Abstract
Background
Computed tomography pulmonary angiography (CTPA) may improve detection of life-threatening pulmonary embolism. But this sensitive test may have a downside: overdiagnosis and overtreatment (finding clinically unimportant emboli and exposing patients to harms from unnecessary treatment).
Methods
To assess the impact of CTPA on national pulmonary embolism incidence, mortality, and treatment complications, we conducted a time trend analysis using the Nationwide Inpatient Sample and Multiple Cause-of-Death databases. We compared age-adjusted incidence, mortality, and treatment complications (in-hospital gastrointestinal or intracranial hemorrhage or secondary thrombocytopenia) of pulmonary embolism among United States adults before (1993–1998) and after (1998–2006) CTPA was introduced.
Results
Pulmonary embolism incidence was unchanged before CTPA (p=0.63), but increased substantially after CTPA (81% increase: from 62.1 to 112.3 per 100,000, p<0.001). Pulmonary embolism mortality decreased during both periods: more so before CTPA (8% reduction: from 13.4 to 12.3 per 100,000, p<0.001) than after (3% reduction: from 12.3 to 11.9 per 100,000, p=0.02). Case-fatality improved slightly before (8% decrease, from 13.2% to 12.1%, p=0.02) and substantially after CTPA (36% decrease: from 12.1% to 7.8%, p<0.001). Meanwhile, CTPA was associated with an increase in presumed complications of anticoagulation for pulmonary embolism: pre-CTPA, the complication rate was stable (p=0.24), but post-CTPA it increased by 71% (from 3.1 to 5.3 per 100,000, p<0.001).
Conclusions
The introduction of CTPA was associated with changes consistent with overdiagnosis: rising incidence, minimal change in mortality, and lower case-fatality. Better technology allows us to diagnose more emboli, but to minimize harms of overdiagnosis we must learn which ones matter.
The introduction in 1998 of multidetector row computed tomography pulmonary angiography (CTPA) revolutionized the way physicians approach pulmonary embolism. Many assumed this highly sensitive test would improve outcomes of this deadly disease by detecting and allowing treatment of emboli that were previously missed. CTPA rapidly spread into practice, largely replacing other tests for pulmonary embolism such as ventilation-perfusion scans and invasive pulmonary angiography.1 Several institutions reported a 7-to-13-fold increase in use of CTPA by 2006,1–4 and nationally there was an 11-fold rise in chest CT angiography from 2001–2006 in the Medicare fee-for-service population [personal communication: 2010 letter from Gottlieb DJ to Woloshin S]. In 2007, 2.6 million chest CT angiography scans were performed in the United States (US).5 CTPA is now preferred as the first-line test for pulmonary embolism by both professional societies6 and practicing physicians.7
But the increased sensitivity of CTPA may have a downside: the detection of emboli that are so small as to be clinically insignificant.8, 9 This phenomenon has been called “overdiagnosis,” defined as the detection of an abnormality that will never cause symptoms or death.10 Overdiagnosis matters because it can lead to iatrogenic harm. While a clinically insignificant pulmonary embolism is by definition not harmful, treating such an embolism can cause harm (e.g., bleeding from anticoagulation, which can in the worst case be fatal). Many are aware of overdiagnosis from the recent controversy over prostate and breast cancer screening,11, 12 but there has been limited consideration of this possibility in other contexts such as pulmonary embolism.13, 14 Typically, as in a recent study reporting a national increase in the incidence of pulmonary embolism, the possibility of overdiagnosis is not seriously addressed.15
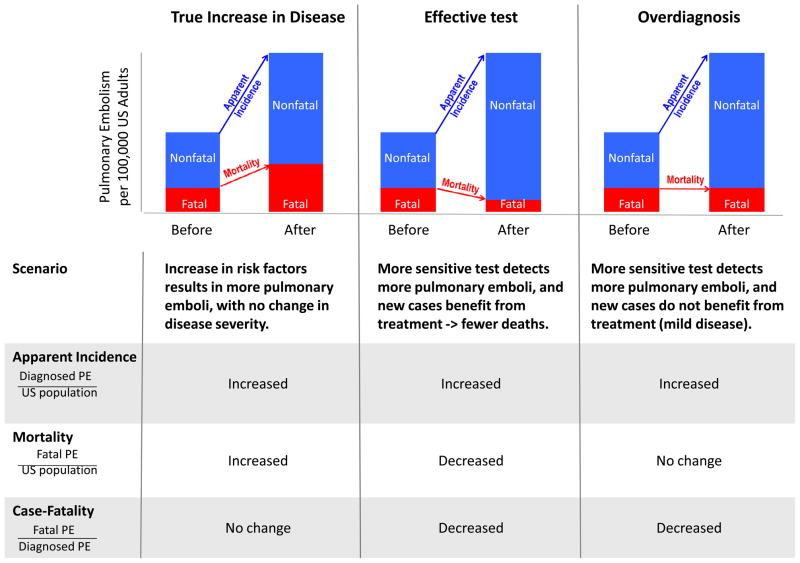
