Abstract
β-Adrenoceptor (β-AR) subtypes act through different signaling pathways to regulate cardiac function and remodeling. Previous in vivo data show a markedly enhanced cardiotoxic response to doxorubicin in β2−/− mice, which is rescued by the additional deletion of the β1-AR. We determined whether this differential response was myocyte specific by examining the effects of doxorubicin in myocytes and fibroblasts from WT and β1, β2 and β1/β2−/− mice. Cells were exposed to doxorubicin at 1–50 µM and viability and apoptosis assessed at 6, 24 and 48 h. WT myocytes showed a time and dose-dependent decrease in viability (42% decrease at 1 µM after 24 h). β2−/− Myocytes showed a greater decrease in viability vs. WT (20.8% less at 6 h; 14% less at 24 h, P < 0.05); β1−/− and β1/β2−/− myocytes showed enhanced survival (β1−/− 11%; β1/β2−/− 18% greater than WT, P < 0.05). TUNEL staining demonstrated a similar differential susceptibility (WT 26% apoptotic nuclei, β2−/− 45.9%, β1/β2−/− 16.8%, P < 0.05). β2−/− Fibroblasts also showed enhanced toxicity. Pertussis toxin pretreatment of WT cells decreased survival similar to the β2−/−, suggesting a role for Gi signaling. JNK was differentially activated in β2−/− myocytes after doxorubicin and its inhibition increased cardiotoxicity. In conclusion, the differential cardioprotective/cardiotoxic effects mediated by β1 vs. β2-AR subtypes in knockout mice are recapitulated in myocytes isolated from these mice. β2-ARs appear to play a cardioprotective role, whereas β1-ARs a cardiotoxic role.
Keywords: Cardiomyopathy, Signal transduction, Apoptosis, Adrenergic receptors, Anthracyclines
1. Introduction
Chronic activation of the sympathetic nervous system plays an important role in the pathogenesis of dilated cardiomyopathy [1–4]. A central signaling abnormality in most forms of dilated cardiomyopathy is the selective downregulation of β1-adrenoceptors (ARs), usually sparing the β2-subtype [5]. Restoration of normal β-AR signaling has thus been the basis for successful pharmacologic therapies of cardiomyopathy, such as β-AR antagonists. However, there is considerable heterogeneity between β1 and β2-ARs beyond their different ligand affinities: their localization in subcellular domains, ability to internalize after agonist stimulation, binding to different G proteins, and crosstalk with other signaling pathways [6–16].
The exact balance between stimulatory/inhibitory and functional/structural signaling pathways regulated by β-ARS and how this homeostasis is altered by the disease processes resulting in cardiomyopathy is still not well understood. One such process that has been studied extensively in vitro is myocyte apoptosis. Evidence points to a differential role for β-AR subtypes in apoptosis and cell survival. In rat myocytes, stimulation of β1-ARs increases apoptosis via a cAMP-dependent mechanism [17,18], whereas β2-ARs inhibit apoptosis via a pertussis toxin (Gi) sensitive pathway [19]. β2-ARs may play a dual role: acute β2-AR stimulation activates the Akt anti-apoptotic pathway via Gi-Gβγ-PI3K. However, inhibition of this cascade converts β2-AR signaling from anti- to pro-apoptotic [20]. Taken together these studies indicate that the balance between cell death and survival could be mediated by differential activation of β-AR subtype-dependent pathways.
Doxorubicin is a quinone-containing anthracycline, which has been effective in treating a wide range of malignancies [21]. However, the clinical utility of doxorubicin therapy is severely limited by its cardiotoxicity [22,23]. Doxorubicin has been widely utilized as a model to study the mechanisms of acquired, as opposed to genetic, cardiomyopathy. We have previously shown a differential response to doxorubicin in mice lacking one or both β1 and β2-AR subtypes [24]. Wild-type (WT) or β1−/− mice exposed to a single high dose (15 mg/kg) of doxorubicin manifest no acute cardiotoxicity, whereas β2−/− mice develop severe acute cardiotoxicity and die within 30 min. This increased lethality was associated with differential activation of several MAPK family members. The additional deletion of the β1-receptor (β1/β2 double knockout mice) rescues this lethality completely.
To further study the signaling pathways that are involved in the differential regulation by β-ARs of doxorubicin cardiotoxicity, we sought to evaluate if the differential cardiotoxicity/cardioprotection observed in vivo was recapitulated in cardiomyocytes isolated from β-AR knockout mice, and whether this response was myocyte specific or was also present in fibroblasts from these same mice.
2. Materials and methods
2.1. Cell culture
Primary cardiac myocytes and fibroblasts were obtained from β1−/−, β2−/−, β1/β2−/− and WT neonatal mice at 1–2 d of age. β1−/− Mice were on a mixed C57BL/6, DBA/2, 129/Sv background, β2−/− mice were on a congenic FVB or mixed 129/Sv, FVB background, whereas β1/β2−/− mice were on a mixed C57BL/6, 129/Sv, FVB, DBA/2 background. All comparisons between knockout mice and WT were performed using littermate mice from matched strain backgrounds. One litter was used for each experiment. Mice were sacrificed, hearts were extracted and chopped into small fragments (3–4 mm each) that were subject to enzymatic digestion in a solution of calcium and bicarbonate free Hanks with HEPES (in mM, NaCl 136.9, KCl 5.36, MgSO4·7H2O 0.81, glucose 5.5, KH2PO4 0.44, Na2HPO4·7H2O 0.31, HEPES (pH 7.4) 20) and collagenase type 2 (Worthington Biochemical Corp., Lakewood, NJ). Serial cycles of agitation at 37 °C for 10 min were performed. After each cycle, cells were resuspended in DMEM with 10% fetal bovine serum and 5% horse serum and plated into a 100 mm culture dish. Cells were preplated for 1.5 h at 37 °C, to obtain cardiomyocytes and non-myocyte cells, predominantly cardiac fibroblasts. All procedures on animals were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
2.2. Cell treatments
2.2.1. Doxorubicin treatment
Cells were exposed to doxorubicin (Ben Venue laboratories, Bedford, OH) 0.1–50 µM for 3–48 hours to obtain dose and time response curves. These studies were performed both in the presence and absence of fetal bovine serum.
2.2.2. MAPK inhibition
Cells were exposed to MAPK Inhibitors (Calbiochem, La Jolla, CA) as follows: SB203580 (p38) 10 µM, PD98059 (ERK) 10 µM or SP600125 (JNK) 10 µM 30 min before doxorubicin 1 uM for 24 h.
