJ/d (80.9% RDA). The other 19 mothers continued to EHT through the end of the 8-wk support period of follow-up.
Infant growth.
Infants had low attained growth at recruitment as assessed by WAZ, WLZ, and LAZ (Table 4). However, during the 8-wk period of support visits, there was no further decline in either ponderal or linear growth. The change in WAZ ranged from −0.58 to +1.22 [+0.03 ± 0.50], the change in WLZ ranged from −3.46 to +2.76 [+0.77 ± 1.59], and the change in LAZ ranged from −1.56 to +1.51 [+0.02 ± 0.85]. Between recruitment and the final visit conducted at 12–18 mo, the changes in WAZ, WLZ, and LAZ were −0.01 ± 0.79, +0.24 ± 1.21 and −0.93 ± 1.34, respectively.
TABLE 4.
WAZ, WLZ, and LAZ of study children at recruitment, after 1 and 2 mo, and at the final visit1
| Time | WAZ | WLZ | LAZ |
|---|---|---|---|
| Recruitment | −1.10 ± 1.13 | −0.39 ± 0.92 | −1.15 ± 1.52 |
| 1 mo after EHT initiation | −1.14 ± 1.36 | −0.49 ± 1.11 | −1.20 ± 1.37 |
| 2 mo after EHT initiation | −1.04 ± 1.30 | −0.38 ± 1.05 | −1.12 ± 1.62 |
| Final visit | −1.07 ± 1.16 | −0.15 ± 1.16 | −2.14 ± 1.20 |
Values are means ± SD, n = 20 (recruitment, final) or 19 (1 and 2 mo).
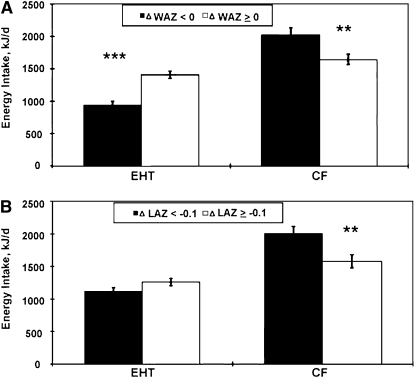
Infants of unmarried mothers, those with higher EHT milk intakes, and those with a latrine in the household were more likely to experience a positive change in WAZ between recruitment and the 8-wk visit. The only factors that were significant for more positive change in WAZ (≥0) and change in LAZ (≥ −1.0) between recruitment and the final visit were higher EHT milk energy intake and lower complementary food energy intake (Fig. 3). Birth weight and recent morbidity were not associated with more positive change in WAZ and change in LAZ.
FIGURE 3 .
Differences in energy intakes from EHT milk and complementary foods (CF) between children whose ponderal (A) and linear (B) growth did (ΔWAZ < 0, n = 10; ΔLAZ < −0.1, n = 10) and did not falter (ΔWAZ ≥0, n = 10; ΔLAZ ≥−0.1, n = 10) between recruitment and the final visit. Bars represent means ± SE. Asterisks indicate that means differed: ** P < 0.01; *** P < 0.001.
Discussion
This study demonstrates that given home-based support and modest material inputs, HIV-positive mothers in a rural resource-constrained Zimbabwean setting were able to express, heat-treat, and feed ∼400 mL/d breast milk to their children for ∼4 mo. Together with complementary foods and 20 g Nutributter, children received nutritionally adequate diets that maintained ponderal and linear growth over at least the first 2 mo of EHT. Our findings suggest that EHT may be a useful strategy for HIV-positive mothers to extend safer breast-feeding beyond the first 6 mo of EBF.
These findings address 2 of the research questions posed in the November 2009 WHO HIV and Infant Feeding Revised Principles and Recommendations (4). First, “Can mothers sustain the practice” The mothers in our study sustained EHT along with complementary feeding for an average of 4.5 mo (range: 1–11 mo). EHT may be more difficult to sustain compared with direct breast-feeding, because manual milk expression elicits lower and less consistent prolactin and oxytocin response, both of which are associated with breast-feeding duration (20) compared with infant suckling (21). In our study, mean volume of milk expressed daily declined over time, although mothers increased the frequency of expressing episodes (Fig. 2A,B). Further research may be helpful in determining whether other interventions, such as massage (22), could increase milk production during manual expression. EHT duration was apparently also limited in our particular study population, because they were enduring severe food insecurity. Indeed, most households in the study area were relying on foraging for wild fruits, notably chakata (Parinari curatellifolia) (23), and all mothers attributed EHT cessation, at least in part, to their own hunger.