In this paper, we investigate whether CTPA has resulted in overdiagnosis of pulmonary embolism in the US. Because there is no direct way to prove that a pulmonary embolism has been “overdiagnosed” (unless patients are observed without treatment until they die from an unrelated cause), we looked for indirect evidence by comparing trends in pulmonary embolism incidence and mortality before and after the introduction of CTPA. As shown in Figure 1, if increasing use of CTPA were improving our ability to find and successfully treat clinically important pulmonary emboli, we would expect to see an increase in incidence (since highly sensitive CTPA finds pulmonary emboli that were previously missed) and a reduction in mortality (due to successful treatment of the “new” pulmonary emboli). On the other hand, if CTPA primarily improves our ability to find pulmonary emboli of minimal clinical significance, we would expect to see rising incidence, but little change in mortality.
Figure 1.
Expected change in mortality and case-fatality in various scenarios of rising apparent incidence
Abbreviations: PE, pulmonary embolism; US, United States.
METHODS
Design Overview
We conducted a time trend analysis of pulmonary embolism incidence, mortality, and treatment complications in US adults (age ≥18 years) from 1993–2006. This timeframe encompasses the five years prior to the introduction of CTPA (1993–1998) and the available years of data following its introduction (1998–2006). Studies using de-identified, publicly available data are exempt from institutional board review at Boston University and Dartmouth College.
Data Sources
We used the Nationwide Inpatient Sample16 (NIS) to determine national estimates of hospitalization for pulmonary embolism. The NIS includes all discharges from a 20% stratified sample of non-federal hospitals in the US. Strata are based on geographic region, public/private status, urban/rural designation, teaching status, and hospital bed size. Hospitals included in the NIS may vary from year to year. Each record contains patient demographics, up to 15 International Classification of Diseases (ICD), 9th revision procedure and diagnosis codes, vital status at hospital discharge, and a discharge weight to allow national estimates. Although the first year of available data in the NIS is 1988, HCUP recommends conducting time trend analyses beginning in 1993 because of the small number of participating states prior to 1993.
We used the Multiple Cause-of-Death files17 to determine national mortality from pulmonary embolism. This comprehensive database compiled by the National Center for Health Statistics contains data from all death certificates filed in the US each year. Each record includes information on the decedent’s demographics and up to 20 contributing causes of death recorded as ICD-9 (1993–1998) or ICD-10 (1999–2006) codes.
Primary Outcomes: Pulmonary Embolism Incidence and Mortality
Incidence
We calculated the annual number of hospital discharges with a diagnosis of pulmonary embolism per 100,000 US adults as our measure of incidence.
The numerator for the incidence rate includes all adults with a pulmonary embolism based on ICD-9 codes for acute (415.11, 415.19) or obstetric (673.2) pulmonary embolism in any of the 15 diagnosis fields on the hospital discharge record. Because the specificity of these codes (e.g., for distinguishing current versus historic embolism) has been questioned,18 we also reported the incidence of pulmonary embolism among patients for whom this was the primary discharge diagnosis, which should have accuracy approaching 95%.19
The denominator for the incidence rate (and all other population rates) is the corresponding mid-year US estimated adult population from the US Census survey.
Mortality
Pulmonary embolism mortality was defined as the annual number of deaths in which pulmonary embolism was listed as a contributing cause per 100,000 US adults. Pulmonary embolism deaths were identified by the presence of the same acute or obstetric pulmonary embolism ICD-9 or ICD-10 (I26, O88.2) codes in any of the 20 diagnosis fields on the death certificate.