2.2.3. Gi signaling inhibition
Cells were pretreated with 1 µg/ml Pertussis Toxin (Sigma, St. Louis, MO) for 3 h hours before doxorubicin treatment, as previously described in [19].
2.3. Methyl thiazol tetrazolium (MTT) assay
After doxorubicin treatment, cells were incubated in DMEM containing 2 mM MTT (Sigma, St. Louis, MO) for 60 min at 37 °C in room air containing 5% CO2. During this incubation the tetrazolium component of the dye is reduced in metabolically active cells to a formazan dye that will be solubilized in DMSO. The absorbance of formazan is then measured at 540 nm by spectrophotometry [25].
2.4. TUNEL assay
TUNEL staining (Roche Molecular Biochemicals, Indianapolis, IN) was performed in cardiomyocytes treated with doxorubicin. Cells were plated on glass coverslips, fixed and permeabilized, and TUNEL mixture was added. Co-staining was performed using DAPI and cells were visualized with a fluorescence microscope. TUNEL-positive-staining cells were counted at 60× magnification in 10 randomly chosen fields in triplicate plates and expressed as percentage of total cells then compared to WT.
2.5. Western blot analysis
Cells were lysed, total protein content was measured by Lowry assay, then proteins were electrophoretically separated and transferred to nitrocellulose membranes, and probed against rabbit polyclonal anti-phospho-specific p38 MAPK, p44/42 (Cell Signaling Technology, Beverly, MA), JNK (Santa Cruz Biotechnology, Santa Cruz, CA) and Bad Ser-128 (Bio-source, Camarillo, CA) antibodies. Protein loading was normalized using anti-GAPDH (Advanced Immunochemical, Long Beach, CA). LumiGLO chemiluminescent reagents (Cell Signaling Technology) were used for signal detection.
2.6. Statistical analysis
Comparisons of the effects of specific treatments were performed using Student’s t-test. Comparisons of effects across multiple genotypes were performed by repeated measures ANOVA with post-hoc testing by Scheffe’s test. All values are expressed as mean ± 1 S.D. The accepted level of significance was P < 0.05.
3. Results
3.1. Effect of doxorubicin on myocyte and fibroblast viability in cells derived from WT mice
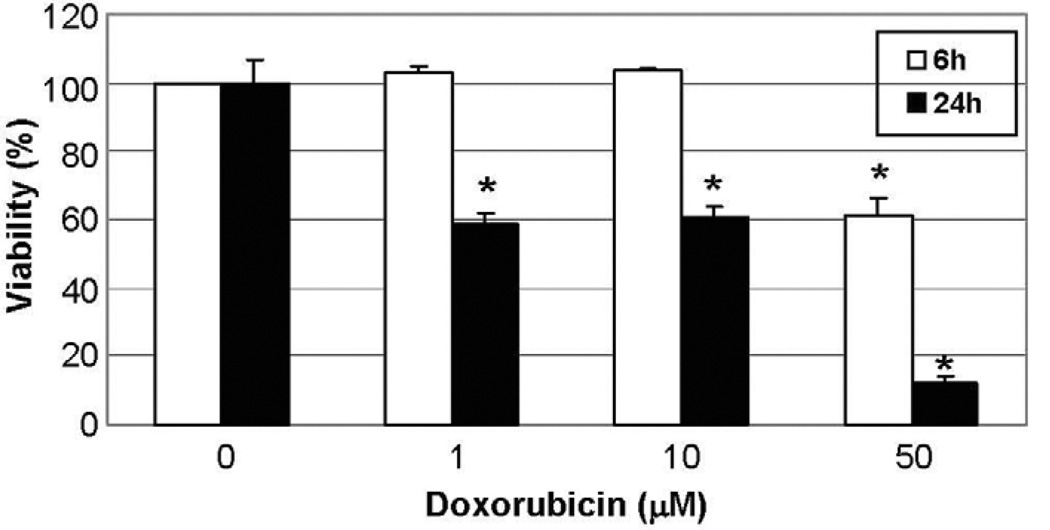
To determine whether the cardiotoxic/cardioprotective effects of β-AR signaling in doxorubicin-induced cardiomyopathy were myocyte-specific, doxorubicin was administered to myocytes directly. One hour after a single intravenous dose of 15–90 mg/m2, peak serum concentrations of doxorubicin reach 0.1–5 µM [26]. However, doxorubicin is highly concentrated within myocardial cells, and at therapeutic plasma doxorubicin concentrations the intramitochondrial concentration is as high as 50–100 µM [26]. Neonatal myocytes were successfully isolated with viability rates of 80–90%. Doxorubicin decreased viability in a time and dose-dependent manner: after 24 h of treatment (N = 3), at both 1 and 10 µM, viability was reduced by 42% (P < 0.01), whereas at 50 µM viability was reduced by 90% (Fig. 1). These results are in concordance with previous reports using similar doses [27,28]. With shorter periods of doxorubicin exposure (3 and 6 h, N = 4), there were no significant decreases in viability at doses up to 10 µM. However, at 50 µM viability at 6 h was decreased by 40%, and at an extremely high dose, 230 µM, viability was decreased by 80%.
Fig. 1.
Doxorubicin decreases viability in WT cardiomyocytes in a time and dose-dependent manner. Doxorubicin 1, 10 and 50 µM was administered to neonatal cardiomyocytes obtained from WT mice. Viability was assessed at 6 and 24 h by MTT assay. At 6 h viability was decreased only at 50 µM, whereas at 24 h viability was decreased at both 1 and 10 µM and severely decreased at 50 µM. * P < 0.05 vs. baseline.
Similar experiments were performed on WT cardiac fibroblasts. Doxorubicin decreased viability in fibroblasts, but the response was delayed compared with the response in myocytes. For example, at 24 h there was only a 20% decrease in viability in fibroblasts compared to the 40% observed in myocytes. At 48 h, viability in fibroblasts was decreased by 40%.
