Abstract
In rodents, severe dietary P restriction increases active phosphate absorption by the intestine. However, it remains unknown if moderate dietary P restriction has a similar effect. Weanling pigs (n = 32; body weight 7.4 ± 0.55 kg) were used in a 2 × 2 factorial design and fed dietary available P (aP) concentrations of 0.23 or 0.40% and Ca concentrations of 0.58 or 1.00% for 14 d. Diets were formulated on an aP basis instead of a total P basis, because pigs are unable to absorb phytate-P present in corn and soybean meal. Jejunal segments were mounted in modified Ussing chambers for determination of Na+-dependent nutrient transport. Intestinal mucosal scrapings were taken for RNA isolation and brush border membrane (BBM) vesicle isolation. Na+-dependent phosphate uptake and gene expression of Na-phosphate cotransporter IIb (NaPi-IIb), SGLT-1 (sodium/glucose cotransporter-1), and calbindin D(9k) and protein expression of NaPi-IIb were evaluated. Na+-dependent phosphate transport increased (P < 0.05) 46% as dietary aP concentration was decreased. However, increased Na+-dependent phosphate uptake was not accompanied by increased NaPi-IIb mRNA expression. Expression of NaPi-IIb protein in the BBM increased (P < 0.01) 84% in pigs fed low-P diets compared with pigs fed adequate-P diets. No dietary Ca effects or aP × Ca interactions were detected for Na-dependent P uptake, mRNA or protein expression of NaPi-IIb, or mRNA expression of calbindin D(9k). These data suggest that restricting dietary aP concentration by only 43% stimulates Na+-dependent phosphate uptake and expression of the NaPi-IIb protein in the BBM of the small intestine and through a post-transcriptional mechanism.
Introduction
Phosphate absorption in the small intestine is a combination of both paracellular and transcellular pathways (1). When dietary P concentrations are lowered below the requirement, transcellular absorption dominates, accounting for 78% of the total transport (2,3). Transcellular absorption occurs through secondary active transport, because it is coupled to the flux of Na and is dependent upon the presence of an inside-negative membrane potential of the cell (4,5). Three families of Na-phosphate (Na-Pi)4 cotransporters have been identified in vertebrates: type I, II, and III. The brush border membrane (BBM) of enterocytes express the type-IIb Na-Pi cotransporter, which is a high affinity, pH-dependent phosphate transport system (6).
Dietary phosphate deprivation is an important physiological regulator of intestinal phosphate uptake as observed in BBM vesicles (BBMV) in rats, mice, and chickens (7–9). Kinetic parameters indicate increases in Na-dependent phosphate uptake are due to a 50% increase in maximal capacity for absorption (Vmax) with no change in affinity, indicating the maximal capacity for phosphate transport is increasing and not the affinity of the transporter for phosphate (10). The increase in Vmax in mice fed a low-P diet has been accompanied by an increase in abundance of membrane-bound NaPi-IIb cotransporter protein in the small intestine (8,10). However, mixed results have been reported on the effect of low-P diets on NaPi-IIb mRNA expression and it appears to be dependent upon both the severity and length of dietary P deprivation.
In an attempt to determine the mechanism through which dietary P regulates intestinal P absorption, diets almost devoid of P [<10% of the total P (tP) requirement] were utilized in rodent models. Severe dietary P deficiency is rare in humans and typically only occurs in situations of severe starvation. However, P deficiency can occur in alcoholics, individuals with malabsorption syndromes, and those taking excessive amounts of Ca supplements (11–13). However, even in these cases, it is unlikely that individuals would be consuming diets almost devoid of dietary P. The severity of dietary P restriction in rodent studies raises questions as to whether the NaPi-IIb is an important factor under physiologically relevant situations. Additionally, severe P deprivation is often conducted without consideration for the dietary ratios of Ca:tP, leading to ratios as extreme as 20:1, which could artificially stimulate Na+-dependent uptake and expression of the NaPi-IIb cotransporter in intestinal BBM compared with feeding a moderately low-P diet with an adequate Ca:tP. In rodent studies, purified diets were fed that contain highly available P (aP) sources and are almost devoid of phytate-P, which is unavailable P to monogastric animals. However, swine diets are often corn-soybean meal based and contain 0.22–0.25% phytate-P and diets must be formulated on aP rather than tP to meet the P needs of the animal. Our objectives were to determine whether modest reductions in dietary aP (∼50% reduction) results in stimulation of Na-dependent phosphate and glucose transport and mRNA expression of NaP-IIb, SGLT-1, and calbindin-D9k in the small intestine of weanling pigs. Additionally, the dietary Ca concentration was altered to achieve a Ca:aP of 1.25–4.3 to evaluate the effects of the Ca:aP on Na-dependent phosphate and glucose transport, mRNA expression, and abundance of the NaPi-IIb protein in the jejunal BBM.
