Abstract
We recently reported long-term organ allograft survival without ongoing immunosuppression in 4 of 5 patients receiving combined kidney and bone marrow transplantation from haploidentical donors following non-myeloablative conditioning. In vitro assays up to 18 months revealed donor-specific unresponsiveness. We now demonstrate that T cell recovery is gradual and is characterized by memory-type cell predominance and an increased proportion of CD4+CD25+CD127−FOXP3+ Treg during the lymphopenic period. Complete donor-specific unresponsiveness in proliferative and cytotoxic assays, and in limiting dilution analyses of IL-2-producing and cytotoxic cells, developed and persisted for the 3-year follow-up in all patients, and extended to donor renal tubular epithelial cells. Assays in 2 of 4 patients were consistent with a role for a suppressive tolerance mechanism at 6 months to 1 year, but later (≥18 months) studies on all 4 patients provided no evidence for a suppressive mechanism. Our studies demonstrate, for the first time, long-term, systemic donor-specific unresponsiveness in patients with HLA-mismatched allograft tolerance. While regulatory cells may play an early role, long-term tolerance appears to be maintained by a deletion or anergy mechanism.
Keywords: mixed chimerism, tolerance, bone marrow transplantation, kidney transplantation
Introduction
Chronic rejection and other complications from long-term immunosuppressive therapies could be overcome by the induction of a state of donor-specific tolerance. Mixed chimerism has shown promise in animal models as an approach to achieving transplantation tolerance (1–4). We have recently reported the successful achievement of long-term acceptance of kidney allografts without maintenance immunosuppression using non-myeloablative conditioning to achieve durable or transient chimerism in two series of patients. The first series of 6 patients, affected by multiple myeloma, received combined kidney and BMT (CKBMT) from HLA-identical related donors (5). Subsequently, renal allograft acceptance induced by mixed chimerism was reported from another center in a single patient without malignancy receiving CKBMT from an HLA-identical related donor (6). Our second series involved 5 patients with end stage renal failure without malignant disease, who received bone marrow and kidney transplantation from haploidentical related donors following non-myeloablative conditioning (Supplementary Table S1). Immunosuppression was discontinued approximately 8–14 months post-transplant in all except Patient 3, who had panel-reactive antibodies pre-transplant and lost the graft at Day 10 to acute humoral rejection. Despite loss of measurable chimerism in all blood lineages by Day 21 post-transplant, long-term renal allograft acceptance (now at 4–8 years) without immunosuppression was achieved in 4 of 5 patients, though low levels of anti-class II donor-specific antibody (DSA) persist in Patient 4. Renal allograft tolerance was associated with donor-specific unresponsiveness in cell-mediated lympholysis (CML) and mixed lymphocyte responses (MLR) assays performed up to 18 months in all four patients (7), suggesting that a state of tolerance was achieved throughout the immune system (systemic tolerance). To gain insight into the mechanisms underlying donor-specific tolerance in these patients, we characterized the phenotype of recovering lymphocytes, followed functional responses to donors and assessed the possible role of regulatory cells.
Materials and Methods
Patients and treatment
Five patients with end stage renal disease were enrolled in this IRB-approved (Massachusetts General Hospital) Immune Tolerance Network (ITN)-sponsored (protocol# ITN036ST, IRB approval# 2008-P002007). As previously described (7), conditioning included cyclophosphamide, MEDI-507 (Medimmune), and, in Patients 4 and 5, pre-transplant rituximab, and thymic irradiation followed by kidney and BM transplantation from the same HLA-haploidentical donor. Postransplant immunosuppression included cyclosporine or tacrolimus(7) and was discontinued within 8 to 14 months (Supplementary Table S1) in all except Patient 3, as noted above.
Flow cytometry
Fresh blood cells were stained with fluorochrome-labelled mAbs against CD3, CD4, CD8, CD19, CD45RO, CD45RA, CD62L and CD25 (Beckton Dickinoson [BD], San Jose, CA). For Treg analysis, cryopreserved patient PBMC were thawed and stained with fluorochrome-conjugated mAbs against CD4, CD25, CD127, HLADR, CD45RO and CD45RA (BD), subsequently permeabilized, fixed and then stained with FOXP3 mAb (eBioscience, San Diego, CA). Polychromatic flow cytometric analysis was performed on a custom 7-laser high resolution LSRII (BD) as described (8) and further analyzed with FlowJo (Tree Star, Inc., Ashland, OR) software.
NK cell depletion
Fresh PBMC were depleted of NK cells and subsequently cryopreserved as previously described (5).
Renal tubular epithelial cell (RTEC) culture
RTECs obtained before transplant from the donor kidney for Patients 1, 2 and 5 were cultured as previously reported(5). Epithelial origin was confirmed by expression of cytokeratin.
Mixed lymphocyte response (MLR) and cell mediated lysis (CML) assays
PBMC were aliquoted and cryopreserved or underwent further separation of T cells, CD56− cells and CD25− cells before cryopreservation and use in MLR and CML assays as previously described (5;9;10). The number of responder cells was reduced by 50% when isolated T cells or CD25− T cells were used instead of whole PBMC so that approximately similar numbers of T cells were present in each culture. Briefly, MLR involved 5-day triplicate cocultures of 2×105 responder cells with equal numbers of 30 Gy-irradiated stimulator cells in AIM V medium supplemented with 10% AB serum, followed by pulsing with 3H thymidine. CML involved cocultures of similar cell combinations for 7 days in RPMI 1640 with 6% FCS and standard additives, with the addition of IL-15, 5U/ml (Immunex, Seattle, WA) on Day 5. Serially diluted responders were incubated with 4000 51Cr-labelled PHA blast targets and lysis was determined by supernatant gamma counting.