3.2. Effect of β-AR subtypes on doxorubicin-induced myocyte toxicity
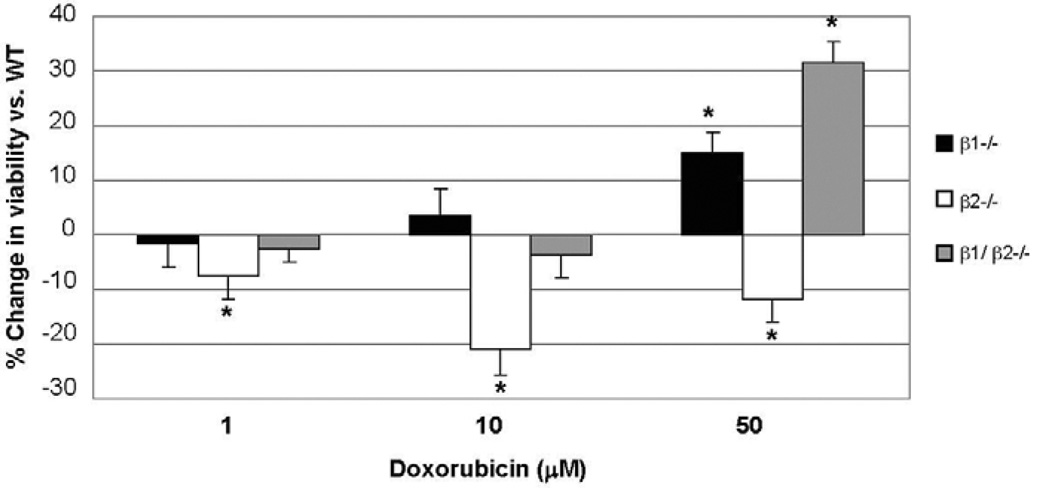
In β1−/− cardiomyocytes, there was a dose and time-dependent decrease in doxorubicin toxicity: after 6 h of treatment at 50 µM, survival was improved by 15 ± 4% compared to WT (N = 4) (Fig. 2). A similar response was observed in cells treated for 24 h (N = 3); survival was increased by 11% at 1 µM (P < 0.05) and by 18% at 10 µM (P < 0.05).
Fig. 2.
Disruption of β1 vs. β2-AR has opposite effects on viability of cardiomyocytes exposed to doxorubicin. Doxorubicin 1, 10 and 50 µM was administered to neonatal cardiomyocytes obtained from WT and β-AR knockout mice. After 6 h viability was assessed by MTT assay. A differential response to doxorubicin was observed in β-AR knockout myocytes: slightly improved survival in β1−/− myocytes, markedly decreased survival in β2−/− myocytes, and markedly improved survival in β1/β2−/− myocytes. *P < 0.05.
In contrast, in β2−/− myocytes there was a time and dose-dependent increase in doxorubicin toxicity: after 6 h this increased toxicity was present even at low doses which did not decrease viability in WT myocytes. At concentrations of 1, 10 and 50 µM, doxorubicin resulted in a decrease in viability of 7.4 ± 4%, 20.8 ± 4% and 12 ± 4%, respectively, in β2−/− myocytes compared to WT (P < 0.05) (Fig. 2). At 24 h, this effect was present at 1 µM, with viability decreased by 14.1 ± 1% (P < 0.05) compared to WT. However, at higher doses, the level of viability was similar between β2−/− and WT, potentially due to the prolonged higher level of toxicity at these concentrations of drug.
Similar to our observations in vivo, the additional deletion of the β1-AR (in cells derived from β1/β2−/− mice) protected against doxorubicin toxicity. At 6 h and 50 µM there was a 31.4 ± 4% increase in survival in β1/β2−/− myocytes compared to WT (P < 0.01) (Fig. 2). This effect persisted at 24 h with an increase in survival of 18.1 ± 1% and 39.9 ± 1% (P < 0.01) at 1 and 10 µM, respectively.
3.3. Effect of Gi inhibition on doxorubicin-induced cardiomyocyte toxicity
WT myocytes were pretreated with pertussis toxin (PTX) 1 µg/ml for 3 h before doxorubicin was administered. When PTX-treated cells were then exposed to doxorubicin 50 µM, after 6 h there was a 13 ± 3.7% (P = 0.05) decrease in viability compared to cells that received doxorubicin alone. When PTX-treated cells were exposed to doxorubicin 10 µM, after 24 h there was a 17.9 ± 3.6% (P < 0.01) decrease in viability compared to cells that received doxorubicin alone.
3.4. Effect of β-AR subtypes on doxorubicin-induced fibroblast toxicity and effect of conditioned media
Similar to results in cardiomyocytes, cardiac fibroblasts isolated from β2−/− mice showed enhanced doxorubicin toxicity, with a 60% decrease in viability at 48 h compared to WT. Previous reports have shown that conditioned media from fibroblasts can influence the response of myocytes to different stimuli, e.g. the hypertrophy associated with β-agonist stimulation [29,30]. Thus, we tested whether conditioned media from doxorubicin-treated fibroblasts could enhance the effect of doxorubicin on cardiomyocytes. Conditioned media was obtained from β2−/− fibroblasts treated for 48 h with 10 µM doxorubicin, and added to β2−/− myocytes also exposed to 10 µM doxorubicin. There was no difference in viability compared to β2−/− myocytes not exposed to conditioned media.