Materials and Methods
Animals and treatments.
Thirty-two crossbred pigs (equal barrows and gilts) of 7.4 ± 0.55 kg body weight were blocked by body weight and sex, randomly assigned to dietary treatments (Table 1), and fed for 14 d. Each block consisted of 4 pigs of the same sex for a total of 4 blocks per sex. Pigs were weaned at ∼17 d of age and placed onto a commercial nursery diet (22.2% crude protein, 14.5 MJ/kg, 0.83% Ca, 0.77% P, 0.55% aP) for 5–12 d before being given treatment diets. After an initial adaptation to the commercial diet for 5 d, the first block was placed on test with a new block starting on test for each of the next 8 consecutive days. Each block was fed the dietary treatments for a total of 14 d. Dietary treatments consisted of: 1) control diet (0.40% aP and 1.00% Ca); 2) low P-adequate Ca diet (0.23% aP and 1.00% Ca); 3) low P-low Ca diet (0.23% aP and 0.58% Ca); and 4) adequate P-low Ca diet (0.40% aP and 0.58% Ca). Diets were formulated on an aP basis instead of a tP basis, because pigs are unable to absorb the phytate-P present in both corn and soybean meal. Pigs were individually housed in pens (0.5 m2) and allowed ad libitum access to feed and water. The study was conducted at the Purdue University Swine Center and all protocols were approved by the Purdue Animal Care and Use Committee.
TABLE 1.
Composition of dietary treatments
| Dietary treatments |
||||
|---|---|---|---|---|
| aP, %: 0.40 |
0.23 |
0.23 |
0.40 |
|
| Ca, %: 1.00 | 1.00 | 0.58 | 0.58 | |
| Ingredients | g/kg | |||
| Soybean meal, 48% | 393.4 | 385.0 | 382.3 | 392.2 |
| Corn | 329.5 | 345.4 | 360.4 | 343.1 |
| Whey | 200 | 200 | 200 | 200 |
| Monosodium phosphate | 7.9 | — | — | 7.9 |
| Choice white grease | 40 | 40 | 40 | 40 |
| Limestone | 19.7 | 19.8 | 7.4 | 7.4 |
| Salt | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin premix1 | 2.5 | 2.5 | 2.5 | 2.5 |
| Trace mineral premix2 | 1.3 | 1.3 | 1.3 | 1.3 |
| Lysine-HCl | 0.5 | 0.5 | 0.5 | 0.5 |
| Na selente premix3 | 0.5 | 0.5 | 0.5 | 0.5 |
| l-Threonine | 0.3 | 0.3 | 0.3 | 0.3 |
| d,l-Methonine | 0.9 | 1.0 | 1.0 | 1.0 |
| Calculated composition | ||||
| ME, MJ/kg | 15.00 | 15.10 | 15.36 | 15.20 |
| P, % | 0.66 | 0.49 | 0.50 | 0.67 |
| aP4, % | 0.40 | 0.23 | 0.23 | 0.40 |
| Ca, % | 1.00 | 1.00 | 0.58 | 0.58 |
| L-lysine, % | 1.50 | 1.50 | 1.50 | 1.50 |
| Analyzed composition | ||||
| P, % | 0.69 | 0.46 | 0.45 | 0.70 |
| Ca, % | 1.15 | 1.19 | 0.64 | 0.67 |
| Dry matter, % | 89.2 | 89.5 | 89.6 | 90.1 |
Vitamin premix provided the following guaranteed minimums per kg diet: retinyl acetate, 17.5 mg; cholecalciferol, 24.2 mg; α-tocopheryl acetate, 44 mg; menadione, 2.0 mg; vitamin B-12, 34.98 μg; riboflavin, 7.08 mg; d-pantothenic acid, 21.9 mg; niacin, 32.93 mg.
Trace mineral premix provided the following guaranteed minimums per kg diet: iron, 84.7 mg; zinc, 84.7 mg; manganese, 10.5 mg; copper, 7.87 mg; iodine, 0.32 mg.
Premix provided 0.3 mg Se/kg diet.
Calculated as tP-phytate bound P.
Intestinal segments.
On d 14, pigs were killed by CO2 asphyxiation. Jejunal samples (200 cm posterior to the pyloric junction) were taken for flux rate measurements in modified Ussing chambers (Physiologic Instruments). Segments taken for the Ussing chambers were immediately placed in a buffer solution (50 mmol/L mannitol, 2 mmol/L Tris-HCl, pH 7.4) and continuously aerated at 4°C until mounting in Ussing Chambers. Mucosal scrapings were also taken for RNA and BBMV isolation. Samples for RNA isolation were rinsed with ice-cold sterile saline (0.90% NaCl, wt:v), scraped with a glass slide, and ∼2.0 g mucosa was flash frozen with liquid nitrogen. In addition, ∼4.0 g of mucosal scrapings were placed on ice in a buffer solution (50 mmol/L mannitol, 2 mmol/L Tris-HCl, pH 7.4) for BBMV preparation.