The standard calculation of pulmonary embolism mortality includes all deaths in which pulmonary embolism is listed as a contributing cause of death rather than only cases in which it is reported as the underlying cause of death.20 We followed this precedent for two reasons. First, relying only the underlying cause of death reported on death certificates to determine deaths related to pulmonary embolism results in sensitivity as low as 27%.21 Second, the US Department of Health & Human Services’ instructions on completing the death certificate direct that pulmonary embolism should not be coded as the underlying cause of death if there is a more specific etiology that precipitated the embolism (e.g., cancer, recent surgery).22 In these cases pulmonary embolism is listed as a contributing cause, even if it is the immediate cause of death.
Secondary outcomes: Case-fatality and treatment complications
We used the NIS to capture all secondary outcomes. Case-fatality was defined as the proportion of hospital deaths among patients with a pulmonary embolism.
We recorded potential in-hospital complications of anticoagulation for pulmonary embolism if these ICD-9 codes appeared in any of the 14 secondary diagnosis fields: gastrointestinal hemorrhage (65 codes shown in the eTable),23 intracranial hemorrhage (430–432), and secondary (e.g., drug-induced) thrombocytopenia (287.4). While these codes may overestimate in-hospital complications related to anticoagulation, trends over time should not be affected.
Statistical Analysis
We derived national estimates from the NIS by applying each record’s discharge weight using the SVY commands in Stata, release 10.1 (StataCorp, College Station, TX). We calculated annual percent change using the Joinpoint Regression Program, version 3.4.2 (Statistical Research and Applications Branch, National Cancer Institute). We age standardized all rates to account for changing demographics. Outcomes reported per 100,000 US adults were standardized using the 2000 US Census as the standard population; outcomes reported as a percentage of patients with pulmonary embolism (e.g., case-fatality) were standardized using all pulmonary embolism patients in the study period as the standard population.
RESULTS
Incidence and Mortality
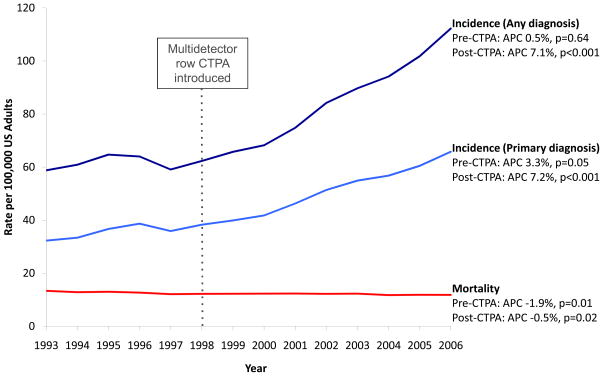
As shown in Figure 2, overall age-adjusted incidence of pulmonary embolism did not significantly change in the period before CTPA (58.8 to 62.3 per 100,000; annual percent change 0.5%, p=0.64), but increased by 81% after CTPA was introduced, rising from 62.3 to 112.3 per 100,000 US adults (annual percent change 7.1%, p<0.001). In the subset of patients with a primary diagnosis of pulmonary embolism, incidence rose 19% before CTPA (32.3 to 38.3 per 100,000; annual percent change 3.3%, p=0.05), but showed a more dramatic rise of 72% after CTPA was introduced, increasing from 38.3 to 65.8 per 100,000 (annual percent change 7.2%, p<0.001).
Figure 2.
Incidence and mortality of pulmonary embolism in the United States, 1993–2006
Abbreviations: APC, annual percent change; CTPA, computed tomography pulmonary angiography; US, United States.
The pattern of stable pulmonary embolism incidence before CTPA and a large rise after the introduction of CTPA was consistent for all admission types. Specifically, after the introduction of CTPA, incidence rose by 86% among medical admissions (45.9 to 85.5 per 100,000, annual percent change 8.1%, p<0.001); by 60% among surgical admissions (16.0 to 25.6 per 100,000, annual percent change 6.5%, p<0.001), and increased 2.7-fold among obstetric admissions (0.7 to 1.9 per 100,000, annual percent change 13.6%, p<0.001).
As shown in Figure 2, age-adjusted pulmonary embolism mortality decreased throughout the study period. The decrease was more pronounced before CTPA (13.4 to 12.3 per 100,000; 8% decrease, annual percent change −1.9%, p=0.01) than afterwards, when mortality fell by 3% from 12.3 to 11.9 per 100,000 (annual percent change −0.5%, p=0.02).