3.5. Effect of doxorubicin on cardiomyocyte apoptosis
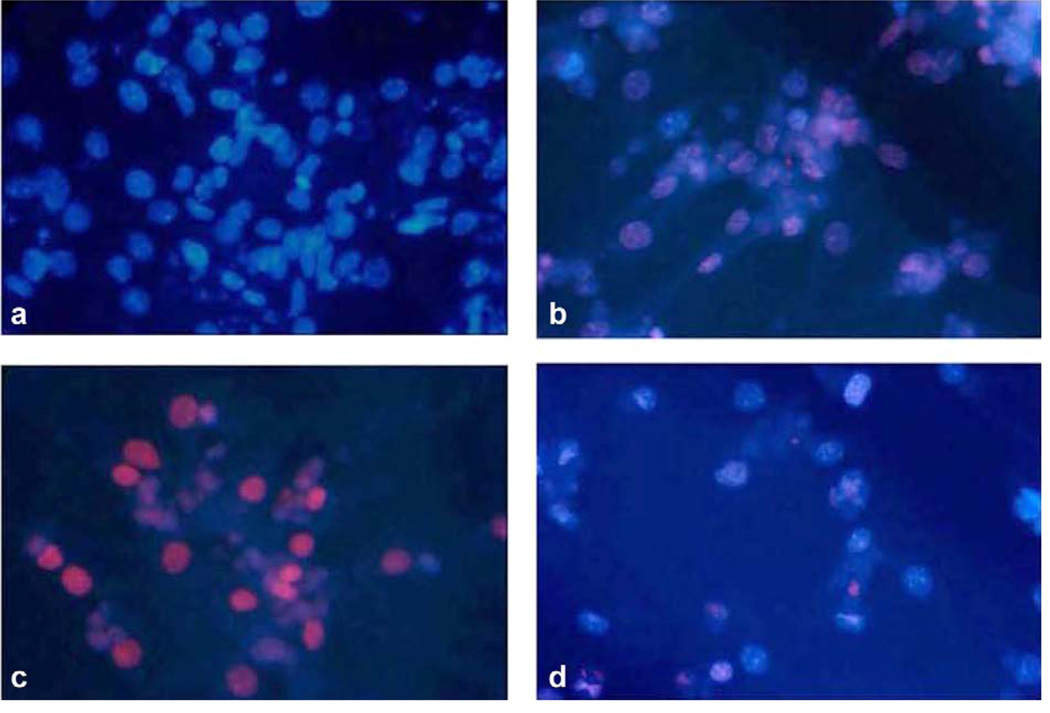
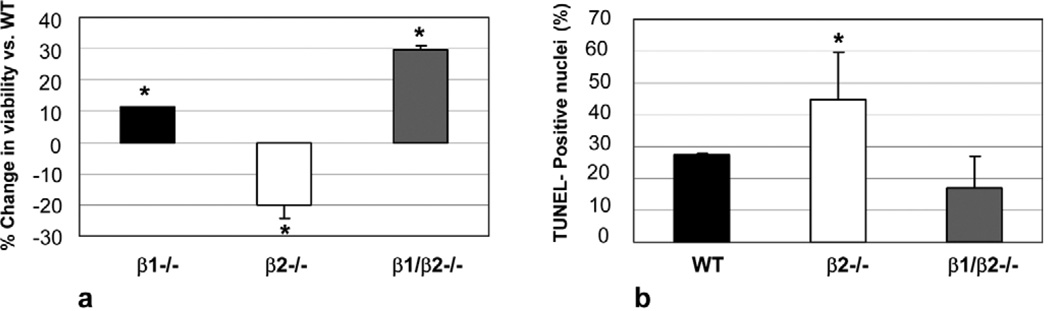
Cardiomyocyte apoptosis was quantified by TUNEL assay after 6 h of doxorubicin exposure (N = 3 for each genotype). At this time point, 1 µM doxorubicin was sufficient to induce a significant number of TUNEL positive cells. Fig. 3a through d are micrographs showing co-staining of TMR Red with DAPI. WT myocytes, with DAPI staining, in the absence of doxorubicin were used as control (Fig. 3a). When doxorubicin was administered to WT cardiomyocytes, 26 ± 10% of nuclei were co-stained red, indicative of apoptosis (Fig. 3b). In concordance with our survival data, in β2−/− myocytes treated with doxorubicin, there was a marked increase in TUNEL positive nuclei (Fig. 3c) with 44.9 ± 1.5% positive for TUNEL staining (P < 0.05 vs. WT). However, in β1/β2−/− myocytes treated with doxorubicin, there was a marked decrease in TUNEL staining (16.8 ± 11.4; Fig. 3d). Taken together, these results demonstrate that myocytes lacking the β2-AR have enhanced apoptosis after exposure to doxorubicin and that myocytes lacking the β1-AR have enhanced survival (Fig. 4). Absence of both receptors, in the β1/β2 double knockout, rescued the enhanced toxicity seen in the β2−/− and these myocytes were the least affected by doxorubicin. These results were independent of the presence or absence of serum in the medium.
Fig. 3.
Effect of β-AR disruption on doxorubicin-induced cardiomyocyte apoptosis. TUNEL staining of isolated cardiomyocytes from WT and β-AR knockout mice shows a differential susceptibility to doxorubicin. Cells were exposed to doxorubicin 1 µM for 6 h. (a) WT myocytes without doxorubicin (control), (b) WT myocytes with doxorubicin, (c) β2−/− show increased apoptotic nuclei with doxorubicin, (d) β1/β2−/− myocytes show decreased apoptotic nuclei with doxorubicin.
Fig. 4.
Cardiotoxic/cardioprotective effects of β-AR subtypes during exposure of cardiomyocytes to doxorubicin. (a) Changes in cell viability compared to WT. Cardiomyocytes were exposed to 1 µM doxorubicin for 24 h. Myocytes lacking the β2-AR showed increased toxicity; in contrast, myocytes lacking the β1-AR showed enhanced survival. Absence of both receptors (β1/β2−/−), rescued the increased toxicity in the β2−/−. (b) Changes in apoptosis compared with WT. Cardiomyocytes were exposed to 1 µM doxorubicin for 24 h. Myocytes lacking the β2-AR showed increased apoptotic nuclei after doxorubicin compared with WT; in contrast, myocytes lacking both β1 and β2-receptors showed decreased apoptosis compared with both WT and β2−/−. * P < 0.05.
3.6. Differential activation of MAPK in β2−/− cardiomyocytes
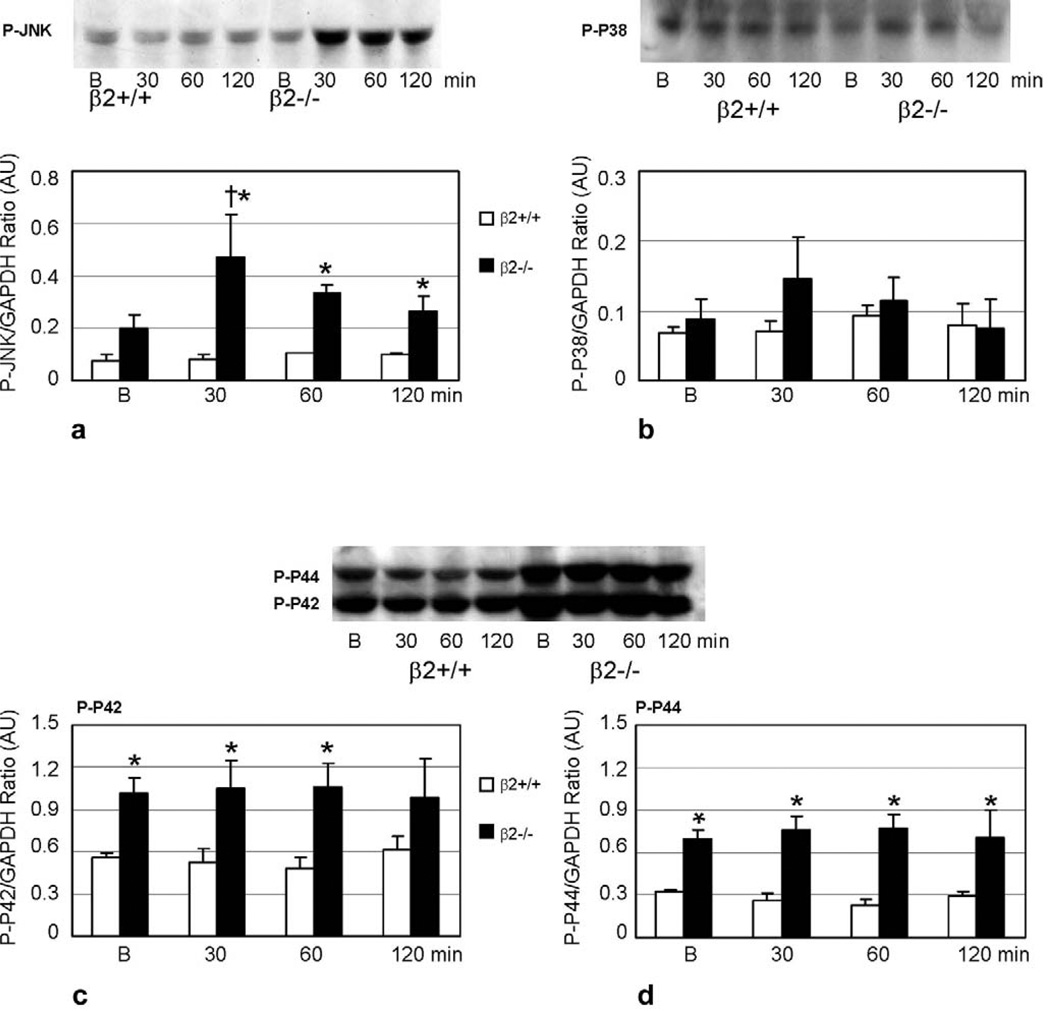
