The effects of chemical (DPP-4) inhibition and genetic reduction of DPP-4 activity on bone quality were studied in wild-type and ovariectomized mice.
Abstract
L-glutamine stimulates glucagon-like peptide 1 (GLP-1) secretion in human subjects and cell lines. As recent advances have enabled the study of primary GLP-1–releasing L cells, this study aimed to characterize glutamine-sensing pathways in native murine L cells. L cells were identified using transgenic mice with cell-specific expression of fluorescent markers. Cells were studied in primary colonic cultures from adult mice, or purified by flow cytometry for expression analysis. Intracellular Ca2+ was monitored in cultures loaded with Fura2, and cAMP was studied using Förster resonance energy transfer sensors expressed in GLUTag cells. Asparagine, phenylalanine, and glutamine (10 mm) triggered GLP-1 release from primary cultures, but glutamine was the most efficacious, increasing secretion 1.9-fold with an EC50 of 0.19 mm. Several amino acids triggered Ca2+ changes in L cells, comparable in magnitude to that induced by glutamine. Glutamine-induced Ca2+ responses were abolished in low Na+ solution and attenuated in Ca2+ free solution, suggesting a role for Na+ dependent uptake and Ca2+ influx. The greater effectiveness of glutamine as a secretagogue was paralleled by its ability to increase cAMP in GLUTag cells. Glutamine elevated intracellular cAMP to 36% of that produced by a maximal stimulus, whereas asparagine only increased intracellular cAMP by 24% and phenylalanine was without effect. Glutamine elevates both cytosolic Ca2+ and cAMP in L cells, which may account for the effectiveness of glutamine as a GLP-1 secretagogue. Therapeutic agents like glutamine that target synergistic pathways in L cells might play a future role in the treatment of type 2 diabetes.
Glucagon-like peptide 1 (GLP-1) is synthesized in and secreted from enteroendocrine L cells, which are located throughout the intestine but predominantly found more distally in the ileum and colon (1). GLP-1 is released after nutrient ingestion and mediates a number of effects that help maintain euglycemia (reviewed in Ref. 2), including the incretin effect which enhances insulin secretion but which is impaired in type 2 diabetes (3).
Type 2 diabetes treatments have recently entered the market, aimed at targeting the GLP-1 axis by either inhibiting its rapid clearance by dipeptidyl-peptidase IV (DPP4) or using DPP4-resistant GLP-1 mimetics (4). Current research is now also focusing on developing treatments that could potentially hijack the endogenous secretory mechanisms in L cells and increase intestinal GLP-1 release. This may have the advantage that hormones levels would be elevated in the neighborhood of the intestinal epithelium, where GLP-1 is also believed to act on vagal afferent neurones mediating GLP-1–dependent central effects and reflexes (5).
A range of nutrients stimulate the release of GLP-1 both in vitro and in vivo (reviewed in Ref. 6), and the underlying molecular mechanisms are now starting to become clearer. To date, the cellular biology of enteroendocrine cells, because of their low density (<1%) within the intestinal epithelium and problems associated with distinguishing them from their enterocyte neighbors, has largely been investigated using cell lines. However, the recent development of primary culture protocols and the generation of transgenic mice with cell-specific fluorescent protein expression driven by gut hormone promoters (7, 8) have allowed these rare cell types to be studied at the single cell level in real time. Hypotheses arising from the study of cell lines can now therefore be further explored in primary tissue.
Previously, we reported that L-glutamine (Gln) stimulated secretion from the GLP-1 producing cell line, GLUTag, and interestingly, that it was by far the most effective of the L-amino acids tested (9). The Gln-mediated response appeared to comprise two components: an electrogenic pathway generating action potentials and calcium influx, proposed to be driven by Na+-dependent amino acid uptake by SLC38A2 (SNAT2, ATA2), and a second amplifying pathway acting downstream or independent of membrane depolarization, the nature of which remained unclear. Further studies in human subjects confirmed the relevance of this finding, as oral supplements of Gln, given to lean healthy human subjects, were found to elevate plasma GLP-1 levels within 30 min of ingestion. Importantly, this effect was also observed in obese type 2 diabetic subjects (10), and therefore the GLP-1 secretory pathway triggered by Gln may represent a novel therapeutic target.
The potential of Gln-based therapies to increase GLP-1 secretion in human subjects has increased interest in the signaling pathways triggered by this amino acid in L cells. The current study initially verified that the effectiveness of Gln as a GLP-1 secretagogue is observed in primary colonic cultures as well as GLUTag cells, and subsequently demonstrated that this is attributable to the activation of both a triggering pathway that elevates intracellular calcium (Cai2+), and an amplifying pathway mediated by elevated cAMP. Synergy between these pathways accounts for the effectiveness of Gln at stimulating GLP-1 release.
Materials and Methods
Salines
The standard saline used for all experiments contained (in mm): 4.5 KCl, 138 NaCl, 4.2 NaHCO3, 1.2 NaH2PO4, 2.6 CaCl2, 1.2 MgCl2, and 10 HEPES (pH 7.4, NaOH). In secretion experiments the saline was supplemented with 0.1% BSA (fatty acid free). Ca2+ free solutions were prepared by omitting CaCl2 and adding 0.5 mm EGTA to the standard saline. In Na+-free studies (GLUTag), NaCl was replaced with the large impermeant cation N-methyl D glucamine (NMDG+), and NaHCO3 and NaH2PO4 were substituted with their equivalent K+ salts. In primary culture studies only NaCl was replaced (low Na+ saline). Where possible all drugs were prepared as a 1000 × stock. All reagents were supplied by Sigma (Poole, UK) unless otherwise stated.
