Abstract
The omentum, external oblique musculocutaneous, and thoracoepigastric flaps are uncommonly used for chest wall reconstruction. Nevertheless, awareness and knowledge of these flaps is essential for reconstructive surgeons because they fill specific niche indications or serve as lifeboats when workhorse flaps are unavailable. The current report describes the anatomic basis, technical aspects of flap elevation, and indications for these unusual flaps.
Keywords: Omental flap, external oblique muscle flap, thoracoepigastric flap, chest wall
Chest wall defects arise in the setting of trauma, infection, neoplasm, or radiation-induced skin damage. The defect may involve the skin only or include all the chest wall lamina such as muscle and bone. The pectoralis major, latissimus dorsi, and vertical rectus abdominis (VRAM) are established workhorse flaps used in 63 to 78% of chest wall reconstructions.1,2 Preferential use of these flaps is based on anatomic considerations, reliability, and simplicity. Although used less frequently, alternative pedicle flaps may be employed. Herein, the anatomic basis, technical aspects of flap elevation, and indications for the omental, external oblique musculocutaneous, and thoracoepigastric flaps in chest wall reconstruction are described.
OMENTAL FLAP
Kiricuta was the first to report use of the omentum for extraperitoneal reconstruction such as in repair of bronchopleural fistula, chest wall defects, and extremity lymphedema.3,4,5 Since then, its effectiveness as either a pedicle or free flap for reconstruction around the body has been widely recognized. Formed by the communication of the right and left gastroepiploic arteries, the gastroepiploic arch of the stomach's greater curvature provides the blood supply to the omentum. Within the substance of the omental apron, the gastroepiploic arcade gives off the right, middle, and left omental vessels. Although anatomic variants exist, as the omental vessels terminate distally they join forming right and left arterial arcades.6
Flap elevation can be performed either through a laparotomy or laparoscopically.7,8 Benefits of the laparoscopic approach include examination of omentum size without formal laparotomy, smaller fascial incision, and reduced rates of postoperative ileus. The flap is elevated from the antimesenteric side of the transverse colon in the avascular plane. As a Mathes and Nahai type III flap with two dominant pedicles, the omental flap can be based on either gastroepiploic vessel; however, due to its larger caliber, the flap is preferentially elevated on the right gastroepiploic artery.9 The omentum is separated from the stomach through serial division of short arteries between the gastroepiploic arcade and greater curvature. Based on knowledge of its arterial anatomy, the omentum can be used in either its native configuration or extended by dividing the apron according to the orientation of the gastroepiploic and omental vessels. Using lengthening techniques, the omentum has been reported to reach the skull vault, mid-leg, and mid-forearm in all cases.6,10 Based on surgeon preference and defect location, the flap is tunneled either subcutaneously or transdiaphragmatically.
The greater omentum has many properties that make it valuable in chest wall reconstruction. The dual dominant blood supply and vascular anastomoses of the omental arcades allow customized flap tailoring according to the defect present. As a vestigial organ, the omentum is the ideal donor tissue for reconstruction because patients suffer no functional loss. The large surface area size of the omentum, averaging 25 cm in length and 34 cm in width, is a great advantage compared with other commonly used flaps.10 The long flap pedicle provides a large arc of rotation permitting it to reach either side of the body. The omentum reliably heals wounds because of its rich blood supply and intrinsic immunologic properties.11,12 Lastly, the malleable and pliable nature of the omentum allows complete obliteration of small dead-space areas not routinely filled by other donor tissues.
Disadvantages associated with the omentum have relegated it to a second-line flap by some reconstructive surgeons. Unlike fasciocutaneous or musculocutaneous flaps, which lie outside major body cavities, harvest of the omentum flap involves entering the peritoneal space. As a result, intra-abdominal organs such as the spleen, stomach, and intestine are subject to bleeding, perforation, and ileus. An inability to predict the omentum size is another barrier to its use in reconstruction. Das found weak correlations between patient weight, height, and gender and omental length in 200 cadavers and at 100 laparotomies.10 Studies using modern imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) to assess omental size are not reported, but these modalities are not thought to be helpful either. Because the omentum has no cutaneous paddle, skin grafting is required unless used as a buried flap. When the flap is used to cover prosthetic materials such as polytetrafluoroethylene or methyl methacrylate, absent skin-to-skin apposition with a watertight closure may contribute to infection. Unless used as a free flap, tunneling of the omentum pedicle creates a hernia at either the abdominal fascia or diaphragmatic rent (Fig. 1). Symptomatic hernia rates range from 0 to 21%, leading some authors to advocate the transdiaphragmatic route.13,14,15 Techniques to reduce symptomatic hernia formation include limited fascial opening through laparoscopic approaches or creation of a partial omental wrap at the diaphragm defect.13,16,17 In cases of delayed hernia presentation, the simplest method of repair is omental division with defect closure.