In our previous in vivo experiments, MAPK activity was increased after doxorubicin exposure. Marked activation of p38 compared to WT was observed in β2−/− mice given a single dose of 15 mg/kg doxorubicin. There were also increases in activation of p44 (ERK1), p42 (ERK2) and JNK, predominantly in the β2−/− mice, although to a lesser extent than p38. To determine whether these same signaling pathways played a role in the cardiotoxic/cardioprotective effects mediated by β-AR subtypes in myocytes, activation of these MAPKs was assessed during the first 2 h after 1 µM doxorubicin administration, which has been documented as the time course of MAPK activation in previous studies [31]. In β2−/− myocytes, JNK activation was increased 2.4-fold (P < 0.05) compared to baseline at 30 min after doxorubicin, and this activation was persistent at 2 h. In comparison, in WT myocytes there was no difference in JNK activation at any time during the 2 hours post-doxorubicin (Fig. 5a). Differential phosphorylation of downstream signaling components associated with JNK could be involved in its intricate regulation of cardiotoxicity/cardioprotection. We examined phosphorylation of the pro-apoptotic protein Bad at Ser-128 [32,33]. There was no baseline difference in Bad phosphorylation between WT and β2−/− myocytes. Furthermore, there was no change when the cells were treated with doxorubicin at the same time points previously chosen to assess JNK activation (data not shown).
Fig. 5.
Differential activation of MAPK in β-AR knockout cardiomyocytes after exposure to doxorubicin. Western blot and graphic representation for phospho-JNK (a), p38 (b), and ERK1/2 (p44/p42) (c, d) showing increased phosphorylation of JNK in β2−/− cardiomyocytes after doxorubicin compared to baseline (B), no significant change in p38 activity, and increase in ERK1/2 (p44/p42) activity compared to WT. † P < 0.05, compared to baseline, * P < 0.05, compared to WT. AU = Arbitrary Units.
Activation of ERK1 (p44) and ERK2 (p42) was increased two to threefold in β2−/− cells compared to WT at baseline (before doxorubicin) and at any time observed after doxorubicin, but the activation of ERK1/2 did not increase with doxorubicin in either WT or β2−/− myocytes (Fig. 5c, d). In contrast, there were no differences in the activation of p38 between WT and β2−/− myocytes and in neither genotype did p38 increase with doxorubicin although there was a trend towards an increase at 30 min in β2−/− cells which did not reach statistical significance (Fig. 5b).
3.7. MAPK inhibition in β2−/− cardiomyocytes
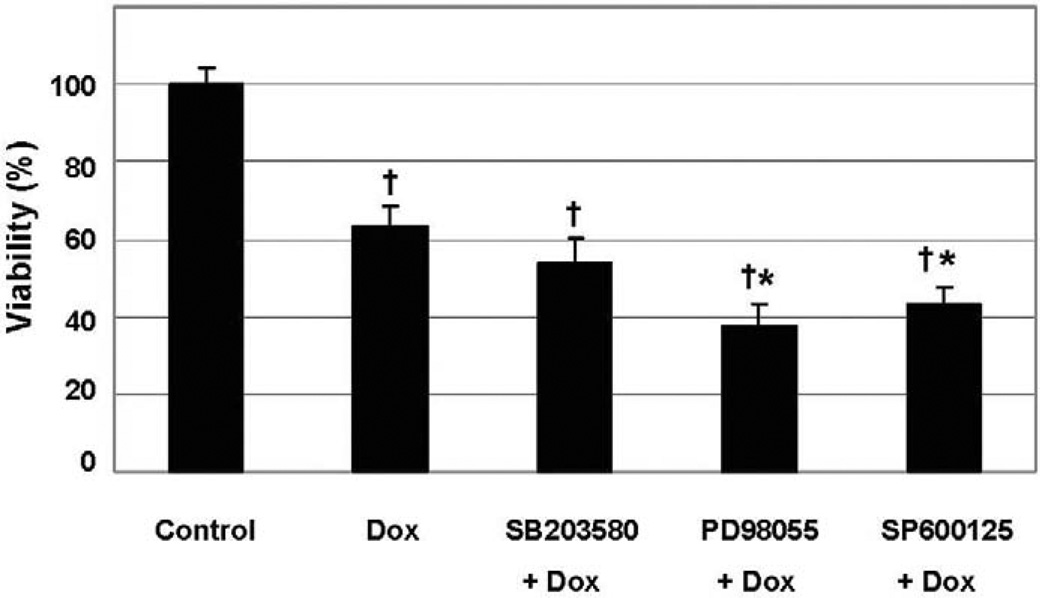
In order to test if the differential MAPK activation in β2−/− myocytes had an effect on cell survival, MAPK inhibitors were administered prior to 1 µM doxorubicin. Inhibition of either ERK (PD98055) or JNK (SP600125) activity resulted in a further decrease in viability in β2−/− myocytes compared to cells without addition of the inhibitor. In contrast, p38 inhibition with SB203580 did not affect viability when β2−/− cells were treated with doxorubicin (Fig. 6).
Fig. 6.
Effect of MAPK inhibitors on doxorubicin-induced toxicity in β2-AR knockout cardiomyocytes. Myocytes derived from β2−/− mice were exposed to MAPK inhibitors: SB203580 10 µM for p38, PD98059 10 µM for ERK and SP600125 10 µM for JNK. Inhibition of both ERK and JNK further decreased viability. † P < 0.05, compared to control, * P < 0.05, compared to doxorubicin-treated cells.