BBMV isolation.
Samples for BBMV isolation were homogenized and isolated by the polyethylene glycol method (14,15). Final membranes were suspended in 1 mL of a solution of 300 mmol/L mannitol and 2 mmol/L Tris-HCL, pH 7.4, 2.0 mg/L leupeptin, 2.0 mg/L soybean trypsin inhibitor, and 1.25 MIU/L apoprotein and immediately frozen in liquid nitrogen and stored at −80°C. Protein content was determined using a BCA Protein Reagent kit (23224, Pierce). The activity of alkaline phosphatase was used as a BBM marker (Sigma, MT-1000 Starbright Alkaline Phosphatase kit) and the activity of ATPase was used as a BLM marker (16). The ratio of enzyme per gram of protein in the BBMV preparation to the original homogenate was used to assess enrichment and purity.
Ussing Chambers.
Techniques assessing gastrointestinal function through ion flux measurements in modified Ussing chambers have been previously described (17,18). Jejunal segments were stripped of the external serosal layer and mounted in Ussing chambers equipped to measure transmural potential difference, short-circuit current, and resistance. The area of tissue exposed was 1.00 cm2. Tissues were mounted in duplicate and placed in modified Ussing chambers (Physiologic Instruments). Tissues were bathed in 8 mL of modified Krebs buffer solution containing 124 mmol/L NaCl, 5 mmol/L KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L CaCl2, 26 mmol/L NaHCO3, and 5 mmol/L glucose. Tissues were maintained at 37°C with a circulating water bath and oxygenated in 95% O2/5% CO2. Basal transepithelial short-circuit current and resistance were measured after a 15-min equilibration period. Na-dependent nutrient transport was determined by measuring changes in short-circuit current induced by the addition of 10 mmol/L inorganic phosphate (Na2HPO4) or glucose to the mucosal side and was osmotically balanced on the serosal side by 10 mmol/L mannitol. Chloride secretion was induced by the addition of 10 mmol/L carbachol to the serosal side and was osmotically balanced by the addition of 10 mmol/L mannitol to the mucosal side of the tissue. The modified Ussing chambers were connected to dual channel voltage/current clamps (VCC MC2; Physiologic Instruments) with a computer interface allowing for real-time data acquisition and analysis (Acquire and Analyze software, Physiologic Instruments).
Real-time PCR.
Total RNA was isolated from jejunal mucosa using Tri-Reagent per the manufacturer's directions (Molecular Research Center). Total RNA (1.0 μg) was reverse transcribed into first-strand cDNA with an oligo-dT primer using Moloney murine leukemia virus transcriptase (Invitrogen) as previously described (19). Real-time PCR was conducted on samples using the Bio-Rad My iQ RT-PCR system containing SYBR green (Bio-Rad). NaPi-IIb, SGLT-1, calbindin D9k, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA concentrations were determined from the threshold cycle value (20) and were normalized to the expression of GAPDH within the sample. These values were then standardized such that a value of 1.0 was assigned to the control group. Primers and annealing temperatures (Ta) were: NaPi-IIb, forward: 5′-CTCTGTAGCTGCCTGGTCCTAA-3′, reverse: 5′-GGTCAGAGTCGACGAGAACAC-3′, Ta = 53°C; SGLT-1, forward: 5′-CGTGCTGTTTCCAGATGATG-3′, reverse: 5′-ATCAGCTCCATGACCAGCTT-3′, Ta = 55°C; calbindin D9k, forward: 5′-CAAACCAGCTGTCGAAGGA-3′, reverse: 5′-TAGGGTTCTCGGACCTTTCAG-3′, Ta = 54°C; GAPDH forward: 5′-TCACCATCTTCCAGGAGCG-3′, reverse: 5′-CTGCTTCACCACCTTCTTGA-3′, Ta = 54°C.
PCR were conducted for 42, 35, 35, and 32 cycles for NaPi-IIb, SGLT-1, calbindin D9k, and GAPDH, respectively, to ensure amplification efficiency in the linear range of each primer set. Product identities were confirmed by melting curve analysis. Gene expression was quantified relative to a standard curve generated from a serially diluted sample.
Western blotting.
The protein content of the jejunal BBMV samples was quantified using BCA assay (Pierce) and frozen at −80°C until analysis. Samples of the BBMV and control samples (pool of jejunal BBMV from pigs fed adequate P and Ca concentrations) were placed in Laemmli buffer (Sigma-Aldrich) and boiled for 5 min at 95°C. Briefly, 300 μg of BBMV protein (300 μg) was loaded in lanes and separated on 9% polyacrylamide gels and electrotransfered onto nitrocellulose paper. Western blots were performed with a custom-designed affinity purified NaPi-IIb pig anti-rabbit polyclonal antibody (GenScript). Membranes were probed with the primary antibody (9000 μg/L) for 1 h at room temperature. Primary antibody binding was visualized using goat anti-rabbit Ig G-horseradish peroxidase antibody (Pierce) at 1:50,000 for 1 h at room temperature. Blots were developed using chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate kit) and the membrane was exposed to X-ray film/imaging instrument and densitometry of the protein was determined (Kodak 1D analysis software). Protein expression is expressed relative to the control sample, which was included to verify equal protein loading and transfer.