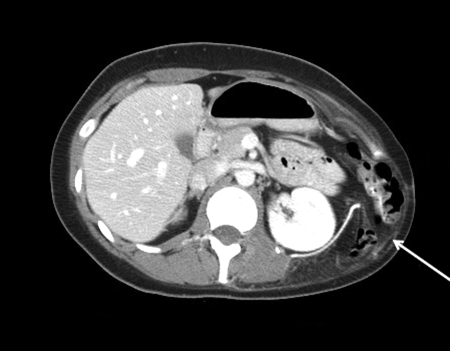
Figure 1.
Reconstruction of an inferolateral chest wall defect with Gore-Tex mesh and an omental flap. CT scan 3 months postoperatively demonstrates herniation of bowel through the omental flap tunnel.
Rates of omental flap use in chest wall reconstruction vary by surgeon preference and indication. In an account of 500 chest wall reconstructions for oncologic, infectious, traumatic, and radiation-induced defects, Arnold and Pairolero used the omentum flap in 10% of patients.1 At our oncologic reconstructive unit, the greater omentum was used for chest wall reconstruction in only 5% of patients.2 We find the omentum is most beneficial in coverage of large central chest defects, particularly when the VRAM flap is unavailable (Fig. 2). For defects located lower around the costal margin, the omentum is an alternative to the external oblique flap. In general, musculocutaneous flaps are preferable to an omentum with overlying skin graft when a prosthetic material is being covered to ensure a watertight seal. Although not a workhorse flap, the omentum is a robust, reliable flap that readily achieves closure of chest wall and other defects around the body.
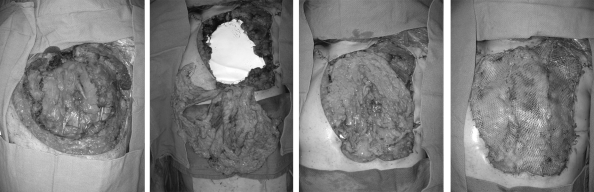
Figure 2.
Reconstruction of a central chest wall defect after a sternectomy. Exposed mediastinal structures (left); placement of Gore-Tex mesh to prevent evisceration along with transdiaphragmatic harvest of an omental flap (center left); the omental flap easily covers the defect while sealing small areas of dead space (center right); a meshed skin graft was covered with a negative-pressure sponge (not shown) until postoperative day 5 (right).
EXTERNAL OBLIQUE FLAP
The external oblique is a type V muscle with both dominant and multiple segmental vascular pedicles.9 The dominant deep circumflex iliac artery (DCIA) originates from the lateral aspect of the external iliac artery, enters the transversus abdominis muscle 6 to 10 cm lateral to the anterior superior iliac spine, and courses superomedially along the abdominal wall to provide perforating branches to the external oblique muscle and overlying skin.18 In 6% of cadavers, the iliac branch of the iliolumbar artery, traveling in a similar path to the DCIA branch, was the only artery entering the external oblique muscle near the iliac crest. The segmental supply to the external oblique muscles is derived from the 5th through 12th posterior intercostal arteries. As the intercostal vessels course circumferentially around the torso between the internal oblique and transversus abdominis muscles, lateral perforators are given off in the midaxillary line that pierce the underside of the external oblique muscle.19
The laterally based external oblique musculocutaneous flap is designed on the abdominal wall with a vertical incision just beyond the midline and horizontal incision at the level of the umbilicus. Alternatively, a V-shaped pennant extending inferior to the umbilicus can be included with the flap to eliminate a dog-ear that tends to develop in this area.20 The superior aspect of the flap lies adjacent to the chest wall defect. Flap elevation commences with a midline skin incision and proceeds laterally either with or without inclusion of the anterior rectus sheath. The anterior rectus sheath is not crucial for flap survival as the pedicles enter laterally; however, this fascia provides a sturdy layer for insetting. Dissection continues laterally in the plane between the external and internal oblique muscles until intercostal vessel perforators are visualized. To facilitate cephalad flap transposition, the external oblique muscle can be detached from the ribs and/or iliac crest, but this maneuver should be performed cautiously to preserve the DCIA pedicle. The flap can reach up to the second intercostal space and 5 cm beyond the midline.20,21 When the anterior rectus sheath is harvested with the flap, fascial closure with either mesh or plication of the internal oblique muscle sheath to the linea alba is recommended to avoid hernia formation.20