4. Discussion
β-AR subtypes play an important role in regulating cardiac function and in regulating cardiac remodeling in conditions of cardiac stress. The differential role of β-AR subtypes in regulating cell death and survival has been reported previously [20,34,35], however, the present study shows the differential cardiotoxic/cardioprotective role of β-AR subtypes in the development of a clinically relevant toxic cardiomyopathy, induced by the anthracycline anti-tumor agent doxorubicin. Cardiomyocytes isolated from β2−/− mice showed significantly increased apoptosis and decreased viability compared to cells isolated from WT mice. In contrast, cardiomyocytes isolated from β1−/− and β1/β2−/− mice showed less apoptosis and enhanced survival compared with β2−/− myocytes, with cells from the double knockout the least affected by doxorubicin. Cardiac fibroblast viability is also decreased by doxorubicin, although this response was delayed and less severe than that in cardiomyocytes. Similar to myocytes, β2−/− fibroblasts also showed enhanced doxorubicin toxicity. These data demonstrate that both cell types may play a role in chronic doxorubicin cardiotoxicity, however that cardiomyocytes are clearly the more vulnerable. Although previous reports have shown that conditioned media from fibroblasts can influence the response of myocytes to remodeling stimuli, such as β-agonists [29,30] we have ruled out a role for such diffusible factors in doxorubicin cardiotoxicity.
Inhibition of Gi with pertussis toxin enhanced doxorubicin toxicity in WT cardiomyocytes to a magnitude similar to that observed with doxorubicin alone in β2−/− cardiomyocytes. These results suggest that the differential toxicity related to β-AR subtypes is at least partially mediated through a Gi-dependent pathway. Human ventricular myocytes have probably the strongest contribution of β2-ARs to contractility of any species, and this effect seems to be predominantly mediated through Gs coupling. Gong et al. found that the β2-AR inverse agonist ICI 118,551 had a negative inotropic effect in myocytes from failing human hearts. This effect was blocked by pertussis toxin, suggesting the involvement of Gi. However, this was not the case in myocytes isolated from non-failing hearts [36,37]. While this area remains controversial, we urge caution in the extrapolation of our findings in the murine model to human cardiomyopathy.
Anthracycline cardiotoxicity is a well characterized model of dilated cardiomyopathy [38,39]. There are several mechanisms by which doxorubicin is thought to induce cell damage, including interference with DNA synthesis, generation of free radicals, DNA adduct formation and cross-linking, interference with DNA helicase activity, direct membrane effects, inhibition of enzymes such as topoisomerase II and the induction of apoptosis [40]. The direct effects of doxorubicin on DNA are thought to mediate its anti-tumor toxicity, whereas the generation of free radicals and membrane effects have been thought to mediate its cardiotoxicity [40,41]. There is evidence that β-AR signaling alterations are present in doxorubicin cardiomyopathy as they are in other forms of dilated cardiomyopathy. Animal models show increased circulating norepinephrine, downregulation of β-ARs, downregulation of Gs and upregulation of Gi [42,43]. β-Blockers have been shown to improve cardiac function in both rats and humans with established doxorubicin cardiomyopathy [44,45]. However, no previous studies have examined the role of β-AR subtypes in the pathogenesis of doxorubicin cardiotoxicity.
One potential pathway for mediation of doxorubicin cardiotoxicity, and one which has been studied previously, is the activation of members of the MAPK family. MAPKs are involved in many aspects of eukaryotic cellular regulation, including cell survival and adaptation to stress [46,47]. There is ample evidence linking β-ARs to MAPK signaling. ERK1/2 are activated by β2-ARs through activation of Gi, dissociation of Gβγ [48] and recruitment of β-arrestin to the sarcolemmal membrane as part of a Src-protein kinase complex [49]. Others have shown that β2-AR activation of p38 MAPK is mediated by PKA rather than by Gi or Gβγ [50]. p38 plays a role in both ischemia/reperfusion injury [51–53] and in preconditioning [54,55], although studies have differed as to whether p38 mediates cardiotoxicity or cardioprotection [28,56–59]. In β-agonist-mediated myocyte apoptosis, β2-ARs may play a dual role: under basal conditions, β2-ARs stimulate anti-apoptotic Akt (through Gi, Gβγ and PI3K), phosphorylating GSK3β, inhibiting caspase 9, and activating Bcl [20]. However, inhibition of Gi with pertussis toxin switches β2-AR signaling to pro-apoptotic, mediated through p38 [20]. In mouse cardiomyocytes exposed to anthracyclines, Kang et al. [28] found that activation of p38 mediated apoptosis, as this effect was blocked by p38 inhibitors.
In β2−/− mice given a single dose (15 mg/kg) of doxorubicin in vivo, we found that p38 MAPK activation was increased 20-fold [24]. However we were not able to confirm a similar differential activation of p38 MAPK in cardiomyocytes isolated from β2−/− mice, and this result was in concordance with the inability of SB203580 to improve survival in cells treated with doxorubicin. There are several possible explanations for this discrepancy between our in vivo and in vitro experiments: the possibility of involvement of other cardiac cells types in vivo, the effect of cell culture conditions on p38 activation, and the possibility of developmental differences in p38 activation in response to doxorubicin. However, other members of the MAPK family (ERK and JNK) were elevated in β2−/− myocytes, either at baseline or with doxorubicin stimulation, indicating activation of pathways that have been implicated in the modulation of survival or apoptosis. ERK1/2 were increased at baseline in β2−/− myocytes compared to WT, and did not increase further with the administration of doxorubicin, as we had observed in vivo. The increase in baseline activation of ERK1/2 suggests that this may be due to cell isolation, with the β2−/− myocytes more sensitive to the stress of cell culture. ERK activation may act as a compensatory cardioprotective mechanism in the absence of β2-ARs, since when ERK was inhibited further, cardiotoxicity was enhanced.
In contrast to the other MAPKs, JNK is differentially activated in β2−/− myocytes after doxorubicin. Furthermore, JNK inhibition increases toxicity, suggesting that JNK is cardioprotective in this model. In accord with these results, our previous in vivo studies showed that inhibition of JNK did not rescue the β2−/− mice, evidence that JNK was not mediating cardiotoxicity. Previous studies have implicated activation of JNK in both pro- and anti-apoptotic signaling [46,47,60–63] and JNK activation has also been implicated in doxorubicin toxicity. In H9c2 cardiac muscle cells, Kim et al. [64] found that increased JNK activity was associated with doxorubicin-induced apoptosis. In Jurkat cells, Panaretakis et al. [65] found that δPKC lies upstream of JNK in doxorubicin-induced cell death. In contrast, the involvement of JNK in activation of anti-apoptotic pathways has been suggested in both hematopoietic [62] and tumor cells [63].