When RTECs were used as targets, CML were performed as previously described, after incubating RTECs with rhIFN-γ to upregulate HLA-class I (otherwise expressed at only low levels on RTEC) (5)
Stimulated suppression assay
These assays were performed in two sequential phases. In phase 1 (priming phase, Days 0–6), 4×106 PBMCs per well from tolerant patients were cultured with 4×106 irradiated donor or third party PBMC for 6 days at 37°C using 24-well flat-bottom plates (Costar, Cambrige, MA). Bulk cultures were harvested, washed and resuspended in fresh medium on Day 6 and allowed to rest overnight. In Phase 2 (coculture phase, Days 7–13), 1×105 fresh pre-transplant PBMC per well from tolerant patients were added in 96-well plates with 2×105 per well irradiated donor or third-party stimulators and 1×105 primed responders from Phase 1 bulk cultures. After 6 days’ culture at 37°C, cytotoxicity was tested as described (9)
Limiting dilution assays (LDA)
LDA to quantify cytotoxic T-lymphocyte precursor (CTLp) frequencies and IL-2-producing helper T cell (Th) frequencies were performed as described on replicates of 12–24 wells per dilution, using 51Cr-labelled lymphoblast targets and proliferation of the IL-2-dependent CTLL-2 cell line, respectively, as readouts for activity in each well (10). IL-15 was added to the CTLp cultures after removal of supernatants for IL-2 analysis on Day 2 and cultures were continued for 7 additional days before cytotoxicity was assayed. LDA analysis was performed as previously described (11); briefly, deviation from the single hit Poisson model (SHPM) was tested by a standard Wald test and only frequencies fitting the SHPM were reported.
Statistical analysis
Statistical analyses were performed using the non-parametric Mann-Whitney U Test. Analyses were performed on triplicate values for each point for comparisons of unfractionated vs separated cell populations in CML and MLR assays.
Results
Lymphocyte subset recovery
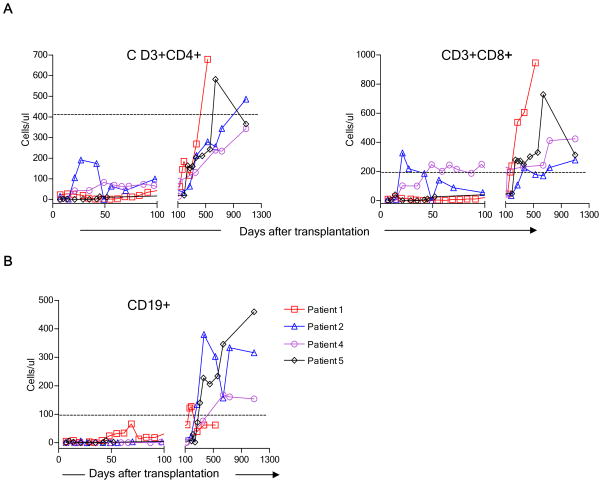
Flow cytometry was used to assess lymphocyte subset recovery. CD3+CD4+ counts recovered slowly, while CD3+CD8+ cells recovered more quickly, resulting in inversion of the normal CD4/CD8 ratio throughout the 3-year follow-up period. The peripheral blood CD19+ cell counts recovered more rapidly in the first 2 patients, who did not receive Rituximab, than in Patients 4 and 5, whose conditioning included Rituximab (Fig. 1). While “memory-type” CD45RO+ cells were most prevalent among CD4+ cells, recovery of “naïve-type” CD4+CD45RA+ cells, presumably arising from the recipient thymus, was variable, beginning to increase between about 150 and 350 days post-transplant, and eventually reaching the normal range in all evaluable patients (Fig. S1). Absolute numbers of naïve-type and memory-type cells are shown in Supplementary Figure 2. In one patient (Patient 5), CD45RA+CD4+ cells persistently expressed CD45RO, making naïve/memory phenotype uninterpretable. We have observed this apparently stable CD45RA/RO “double positive” phenotype in a small proportion of donors in many phenotypic studies performed over the years (M. Sykes et al, unpublished data). Among CD3+CD8+ T cells, the proportion of “naïve-type” CD45RA+RO−CD62L+ cells fluctuated in Patients 1 and 5, showed a steady increase over time in Patient 2, and remained low for the duration of follow-up in Patient 4 (Fig. S3).
Figure 1.
Time course of T and B cell recovery in four patients following non-myeloablative conditioning. (A) CD3+CD4+, CD3+CD8+ and (B) CD19+ recovery. Peripheral blood concentrations of each cell type in fresh blood samples are shown at the indicated time point. The horizontal dotted lines denote the 10th percentile for normal adults.
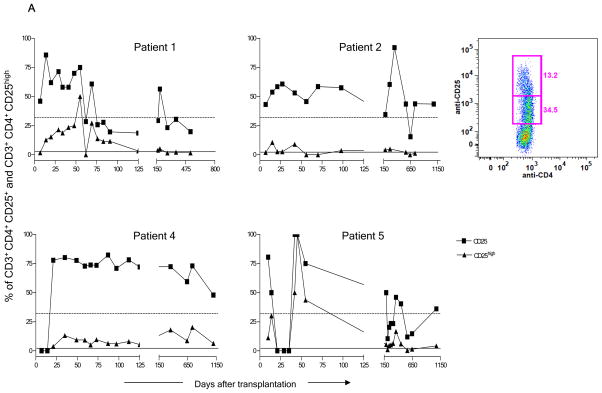
As shown in Figure 2, a very high proportion of initially recovering CD3+CD4+ T cells in all patients expressed CD25. The reduction in CD25+ cells in Patient 5 between Days 21 and 42 occurred in association with treatment with rabbit anti-thymocyte globulin (ATG), plasmapheresis and rituximab for a suspected acute humoral rejection episode. Figure 2A shows that the percentage of CD25high cells was also elevated compared to normal to varying degrees in all 4 patients during the first 150 days post-transplant and remained elevated for the duration of follow-up in Patient 4. In Patient 5 at POD 45, all gated CD3+CD4+ cells were CD25high. The most markedly increased percentages of CD25high cells were seen in the context of the most marked CD4 T cell lymphopenia (Figure 1) and probably do not represent increased absolute concentrations over pre-transplant values, which were not available. Absolute counts of CD25+ CD4 T cells are shown in Supplementary Figure S4.
Figure 2.