Tissue and cell preparation
The generation of transgenic mice, using a bacterial artificial chromosome construct to drive Venus expression by the proglucagon promoter is described in detail elsewhere (7). All animal procedures were approved by the local ethical committee and conformed to UK Home Office regulations. Colonic intestinal tissue, from mice aged between 3 and 6 months, was collected in Leibovitz L-15 medium, chopped into fine pieces, and digested in 0.4 mg/ml collagenase XI in DMEM (25 mm glucose) for an initial 10 min. The supernatant was discarded and replaced with fresh collagenase and incubated for a further 15 min and the supernatant collected. This was repeated twice more. The supernatants were centrifuged at 300 g and the pellets resuspended in DMEM supplemented with 10% fetal bovine serum, 2 mm L-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Aliquots were plated on matrigel- (BD Bioscience, Oxford, UK) coated 24-well plates or glass-bottom culture dishes (Mattek Corporation, MA, USA) for secretion and imaging studies, respectively, and incubated for 1 to 7 d at 37 C, 5% CO2.
GLUTag cells were cultured in DMEM (5.5 mm glucose) supplemented with 10% fetal bovine serum, 2 mm L-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin.
GLP-1 secretion assay
Secretion studies were performed on primary cultures 24 h after plating. Wells were incubated with test substances for 2 h at 37 C. Cultures were preincubated for 10 min or 30 min if being cotreated with channel toxins or antagonists, respectively. Secreted and cellular GLP-1 were extracted as previously described (7), and active GLP-1 was quantified using an ELISA (Millipore, Watford, UK). GLP-1 secretion was expressed as a fraction of the total hormone (secreted + extracted) measured from a well and was normalized to the basal secretion measured under control conditions in parallel in the same set of experiments.
Calcium imaging
Experiments were performed on 3- to 7-d-old cultures. Cells were loaded with 7 μm Fura2-AM (Invitrogen, Paisley, UK) and 0.01% pluronic in standard bath solution containing 10 mm glucose and 300 μm eserine, for 30 min at 37 C. Experiments were performed using an inverted fluorescence microscope (Eclipse TE2000, Nikon, UK) with a ×40 oil-immersion objective. Fura2 was excited at 340/8 and 380/4 nm, and Venus at 475 nm, using a 75 W xenon arc lamp and a monochromator (Cairn Research, Faversham, UK) controlled by MetaFluor software (Molecular Devices, Wokingham, UK). Emission was recorded with a QuantEM CCD camera (Photometrics, Roper Scientific, UK) using a dichroic mirror and a 510-nm long pass filter. Fura2 fluorescence measurements were taken every 2 sec, background corrected, and expressed as the 340/380 nm ratio. Agents were added to the bath solution, perfused at ∼1 ml/min. Average fluorescence ratios were determined over 20-s periods, before addition of a test agent, during its perfusion, and after washout. Responses to a test agent were expressed as the mean ratio in test agent divided by the mean of the ratios measured before and after washout.
cAMP-measurements using Förster resonance energy transfer (FRET) probes
GLUTag cells were transfected with 3 μg of the FRET cAMP probe, Epac2-camps (11) using lipofectamine 2000 reagent (Invitrogen, Paisley, UK), overnight. The cells were then seeded onto matrigel-coated glass dishes and imaged 24–48 h later. Experiments were performed using an inverted fluorescence microscope (Nikon, UK) with a ×40 oil-immersion objective. The FRET probe was excited at 435/10 nm using a 75 W xenon arc lamp and a monochromator (Cairn Research) controlled by MetaFluor software (Molecular Devices). CFP and YFP emissions were separated using a Cairn Optosplit II Image splitter and acquired with an Orca-ER digital camera (Hamamatsu, Welwyn Garden City, UK) at 470/24 nm and 535/30 nm, respectively. Cells were excited every 5 sec, and fluorescence was recorded from individual L cells, background corrected, and expressed as the ratio of CFP over YFP. Reagents were prepared in standard saline and perfused at ∼1 ml/min. At the end of each experiment, cAMP was maximally elevated by coapplication of 10 μm forskolin (fsk) and 10 μm 3-isobutyl 1-methylxanthine (IBMX) (fsk/IBMX). Average fluorescence readings were determined over 20 sec before and at the peak of the response. Responses were calculated by subtraction of the baseline from the averaged peak response. All responses are expressed relative to the maximal response (fsk/IBMX) in that individual cell.
Flow cytometry
Venus positive cells were purified from GLU-Venus colonic tissue, digested as above but with a higher collagenase concentration of 1 mg/ml in Hanks' Balanced Salt Solution, to obtain single cells. Cell suspensions were separated using a MoFlo Beckman Coulter Cytomation sorter (488 nm excitation) to obtain populations of >95% pure Venus positive or negative cells, as described previously (7). Cells were sorted at numbers of up to 30,000 into lysis buffer for mRNA extraction.
RNA extraction and microarray analysis
Total RNA from FACS-sorted L and control cells (purified from three to four animals per run) and GLUTag cells was isolated using a micro scale RNA isolation kit (Applied Biosystems, Warrington, UK) and a TRI Reagent protocol, respectively. This then underwent two rounds of in vitro amplification (MessageAmp mRNA amplification kit, Applied Biosystems), during which target RNA was labeled with biotin-16-UTP (Enzo Life Sciences, Exeter, UK). RNA from GLUTag cells (n = 2) and FACS-purified L (n = 2) and non–L cells (n = 3) was hybridized against Affymetrix mouse 430 2.0 expression arrays. Expression levels of each probe were determined by robust multi-chip average analysis. Our experience is that robust multi-chip average values >100 represent expression levels that can be robustly verified by quantitative RT-PCR.