Case-fatality
As shown in the Table, age-adjusted pulmonary embolism case-fatality improved slightly before (8% decrease, from 13.2% to 12.1%, p=0.02) and substantially after CTPA was introduced (36% decrease: from 12.1% to 7.8%, p<0.001). For context, the annual percent change in case-fatality among pulmonary embolism patients was similar to that among all medical admissions before CTPA (roughly −2.0%). But after CTPA was introduced, case-fatality decreased by a third for all patients with pulmonary embolism and by half for patients with a primary diagnosis of pulmonary embolism, while falling only 20% among all medical admissions.
Table.
Characteristics and case-fatality of US adults with pulmonary embolism
| Year | Annual Percent Change (P-value) | ||||
|---|---|---|---|---|---|
| 1993 | 1998 | 2006 | Before CTPA (1993–1998) | After CTPA (1998–2006) | |
| Pulmonary embolism (any diagnosis) | n=110,726 | n=126,887 | n=258,602 | ||
| Characteristics | |||||
| Mean age, yearsa | 64.8±0.3 | 64.9±0.2 | 63.6±0.2 | ||
| Female | 53.7% | 57.4% | 55.0% | ||
| Admission type | |||||
| Medical | 74.9% | 73.7% | 76.1% | ||
| Surgical | 24.2% | 25.5% | 22.9% | ||
| Obstetric | 0.8% | 0.8% | 1.0% | ||
| Case-fatality (i.e., hospital mortality) | 13.2% | 12.1% | 7.8% | −2.0% (p=0.02) | −5.5% (p<0.001) |
|
| |||||
| Pulmonary embolism (primary diagnosis) | n=60,849 | n=77,990 | n=151,345 | ||
| Characteristics | |||||
| Mean age, years* | 64.6±0.3 | 64.2±0.2 | 62.5±0.2 | ||
| Female | 55.8% | 58.8% | 55.9% | ||
| Admission type | |||||
| Medical | 87.6% | 84.8% | 84.2% | ||
| Surgical | 11.7% | 14.5% | 14.9% | ||
| Obstetric | 0.7% | 0.7% | 0.8% | ||
| Case-fatality (i.e., hospital mortality) | 7.1% | 6.7% | 3.7% | −2.1% (p=0.14) | −7.7% (p<0.001) |
|
| |||||
| All medical admissions | |||||
| Hospital mortality | 3.7% | 3.4% | 2.7% | −1.6% (p<0.001) | −3.0% (p<0.001) |
Plus-minus values indicate means ± standard error.
Treatment Complications
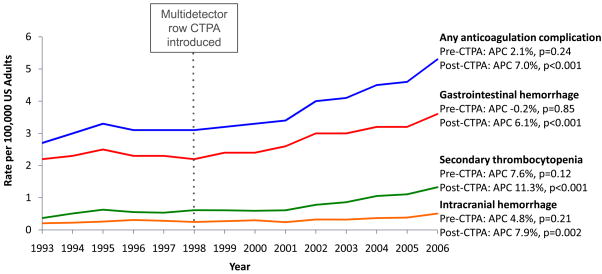
As shown in Figure 3, the introduction of CTPA was associated with an increase in presumed in-hospital complications of anticoagulation for pulmonary embolism. Before CTPA, the age-adjusted complication incidence rate did not significantly change (2.7 to 3.1 per 100,000; p=0.24), but after CTPA was introduced, it increased by 71% from 3.1 to 5.3 per 100,000 (annual percent change 7.0%, p<0.001). Among patients with a primary diagnosis of pulmonary embolism, we observed the same pattern: stable complication rates before CTPA (1.2 to 1.5 per 100,000; p=0.07) and increasing rates after CTPA was introduced, when complications rose by 47% from 1.5 to 2.2 per 100,000 (annual percent change 5.2%, p<0.001).
Figure 3.
Rates of potential complications of anticoagulation treatment among US adults hospitalized with a pulmonary embolism, 1993–2006
Abbreviations: APC, annual percent change; CTPA, computed tomography pulmonary angiography; US, United States.
COMMENT
The epidemiology of pulmonary embolism has changed since CTPA was introduced. Compared to the pre-CTPA era, pulmonary embolism incidence rose, mortality changed little, and case-fatality decreased.