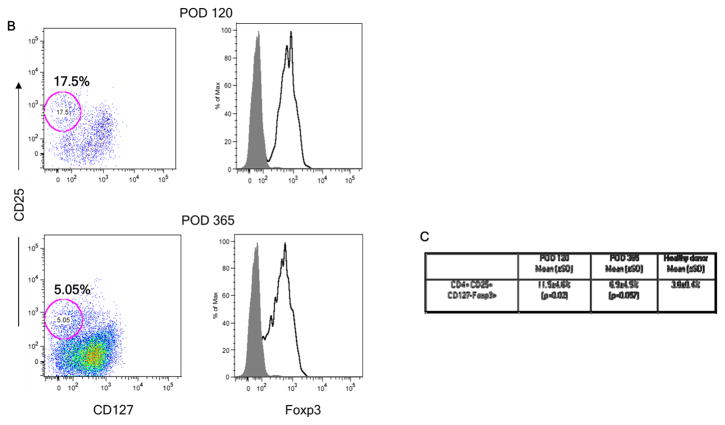
Enrichment of CD4+CD25+ cells after transplant. (A) Recovery of CD25+ and CD25high cells in fresh blood samples in each of the four patients among total gated CD3+CD4+ T cells. Dotted and solid lines, respectively, denote the median value of CD4+CD25+ and of CD4+CD25high cells found in normal controls. The inset panel shows CD25low and CD25high populations in a representative sample from Patient 5. (B) Expression of FoxP3 (right panel), open histograms. Shaded histograms represent unstained controls on gated cryopreserved/thawed CD4+CD25+CD127− cells (left panel), at POD 120 and 365. Plots from Patient 5 are representative of results from all 4 patients. (C) Percentages of CD25+CD127−FOXP3+ cells among gated cryopreserved/thawed CD4+ cells in patients’ peripheral blood at POD 120 and 1 year compared to healthy donors (HD) (n=4 patients at POD 120 and n=3 at 1 year and 5 normal controls).
To determine whether the above changes indicated enrichment of Treg cells, we characterized samples that had been cryopreserved at approximately POD 120 and 365 for expression of CD127, FOXP3, CD45RO, CD45RA, and HLA-DR. At POD +120, the patients showed significantly increased frequencies of CD25+CD127−FOXP3+ regulatory T cells (Treg) within the CD4 population compared to healthy subjects (Fig. 2B–C). By 1 yr post-transplant, when only 3 patient samples were evaluable, the increased frequencies of Treg in patient CD4 cells narrowly missed achieving statistical significance compared to normal controls (P=0.057) (Fig 2C). Expression of CD45RO, CD45RA and HLA-DR on CD25+CD127−CD4+ T cells was variable (not shown) but not significantly different from that in Tregs in healthy controls. A significantly increased proportion of CD4+CD25−CD127− cells compared to normal was also detected in the patients at POD 120 (p<0.05) (data not shown).
Role of suppression in donor-specific CML and MLR unresponsiveness
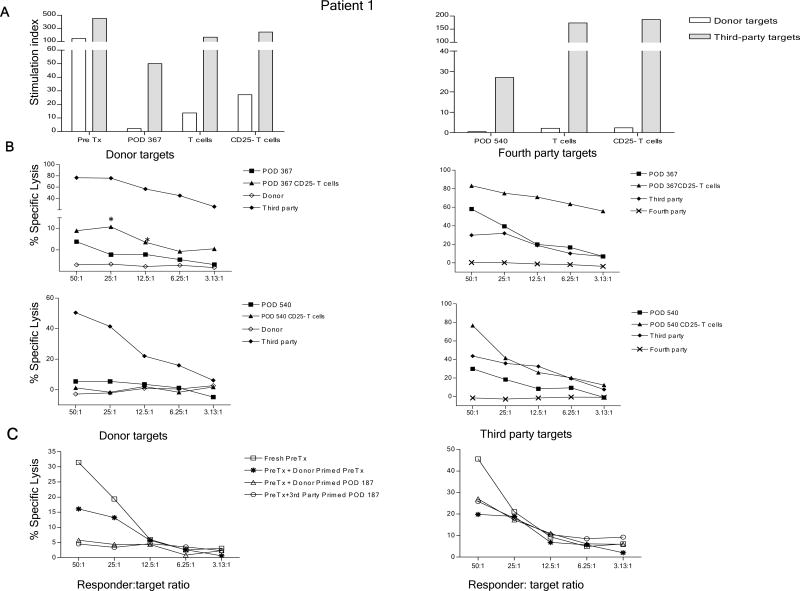
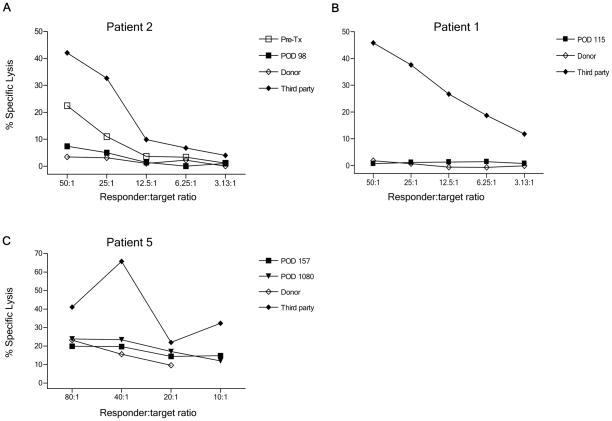
As previously published, in vitro assays for CD8 and CD4 T cell-mediated alloreactivity (CML/MLR) demonstrated donor-specific unresponsiveness by 3 months after transplant in Patients 2, 4, and 5, and by 9 months in Patient 1, which persisted up to 9–18 months post-transplant, with recovery of third party responses (7). Further follow-up (to 3 years post-transplant) revealed the donor-specific unresponsiveness to be durable in all 4 patients (Fig. S5). To assess a possible role for regulatory cells in this unresponsiveness, whole PBMC, isolated T cells and CD25-depleted T cells were compared in MLR and CML assays. As shown in Figure 3A, depletion of CD25+ T cells revealed an anti-donor response in Patient 1 at POD 367 in both MLR and CML assays (Fig. 3A–B). In contrast, anti-donor responses did not become apparent among CD25− T cells at POD 540 (Fig. 3A–B). Nevertheless depletion of CD25+ cells enhanced the anti-third party CML and MLR response at both time points (Fig. 3A–B). Similarly, CD25+ depletion failed to reveal an anti-donor response at POD 1080 in this patient (data not shown).