A second question posed by the 2009 WHO Recommendations (4) was: “What is needed from health systems to effectively support mothers in this approach” Our study provided: 1) material inputs of pot, soap, and glass jars; 2) intensive individual support and counseling; and 3) social marketing to normalize the practice in the general community, regardless of HIV status. In addition, our study was conducted within an existing EID service. A substantial level of support and follow-up was provided by our study for ethical reasons, because early cessation can result in serious adverse events (24–26) and because our goal was to learn as much as possible about the feasibility of this method from the study mothers. However, it would not be possible for nurses to provide this level of support at a larger scale because of the nursing shortage in Zimbabwe and throughout Africa. In a similar study in Tanzania (27), home-based support for EHT was provided by community health workers. Further research is required to ascertain the minimum additional training and skills required for community health workers to adequately counsel and support mothers to EHT in the context of their other roles and responsibilities.
The other health system component required is an EID service with efficient communication and transportation networks between health institutions and PCR laboratories to ensure timely receipt of infant HIV status by mothers. In the context of this study, there was a mean 19-d time lapse between the collection of infant blood and maternal receipt of the test result, with 4 mothers receiving the result more than 1 mo after blood collection. Long lag periods increase the possibility of the infant acquiring HIV during breast-feeding after blood collection and of the mother being incorrectly advised to stop direct breast-feeding and initiate EHT.
In our study, neither stigma nor time burden were perceived as constraints for EHT by the study mothers. At least 2 factors may have contributed to the lack of reported stigma. First, selection bias; ∼31% of the eligible mothers declined to participate due to concern it would lead to disclosure of their HIV status or discomfort with either practicing EHT or participating in a study of HIV-exposed children. This suggests that EHT is unlikely to be a feeding solution for all HIV-positive mothers, but may be for most. Second, our study was conducted within the context of an EBF social marketing campaign that promoted EHT as a means of supporting breast-feeding for all mothers when they must be apart from their infants for several hours and do not have a refrigerator. Several mothers commented that community knowledge about EHT and acceptance that it was a practice for all women facilitated their adoption of the practice. With regard to time burden, mothers in our study perceived that the time burden of EHT was comparable to that for preparation of other foods and meals and also reported that it declined with practice. These factors have implications for the generalizability of our findings.
One of the 20 babies in the study tested PCR-HIV-positive at the final visit. This child had a 41-d lag period between blood collection and EHT initiation, so it is possible the child became infected through direct breast-feeding prior to joining the study. However, we cannot rule out the possibility that EHT, as implemented by this mother, did not completely inactivate milk HIV and that the infant acquired the infection through EHT milk that still contained active virus. A second infant enrolled in the study died 1 mo following his final study visit at 15 mo of age following a 6-d hospitalization for a diarrhea-related illness. This child had successfully transitioning from EBF to exclusive EHT within 1 d of recruitment, had a mean daily intake of 775 mL EHT milk, and grew well during the study (ΔWAZ between recruitment and 8-wk follow-up = +0.76 and between recruitment and final visit at 14 mo = +0.17). Following review of the hospital records and interviews with family members, the external safety monitor pediatrician deemed that the death was unrelated to study participation and attributed the death to shock secondary to dehydration, electrolyte disturbance, renal failure, and/or sepsis.
Despite a severe food shortage during the time of the study, mean energy intake by the children in our study met or exceeded their requirement within 2 wk of initiating EHT and continued throughout EHT. EHT milk contributed about one-half of total energy intake during the first month of EHT and one-third during the second month of EHT. Moreover, this diet supported normal growth velocity; children maintained WAZ, WLZ, and LAZ during these 2 mo. Finally, total energy intake did not vary between infants whose ΔWAZ and LAZ was more positive compared with negative or less positive. Instead, the proportion of total energy contributed by EHT milk distinguished these groups: kJ for kJ, EHT milk promoted better growth than complementary foods. Whereas ponderal growth velocity continued between the 8-wk and final visits, linear growth fell by nearly 1 Z-score. This may be due to EHT cessation during this period, or the chronic absence of other factors apparently important to growth in these children, like sanitation. Although these analyses were post hoc, and performed on data from a small sample of intensively studied mothers and babies, they suggest that EHT may have been particularly important for supporting the growth relative to the other components of the diet. The Nutributter supplement contributed to macro- and micronutrient intake but was only essential for meeting iron and calcium requirements. Nutributter may have also contributed to growth by making foods taste better so that infants consumed larger quantities of food (12) or through other components like long-chain fatty acids (28).