What explains these findings (Figure 1)? At first glance, the rapid increase in incidence seems alarming – an apparent epidemic of pulmonary embolism. But the epidemic is unusual because it has only occurred among non-fatal emboli: despite increased incidence, population mortality from pulmonary embolism has not shown a parallel increase. Moreover, an epidemic (or true increase in disease incidence) is unlikely without a corresponding increase in risk factors. Risk of pulmonary embolism may actually be decreasing: in the past several years, quality improvement efforts have focused on increasing prophylaxis against venous thromboembolism in hospitalized patients. Despite the fact that most surgical patients now receive prophylaxis, pulmonary embolism incidence nonetheless has risen substantially in the surgical population. Incidence has risen even more dramatically among obstetric patients, nearly tripling in the eight years after CTPA was introduced. Although the major underlying risk factor (pregnancy) has remained constant, use of CT in the obstetric population has risen by 25% per year.24
The widespread adoption of CTPA points to an alternative explanation. Rather than an epidemic of disease, we think the increased incidence of pulmonary embolism reflects an epidemic of diagnostic testing that has created overdiagnosis. In this scenario, much of the increased incidence in pulmonary embolism consists of cases that are clinically unimportant, cases that would not have been fatal even if left undiagnosed and untreated.
Overdiagnosis explains the increased incidence, decreased case-fatality, and minimal change in mortality we observed (Figure 1). If the extra emboli diagnosed were clinically important and benefited from treatment, mortality (i.e., number of fatal pulmonary emboli / population at risk) would show a parallel decrease. This is exactly what happened in the 20 years prior to the introduction of CTPA: with improved prevention and treatment, pulmonary embolism mortality in the US fell 50%, decreasing by 970 deaths per 100,000.20 By contrast, in the eight years since CTPA was introduced, despite the large increase in new cases, mortality decreased by only another 0.4 deaths per 100,000. Mortality changed little, because many of the extra emboli may not have needed treatment at all.
The concomitant improvement in case-fatality is also explained by overdiagnosis. Case-fatality (i.e., number of deaths / people diagnosed) decreases because the denominator has been inflated with clinically insignificant cases that are only identifiable by highly sensitive tests (corresponding mortality statistic is not distorted since the denominator includes all people at risk, not just those diagnosed). A recent time trend analysis of Pennsylvania residents hospitalized with pulmonary embolism confirms that patients admitted in recent years have a lower disease severity than patients admitted in the past.13
The discussion of overdiagnosis has been largely restricted to the cancer screening literature.11, 12 But the concept is relevant whenever there is a large reservoir of undiagnosed cases and a new, sensitive test to detect them. In the case of pulmonary embolism, both conditions exist. First, there appears to be a large reservoir of unsuspected emboli. Signs of recent or prior pulmonary embolism can be identified in more than half of autopsies if the pulmonary arteries are meticulously examined.9 Moreover, among consecutive patients undergoing contrast chest CT for unrelated reasons (e.g., cancer staging), unsuspected emboli are found in 4% overall,25 in 17% of patients over age 80,26 and in 24% of asymptomatic trauma patients.27 Second, evidence of overdiagnosis of pulmonary embolism initially arose in the randomized trial comparing CTPA to ventilation-perfusion scan: while the CTPA arm detected more patients with pulmonary embolism, there was no apparent improvement in outcomes.8 A recent meta-analysis confirms that many of the additional emboli identified by multidetector row CTPA are subsegmental emboli that do not lead to adverse outcomes even if left untreated.28 In this paper we demonstrate that it was not until after the introduction and rapid adoption of a highly sensitive test (CTPA) that the dramatic rise in pulmonary embolism incidence occurred.
Like any study relying on administrative databases, our study has limitations. While some factors inherent to administrative data may overestimate incidence, others cause an underestimate. Trends may be confounded by “upcoding,” an artifact whereby discharge records in later years contain more thorough ICD-9 coding in an effort to maximize reimbursement.29 While such upcoding could lead to an overestimate of the increase in incidence over time, it is unlikely to explain the magnitude of change we noted (i.e., near doubling in incidence). Because the NIS does not have identifiers to track individuals after hospital discharge, patients who are re-admitted may be erroneously counted as two unique individuals with pulmonary embolism. There are reasons, however, to suspect that incidence is actually underestimated. Pulmonary embolism is considered to be one of the most common missed diagnoses; thus, our estimate of pulmonary embolism incidence is likely to be falsely low. Furthermore, the NIS did not allow us to capture emboli diagnosed and treated solely as an outpatient. However, over 90% of pulmonary embolism patients seen in US emergency departments in 2006 were admitted to the hospital.30 This proportion was likely even higher early in the study period, when outpatient management of pulmonary embolism with low molecular weight heparin was unusual.31 Hence, the overall rise in pulmonary embolism incidence may be even greater than what we captured among inpatients.