Figure 3.
Role of CD25+ T cells in donor-specific unresponsiveness in Patient 1. (A) Pre-transplant and post-transplant (POD 367 and POD 540) whole PBMC, T cell and CD25− fractions were tested against irradiated donor and third party targets in MLR; (B) Whole PBMC and CD25− fraction from POD 367 and POD 540 were tested at various ratios against irradiated donor and fourth party stimulators and targets (unrelated to third party or patient) in CML assay. CD25+ cell depletion reveals an anti-donor CML response (*p<.05) in Patient 1 at POD 367 but not at POD 540 (Panel B, left). Normal third-party control killing of donor targets is seen in the same assay; (C) Suppression of anti-donor CTL responses by Patient 1 cells at POD 187 in co-culture assay. Pre-transplant or POD 187 PBMC were stimulated for 5 days with donor or third party PBMC stimulators, then tested for the ability to suppress anti-donor or 3rd party CML responses.
An additional assay, which we term a “stimulated suppression assay”, described elsewhere (12), was performed on Patient 1. Following restimulation in vitro with donor antigens, this patient’s POD 187 post-transplant PBMC completely suppressed the anti-donor response (Fig. 3C, left panel) of pre-transplant cells, but did not markedly suppress the anti-third party response (Fig. 3C, right panel). Pre-transplant patient cells did not have donor-specific suppressive activity (Fig. 3C), suggesting that donor-specific regulatory cells may have been specifically activated or expanded following the transplant. Priming of POD 187 post-transplant PBMC with third party stimulators also led to specific suppression of the pre-transplant anti-donor response, with no significant suppression (compared to pre-transplant cells) of the anti-third party response (Fig. 3C).
In Patient 4, donor-primed POD 185 PBMC suppressed the pre-transplant anti-donor response completely. In contrast, neither the donor-primed pre-transplant sample nor the third party-primed POD 185 patient PBMC suppressed this response (Fig. S6). A similar assay on Patient 5 at POD 240 did not provide evidence for suppression.
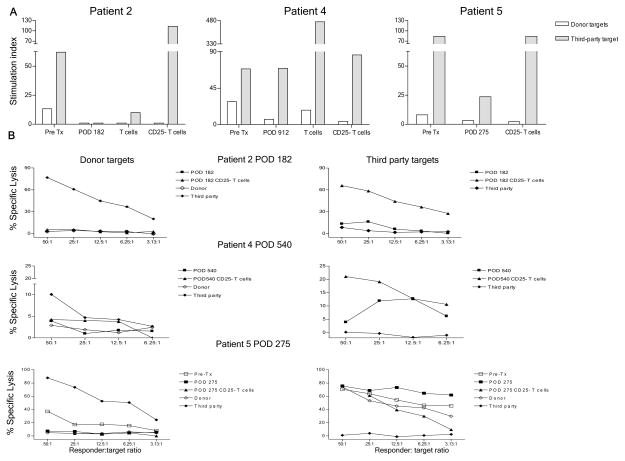
Depletion of CD25+ cells did not reveal an anti-donor alloresponse in Patient 2 or 5 at similar early time points (POD 182 and 275, respectively), or in Patient 4 at POD 540, despite enhancement of anti-third party responses (Fig. 4A–B). CD25+ cell depletion also failed to reveal an anti-donor response at POD 1080 in MLR and/or CML in these patients (data not shown).
Figure 4.
Donor-specific MLR and CML unresponsiveness in Patients 2, 4, and 5 is not dependent on CD25+ T cells. (A) Pre-transplant and POD 182 whole PBMC, T cells and CD25− fractions in Patient 2, pre-transplant and POD 912 whole PBMC, T cells and CD25− fractions in Patient 4, pre-transplant and POD 275 whole PBMC and CD25− fractions in Patient 5 were tested against irradiated donor and third party stimulators in MLR; (B) POD 182 whole PBMC and CD25− fraction in Patient 2, POD 540 whole PBMC and CD25− fraction in Patient 4, pre-transplant and POD 275 whole PBMC and CD25− fraction in Patient 5 were stimulated for 5 days with irradiated PBMC, then tested at different ratios for cytotoxicity against donor and third party targets in CML assays.
Absence of killing of donor RTECs by recipient PBMC
In Patients 1, 2 and 5, RTECs from the donor kidney were cultured and tested as targets following stimulation with NK cell-depleted donor PBMC. In Patient 2, loss of killing activity against donor RTECs was observed post-transplant compared to pre-transplant levels (Fig. 5A). In Patient 1 and 5, for whom a pre-transplant sample was not available, we also observed an absence of killing of donor RTECs post-transplant (Fig. 5B–C).
Figure 5.
Lack of killing of donor RTECs by patient NK cell-depleted PBMC in a Cr51 realease cytotoxicity assay. (A) Patient 2, third party and donor responses to donor RTEC targets; (B) Patient 1, third party and donor responses to donor RTECs. (C) Patient 5, third party and donor responses to donor RTECs.