Chemical analysis.
Diet samples were ground to pass through a 1-mm screen. Dry matter content of diets was determined at 105°C according to standard AOAC (21) procedures. Diet samples were digested using a 1:3 ratio (v:v) of 60% perchloric acid and 70% nitric acid in tubes at 100°C for 1.5 h and 200°C for 3.5 h. The P content was determined colorimetrically using a molybdovandate assay (21). Ca and Cr concentrations were determined with an atomic absorption spectrophotometer (Spectra AA 220Z, Varian).
Statistical analysis.
The experimental design was a 2 × 2 factorial and the main effects of P, Ca, and the P × Ca interaction were analyzed using the GLM procedure in SAS (22). Data were analyzed with the pig as the experimental unit. Differences were considered significant at P < 0.05 and trends were considered at P < 0.10. Normality was assessed using the proc univariate procedure in SAS and homogeneity of the variance using residual plots. All data are reported as means ± SE.
Results
Animal performance.
Pigs consuming the adequate-P diet grew 50 g/d faster and were 640 g heavier (P < 0.10) at the end of the trial (Table 2). Feed intake did not differ between treatment groups. Feed efficiency improved (P < 0.05) 26.3% as the concentration of P was increased in the diet. However, as the concentration of Ca was increased in the diet from 0.58 to 1.00%, feed efficiency declined (P < 0.05) 19.3%. As expected, daily P intake was reduced (P < 0.0001) 34% in pigs fed the low-P diet compared with pigs fed the adequate-P diet. In pigs fed the P-adequate diets, decreasing dietary Ca level increased P intake. However, in pigs fed the P-deficient diets, decreasing the dietary Ca level decreased P intake (P × Ca, P < 0.10).
TABLE 2.
Effects of dietary P and Ca concentrations on pig performance1
| Dietary treatment |
P-value | ||||||
|---|---|---|---|---|---|---|---|
| P, %: 0.40 |
0.40 |
0.23 |
0.23 |
||||
| Item | Ca, %: 1.00 | 0.58 | 1.00 | 0.58 | P effect | Ca effect | P x Ca |
| Initial body weight, kg | 7.38 ± 0.15 | 7.37 ± 0.14 | 7.44 ± 0.14 | 7.50 ± 0.14 | 0.641 | 0.893 | 0.894 |
| Final body weight, kg | 10.92 ± 0.38 | 11.50 ± 0.36 | 10.33 ± 0.34 | 10.92 ± 0.38 | 0.086 | 0.122 | 0.910 |
| Weight gain, kg/d | 0.250 ± 0.03 | 0.294 ± 0.02 | 0.206 ± 0.02 | 0.250 ± 0.03 | 0.078 | 0.115 | 0.906 |
| Feed intake, kg/d | 0.537 ± 0.04 | 0.567 ± 0.03 | 0.610 ± 0.03 | 0.573 ± 0.02 | 0.330 | 0.601 | 0.170 |
| Gain:feed | 0.461 ± 0.04 | 0.527 ± 0.04 | 0.329 ± 0.04 | 0.453 ± 0.05 | 0.027 | 0.028 | 0.498 |
| P intake, mg/d | 3.75 ± 0.24 | 4.02 ± 0.22 | 2.82 ± 0.21 | 2.23 ± 0.24 | <0.0001 | 0.500 | 0.071 |
Means ± SE, n = 8 except P 0.40%, Ca 1.00%, n = 7.
Transepithelial ion transport measurements.
Transepithelial resistance and short-circuit current were measured in intestinal samples as a direct assessment of ion transport. Initial transepithelial R, a measure of total passive ion transport, was not different among treatment groups (data not shown). Basal Isc, a measure of total active ion transport, also did not differ among treatment groups.
Na-dependent nutrient transport.