Analysis
Comparisons between conditions were made using regression analysis or by Student's t tests (Microsoft Excel), as indicated, with a threshold for significance of P < 0.05. The dose-response curve for Gln-triggered GLP-1 release was fit with a logistic equation y = A2 + [(A1 − A2)/(1 + [Gln]/EC50)]p̂ using Microcal Origin software. All data are expressed as mean ± se of the mean.
Results
L-Glutamine stimulates GLP-1 release from primary L cells
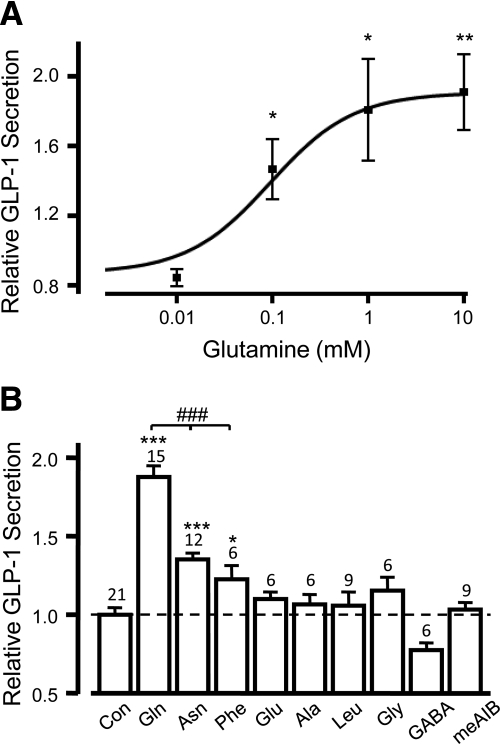
Gln dose-dependently stimulated the release of GLP-1 from primary murine colonic cultures, with an EC50 of 0.19 mm (n = 3–6, Fig. 1A), comparable to previous findings in GLUTag cells (9). The maximal response induced by 10 mm Gln was a 1.88 ± 0.07-fold elevation in GLP-1 secretion above basal (P < 0.001, n = 15, Fig. 1B). Other L-amino acids were tested, including the nonmetabolizable analog, 2-(methylamino)-isobutyric acid (meAIB), of which, only 10 mm asparagine (Asn) and 10 mm phenylalanine (Phe) significantly elevated GLP-1 release, by 1.35 ± 0.04 (P < 0.001, n = 12) and 1.22 ± 0.09 (P < 0.5, n = 6) fold, respectively (Fig. 1B). However, these responses were significantly smaller than that evoked by Gln (P < 0.001).
Fig. 1.
L-glutamine stimulates GLP1 secretion from primary intestinal cultures. Mixed primary cultures from the colon were incubated in bath solution containing amino acids as indicated. The percentage of GLP-1 secretion in each well is expressed relative to the basal secretion measured in parallel on the same day. A, A range of concentrations of glutamine (n = 3–6) was tested, and a dose-response curve was fitted through the entire data set using a logistic equation, giving an EC50 of 0.19 mm. Error bars represent 1 se, and significance is shown relative to baseline using a single-factor t test. *, P < 0.05; **, P < 0.01. B, GLP-1 secretion from primary colonic cultures triggered by a range of amino acids, all tested at 10 mm, as indicated. Data represent the mean and sem of the number of wells indicated, and significance is shown relative to baseline, tested by a single-factor t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The additional effect of glutamine was analyzed using Student's t test; ###, P < 0.001.
Elevation of Ca2+ in primary L cells
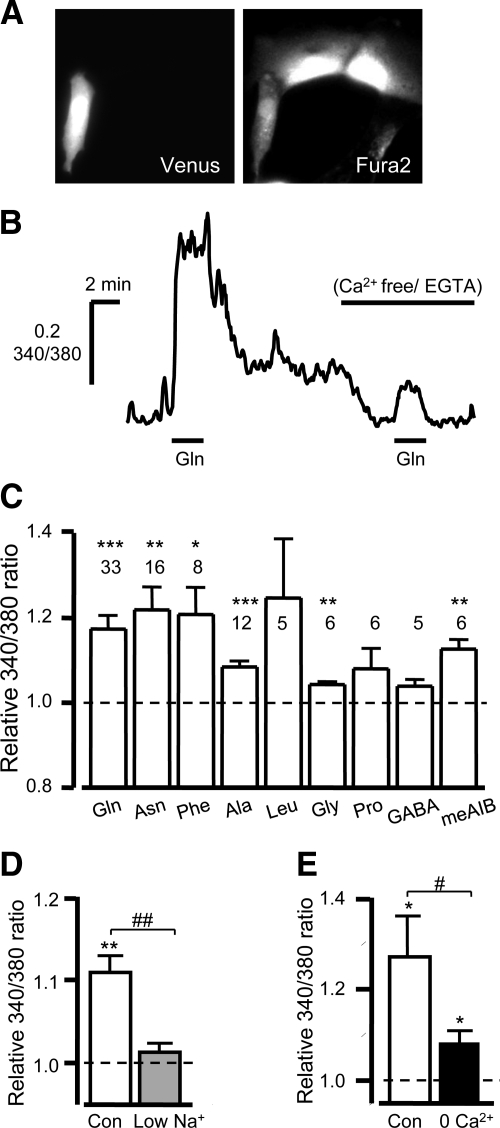
Given that elevation of Cai2+ is a key requirement for glutamine-stimulated secretion in GLUTag cells (9), we investigated the ability of Gln to mobilize Ca2+ in primary L cells. Cai2+ was measured in real time from individual L cells identified within a mixed colonic culture based on their Venus fluorescence and loaded with Fura2, as illustrated in Fig. 2, A and B. Gln (10 mm) induced a transient rise in the Fura2 340/380 fluorescence ratio (Fig. 2B), indicative of elevated Cai2+ (1.17 ± 0.03-fold change compared with baseline; P < 0.001, n = 33), similar to the fold increases of 1.22 ± 0.05 (P < 0.01, n = 16) and 1.21 ± 0.06 (P < 0.05, n = 8) triggered by 10 mm Asn and 10 mm Phe, respectively (Fig. 2C). In contrast to the negative findings of the secretion experiments, 10 mm alanine (Ala) and 10 mm glycine (Gly) also triggered small transient rises in Cai2+ (1.08 ± 0.01; P < 0.001, n = 12) and 1.04 ± 0.01 (P < 0.01, n = 6), respectively), whereas 10 mm leucine (n = 5), proline (n = 6), or γ-aminobutyric acid (n = 5) failed to elevate the Fura2 340/380 ratio significantly (Fig. 2C).