LDA
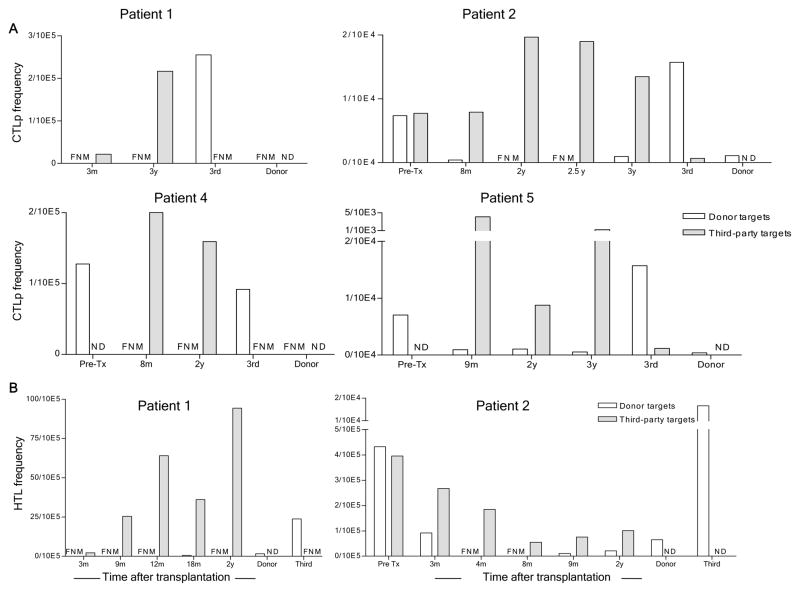
To quantify donor-reactive T cells and probe further for evidence of suppression, LDA were performed. As shown in Figure 6A, anti-donor CTLp were undetectable at 3 months and 3 years post-transplant in Patient 1. Consistent with bulk CML assays, the anti-third party CTLp frequency was low at 3 months but had recovered at 3 years. Patients 2, 4 and 5 similarly showed specifically reduced anti-donor CTLp frequencies (often below the limit of detection) compared to pre-transplant levels, with recovery of anti-third party CTLp (Fig. 6A). All of these assays demonstrated single-hit kinetics, with no evidence for a suppressive pattern.
Figure 6.
CTL precursor and IL-2-producing cell frequencies as detected by limiting dilution assays against donor and third party PBMC stimulators and targets at various time points after transplant. (A) Summary of CTL precursor frequencies as calculated with the Poisson distribution. Responders and time points are indicated on the x axes. Stimulators and targets were donor and third party PBMC; (B) Frequencies of anti-donor IL-2-producing cells (helper T lymphocyte, HTL) in Patients 1 and 2 at the indicated time points, as calculated by Poisson distribution from IL-2 production measured in supernatants from limiting dilution cultures of patient and control PBMC stimulated with donor or 3rd party PBMC. FNM (Frequency not measurable), ND (Not Done)
Patient 1 showed low frequencies of IL-2-producing cells (T helper cells, Th) recognizing third party at 3 months but recovered responses at 9 months, 1 year, 18 months and 2 years. Anti-donor responses were undetectable at all of these time points (Fig. 6B). Patient 2 showed a markedly reduced anti-donor Th frequency with preserved 3rd party response at 3 months and undetectable anti-donor responses at 4, 8, 9 months and 2 years with weak but measurable anti-third party responses. No evaluable data are available for Patient 4. Patient 5 revealed marked reductions in donor-reactive IL-2-producing cells at 6 and 9 months post-transplant (Fig. 6B). The fit with the single hit Poisson model is consistent with deletion or anergy and not suggestive of suppression as a mechanism of tolerance (13).
We analyzed Patient 5 samples in LDA with and without Treg depletion. As shown in Figure 7(A–B), Treg removal did not reveal an increased frequency of donor-reactive Th or CTLp at relatively early time points (6 and 9 months), when donor-reactive T cells had not yet undergone complete deletion.
Figure 7.
Donor-specific unresponsiveness in Patient 5 at 6 and 9 months is not dependent on CD25+ T cells. (A) Frequencies of anti-donor and third party CTLp of indicated cell populations as calculated from Poisson distribution analysis of the limiting dilution cultures; (B) Frequencies of anti-donor and third party IL-2-producing cell (helper T lymphocyte, HTL) of indicated cell populations as calculated from Poisson distribution analysis of the IL-2 content of supernatants from limiting dilution cultures. FMN (Frequency not measurable), ND (Not Done)
Discussion
We present here the first long-term demonstration of complete, systemic donor-specific unresponsiveness in human organ allograft recipients. These data were obtained in a unique series of patients exhibiting allograft tolerance. Our mechanistic data suggest that long-term tolerance in CKBMT recipients is due to deletion or anergy of donor-reactive T cells. While Tregs were enriched in initially-recovering CD4 cell populations of all patients, evidence for active suppression of anti-donor reactivity was obtained in only 2 of 4 patients at 6–12 months and in 0 of 4 patients after 1 year.
Following transplantation, T cell lymphopenia was prolonged, but none of our patients developed serious opportunistic infections. Most CD4 cells had a previously activated/memory phenotype, but CD45RA+RO− “naïve-type” CD4 cells eventually recovered. The predominant “memory” T cell phenotype is consistent with our previous results in patients receiving a similar non-myeloablative BMT regimen without kidney transplantation for the treatment of hematologic malignancies (10) and reported in other protocols involving T cell-depleting reagents, both with (14;15) and without (16–18) conditioning for hematopoietic cell transplantation (HCT). However, patients with malignancies had received multiple prior chemotherapies and radiotherapies and often experienced infectious complications following transplant (19). Recovery of CD45RA+CD4+ cells in the current patient series, which did not occur in the patients with hematologic malignancies (10), most likely reflects more robust thymic recovery (15;20–23).
Early predominance of “memory” T cells may reflect resistance to mAb-induced depletion of memory cells (24) or expansion from a small residual pool of peripheral T cells escaping depletion with conditioning therapy. While T cells undergoing lymphopenia-driven expansion have been reported to have stronger rejection capacity (25) and to resist tolerance induction in mice (26), our patients have achieved tolerance of an HLA-mismatched organ allograft despite presumed lymphopenia-driven expansion.
We considered a possible role in tolerance induction for the enriched Treg population, which has been previously noted in patients receiving HCT alone following a similar non-myeloablative regimen (10), and was not observed in patients receiving alemtuzemab (16). Rabbit ATG causes Treg expansion and/or conversion of conventional T cells to Tregs (27–29) and spares Tregs in vivo in mice (30;31).