Our study was conducted when infant feeding guidance recommended EBF to 6 mo for HIV-exposed infants without an AFASS replacement feeding, followed by continued breast-feeding until an AFASS diet was available (1). In light of the new WHO recommendations for direct breast-feeding to 12 mo covered by either maternal or infant ARV, we propose 3 situations where EHT may be a useful intervention for HIV-positive mothers: 1) mothers who want to stop breast-feeding early where the 2009 WHO ARV recommendations have not yet been implemented; 2) as an interim strategy where ARV are temporarily not available due to clinic stock-outs or where mothers have been unable to go to the clinic to collect them. In this regard, our findings suggest that all HIV-positive breast-feeding mothers should be taught to EHT breast milk as part of implementing the new WHO guidelines; and 3) because breast milk consumption beyond 12 mo will improve child health and growth if it can be provided with minimal HIV transmission risk, EHT may be useful in extending safer breast-feeding for HIV+ women beyond 12 mo for mothers who stop receiving ARV and direct breast-feeding at that time.
Although these findings demonstrate that EHT is an acceptable and feasible feeding option for mothers of HIV-exposed but uninfected children given intense support, further field research in the context of routine health services is required to establish its effectiveness on growth, morbidity, and infection-free survival.
Acknowledgments
We thank research nurses Idaishe Shumba, Dorothy Chagwiza, and Tariro Wande who delivered the study interventions. M.N.N.M., J.H.H., A.J., R.C.M., and K.I-B. designed research; F.M., M.M., R.C.M., and M.N.N.M. conducted research; L.H.M., B.C., K.H.P., and M.N.N.M. performed statistical analysis; M.N.N.M., J.H.H., and R.J.S. wrote the paper; and M.N.N.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by the Department for International Development (DFID), United Kingdom under the grant: “Saving Maternal and Newborn Lives in the Context of HIV and AIDS in Zimbabwe” Grant # AG 4996 (MIS code 073-555-013 CA 007). Nutriset provided Nutributter at no cost to the study team.
Author disclosures: M. N. N. Mbuya, J. H. Humphrey, F. Majo, B. Chasekwa, A. Jenkins, K. Israel-Ballard, M. Muti, K. H. Paul, R. C. Madzima, L. H. Moulton, and R. J. Stotzfus, no conflicts of interest.
Abbreviations used: AFASS, acceptable, feasible, affordable, sustainable, and safe; ARV, antiretroviral; EBF, exclusive breast-feeding; EHT, expressing and heat-treating; EID, Early Infant Diagnosis; PMTCT, prevention of mother to child transmission of HIV; WAZ, Weight-for-age Z score; WLZ, weight-for-length Z score; LAZ, length-for-age Z score; RDA, Recommended Dietary Allowance.
References
- 1.WHO. HIV and infant feeding: new evidence and programmatic experience: report of a technical consultation held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women. Geneva: WHO; 2006.
- 2.Doherty T, Chopra M, Jackson D, Goga A, Colvin M, Persson LA. Effectiveness of the WHO/UNICEF guidelines on infant feeding for HIV-positive women: results from a prospective cohort study in South Africa. AIDS. 2007;21:1791–7. [DOI] [PubMed] [Google Scholar]
- 3.Lunney KM, Jenkins AL, Tavengwa NV, Majo F, Chidhanguro D, Iliff P, Strickland GT, Piwoz E, Iannotti L, et al. HIV-Positive poor women may stop breast-feeding early to protect their infants from HIV infection although available replacement diets are grossly inadequate. J Nutr. 2008;138:351–7. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Rapid advice: revised WHO principles and recommendations on infant feeding in the context of HIV – November 2009. Geneva: WHO; 2009.
- 5.WHO. Rapid advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants – November 2009. Geneva: WHO; 2009.
- 6.Israel-Ballard K, Donovan R, Chantry C, Coutsoudis A, Sheppard H, Sibeko L, Abrams B. Flash-heat inactivation of HIV-1 in human milk: a potential method to reduce postnatal transmission in developing countries. J Acquir Immune Defic Syndr. 2007;45:318–23. [DOI] [PubMed] [Google Scholar]
- 7.Israel-Ballard KA, Abrams BF, Coutsoudis A, Sibeko LN, Cheryk LA, Chantry CJ. Vitamin content of breast milk from HIV-1-infected mothers before and after flash-heat treatment. J Acquir Immune Defic Syndr. 2008;48:444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chantry CJ, Israel-Ballard K, Moldoveanu Z, Peerson J, Coutsoudis A, Sibeko L, Abrams B. Effect of flash-heat treatment on immunoglobulins in breast milk. J Acquir Immune Defic Syndr. 2009;51:264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israel-Ballard K, Coutsoudis A, Chantry CJ, Sturm AW, Karim F, Sibeko L, Abrams B. Bacterial safety of flash-heated and unheated expressed breastmilk during storage. J Trop Pediatr. 2006;52:399–405. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health and Child Welfare. Prevention of mother to child transmission of HIV in Zimbabwe: a trainer's manual for the integrated approach to HIV and AIDS prevention, care, treatment and follow up for pregnant women, their babies and families. 2nd ed. Harare (Zimbabwe): MOH&CW; 2006.