Fig. 2.
Glutamine evoked Cai2+ responses in cultured colonic L cells. A, Mixed colonic cultures were loaded with Fura2-AM, and L cells were identified by their Venus fluorescence (475 nm excitation, left). The image of Fura2-loaded cells was obtained by excitation at 340 nm (right). B, Representative trace showing the 340/380 nm fluorescence ratios (reflecting [Ca2+]i) recorded from an individual L cell after addition of 10 mm glutamine in the presence and absence of extracellular calcium. C, Mean calcium changes in L cells in response to a range of L amino acids (10 mm of each), as indicated. 340/380 fluorescence ratios in the presence of the test agent were normalized to the mean of the background ratios of each cell measured before addition and after washout of the test compound. D and E, Mean calcium changes in L cells in response to 10 mm L-Glutamine under control conditions (con) or when the majority of extracellular sodium (low Na+, gray bar, n = 8) or all calcium (0 Ca2+, black bar, n = 10) was removed. Data were normalized as described in C. Data represent the mean and sem of the number of cells indicated above each bar. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with baseline. #, P < 0.05; ##, P < 0.01 for comparison between control and test extracellular saline by Student's t test.
Stimulation of an electrogenic pathway
Interestingly, meAIB, known to be transported across the membrane by the Na+-dependent system A transporters SLC38A1 (SNAT1, ATA1) and SLC38A2 (SNAT2, ATA2) (12), elevated the Cai2+ signal by 1.13 ± 0.02 fold (P < 0.01, n = 6, Fig. 2C). Furthermore, the Gln-mediated elevation in Cai2+ was dependent on extracellular Na+ as the Ca2+ response was largely abolished when the majority of the Na+ was removed (P < 0.01 vs. Na+ containing solution, n = 8, Fig. 2D). Previously, in GLUTag cells, it was hypothesized that the Na+ influx via SNAT2 was sufficient to depolarize the membrane and activate voltage-gated Ca2+ channels (9), and indeed in the primary L cell the Gln-evoked Ca2+ response is largely dependent on extracellular Ca2+, as the Gln-induced Ca2+ response was reduced from 1.27 ± 0.09- to 1.08 ± 0.03-fold (P < 0.05, n = 8, Fig. 2, B and E) in Ca2+ free solution. A small but significant (P < 0.05) rise in Cai2+ remained, but the majority of the Gln-mediated Ca2+ rise appears to result from Ca2+ entry across the plasma membrane.
A number of Gln carrying transporters and exchangers are expressed in primary L cells (Table 1) which may contribute to Na+-dependent electrogenic Gln entry across the plasma membrane and could potentially trigger depolarization. The most highly expressed transporters in L cells are SNAT2 and B0AT1, the latter being specific to the L cell population in the colon.
Table 1.
Relative expression of glutamine-carrying members of the slc family, assessed by RMA analysis of microarray data
| Gene | Protein | L cell | RMA values |
Na+ | Electrogenic | ||
|---|---|---|---|---|---|---|---|
| Non–L cell | GLUTag | Mode | |||||
| Slc1a5 | ASCT2 | 903 | 786 | 289 | C/E | Na+a | − |
| Slc3a1 | rBAT | 113 | 167 | 38 | Heavy subunit of heteromeric transporter dimerises with Slc7a9 | ||
| Slc3a2 | 4F2hc | 704 | 1298 | 3129 | Heavy subunit of heteromeric transporter dimerises with Slc7a5-8 & 10 | ||
| Slc6a14 | ATB0,+ | 691 | 655 | 9 | C | Na+ Cl− | + |
| Slc6a15 | SBAT1 | 7 | 7 | 3991 | C | Na+ | + |
| Slc6a19 | b0AT1 | 2270 | 38 | 176 | C | Na+ | + |
| Slc7a5 | LAT1 | 124 | 75 | 865 | E | − | − |
| Slc7a6 | y+LAT2 | 240 | 151 | 1260 | E | Na+ | − |
| Slc7a7 | y+LAT1 | 313 | 465 | 76 | E | Na+ | − |
| Slc7a8 | LAT2 | 27 | 22 | 260 | E | − | − |
| Slc7a9 | b0,+AT | 83 | 104 | 32 | E | − | − |
| Slc7a10 | Asc-1 | 14 | 15 | 28 | E | − | − |
| Slc38a1 | SNAT1 | 560 | 420 | 5192 | C | Na+ | + |
| Slc38a2 | SNAT2 | 959 | 1136 | 6700 | C | Na+ | + |
| Slc38a3 | SNAT3 | 70 | 72 | 147 | C/E | Na+a | − |
| Slc38a4 | SNAT4 | 9 | 9 | 11 | C | Na+ | − |
| Slc38a5 | SNAT5 | 11 | 14 | 22 | C/E | Na+a | − |
| Slc43a1 | LAT3 | 54 | 54 | 87 | F | − | − |
| Slc43a2 | LAT4 | 94 | 95 | 149 | F | − | − |
| Slc43a3 | EEG1 | 71 | 276 | 21 | Orphan transporter | ||
Final three columns adapted from http://www.bioparadigms.org/slc/menu.asp.