A second limitation is that death certificates undercount pulmonary embolism mortality. Since there is no national autopsy database, the Multiple Cause-of-Death database contains the most comprehensive data available on deaths in the US population. Studies have found that death certificates have a sensitivity less than 40% compared to autopsy results for identifying deaths related to pulmonary embolism.32 To minimize this problem, we counted any diagnosis of pulmonary embolism listed on the death certificate (whether listed as the immediate or a contributing cause of death) as a pulmonary embolism death – a standard strategy in this area of research.20 Although death certificate data may underestimate pulmonary embolism mortality, it can accurately estimate time trends and is routinely used for this purpose.20, 33, 34
While our findings suggest there may be substantial overdiagnosis of pulmonary embolism, we cannot conclude that overdiagnosis explains the entire increase. Some of these “emboli” may represent false positive results; the PIOPED II trial found the positive predictive value of CTPA to be <60% in cases of low clinical suspicion for pulmonary embolism.35 Increasing indiscriminate use of both CTPA itself and d-dimer testing prompting follow-up CTPA4, 36 may have led over time to more false positive CTPA results in patients with low clinical pre-test probability of pulmonary embolism. Patients treated for a false positive pulmonary embolism – just like those treated for a clinically unimportant one – can only be harmed. Among true positive emboli detected by CTPA, there may be both clinically relevant and irrelevant emboli. The small decrease in population mortality may indicate there has been some increase in the detection and successful treatment of clinically meaningful embolism. In the “best case scenario” (i.e., assuming decreased deaths are from increased detection alone), there appear to be 128 extra patients diagnosed with a pulmonary embolism for every death avoided. CTPA, however, may have nothing to do with better outcomes – efforts at improved prevention and treatment of pulmonary embolism may be the explanation. Even if the increased diagnosis and treatment of the “new” pulmonary emboli detected by CTPA did not reduce death, it might reduce morbidity (e.g., hemodynamic compromise at presentation or subsequent complications like recurrent embolism or chronic thromboembolic pulmonary hypertension). However, we believe that the increased incidence following introduction of CTPA is unlikely to be attributable to increased detection of massive pulmonary emboli with hemodynamic compromise; these massive emboli could easily be detected in the pre-CTPA era by less sensitive tests like ventilation-perfusion scanning. While it is possible that recognition and treatment of some pulmonary emboli may have prevented subsequent non-fatal complications (e.g., pulmonary hypertension), which we could not detect using our databases, many patients will have received unnecessary treatment without any obvious benefit.
Overdiagnosis of these extra patients matters because treatment of pulmonary embolism can cause real harm. Anticoagulation, the current standard of care for all pulmonary emboli, is not benign. Even in the short-term context of the hospital stay we found significant increases in presumed complications of anticoagulation for pulmonary embolism. The true danger of anticoagulation, however, lies in its longer term use: 12% of patients anticoagulated for 3–6 months experience clinically significant bleeding.31 A recent study suggested that patients with subsegmental emboli detected by multidetector row CTPA are far more likely to experience complications of anticoagulation than adverse outcomes from the embolism itself.37 While newer treatments for pulmonary embolism, such as dabigatran, may be as effective but somewhat safer than warfarin,38 these agents are not yet standard of care. In addition to the harms of anticoagulation, IVC filters, which are increasingly used in the management of pulmonary embolism,39 can cause substantial morbidity, both during insertion (e.g., bleeding) and while in place (e.g., clotting of filter, fracture and migration of filter, increased incidence of subsequent deep vein thrombosis).40, 41
As use of CT scans continues to rise,5 the problem of overdiagnosis and overtreatment of pulmonary embolism will likely continue to grow. Since the harms of treatment can be substantial, including in the worst case death, it is imperative that we do not turn the problem of underdiagnosis into one of overdiagnosis. It is time to strengthen the evidence base: a trial randomizing stable patients with small emboli to observation versus anticoagulation would help determine whether all patients with pulmonary embolism require treatment.
Supplementary Material
Acknowledgments
Funding/Support: Dr. Wiener is supported by a career development award through the National Cancer Institute (K07 CA138772) and the Department of Veterans Affairs. Drs. Schwartz and Woloshin are supported by the Robert Wood Johnson Foundation and the Department of Veterans Affairs.