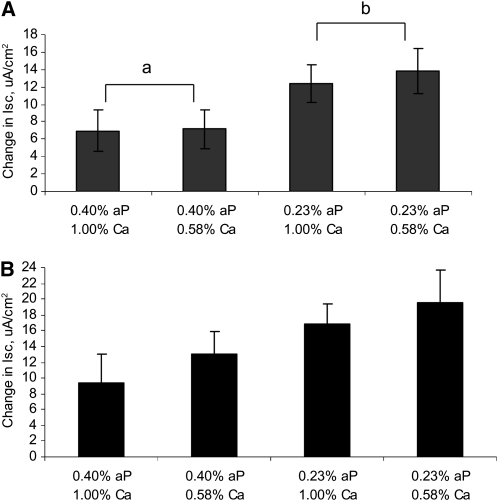
Na-dependent phosphate and glucose transport were measured in intestinal samples and results are reported as the change in short-circuit current relative to baseline values. No P × Ca interactions were detected, so only the main effects are reported. Na-dependent phosphate transport increased 46% as the P concentration in the diet was lowered from 0.40 to 0.23% (P < 0.05) (Fig. 1A). Ca concentration in the diet did not affect Na+-dependent phosphate uptake. Na-dependent glucose uptake was also altered by the feeding of a low-P diet and increased (P < 0.10) 36% as the concentration of P in the diet was lowered (Fig. 1B). Similar to phosphate uptake, this change occurred independently of the dietary Ca concentration.
FIGURE 1 .
Effects of dietary P and Ca concentrations on Na-dependent phosphate (A) and glucose (B) transport as measured by change in short-circuit current in weanling pigs. Changes in Na-dependent phosphate transport induced by the addition of 10 mmol/L phosphate or glucose to the luminal buffer. Values are means ± SE, n = 8. Means without a common letter differ, P < 0.05, for the main effect of dietary aP level.
Jejunal gene expression.
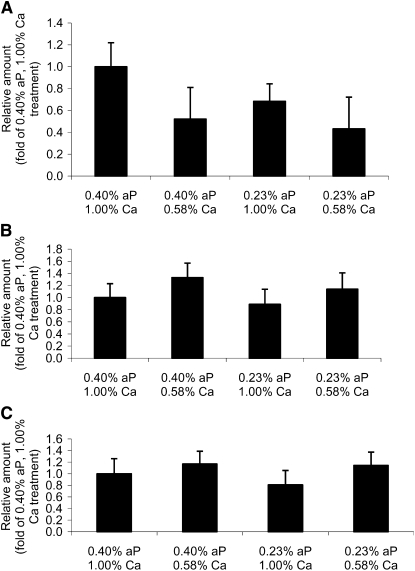
Neither dietary P nor Ca concentration had a significant effect on NaPi-IIb mRNA expression (Fig. 2A). Pigs fed the low-P diet did not have greater expression of NaPi-IIb compared with pigs fed the adequate-P diet, indicating the change in Na-dependent phosphate uptake occurred independent of alterations in gene expression. Pigs fed the adequate-Ca diet had a 41% greater expression of NaPi-IIb compared with pigs fed the low-Ca diet; however, due to large variation, this difference was not significant (P = 0.15). Similarly, SGLT-1 (Fig. 2B) and calbindin D9k (Fig. 2C) mRNA expression did not differ among dietary treatments.
FIGURE 2 .
The effect of dietary P and Ca levels on jejunal NaPi-IIb (A), calbindin D9k (B), and SGLT-1 (C) mRNA in weanling pigs. Values are means ± SE, n = 8.
Western blots.
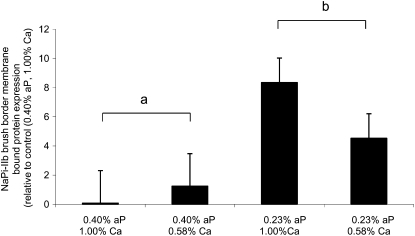
Expression of the NaPi-IIb cotransporter protein was determined in BBMV isolated from the jejunum. Pigs fed the low-P diet had an 84% greater expression of the NaPi-IIb protein on the BBM compared with pigs fed the adequate-P diet (P < 0.01; Fig. 3). Expression was not affected by the dietary Ca concentration.
FIGURE 3 .
Jejunal NaPi-IIb protein expression in weanling pigs fed various concentrations of dietary aP and Ca. Values are means ± SE, n = 4 for 0.40% P, 1.00% Ca and 0.40% P, 0.58% Ca; n = 7 for 0.23% P, 1.00% Ca and 0.23% P, 0.58% Ca. Means without a common letter differ, P < 0.01, for the main effect of dietary aP level.
Discussion
The efficiency of intestinal phosphate absorption is greatest when dietary concentrations of phosphate are limiting and efficiency declines as the requirement is met and exceeded (23). Increases in intestinal phosphate absorption in the presence of low dietary P intakes have been reported in mice, rats, and chickens (6–8). The increase in absorption efficiency in the presence of low dietary concentrations was due to a 55–70% increase in Na+-dependent phosphate uptake (8,9). Analysis of kinetic parameters of isolated BBMV indicate the adaptive response was due to an increase in Vmax and not the affinity of phosphate for the NaPi-cotransporter (7,8). Net phosphate flux experiments conducted in modified Ussing chambers with rat small intestines reported active phosphate transport was 4-fold greater in rats fed a low-P diet (0.03%) compared with rats fed a control diet containing 0.30% P (24). Our results confirm these earlier findings on the impact of dietary P restriction and extend them by showing an increase in active-phosphate transport with only a modest, 43% reduction in dietary P concentration. The greater increase in active P uptake in rats is most likely due to differences in the severity of P restriction. Dietary P intake was reduced 53% in pigs fed the low-P diet and resulted in a decreased feed efficiency and tended to reduce weight gain and final body weight. As expected, reducing dietary P intake resulted in a stimulation of active P uptake; however, this was not enough to maintain similar performance to pigs fed the adequate-P diet. This indicates that only moderate P restriction is necessary to stimulate Na+-dependent phosphate transport in the small intestine.