C, cotransporter; E, exchanger; F, facilitated transporter.
Cotransporter mode dependent on Na+.
Glutamine stimulates secretion via an additional nonelectrogenic pathway
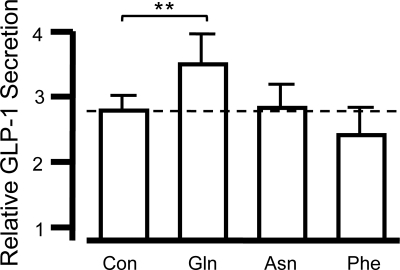
In GLUTag cells, Gln was previously found to potentiate GLP-1 secretion by an additional pathway acting downstream or independent of membrane depolarization and Ca2+ entry (9). This was further examined in primary murine L cells, by performing static secretion experiments in the presence of 75 mm KCl and 340 μm diazoxide. Diazoxide, a KATP channel activator, is added to help ‘clamp’ the membrane at the potential predicted by the Nernst equation for K+, resulting in a sufficiently depolarized membrane potential to activate voltage-gated Ca2+ entry. As expected, given the excitable nature of L cells, 75 mm KCl/diazoxide triggered significant release of GLP-1 (2.78 ± 0.23 fold increase, compared with 4.5 mm KCl in diazoxide; P < 0.001, n = 12; Fig. 3). Under these conditions, 10 mm Asn or Phe failed to stimulate any further secretion (n = 6), but 10 mm Gln significantly enhanced GLP-1 release by a further 37% (P < 0.01, n = 6), indicative that a nonelectrogenic pathway is activated by Gln.
Fig. 3.
Glutamine stimulates a nonelectrogenic pathway. Mixed primary cultures from the colon were incubated in bath solution containing 75 mm KCl and 340 μm diazoxide (con, n = 12) alone or in combination with 10 mm amino acid as indicated (each n = 6). The percentage of GLP-1 secretion in each well is expressed relative to the basal secretion measured in parallel on the same day. Data represent the mean and sem. **, P < 0.01 compared with KCl/diazoxide control by Student's t test.
Potential intracellular signaling pathways
Gln is involved in a number of metabolic pathways and is the primary metabolite for enterocytes in the small intestine (13). Potentially, these metabolic pathways or other intracellular amino acid targets may be responsible for the additional pathway evoked by Gln. Inhibition of metabolism to glutamate or of pyrimidine synthesis using 50 μm 6-diaza-5-oxo-L-norleucine (DON), or purine synthesis using 100 μm mycophenolic acid, had no effect on the Gln-evoked GLP-1 response (Table 2). Another potential fate of glutamine is metabolism to glucosamine, and so in the absence of a specific inhibitor of glucosamine synthesis, we examined the effect of 10 mm glucosamine itself. Glucosamine should be transported directly into L cells via GLUT2, which is expressed in the L cell (7). However, direct application of glucosamine failed to stimulate secretion of GLP-1 release and actually decreased GLP-1 levels below basal (Table 2). Gln (1 mm) was also tested in the presence of 0.2 μg/ml rapamycin to investigate any effect of the mTOR pathway, another known target of intracellular amino acids (14). Rapamycin did not interfere with the response to Gln (Table 2).
Table 2.
Effects of inhibition of intracellular enzyme targets and components of the Gq pathway on basal and stimulated GLP1 release
| Inhibitor | Baseline | 1 mm Glutamine |
|---|---|---|
| 50 μmol/liter DON | 1.15 ± 0.14 ns n = 3 | 1.77 ± 0.08 ns n = 3 |
| 100 μmol/liter MPA | 0.97 ± 0.05 ns n = 3 | 1.93 ± 0.13 ns n = 3 |
| 10 mmol/liter glucosamine | 0.59 ± 0.04* n = 3 | n.t. |
| 0.2 μg/ml rapamycin | 0.97 ± 0.08 ns n = 6 | 1.50 ± 0.12 ns n = 3 |
|
10 mm Glutamine |
||
| 10 μmol/liter U73122 | 1.27 ± 0.07** n = 9 | 2.29 ± 0.13 ns n = 9 |
| 10 μmol/liter U73343 | 1.25 ± 0.11 ns n = 6 | 1.92 ± 0.14 ns n = 3 |
| 1 μmol/liter Gö6893 | 1.00 ± 0.07 ns n = 6 | 2.22 ± 0.14 ns n = 6 |
| 10 μmol/liter 2-APB | 0.93 ± 0.09 ns n = 6 | 3.02 ± 0.55 ns n = 6 |
| 10 μmol/liter Thapsigargin | 1.91 ± 0.08*** n = 6 | 4.03 ± 0.19** n = 6 |
| Thapsigargin & 2-APB | 1.36 ± 0.15 ns n = 3## | n.t. |
GLP-1 release is expressed relative to that measured in parallel control wells containing no glutamine or inhibitor. Data are presented as means ± sem, significance, n. Significance was evaluated using Students t test comparing either baseline secretion in the absence and presence of inhibitor, or glutamine-triggered secretion in the absence and presence of inhibitor; ns, not significant;
, P < 0.05;
, P < 0.01;
, P < 0.001;
, P < 0.01 when compared to thapsigargin alone. n.t., Not tested.