We would like to recognize the voluntary contributions of Dan Gottlieb, MS of the Dartmouth Institute for Health Policy & Clinical Practice for graciously sharing data on use of chest CT angiography among Medicare patients and Daniel Witt, PharmD of Kaiser Permanente Colorado for supplying the list of ICD9 codes used to identify patients with anticoagulation-related gastrointestinal hemorrhage. We would also like to thank Dan Berlowitz, MD, MPH and Adam Rose, MD, MSc of the Center for Health Quality, Outcomes, & Economic Research; William C. Black, MD of Dartmouth Medical School; and Michael Gould, MD, MS of the University of Southern California Keck School of Medicine, whose voluntary feedback on early drafts of our manuscript enhanced both our thinking and the presentation of our results.
Footnotes
Financial Disclosure: The authors do not have any conflict of interests to disclose.
Author Contributions: Dr. Wiener had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wiener, Schwartz, Woloshin. Acquisition of data: Wiener. Analysis and interpretation of data: Wiener, Schwartz, Woloshin. Drafting of the manuscript: Wiener, Schwartz, Woloshin. Critical revision of the manuscript for important intellectual content: Wiener, Schwartz, Woloshin. Statistical analysis: Wiener. Obtained funding: Wiener, Schwartz, Woloshin. Study supervision: Schwartz, Woloshin. Review of ethics content: Wiener, Schwartz, Woloshin.
Disclaimer: The views expressed herein do not necessarily represent the views of the funding agencies, the Department of Veterans Affairs, or the United States government.
References
- 1.Wittram C, Meehan MJ, Halpern EF, Shepard JA, McLoud TC, Thrall JH. Trends in thoracic radiology over a decade at a large academic medical center. J Thorac Imaging. 2004;19(3):164–170. doi: 10.1097/01.rti.0000117623.02841.e6. [DOI] [PubMed] [Google Scholar]
- 2.Donohoo JH, Mayo-Smith WW, Pezzullo JA, Egglin TK. Utilization patterns and diagnostic yield of 3421 consecutive multidetector row computed tomography pulmonary angiograms in a busy emergency department. J Comput Assist Tomogr. 2008;32(3):421–425. doi: 10.1097/RCT.0b013e31812e6af3. [DOI] [PubMed] [Google Scholar]
- 3.Auer RC, Schulman AR, Tuorto S, et al. Use of helical CT is associated with an increased incidence of postoperative pulmonary emboli in cancer patients with no change in the number of fatal pulmonary emboli. J Am Coll Surg. 2009;208(5):871–8. doi: 10.1016/j.jamcollsurg.2008.12.030. discussion 878–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir ID, Drescher F, Cousin D, et al. Trends in use and yield of chest computed tomography with angiography for diagnosis of pulmonary embolism in a Connecticut hospital emergency department. Conn Med. 2010;74(1):5–9. [PubMed] [Google Scholar]
- 5.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003;58(6):470–483. doi: 10.1136/thorax.58.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss CR, Scatarige JC, Diette GB, Haponik EF, Merriman B, Fishman EK. CT pulmonary angiography is the first-line imaging test for acute pulmonary embolism: A survey of US clinicians. Acad Radiol. 2006;13(4):434–446. doi: 10.1016/j.acra.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: A randomized controlled trial. JAMA. 2007;298(23):2743–2753. doi: 10.1001/jama.298.23.2743. [DOI] [PubMed] [Google Scholar]
- 9.Goodman LR. Small pulmonary emboli: What do we know? Radiology. 2005;234(3):654–658. doi: 10.1148/radiol.2343041326. [DOI] [PubMed] [Google Scholar]
- 10.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 11.Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–26. W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 13.DeMonaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med. 2008;121(7):611–617. doi: 10.1016/j.amjmed.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burge AJ, Freeman KD, Klapper PJ, Haramati LB. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol. 2008;63(4):381–386. doi: 10.1016/j.crad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Park B, Messina L, Dargon P, Huang W, Ciocca R, Anderson FA. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: Findings from the Nationwide Inpatient Sample. Chest. 2009;136(4):983–990. doi: 10.1378/chest.08-2258. [DOI] [PubMed] [Google Scholar]