Studies investigating the ability of dietary P to alter Na+-dependent phosphate transport have been conducted with severe phosphate restriction and without regard to dietary Ca:aP, as dietary Ca was fed at the Ca requirement. As a result, Ca:aP ratios varied from 5.5–35:1,which are greater than the recommended ratio of 2:1–3:1 (25). High concentrations of dietary Ca are reported to reduce P absorption due to the formation of insoluble tricalcium phosphate in the intestinal tract (26–28). Whereas wide Ca:aP are not typical in human nutrition, they can occur in patients with hyperphosphatemia treated with Ca carbonate as a dietary phosphate binder (29). We hypothesized that feeding a marginal dietary P concentration without altering the Ca concentration, and hence resulting in the Ca:aP exceeding 4:1, would further stimulate Na+-dependent phosphate transport due to binding of the remaining dietary P by excess Ca. However, our results indicate that Na+-dependent P uptake was not altered by the Ca:aP in the diet. This indicates that altering dietary Ca concentration to achieve a Ca:aP as wide as 4.3:1 does not impact Na+-dependent phosphate uptake. However, this data does not imply that feeding a Ca:aP ratio as wide at 35:1 would not cause a reduction in P absorption and hence further stimulate Na+-dependent P uptake.
Severe dietary P restriction in both mice and rats has resulted in a 30–40% increase in expression of the NaPi-IIb cotransporter protein from small intestine BBMV compared with control-fed animals (8,10). Mixed results have been reported on the effect of low-P diets on NaPi-IIb mRNA expression. Studies in mice have resulted in no alterations in NaPi-IIb mRNA to a 66% increase in NaPi-IIb mRNA expression in response to consumption of a low-P diet compared with animals fed a high-P diet (8,30). In the present study, NaPi-IIb mRNA expression did not differ in pigs fed a low-P diet compared with pigs fed an adequate-P diet. These data are in agreement with Katai et al. (9), who reported no differences in NaPi-IIb mRNA in rats fed a low-P diet compared with rats fed a control diet. It is possible that the stimulation of transcription occurred at a point prior to tissue harvesting and had returned to baseline levels at the time of slaughter. In the present study, the abundance of the NaPi-IIb protein in the BBM increased 84% in pigs fed the moderate-P diets compared with pigs fed the adequate-P diets. Severe dietary P restriction in mice and rats resulted in a 30–40% increase in abundance of the NaPi-IIb protein in BBMV isolated from the small intestine compared with control-fed animals (8,10). The greater increase in abundance of NaPi-IIb in the present trial compared with the rodent studies in response to consumption of a low-P diet may be due to the age of the animals, i.e. adult compared with weanling animals. Expression of the NaPi-IIb transcript has been shown to decrease with age. Kirchner et al. (31) reported that mRNA expression of the NaPi-IIb in 20-d- and 2-mo-old rats was 20 and 3%, respectively, of that in 10-d-old rats (31). In the present trial, animal age between blocks varied by 1–7 d; however, no block effects or block by treatment interactions were detected. These data imply that stimulation of P uptake in the jejunum of weanling pigs fed a low-P diet is not occurring through a transcriptional mechanism and is likely occurring via an increase in translation of the NaPi-IIb cotransporter protein or translocation of NaPi-IIb from a subapical pool to the apical surface. However, the severity of dietary restriction and length of time over which restriction is imposed may affect the mechanism through which intestinal Na+-dependent phosphate uptake is stimulated.
Restricting dietary Ca and P by 50–98% of the requirement has been reported to stimulate calbindin D9k mRNA expression in the duodenum of chicks and in both the duodenum and ileum of rats (32,33). In the present experiment, moderate dietary P and Ca restriction did not affect (P > 0.10) calbindin D9k mRNA expression in the jejunum of weanling pigs. We focused on the jejunum in the present trial, because it is the primary site of active P absorption (2), whereas the primary site of Ca absorption is the duodenum (34), so sampling location could explain our failure to detect a difference in calbindin D9k mRNA expression. Studies conducted with intestinal BBMV isolated from rats and chickens reported no alteration in Na+-dependent glucose uptake in response to feeding a low-P diet compared with control animals (7,9). Surprisingly, pigs fed the moderate-P diet had a 36% increase in Na+-dependent glucose uptake compared with pigs fed the control diet. Unlike previous studies, we utilized modified Ussing chambers instead of an isolated BBMV, which could account for the different results.