Given the lack of effect of targeting known intracellular targets or pathways that use Gln, we next considered the possibility that Gln might activate surface receptors on L cells. In the past few years a number of G protein–coupled receptors (GPCR) have been shown to respond to amino acids (for review, see Ref. 15), although none have been found to respond preferentially to Gln. Given that small rises in Cai2+ persisted in the absence of extracellular Ca2+, consistent with Ca2+-release from intracellular stores (Fig. 2, B and E), we examined the potential involvement of Gq-coupled pathways. Gq activation leads to the activation of protein kinase C, a potent secretory stimulus in many cell types including enteroendocrine cells (7). Inhibitors of phospholipase C (10 μm U73122, and its negative control U73343), protein kinase C (1 μm Gö6893), and inositol 1,4,5-trisphosphate receptors (100 μm 2-aminoethoxydiphenyl borate) did not, however, affect GLP-1 release evoked by 10 mm Gln (Table 2).
We also investigated the importance of store-mediated Ca2+ mobilization in the Gln-induced GLP-1 response, using 10 μm thapsigargin. Thapsigargin depletes intracellular Ca2+ stores, thus abolishing any potential Gln-triggered stored Ca2+ release, but may also stimulate a store-operated Ca2+ entry pathway. In line with this idea, thapsigargin itself triggered GLP-1 secretion 1.91 ± 0.08-fold (P < 0.001, n = 6, Table 2), an effect inhibitable by 2-aminoethoxydiphenyl borate. However, thapsigargin did not prevent Gln-triggered secretion, which was further enhanced 2.11 ± 0.06 fold, comparable to the control Gln-evoked response (n = 6, Table 2).
Glutamine elevates intracellular cAMP (cAMPi)
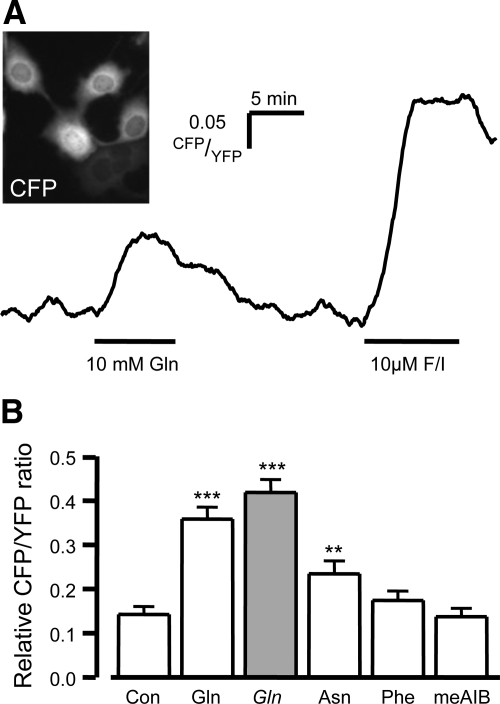
An alternative signaling pathway could involve elevation of cAMPi which is a known potent L cell stimulus (16) (7). We investigated this using a cAMP FRET sensor based on Epac2, which we validated recently for monitoring cAMP levels in single GLUTag cells (11, 17) (Fig. 4A). Gln (10 mm) evoked a significant rise in the CFP/YFP ratio in GLUTag cells compared with vehicle control, indicative of elevated cAMPi (P < 0.001, Fig 4, A and B). The response to Gln was 36 ± 3% (n = 72) of that produced by a maximal stimulus induced by 10 μm fsk/IBMX (Fig. 4, A and B). By comparison, the response to 10 mm Asn was smaller, at 24 ± 3% of maximal (P < 0.01 compared with vehicle, n = 52; Fig. 4B), whereas 10 mm Phe failed to stimulate a significant response (n = 43). In contrast to the Ca2+ mobilization experiments, 10 mm meAIB failed to elevate cAMPi (n = 44), and removing the extracellular Na+ had no effect on the Gln-evoked FRET response (42 ± 3%, n = 36; Fig. 4B).
Fig. 4.
Glutamine-evoked cAMPi responses in GLUTag cells. GLUTag cells were transfected with the cAMP probe, Epac2 camps. FRET was measured as the YFP/CFP ratio by exciting the cells at 435/10 nm (A inset shows example image) A, Representative trace showing the CFP/YFP fluorescence ratio (reflecting [cAMP]i) recorded from an individual cell after addition of 10 mm glutamine and the positive control 10 μm forskolin and 10 μm IBMX (F/I). B, Mean cAMP changes in response to a vehicle control (con) and a range of L amino acids (10 mm of each), as indicated (open bars). Gln (10 mmol/liter) was also tested in the absence of extracellular Na+ (Gln, gray bars). CFP/YFP ratios in the presence of the test agent were expressed relative to the positive control performed in each experiment, as shown in A. Data represent the mean and sem. **, P < 0.01; ***, P < 0.001 compared with vehicle by Student's t test.