- 16.HCUP Nationwide Inpatient Sample (NIS) Healthcare cost and utilization project (HCUP), 1993–2006. Rockville, MD: Agency for Healthcare Research and Quality; Available from: www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Multiple cause-of-death files, 1993–2006. Hyattsville, Maryland: National Center for Health Statistics; [Google Scholar]
- 18.White RH, Sadeghi B, Tancredi DJ, et al. How valid is the ICD-9-CM based AHRQ patient safety indicator for postoperative venous thromboembolism? Med Care. 2009;47(12):1237–1243. doi: 10.1097/MLR.0b013e3181b58940. [DOI] [PubMed] [Google Scholar]
- 19.White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525–1531. doi: 10.1001/archinte.158.14.1525. [DOI] [PubMed] [Google Scholar]
- 20.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: An analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711–1717. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 21.Attems J, Arbes S, Bohm G, Bohmer F, Lintner F. The clinical diagnostic accuracy rate regarding the immediate cause of death in a hospitalized geriatric population; an autopsy study of 1594 patients. Wien Med Wochenschr. 2004;154(7–8):159–162. doi: 10.1007/s10354-004-0057-0. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Physicians’ handbook for medical certification of death, 2003 revision. Hyattsville, Maryland: National Center for Health Statistics; Available from: http://www.cdc.gov/nchs/data/misc/hb_cod.pdf. [Google Scholar]
- 23.Witt DM, Delate T, Clark NP, et al. Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood. 2009;114(5):952–956. doi: 10.1182/blood-2009-02-207928. [DOI] [PubMed] [Google Scholar]
- 24.Chen MM, Coakley FV, Kaimal A, Laros RK., Jr Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol. 2008;112(2 Pt 1):333–340. doi: 10.1097/AOG.0b013e318180a505. [DOI] [PubMed] [Google Scholar]
- 25.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184(1):264–267. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax. 2007;62(6):536–540. doi: 10.1136/thx.2006.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56(4):727–31. doi: 10.1097/01.ta.0000119687.23542.ec. discussion 731–3. [DOI] [PubMed] [Google Scholar]
- 28.Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: Incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010 doi: 10.1111/j.1538-7836.2010.03938.x. [DOI] [PubMed] [Google Scholar]
- 29.Assaf AR, Lapane KL, McKenney JL, Carleton RA. Possible influence of the prospective payment system on the assignment of discharge diagnoses for coronary heart disease. N Engl J Med. 1993;329(13):931–935. doi: 10.1056/NEJM199309233291307. [DOI] [PubMed] [Google Scholar]
- 30.HCUPnet. Healthcare cost and utilization project. Rockville, MD: Agency for Healthcare Research and Quality; Available from: http://hcupnet.ahrq.gov/ [PubMed] [Google Scholar]
- 31.Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis. 2009;28(4):401–409. doi: 10.1007/s11239-009-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dismuke SE, VanderZwaag R. Accuracy and epidemiological implications of the death certificate diagnosis of pulmonary embolism. J Chronic Dis. 1984;37(1):67–73. doi: 10.1016/0021-9681(84)90127-9. [DOI] [PubMed] [Google Scholar]
- 33.Lilienfeld DE, Chan E, Ehland J, Godbold JH, Landrigan PJ, Marsh G. Mortality from pulmonary embolism in the United States: 1962 to 1984. Chest. 1990;98(5):1067–1072. doi: 10.1378/chest.98.5.1067. [DOI] [PubMed] [Google Scholar]
- 34.Stein PD, Kayali F, Beemath A, et al. Mortality from acute pulmonary embolism according to season. Chest. 2005;128(5):3156–3158. doi: 10.1378/chest.128.5.3156. [DOI] [PubMed] [Google Scholar]
- 35.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 36.Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med. 2009;169(21):1961–1965. doi: 10.1001/archinternmed.2009.360. [DOI] [PubMed] [Google Scholar]
- 37.Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010 doi: 10.1016/j.thromres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 39.Moore PS, Andrews JS, Craven TE, et al. Trends in vena caval interruption. J Vasc Surg. 2010;52(1):118–125.e3. doi: 10.1016/j.jvs.2009.09.067. discussion 125–6. [DOI] [PubMed] [Google Scholar]
- 40.Young T, Tang H, Hughes R. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst Rev. 2010;2:CD006212. doi: 10.1002/14651858.CD006212.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson W, Nicholson WJ, Tolerico P, et al. Prevalence of fracture and fragment embolization of bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010 doi: 10.1001/archinternmed.2010.316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.