Weanling pigs adapt to moderate dietary P restrictions by increasing absorption efficiency of phosphate through stimulation of Na+-dependent phosphate uptake in the small intestine. This adaptation occurs independent of dietary Ca concentrations as low as 0.58% and a Ca:aP as wide as 4.3:1. However, increases in absorption efficiency are not enough to maintain performance to that of pigs fed adequate-P diets. Additionally, stimulation of Na+-dependent phosphate uptake in weanling pigs fed a moderate-P diet corresponds with an increase in expression of the NaPi-IIb protein in the BBM. Na-dependent phosphate uptake is stimulated post-transcriptionally, because there were no alterations in NaPi-IIb mRNA expression as the concentration of P decreased in the diet. Further research is necessary to determine if NaPi-IIb is regulated at the translational or post-translational level following consumption of a moderate-P diet. Additionally, the level of dietary P restriction required to stimulate active P transport in the intestine has yet to be determined.
Acknowledgments
K.L.S. and J.S.R. designed and conducted the research and analyzed the data; J.C.F. helped conduct the research and provided essential reagents; K.L.S. and J.S.R. wrote the paper; and J.S.R. had primary responsibility for the final content. All authors read and approved the final version of the paper.
Author disclosures: K. L. Saddoris, J. C. Fleet, and J. S. Radcliffe, no conflicts of interest.
Abbreviations used: aP, available P; 0.40% available P and 1.00%, control dietary treatment representing an adequate-P and -Ca, corn and soybean meal-based diet; 0.23% available P and 1.00% Ca, dietary treatment representing a low-P, adequate-Ca, corn and soybean meal-based diet; 0.23% available P and 0.58% Ca, dietary treatment representing a low-P, adequate-Ca, corn and soybean meal-based diet; 0.40% available P and 0.58% Ca, dietary treatment representing an adequate-available P, low-Ca, corn and soybean meal-based diet; BBM, brush border membrane; BBMV, brush border membrane vesicle; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NaPi-IIb, Na-phosphate cotransproter IIb; tP, total P; SGLT-1, sodium/glucose cotransporter-1.
References
- 1.Lee DBN, Walling W, Barautbar N. Intestinal phosphate absorption: influence of vitamin D and non-vitamin D factors. Am J Physiol. 1986;250:G369–73. [DOI] [PubMed] [Google Scholar]
- 2.Breves G, Schröder B. Comparative aspects of gastrointestinal phosphorus metabolism. Nutr Res Rev. 1991;4:125–40. [DOI] [PubMed] [Google Scholar]
- 3.Eto N, Tomita M, Hayashi M. NaPi-mediated transcellular permeation in the dominant route in intestinal inorganic phosphate absorption in rats. Drug Metab Pharmacokinet. 2006;21:217–21. [DOI] [PubMed] [Google Scholar]
- 4.Berner W, Kinner R, Murer H. Phosphate transport into brush border membrane vesicles isolated from rat small intestine. Biochem J. 1976;160:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forester IC, Loo DDF, Eskandari S. Stoichiometry and Na+ binding cooperativeity of rat and flounder renal type II Na-Pi cotransporters. Am J Physiol Renal Physiol. 1999;276:F644–9. [DOI] [PubMed] [Google Scholar]
- 6.Hilfiker H, Hattenhauer I, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci USA. 1998;95:14564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quamme GA. Phosphate transport in intestinal brush-border membrane vesicles: effect of pH and dietary phosphate. Am J Physiol. 1985;249:G168–76. [DOI] [PubMed] [Google Scholar]
- 8.Hattenhaur O, Traebert M, Murer H, Biber J. Regulation of small intestinal Na-Pi type IIb cotransporter by dietary phosphate intake. Am J Physiol Gastrointest Liver Physiol. 1999;277:G756–62. [DOI] [PubMed] [Google Scholar]
- 9.Katai K, Miyamoto K, Kishida S, Segawa H, Hii T, Tanaka H, Tani Y, Arai H, Tatsumi S, et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphorus diet and 1,25-dihydroxyvitamin D3. Biochem J. 1999;343:705–12. [PMC free article] [PubMed] [Google Scholar]
- 10.Segawa H, Kaneko I, Tamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K. Intestinal Na-Pi cotransporter adaptation to dietary Pi content in vitamin D receptor null mice. Am J Physiol Renal Physiol. 2004;287:F39–47. [DOI] [PubMed] [Google Scholar]
- 11.Knochel J, Agarwal R. Hypophosphatemia and hyperphosphatemia. In: The kidney. 5th ed. Brenner B, editor. Philadelphia: WB Saunders; 1996. p. 1086–133.