Discussion
In primary murine L cells, L-glutamine is highly effective at stimulating the release of GLP-1. This appears to be attributable to its ability to trigger two independent pathways—a priming step that increases cell excitability and an amplification step acting through elevation of cAMPi.
In GLUTag cells we previously described both an electrogenic triggering pathway likely attributable to SLC38A2 and a Gln-dependent amplification pathway, the molecular details of which remained unclear (9). Given the similarities between the GLUTag and primary L cell results, the GLUTag cell line appears a valid model for investigating Gln-evoked signaling. However, not all GLUTag amino acid responses were reproduced in the primary cells: Ala and Gly for example triggered GLP-1 secretion from GLUTag cells via a pathway involving ionotropic Gly receptors (18), but we found no evidence for this pathway in primary colonic cultures.
L cells in culture display a basal tone of electrical activity, observed as infrequent Na+-dependent action potentials (7), that maintains a background level of GLP-1 secretion. Clamping the membrane potential at a more hyperpolarized level with diazoxide, or inhibiting voltage gated Na+ channels or L- and Q-type voltage-gated Ca2+ channels decreased basal GLP-1 secretion from primary cultures (Rogers et al., unpublished observations), presumably by blocking action potential firing and reducing intracellular Ca2+ levels. The finding that the Gln-evoked Ca2+ response was markedly attenuated by the removal of extracellular Na+ or Ca2+ supports the involvement of Na+-dependent electrogenic transporters and a Ca2+ influx pathway in mediating this initial response to amino acids. In GLUTag cells we showed previously that amino acid transport induced a small Na+-dependent inward current of ∼3 pA/cell, which increased L cell excitability and firing rate (9), potentially also making the cell more responsive to further stimuli.
Amino acids such as glutamine and Ala, which are known substrates of system A transporters including SNAT2, increased Cai2+ to a similar degree in L cells, consistent with the expression of slc38 members by microarray (Table 1). Even though SNAT2 is believed to be located basolaterally (19), given the high level of expression of slc38a2 and the effect of meAIB on Cai2+ in L cells, it is likely that this member of the Slc family is involved in the Gln-mediated response. Interestingly, slc6a19 (B0AT1), a Na+-dependent electrogenic transporter, was the most highly expressed of the Gln-carrying Slc members (Table 1), and was highly enriched in L cells compared with their neighbors. B0AT1 is reported to localize to the apical membrane of intestinal cells (20) where it would be exposed to the luminal contents. Although its role in the Gln-evoked GLP-1 response is currently unclear, given that it is highly enriched in the L cell, it may play a significant role in the response of the L cell to luminal neutral amino acids.
In contrast to the Ca2+ elevation evoked by a number of amino acids, the ability of amino acids to increase cAMP more closely mirrors the results of the GLP-1 secretion experiments. Gln and to a lesser extent Asn, but not Phe or meAIB, elevated intracellular levels of cAMP. This observation suggests that the cAMP-dependent pathway is critical in determining whether an amino acid effectively triggers and maintains GLP-1 secretion over a 2-h incubation period. In GLUTag cells, cAMP modulates plasma membrane ion channels resulting in depolarization and increased excitability (16), thereby tending also to mobilize Cai2+ (7). However, its principal action is probably on downstream targets, resulting in enhanced vesicle exocytosis by Epac- and PKA-dependent pathways, as described in other endocrine cells (reviewed in Ref. 21). Finally, Gln also stimulated a small but significant Ca2+ response in the absence of extracellular Ca2+, suggesting that it may also trigger the release of Ca2+ from an intracellular store.
Given the lack of effect of inhibiting uptake, known intracellular targets or metabolic pathways that use Gln, it would seem that any underlying Gln ‘sensor’ must be localized at an extracellular site, suggesting the possibility that it may involve a GPCR. This is also supported by the finding that removing extracellular Na+ did not prevent the Gln-triggered cAMP rise, indicating that Na+-coupled uptake is not required for activation of this signaling pathway. Three members of the Class C GPCR family have been shown to respond to L-amino acids, although none reportedly show a preference for glutamine: the umami taste receptor, Tas1R1/Tas1R3, the calcium-sensing receptor, CasR, and the less well characterized GPRC6A receptor (for review, see Ref. 15). Involvement of the former two is unlikely, as their amino acid rank potency and known downstream pathways do not match the pattern described in this article. The umami taste receptor and CasR are believed to couple predominantly to α-gustducin and Gq pathways, respectively, leading to the activation of phospholipase C. GPRC6A has been shown to activate Gq pathways and to favor basic amino acids. If GPRC6A were the molecular candidate, however, one would expect to observe GLP-1 secretion in response to a number of other amino acids given the receptor's promiscuity. Furthermore, mRNA data obtained from purified primary L cells suggest that Tas1R1/Tas1R3, CasR and GPRC6A are expressed at such low levels that one might question their functional significance (Table 3). Our data therefore suggest there may be another amino acid receptor/sensor with a preference for Gln, perhaps comprising another family C GPCR, yet to be identified. The recently observed heterodimerization of family C GPCRs, which include the metabotropic glutamate receptors and amino acid receptors (15), raises the possibility that a glutamine-selective receptor might be generated from a combination of different members of this family.
Table 3.