The proportions of CD25+ cells were markedly greater than the proportion of CD25high CD4+ cells in most of our patients. Unfortunately our phenotypic studies on fresh PBMC did not include stains for FoxP3 and CD127, so the latter studies were performed after cryopreservation, which reduces the level of surface CD25 expression (unpublished data), preventing distinction of CD25 high and low populations. Ongoing studies on fresh PBMC of a new patient series demonstrate higher proportions of Tregs early post-transplant than those reported here (T. Morokata et al, unpublished data). It is also possible that the high proportions of CD25low CD4 T cells in the initially reconstituting T cell populations reflect T cell activation.
The current studies demonstrate persistent donor-specific unresponsiveness of both proliferating and cytotoxic T cells for at least 3 years in all patients (Fig. S5). Moreover, LDA studies demonstrate both an absence of IL-2-producing cells and of CTL recognizing donor antigens in long-term tolerant patients. The absence of a “sawtooth” pattern (13;32) in these analyses suggests a deletional or anergy, rather than a suppressive, mechanism of tolerance. The LDA pattern produced by anergic T cells would be indistinguishable from that of a deleted population. However, our CTLp LDA and CML assays include IL-15, which has been shown to overcome anergy of human cytotoxic T cells even more effectively than IL-2 (33;34).
While some, but not other (35) studies have demonstrated relative hyporesponsiveness to the donor in MLR(36–38) and LDA (39) in patients with stable allograft function on immunosuppressive therapy, ours is the first to achieve complete, specific and persistent unresponsiveness to donor antigens in humans. In one study of CKBMT without specific conditioning for BMT or withdrawal of immunosuppresion, half of the patients achieved donor-specific unresponsiveness in MLR and CML assays, but in most cases unresponsiveness was demonstrated at only one time point (40). In contrast to our results, a renal allograft recipient studied 12 years after successful immunosuppression withdrawal demonstrated a vigorous MLR response to the donor (41). The specific, complete and persistent absence of anti-donor reactivity in LDA, MLR and CML assays in our patients denotes a state of systemic tolerance.
One of our patients demonstrated suppression of persistent anti-donor responses by regulatory cells at 1 year post-transplant. This suppression was mediated largely by CD25+ cells, presumably Tregs. A non-T cell suppressive population may also have been present at this point, as purification of T cells revealed a weak anti-donor MLR (Fig. 3). At 6 months post-transplant, the patient’s cells specifically suppressed anti-donor responses in a “stimulated suppression assay”, whereas pre-transplant PBMC lacked such activity. The suppression was detected following exposure in vitro to donor or third party antigens. Patient 4 PBMC also suppressed the anti-donor response at 6 months, and in this case third party stimulation of these cells did not induce the suppressive activity. Collectively, these data suggest that suppression was specific for donor antigens and that the third party used to stimulate Patient 1 cells may have shared antigens with the donor, whereas that used to stimulate Patient 4 cells did not.
CD25 depletion assays performed in the first year did not reveal suppressed anti-donor responses in Patient 2 or 5. These patients developed CML unresponsiveness more rapidly than Patient 1 and the expansion, activation and/or survival of Tregs may require a persistent alloreactive population. Moreover, by 18 months, an anti-donor response was no longer apparent in Treg-depleted PBMC of Patient 1 and was also lacking in Patient 4, suggesting that deletion (or anergy) of donor-reactive cells was complete.. Collectively, evidence for suppressive mechanisms was obtained in 2 of 4 patients in the first year and in 0 of the 4 patients after 1 year. While the possible role of Tregs in this tolerance is thus unclear, preliminary data suggest that Tregs may be in the renal allografts in a new series of patients, consistent with elevated FoxP3 mRNA levels noted in protocol biopsies of patients on this trial (7). We have not addressed a possible role for michrochimerism, which has been associated with CD8 cell-mediated suppression of anti-donor reactivity in patients achieving spontaneous tolerance to HLA-identical kidneys (42).
The loss of chimerism in the present series of patients occurred within a few weeks of transplantation, when T cells were markedly depleted by the conditioning. Since we have observed early development of donor-specific IL-2-producing cell unresponsiveness in a patient in our newer series (43), this loss of chimerism may not reflect a T cell-mediated immune response but instead may reflect inadequate donor hematopoietic stem cell engraftment.
Patients receiving mismatched HCT alone for hematologic malignancies following a similar MEDI-507 conditioning regimen showed generally weak alloresponses but nevertheless had stronger anti-donor than anti-third party responses following loss of chimerism (10), in marked contrast to the specifically reduced anti-donor responses observed in the current CKBMT patients. In combination, our results are consistent with a role for the kidney in the tolerance achieved in CKBMT recipients, through mechanisms that remain to be determined. Revealing the mechanism by which CKBMT leads to tolerance of the kidney allograft is of extreme importance for developing more widely applicable clinical protocols.
Supplementary Material
Figure S1. Time course of naive and memory-type CD3+CD4+ cell recovery. Proportions of naive-type CD45RA+CD45RO− and memory-type CD45RA−CD45RO+ subsets among gated peripheral blood CD3+CD4+ cells are shown. Dotted lines denote the 25th to 75th percentiles for percentages of CD3+CD4+CD45RA+CD45RO− cells in a healthy adult population.
Figure S2. Time course of naïve and memory-type CD3+CD4+ cell recovery. Absolute numbers of naïve-type CD45RA+CD45RO+ and memory-type CD45RA−CD45RO+ subsets among gated CD3+CD4+ cells are shown.
Figure S3 Time course of naïve CD8+CD45RA+CD62L+ T cell recovery. Proportions of naive-type CD45RA+CD62L+ subsets among gated peripheral blood CD3+CD8+ cells are shown
Figure S4 Time course of CD4+CD25+ and CD4+CD25high cell recovery. Absolute numbers of CD4+CD25+ and CD4+CD25high CD3+ cells are shown.
Figure S5. MLR (left) and CML (right) responses at 3 year time point.
Figure S6 Suppression of pre-transplant anti-donor CTL responses by Patient 4 cells at POD 185 in co-culture assay.
Table S1 Patient profiles and outcomes
Acknowledgments
We thank Dr. Susan Saidman for selecting mAbs to be used in chimerism determination and Ms. Gena Coleman and Shavree Washington for secretarial assistance.