- 12.Lotz M, Zisman E, Bratter FC. Evidence for a phosphorus-depleting syndrome in man. N Engl J Med. 1968;278:409–15. [DOI] [PubMed] [Google Scholar]
- 13.Heaney RP, Nordin BEC. Calcium effects on phosphorus absorption: implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. 2002;21:239–44. [DOI] [PubMed] [Google Scholar]
- 14.Prabhu R, Balasubramania KA. A novel method of preparation of small intestinal brush border membrane vesicles by polyethylene glycol precipitation. Anal Biochem. 2001;289:157–61. [DOI] [PubMed] [Google Scholar]
- 15.Basivireddy J, Balasubramanian KA. A simple method of rat renal brush border membrane preparation using a polyethylene glycol precipitation. Int J Biochem Cell Biol. 2003;35:1248–55. [DOI] [PubMed] [Google Scholar]
- 16.Kelly M, Butler KM, Hamilton DN. Jr. Transmissible gastroenteritis in piglets: a model of infantile viral diarrhea. J Pediatr. 1972;80:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro GA, Harari Y, Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987;253:G540–8. [DOI] [PubMed] [Google Scholar]
- 18.Kles KA, Wallig MA, Tappenden KA. Luminal nutrients exacerbate intestinal hypoxia in the hypoperfused jejunum. JPEN J Parenter Enteral Nutr. 2001;25:246–53. [DOI] [PubMed] [Google Scholar]
- 19.Fleet JC, Wood RJ. Specific 1,25 (OH)2D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am J Physiol. 1999;276:G958–64. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Δ Δ Ct) method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 21.AOAC. Official methods of analysis. 15th ed. Arlington (VA): AOAC; 1990.
- 22.SAS. SAS user's guide. Cary (NC): SAS Institute; 2003.
- 23.Anderson JJB. Nutritional biochemistry of calcium and phosphorus. J Nutr Biochem. 1991;2:300–7. [Google Scholar]
- 24.Jungbluth H, Binswanger U. Unidirectional duodenal and jejunal calcium and phosphorus transport in the rat: Effects of dietary phosphorus depletion, ethane-1-hydroxy-1,1-diphopshonate and 1,25 dihydroxycholecalciferol. Res Exp Med (Berl). 1989;189:439–49. [DOI] [PubMed] [Google Scholar]
- 25.NRC. Nutrient requirements of swine. 10th ed. Washington, DC: National Academy Press; 1998.
- 26.Heaney RP, Nordin TEC. Calcium effects on phosphours absorption: implication for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. 2002;21:239–44. [DOI] [PubMed] [Google Scholar]
- 27.Vipperman PE, Poe ER Jr, Cunningham PJ. Effect of dietary calcium and phosphorus level upon calcium, phosphorus and nitrogen balance in swine. J Anim Sci. 1974;38:758–65. [DOI] [PubMed] [Google Scholar]
- 28.Cromwell GL. Metabolism and role of phosphorus, calcium, and vitamin D3 in swine nutrition. In: MB Coelho, ET Kornegay, editors. Phytase in animal nutrition and waste management. BASF reference manual. XXX (NJ): BASF Corp.; 1996.
- 29.Slatopolsky E, Weerts C, Lopez Hilker S, Norwood K, Zink M, Windus D, Delmez J. Calcium carbonate as a phosphate binder in patients with chronic renal failure undergoing dialysis. N Engl J Med. 1986;315:157–61. [DOI] [PubMed] [Google Scholar]
- 30.Radanovic T, Wagner CA, Murer H, Biber J. Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P (i) diet of the type IIb Na+-P(i) cotransport in mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G496–500. [DOI] [PubMed] [Google Scholar]
- 31.Kirchner S, Muduli A, Casirola D, Prum K, Douard V, Ferraria RP. Luminal fructose inhibits rat intestinal sodium-phosphate cotransporter gene expression and phosphate uptake. Am J Clin Nutr. 2008;87:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar A, Shani M, Fullmer CS, Brindak ME, Striem S. Modulation of chick intestinal and renal calbindin gene expression by dietary vitamin D3, 1.25-dihydroxyvitamin D3, calcium and phosphorus. Mol Cell Endocrinol. 1990;72:23–30. [DOI] [PubMed] [Google Scholar]
- 33.Armbrecht HJ, Boltz MA, Bruns MEH. Effect of age and dietary calcium on intestinal calbindin D-9k expression in the rat. Arch Biochem Biophys. 2003;420:194–200. [DOI] [PubMed] [Google Scholar]
- 34.Armbrecht HJ, Zenser TV, Gross CJ, Davis BB. Adaptation to dietary calcium and phosphorus restriction changes with age in the rat. Am J Physiol. 1980;239:E322–7. [DOI] [PubMed] [Google Scholar]