Thus, JNK may be activated in acute doxorubicin cardio-toxicity in vitro as a compensatory, cell-survival promoting, mechanism. Differential phosphorylation of downstream signaling components associated with JNK could be involved in its intricate regulation of cardiotoxicity/cardioprotection. One of these components, the pro-apoptotic protein Bad [60,61], has been implicated in doxorubicin cardiotoxicity [66]. At least two Bad phosphorylation sites have been associated with JNK activation: Thr-201 may be responsible for the anti-apoptotic functions of JNK [62], and Ser-128 responsible for its pro-apototic effects [32,33]. We examined Bad phosphorylation at Ser-128 and found no baseline difference between WT or B2−/− myocytes, and no change when the cells were treated with doxorubicin. Thus, it appears that JNK signaling may function in a cell type-dependent and stimulus-dependent manner in regulating the balance between cardiotoxicity and cardioprotection. Further characterization of crosstalk between β-ARs and members of the MAPK family under different cardiac stress conditions will be required.
One limitation of our study is that our experiments were confined to neonatal cardiomyocytes. Numerous developmental differences in signaling pathways and susceptibility to oxidant stress have been described between neonatal and adult cardiomyocytes that could alter the effect we have described for β-ARs on doxorubicin toxicity [67]. Additional studies in adult cardiomyocytes could be potentially informative, although differences and limitations in adult myocyte culture techniques could confound interpretation of any differences. The best evidence that the differential effect of β-AR subtypes on doxorubicin cardiotoxicity holds true in adult myocytes is that it occurs in adult mice in vivo.
In summary, we have shown that β2-ARs modulate cardio-protection during exposure of isolated cardiomyocytes to doxorubicin, whereas β1-ARs appear to enhance doxorubicin cardiotoxicity. These results are qualitatively similar to our previous studies in vivo. Cardiomyocytes isolated from β-AR knockout mice thus represent an excellent model system in which to examine β-AR crosstalk with other signaling pathways which regulate cardiotoxicity/cardioprotection. Developing a better understanding of the role of β-AR subtypes in cardiotoxic/cardioprotective signaling has important therapeutic consequences: if β1-ARs are cardiotoxic and β2-ARs are cardioprotective, as our data suggest, then future therapy of heart failure could be tailored to address these receptor-specific effects.
Acknowledgments
This work was supported by a grant to D.B. from the National Heart Lung and Blood Institute of the National Institutes of Health (HL61535). G.F. was supported by a Post-Doctoral Fellowship from the American Heart Association, Western States Affiliate.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yjmcc.2005.12.004.
References
- 1.Cohn J, Levine T, Olivari M. Plasma norepinephrine as a guide to prognosis in patients with congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 2.Kaye D, Lambert G, Lefkowits J. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–578. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation. 1990;82:1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- 4.Benedict C, Shelton B, Johnstone D, Francis G, Greenberg B, Konstam M, et al. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation. 1996;94:690–697. doi: 10.1161/01.cir.94.4.690. [DOI] [PubMed] [Google Scholar]
- 5.Bristow M, Ginsburg R, Minobe W, Cubicciotti R, Sageman A, Lurie K, et al. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Nguyen C, Nantel F, Bonin H, Valiquette M, Frielle T, et al. Distinct regulation of β1- and β2-adrenergic receptors in Chinese hamster fibroblasts. Mol Pharmacol. 1992;41:542–548. [PubMed] [Google Scholar]
- 7.Steinfath M, Geertz B, Schmitz W, Scholz H, Haverich A, Breil I, et al. Distinct down-regulation of cardiac β1- and β2-adrenoceptors in different human heart diseases. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:217–220. doi: 10.1007/BF00168613. [DOI] [PubMed] [Google Scholar]
- 8.Brodde O. β1 and β2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- 9.Bristow M, Anderson F, Port J, Skerl L, Hershberger R, Larrabee P, et al. Differences in β-adrenergic neuroeffector mechanisms in ischemic versus idiopathic dilated cardiomyopathy. Circulation. 1991;84:1024–1039. doi: 10.1161/01.cir.84.3.1024. [DOI] [PubMed] [Google Scholar]
- 10.Muntz K, Zhao M, Miller J. Downregulation of myocardial β-adrenergic receptors: receptor subtype selectivity. Circ Res. 1994;74:369–375. doi: 10.1161/01.res.74.3.369. [DOI] [PubMed] [Google Scholar]
- 11.von Zastrow M, Kobilka B. Ligand-regulated recycling of human beta-2 adrenergic receptors between the plasma membrane and endosomes containing transferrin receptor. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 12.Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- 13.Xiang Y, Kobilka B. The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc Natl Acad Sci USA. 2003;100:10776–10781. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao R-P, Avdonin P, Zhou Y-Y, Cheng H, Akhter S, Eschenhagen T, et al. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 16.Gutkind J. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 17.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Communal C, Singh K, Colucci W. Gi protein protects adult rat ventricular myocytes from β-adrenergic receptor-stimulated apoptosis in vitro. Circulation. 1998;98 (I-742) [Google Scholar]
- 19.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 20.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- 22.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 23.Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28:2–7. [PubMed] [Google Scholar]
- 24.Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, et al. Differential cardioprotective/cardiotoxic effects mediated by {beta}-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–H2449. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- 25.Gomez LA, Alekseev AE, Aleksandrova LA, Brady PA, Terzic A. Use of the MTT assay in adult ventricular cardiomyocytes to assess viability: effects of adenosine and potassium on cellular survival. J Mol Cell Cardiol. 1997;29:1255–1266. doi: 10.1006/jmcc.1996.0363. [DOI] [PubMed] [Google Scholar]
- 26.Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234–235:119–124. [PubMed] [Google Scholar]
- 27.Shneyvays V, Mamedova LK, Korkus A, Shainberg A. Cardiomyocyte resistance to doxorubicin mediated by A(3) adenosine receptor. J Mol Cell Cardiol. 2002;34:493–507. doi: 10.1006/jmcc.2002.1532. [DOI] [PubMed] [Google Scholar]
- 28.Kang YJ, Zhou ZX, Wang GW, Buridi A, Klein JB. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. J Biol Chem. 2000;275:13690–13698. doi: 10.1074/jbc.275.18.13690. [DOI] [PubMed] [Google Scholar]