Funding: American Recovery and Reinvestment Act funding NIH R01AI084074, NIH ITN N01AI15416, Multiple Myeloma Research Foundation. G.A. was supported by the American Society of Transplantation and Umberto Veronesi Foundation.
Footnotes
Author contributions: G.A. wrote the manuscript, performed flow cytometry and in vitro assays, analyzed data and prepared specimens. M.C., J.S. and P.C. coordinated sample acquisition, prepared specimens, performed in vitro assays. S.A.L. and T.M. performed in vitro assays and assisted with data analysis. F.I.P. provided expert advice on and performed flow cytometry. K.K. supervised and coordinated research sample acquisition. T.B. aided in the analysis and interpretation of limiting dilution assays. A. B.C. T.K., B.R.D., N.T.R., T.S. and D.H.S. designed, supervised and carried out the clinical protocol and patient care. M.S. participated in the design of the clinical protocol, co-wrote the paper and participated in and oversaw design of all laboratory studies, data analysis and interpretation, and preparation of the manuscript.
Disclosure
The authors of this manuscript have no conflicts of interest as described by the American Journal of Transplantation
Reference List
- 1.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T, Cosimi AB, COLVIN RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomologous monkeys. Transplantation. 1995;59:256–62. [PubMed] [Google Scholar]
- 3.Sharabi Y, Sachs DH, Sykes M. T cell subsets resisting induction of mixed chimerism across various histocompatibility barriers. In: Gergely J, Benczur M, Falus A, Fust Gy, Medgyesi G, Petranyi Gy, et al., editors. Progress in Immunology VIII; Proceedings of the Eighth International Congress of Immunology; Budapest. 1992; Heidelberg: Springer-Verlag; 1992. pp. 801–5. [Google Scholar]
- 4.Ohdan H, Yang Y-G, Shimizu A, Swenson KG, Sykes M. Mixed bone marrow chimerism induced without lethal conditioning prevents T cell and anti-Gala1,3Gal-mediated graft rejection. J Clin Invest. 1999;104:281–90. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma Responses and Tolerance Following Combined Kidney and Nonmyeloablative Marrow Transplantation: In Vivo and In Vitro Analyses. Am J Transplant. 2006 Jun 22;6(9):2121–33. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 6.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008 Jan 24;358(4):362–8. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. New Engl J Med. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preffer F, Dombkowski D. Advances in complex multiparameter flow cytometry technology: Applications in stem cell research. Cytometry B Clin Cytom. 2009 Sep;76(5):295–314. doi: 10.1002/cyto.b.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus AB, Shaffer J, Toh HC, Preffer F, Dombkowski D, Saidman S, et al. Early host CD8 T-cell recovery and sensitized anti-donor IL-2-producing and cytolytic T-cell responses associated with marrow graft rejection following nonmyeloablative bone marrow transplantation. Exp Hematol. 2003;31(7):609–21. doi: 10.1016/s0301-472x(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR, et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp Hematol. 2007;35:1140–52. doi: 10.1016/j.exphem.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnefoix T, Bonnefoix P, Verdiel P, Sotto JJ. Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. J Immunol Methods. 1996 Aug 14;194(2):113–9. doi: 10.1016/0022-1759(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 12.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: DOnor acntigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550–9. [PubMed] [Google Scholar]
- 13.Bonnefoix T, Bonnefoix P, Mi JQ, Lawrence JJ, Sotto JJ, Leroux D. Detection of suppressor T lymphocytes and estimation of their frequency in limiting dilution assays by generalized linear regression modeling. J Immunol. 2003 Mar 15;170(6):2884–94. doi: 10.4049/jimmunol.170.6.2884. [DOI] [PubMed] [Google Scholar]
- 14.Novitzky N, Davison GM, Hale G, Waldmann H. Immune reconstitution at 6 months following T-cell depleted hematopoietic stem cell transplantation is predictive for treatment outcome. Transplantation. 2002 Dec 15;74(11):1551–9. doi: 10.1097/00007890-200212150-00012. [DOI] [PubMed] [Google Scholar]
- 15.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007 Oct;19(5):318–30. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005 Mar;5(3):465–74. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 17.Cox AL, Thompson SA, Jones JL, Robertson VH, Hale G, Waldmann H, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005 Nov;35(11):3332–42. doi: 10.1002/eji.200535075. [DOI] [PubMed] [Google Scholar]
- 18.Trzonkowski P, Zilvetti M, Friend P, Wood KJ. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006 Nov 27;82(10):1342–51. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- 19.Daly A, MCafee S, Dey B, Colby C, Schulte L, Yeap B, et al. Nonmyeloablative bone marrow transplantation: Infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003 Jun;9(6):373–82. doi: 10.1016/s1083-8791(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 20.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 21.Dumont-Girard F, Roux E, van Lier RA, Hale G, Helg C, Chapuis B, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92:4464–71. [PubMed] [Google Scholar]
- 22.Heitger A, Greinix H, Mannhalter C, Mayerl D, Kern H, Eder J, et al. Requirement of residual thymus to restore normal T-cell subsets after human allogeneic bone marrow transplantation [see comments] Transplantation. 2000;69(11):2366–73. doi: 10.1097/00007890-200006150-00026. [DOI] [PubMed] [Google Scholar]
- 23.Malphettes M, Carcelain G, Saint-Mezard P, Leblond V, Altes HK, Marolleau JP, et al. Evidence for naive T-cell repopulation despite thymus irradiation after autologous transplantation in adults with multiple myeloma: role of ex vivo CD34+ selection and age. Blood. 2003 Mar 1;101(5):1891–7. doi: 10.1182/blood-2002-06-1929. [DOI] [PubMed] [Google Scholar]