MicroArray expression of amino acid–sensing GPCRs
| Name | Gene | RMA values |
||
|---|---|---|---|---|
| Non–L cell | Non–L cell | GLUTag | ||
| Calcium-sensing receptor | Casr | 59 | 42 | 103 |
| GPRC6A | GPRC6A | 31 | 34 | 92 |
| T1R Taste receptor 1 | Tas1R1 | 48 | 54 | 117 |
| T1R Taste receptor 3 | Tas1R3 | 60 | 63 | 102 |
Conclusion and physiological perspective
Previously, we showed that Gln triggered electrical activity in GLUTag cells, but we were unable to explain the amplifying pathway responsible for the greater effect of Gln compared with other amino acids. The findings did, however, spark an interest in the potential use of Gln as a GLP-1 secretagogue in human subjects, and it is notable that an effect originally identified in a cell line was subsequently reproduced in type 2 diabetic and obese subjects (10), and has now been further validated in studies on primary L cells. The present results suggest that Gln-triggered membrane depolarization and elevation of cAMP act synergistically on L cells and account for the particular effectiveness of this amino acid on GLP-1 secretion. The findings raise the possibility that there may exist a Gln-responsive membrane receptor coupled to Gs signaling pathways.
The EC50 for glutamine triggered GLP-1 secretion from primary L cells (∼0.2 mm) is close to normal plasma glutamine concentrations, which range from 0.1 to 1 mm (10). Although orally administered glutamine enhanced GLP-1 release in human subjects, it is not clear whether glutamine stimulates the L cell from the apical or basolateral surface. Under physiological conditions, it is likely that L cells would be activated by dietary glutamine, released in the gut lumen by protein digestion.
Agents are currently under development that will stimulate GLP-1 release in vivo by activation of L cell specific adenylate cyclase coupled receptors, such as GPR119 and the bile acid receptor, TGR5. Our data suggest these will be maximally effective when combined with a triggering stimulus, such as luminal signals from ingested nutrients, and that synergy between signaling pathways should be considered when devising strategies to enhance GLP-1 release therapeutically.
Supplementary Material
Acknowledgments
We thank Martin Lohse (Wuerzburg, Germany) for the gift of the Epac2-camps FRET probe, Daniel Drucker (Toronto, Canada) for the GLUTag cell line, and Drs. Giles Yeo and Ian McKenzie (MRC-CORD, Cambridge) for assistance with microarray analysis.
This work was supported by the Wellcome Trust (grant number WT088357 and WT084210) and the Lister Institute for Preventive Medicine.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- Cai2+
- Intracellular calcium
- cAMPi
- intracellular cAMP
- FRET
- Förster resonance energy transfer
- fsk
- forskolin
- GLP-1
- glucagon-like peptide 1
- GPCR
- G protein–coupled receptor
- IBMX
- 3-isobutyl 1-methylxanthine
- meAIB
- 2-(methylamino)-isobutyric acid.
References
- 1. Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Göke B. 1992. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22:283–291 [DOI] [PubMed] [Google Scholar]
- 2. Baggio LL, Drucker DJ. 2007. Biology of Incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 3. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. 1986. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29:46–52 [DOI] [PubMed] [Google Scholar]
- 4. Drucker DJ, Nauck MA. 2006. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 5. Migrenne S, Marsollier N, Cruciani-Guglielmacci C, Magnan C. 2006. Importance of the gut-brain axis in the control of glucose homeostasis. Curr Opin Pharmacol 6:592–597 [DOI] [PubMed] [Google Scholar]
- 6. Reimann F. 2010. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J 20:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. 2008. Glucose sensing in L cells: a primary cell study. Cell Metab 8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. 2009. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. 2004. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47:1592–1601 [DOI] [PubMed] [Google Scholar]
- 10. Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. 2009. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr 89:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. 2004. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279:37215–37218 [DOI] [PubMed] [Google Scholar]
- 12. Mackenzie B, Erickson JD. 2004. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch 447:784–795 [DOI] [PubMed] [Google Scholar]
- 13. Neu J, Shenoy V, Chakrabarti R. 1996. Glutamine nutrition and metabolism: where do we go from here? FASEB J 10:829–837 [DOI] [PubMed] [Google Scholar]
- 14. Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. 2009. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 296:E592–E602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conigrave A, Hampson DR. 2010. Broad-spectrum amino acid-sensing class C G-protein coupled receptors: Molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther 127:252–260 [DOI] [PubMed] [Google Scholar]
- 16. Simpson A, Ward P, Wong K, Collord G, Habib A, Reimann F, Gribble F. 2007. Cyclic AMP triggers glucagon-like peptide-1 secretion from the GLUTag enteroendocrine cell line. Diabetologia 50:2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedlander RS, Moss CE, Mace J, Parker HE, Tolhurst G, Habib AM, Wachten S, Cooper DM, Gribble FM, Reimann F. 3 November 2010. Role of phosphodiesterase and adenylate cyclase isozymes in murine colonic glucagon like peptide 1 secreting cells. Br J Pharmacol 10.1111/j.1476-381.2010.01107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gameiro A, Reimann F, Habib AM, O'Malley D, Williams L, Simpson AK, Gribble FM. 2005. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol 569:761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilde SW, Kilberg MS. 1991. Glutamine transport by basolateral plasma-membrane vesicles prepared from rabbit intestine. Biochem J 277:687–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bröer A, Klingel K, Kowalczuk S, Rasko J, Cavanaugh J, Bröer S. 2004. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. J Biol Chem 279:24467–24476 [DOI] [PubMed] [Google Scholar]
- 21. Szaszák M, Christian F, Rosenthal W, Klussmann E. 2008. Compartmentalized cAMP signalling in regulated exocytic processes in non-neuronal cells. Cell Signal 20:590–601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.