- 11.Israel-Ballard KA, Maternowska MC, Abrams BF, Morrison P, Chipato T, Chitibura L, Chirenje ZM, Padian NS, Chantry CJ. Acceptability of heat treating breast milk to prevent mother-to-child transmission of human immunodeficiency virus in Zimbabwe: a qualitative study. J Hum Lact. 2006;22:48–60. [DOI] [PubMed] [Google Scholar]
- 12.Paul KH, Muti M, Chasekwa B, Mbuya MNN, Madzima RC, Humphrey JH, Stoltzfus RJ. Complementary feeding messages that target cultural barriers enhance both the use of lipid-based nutrient supplements and underlying feeding practices to improve infant diets in rural Zimbabwe. Maternal and Child Nutrition. 2010; in press. [DOI] [PMC free article] [PubMed]
- 13.Chitsiku IC. Nutritive value of foods of Zimbabwe. 1st ed. Harare (Zimbabwe): University of Zimbabwe Publications; 1991.
- 14.PROTA Foundation. Plant resources of tropical Africa, PROTA. Wageningen (The Netherlands): PROTA; 2005.
- 15.van der Walt AM, Ibrahim MIM, Bezuidenhout CC, Loots DT. Linolenic acid and folate in wild-growing African dark leafy vegetables (morogo). Public Health Nutr. 2009;12:525–30. [DOI] [PubMed] [Google Scholar]
- 16.FAO/WHO. Human vitamin and mineral requirements: report of a joint FAO/WHO expert consultation. Rome: FAO; 2002.
- 17.WHO. Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva: WHO; 1998.
- 18.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for height and body mass index-for-age: methods and development. Geneva: WHO; 2006.
- 19.Pelto GH, Levitt E, Thairu L. Improving feeding practices: current patterns, common constraints, and the design of interventions. Food Nutr Bull. 2003;24:45–82. [DOI] [PubMed] [Google Scholar]
- 20.Uvnas-Moberg K, Widstrom AM, Werner S, Matthiesen AS, Winberg J. Oxytocin and prolactin levels in breast-feeding women. Correlation with milk yield and duration of breast-feeding. Acta Obstet Gynecol Scand. 1990;69:301–6. [DOI] [PubMed] [Google Scholar]
- 21.Zinaman MJ, Hughes V, Queenan JT, Labbok MH, Albertson B. Acute prolactin and oxytocin responses and milk yield to infant suckling and artificial methods of expression in lactating women. Pediatrics. 1992;89:437–40. [PubMed] [Google Scholar]
- 22.Jones E, Dimmock PW, Spencer SA. A randomised controlled trial to compare methods of milk expression after preterm delivery. Arch Dis Child Fetal Neonatal Ed. 2001;85:F91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chimhete C. Survival of the fittest. Zimbabwe Standard (Harare). 6 September 2008. [cited 2009 Dec 2]. Available from: http://allafrica.com/stories/200809081375.html.
- 24.Kafulafula G, Hoover DR, Taha ET, Thigpen M, Li Q, Fowler MG, Kumwenda NI, Nkanaunena K, Mipando L, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. [DOI] [PubMed] [Google Scholar]
- 25.Onyango-Makumbi C, Bagenda D, Mwatha A, Omer SB, Musoke P, Mmiro F, Zwerski SL, Kateera BA, Musisi M, et al. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J Acquir Immune Defic Syndr. 2010;53:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, Kasonde P, Scott N, Vwalika C, et al. Effects of early, abrupt weaning for HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young SL, Chantry C, Ngonyani M, Israel-Ballard K, Ash D, Nyambo M. Flash-heating breastmilk is feasible in Dar es Salaam, Tanzania. FASEB J. 2009;23:LB443.
- 28.Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr. 2007;86:412–20. [DOI] [PubMed] [Google Scholar]