- 29.Simpson P, McGrath A, Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and catecholamines. Circ Res. 1982;51:787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- 30.Long CS, Henrich CJ, Simpson PC. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul. 1991;2:1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, You H, Yang Y, Wei L, Zhang X, Yao L, et al. Distinctive regulation and function of PI 3K/Akt and MAPKs in doxorubicin-induced apoptosis of human lung adenocarcinoma cells. J Cell Biochem. 2004;91:621–632. doi: 10.1002/jcb.10751. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Liu J, Yu C, Lin A. BAD Ser128 is not phosphorylated by c-Jun NH2-terminal kinase for promoting apoptosis. Cancer Res. 2005;65:8372–8378. doi: 10.1158/0008-5472.CAN-05-0576. [DOI] [PubMed] [Google Scholar]
- 33.Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- 34.Ponicke K, Heinroth-Hoffmann I, Brodde OE. Role of beta 1- and beta 2-adrenoceptors in hypertrophic and apoptotic effects of noradrenaline and adrenaline in adult rat ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:592–599. doi: 10.1007/s00210-003-0754-z. [DOI] [PubMed] [Google Scholar]
- 35.Shizukuda Y, Buttrick PM. Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:823–831. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]
- 36.Gong H, Sun H, Koch WJ, Rau T, Eschenhagen T, Ravens U, et al. Specific beta(2)AR blocker ICI 118,551 actively decreases contraction through a G(i)-coupled form of the beta(2)AR in myocytes from failing human heart. Circulation. 2002;105:2497–2503. doi: 10.1161/01.cir.0000017187.61348.95. [DOI] [PubMed] [Google Scholar]
- 37.Harding SE, Gong H. Beta-adrenoceptor blockers as agonists: coupling of beta2-adrenoceptors to multiple G-proteins in the failing human heart. Congest Heart Fail. 2004;10:181–185. doi: 10.1111/j.1527-5299.2004.02052.x. (quiz 186-7) [DOI] [PubMed] [Google Scholar]
- 38.Fisher NG, Marshall AJ. Anthracycline-induced cardiomyopathy. Post-grad Med J. 1999;75:265–268. doi: 10.1136/pgmj.75.883.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otero FJ, Boor PJ, Sheahan RG. Doxorubicin-induced cardiomyopathy. Am J Med Sci. 2000;320:59–63. [PubMed] [Google Scholar]
- 40.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 41.Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11:931–936. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- 42.Fu M, Matoba M, Liang Q-M, Sjögren K-G, Hjalmarson A. Properties of G-protein modulated receptor-adenylyl cyclase system in myocardium of spontaneously hypertensive rats treated with adriamycin. Inter J Cardiol. 1994;44:9–18. doi: 10.1016/0167-5273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 43.Robison TW, Giri SN. Effects of chronic administration of doxorubicin on myocardial beta-adrenergic receptors. Life Sci. 1986;39:731–736. doi: 10.1016/0024-3205(86)90021-4. [DOI] [PubMed] [Google Scholar]
- 44.Noori A, Lindenfeld J, Wolfel E, Ferguson D, Bristow MR, Lowes BD. Beta-blockade in adriamycin-induced cardiomyopathy. J Card Fail. 2000;6:115–119. [PubMed] [Google Scholar]
- 45.Fujita N, Hiroe M, Ohta Y, Horie T, Hosoda S. Chronic effects of metoprolol on myocardial beta-adrenergic receptors in doxorubicin-induced cardiac damage in rats. J Cardiovasc Pharmacol. 1991;17:656–661. doi: 10.1097/00005344-199104000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 47.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 48.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 49.Luttrell L, Gerguson S, Daaka Y, Miller W, Maudsley S, Della Rocca G, et al. β-Arrestin-dependent formation of β2-adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 50.Zheng M, Zhang SJ, Zhu WZ, Ziman B, Kobilka BK, Xiao RP. Beta 2-adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or gbeta gamma in adult mouse cardiomyocytes. J Biol Chem. 2000;275:40635–40640. doi: 10.1074/jbc.M006325200. [DOI] [PubMed] [Google Scholar]
- 51.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, et al. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res. 2003;93:254–261. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- 52.Lochner A, Genade S, Hattingh S, Marais E, Huisamen B, Moolman JA. Comparison between ischaemic and anisomycin-induced preconditioning: role of p38 MAPK. Cardiovasc Drugs Ther. 2003;17:217–230. doi: 10.1023/a:1026116022552. [DOI] [PubMed] [Google Scholar]
- 53.Schneider S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK alpha/beta reduces ischemic injury and does not block protective effects of preconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 54.Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383–2391. doi: 10.1006/jmcc.1997.0473. [DOI] [PubMed] [Google Scholar]
- 55.Baines CP, Liu GS, Birincioglu M, Critz SD, Cohen MV, Downey JM. Ischemic preconditioning depends on interaction between mitochondrial KATP channels and actin cytoskeleton. Am J Physiol. 1999;276:H1361–H1368. doi: 10.1152/ajpheart.1999.276.4.H1361. [DOI] [PubMed] [Google Scholar]
- 56.Communal C, Colucci WS, Singh K. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta-adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J Biol Chem. 2000;275:19395–19400. doi: 10.1074/jbc.M910471199. [DOI] [PubMed] [Google Scholar]
- 57.Zechner D, Craig R, Hanford DS, McDonough PM, Sabbadini RA, Glembotski CC. MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J Biol Chem. 1998;273:8232–8239. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]
- 58.Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, et al. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000;275:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- 59.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–699. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]
- 60.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 61.Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 62.Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, et al. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell. 2004;13:329–340. doi: 10.1016/s1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 63.Chen N, Nomura M, She QB, Ma WY, Bode AM, Wang L, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908–3912. [PubMed] [Google Scholar]
- 64.Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ, Chae SW. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005;70:1066–1078. doi: 10.1016/j.bcp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Panaretakis T, Laane E, Pokrovskaja K, Bjorklund AC, Moustakas A, Zhivotovsky B, et al. Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16:3821–3831. doi: 10.1091/mbc.E04-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youn HJ, Kim HS, Jeon MH, Lee JH, Seo YJ, Lee YJ. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005;270:13–19. doi: 10.1007/s11010-005-2541-2. [DOI] [PubMed] [Google Scholar]
- 67.Rybin VO, Pak E, Alcott S, Steinberg SF. Developmental changes in beta2-adrenergic receptor signaling in ventricular myocytes: the role of Gi proteins and caveolae microdomains. Mol Pharmacol. 2003;63:1338–1348. doi: 10.1124/mol.63.6.1338. [DOI] [PubMed] [Google Scholar]