- 24.Chace JH, Cowdery JS, Field EH. Effect of anti-CD4 on CD4 subsets. I. Anti-CD4 preferentially deletes resting, naive CD4 cells and spares activated CD4 cells. J Immunol. 1994;152:405–12. [PubMed] [Google Scholar]
- 25.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, et al. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol. 2008 Mar 15;180(6):3910–8. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2003 Nov 30;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006 Oct;17(10):2844–53. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 28.Lacorcia G, Swistak M, Lawendowski C, Duan S, Weeden T, Nahill S, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation. 2009 Apr 15;87(7):966–74. doi: 10.1097/TP.0b013e31819c84b8. [DOI] [PubMed] [Google Scholar]
- 29.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008 Apr 1;111(7):3675–83. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minamimura K, Gao W, Maki T. CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol. 2006 Apr 1;176(7):4125–32. doi: 10.4049/jimmunol.176.7.4125. [DOI] [PubMed] [Google Scholar]
- 31.Ruzek MC, Neff KS, Luong M, Smith KA, Culm-Merdek K, Richards SM, et al. In vivo characterization of rabbit anti-mouse thymocyte globulin: a surrogate for rabbit anti-human thymocyte globulin. Transplantation. 2009 Jul 27;88(2):170–9. doi: 10.1097/TP.0b013e3181abc061. [DOI] [PubMed] [Google Scholar]
- 32.Eichmann K, Falk I, Melchers I, Simon MM. Quantitative studies on T cell diversity. I. Determination of the precursor frequencies for two types of streptococcus A specific helper cells in non-immune, polyclonally activated splenic T cells. J Exp Med. 1980;152:477. doi: 10.1084/jem.152.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu XX, Yue FY, Kovacs CM, Ostrowski MA. The role of cytokines which signal through the common gamma chain cytokine receptor in the reversal of HIV specific CD4(+) and CD8(+) T cell anergy. PLoS One. 2007;2(3):e300, 1–7. doi: 10.1371/journal.pone.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YZ, Lai ZF, Nishimura Y. Coculture of Th cells with interleukin (IL)-7 in the absence of antigenic stimuli induced T-cell anergy reversed by IL-15. Hum Immunol. 2005 Jun;66(6):677–87. doi: 10.1016/j.humimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Hathaway M, Adams DH. Demonstration that donor-specific nonresponsiveness in human liver allograft recipients is both rare and transient. Transplantation. 2004 Apr 27;77(8):1246–52. doi: 10.1097/01.tp.0000121136.84965.35. [DOI] [PubMed] [Google Scholar]
- 36.Reinsmoen NL, Matas AJ. Evidence that improved late renal transplant outcome correlates with the development of in vitro donor antigen-specific hyporeactivity. Transplantation. 1993;55:1017–23. doi: 10.1097/00007890-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Reinsmoen NL, Jackson A, McSherry C, Ninova D, Wiesner RH, Kondo M, et al. Organ-specific patterns of donor antigen-specific hyporeactivity and peripheral blood allogeneic microchimerism in lung, kidney, and liver transplant recipients. Transplantation. 1995;60:1546–54. doi: 10.1097/00007890-199560120-00029. [DOI] [PubMed] [Google Scholar]
- 38.Raju GP, Belland SE, Eisen HJ. Prolongation of cardiac allograft survival with transforming growth factor-b1 in rats. Transplantation. 1994;58:392–6. [PubMed] [Google Scholar]
- 39.Van der Mast BJ, Rischen-Vos J, de Kuiper P, Vaessen LM, van Besouw NM, Weimar W. Calcineurin inhibitor withdrawal in stable kidney transplant patients decreases the donor-specific cytotoxic T lymphocyte precursor frequency. Transplantation. 2005 Nov 15;80(9):1220–5. doi: 10.1097/01.tp.0000179642.03665.dd. [DOI] [PubMed] [Google Scholar]
- 40.Mathew JM, Garcia-Morales R, Fuller L, Rosen A, Ciancio G, Burke GW, et al. Donor bone marrow-derived chimeric cells present in renal transplant recipients infused with donor marrow. I. Potent regulators of recipient antidonor immune responses. Transplantation. 2000 Dec 27;70(12):1675–82. doi: 10.1097/00007890-200012270-00003. [DOI] [PubMed] [Google Scholar]
- 41.Strober S, Benike C, Krishnaswamy S, Engleman EG, Grumet FC. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: Studies of chimerism and anti-donor reactivity. Transplantation. 2000;69:1549–54. doi: 10.1097/00007890-200004270-00005. [DOI] [PubMed] [Google Scholar]
- 42.Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, et al. Minor H Antigen HA-1-specific Regulator and Effector CD8+ T Cells, and HA-1 Microchimerism, in Allograft Tolerance. J Exp Med. 2004 Apr 5;199(7):1017–23. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Locascio SA, Morokata T, Chittenden M, PREFFER FI, Dombkowski DM, Andreola G, et al. Mixed Chimerism, Lymphocyte Recovery, and Evidence for Early Donor-Specific Unresponsiveness in Patients Receiving Combined Kidney and Bone Marrow Transplantation to Induce Tolerance. Transplantation. 2010 Nov 16;90(12):1607–15. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Time course of naive and memory-type CD3+CD4+ cell recovery. Proportions of naive-type CD45RA+CD45RO− and memory-type CD45RA−CD45RO+ subsets among gated peripheral blood CD3+CD4+ cells are shown. Dotted lines denote the 25th to 75th percentiles for percentages of CD3+CD4+CD45RA+CD45RO− cells in a healthy adult population.
Figure S2. Time course of naïve and memory-type CD3+CD4+ cell recovery. Absolute numbers of naïve-type CD45RA+CD45RO+ and memory-type CD45RA−CD45RO+ subsets among gated CD3+CD4+ cells are shown.
Figure S3 Time course of naïve CD8+CD45RA+CD62L+ T cell recovery. Proportions of naive-type CD45RA+CD62L+ subsets among gated peripheral blood CD3+CD8+ cells are shown
Figure S4 Time course of CD4+CD25+ and CD4+CD25high cell recovery. Absolute numbers of CD4+CD25+ and CD4+CD25high CD3+ cells are shown.
Figure S5. MLR (left) and CML (right) responses at 3 year time point.
Figure S6 Suppression of pre-transplant anti-donor CTL responses by Patient 4 cells at POD 185 in co-culture assay.
Table S1 Patient profiles and outcomes