Beyond chest wall reconstruction, other applications of the external oblique muscle flap are described. For example, it may be rotated caudally to cover hemipelvectomy defects or used as a turnover flap to cover infero-posterior back wounds.22,23,24 Finally, the external oblique muscle or musculocutaneous flap can be used in free tissue transfer when elevated on the dominant DCIA pedicle.18
Inherent advantages and unique features of the external oblique flap ensure its place in the reconstructive armamentarium. Flap elevation can be performed expeditiously due to an absence of critical structures in the region and multiple supplying vessels. Maybe the greatest advantage of this flap is the large surface area it provides for defect coverage. In a series of oncologic chest wall reconstructions, the external oblique flap achieved closure of the largest chest wall defects measuring an average area of 391 sq cm.2 Furthermore, the donor area is closed primarily without skin grafting in all instances by undermining the remaining abdominal wall. Because segmental nerves travel with the intercostal pedicles, some sensation to the flap is preserved. Finally, compared with other commonly used flaps, such as the latissimus dorsi, the patient does not need repositioning during the procedure. Undesirable features, such as a lack of bulk, may limit use of the external oblique flap. As such, the flap is not routinely used for breast mound reconstruction, although this indication is reported.25 The large area of the external oblique muscle necessitates numerous and lengthy incisions creating opportunities for wound breakdown. Lastly, hernia development can occur when the anterior rectus fascia is harvested in conjunction with the flap.
Although there are limited applications for the external oblique musculocutaneous flap, it fills a niche indication in patients undergoing radical mastectomy for locally advanced or recurrent breast cancer (Fig. 3). The largest series by Bogossian et al in 20 patients reported only one partial flap loss that required revision.21 The flap is also useful in elderly or comorbid patients where the primary goal is rapid defect closure. For the subgroup of patients with lower chest defects, when other workhorse flaps are unavailable, the external oblique flap is a valuable alternative.
Figure 3.
Reconstruction of a radical mastectomy defect with an external oblique musculocutaneous flap. Design of an ipsilateral flap extending inferior to the umbilicus (left); flap elevation (center); final defect closure (right).
THORACOEPIGASTRIC FLAPS
Thoracoepigastric flaps, also referred to as thoracoabdominal flaps, have a segmental blood supply. Relevance of segmental abdominal wall anatomy to transverse thoracoepigastric flaps was delineated by Brown et al in 1975.19 Three main sources of vessels supply the anterior and lateral abdominal wall: (1) perforating branches of the intercostal and lumbar arteries; (2) perforators of the epigastric arcade; and (3) the superficial inferior epigastric artery. Therefore, medially based thoracoepigastric flaps receive perforating vessels from the epigastric arcade and are considered analogous to the more cephalad deltopectoral flap.26 Laterally based flaps are nourished by intercostal and lumbar artery perforators. The segmental blood supply at the base of thoracoepigastric flaps distinguishes them from and makes them more reliable than random pattern flaps.
Thoracoepigastric flaps are designed as transposition flaps. An oblique or transverse flap configuration ensures inclusion of multiple rows of segmental perforators at the base. Widening the base improves vascularity through inclusion of more perforators. Determination of flap length is uncertain. Posterior or laterally based flaps crossing the midline are reported, but the blood supply is tenuous.27 Conversely, anteriorly or medially based flaps become unreliable beyond the anterior axillary line.28 Despite the axial blood supply to thoracoepigastric flaps, they behave more like random flaps, so length-width ratios exceeding 1.5:1 are not recommended.27,29 Flaps can be raised either above or below the level of the rectus fascia and investing fascia of the external oblique musculature. Donor site closure is achieved primarily for laterally based flaps through abdominal wall undermining, whereas skin grafting is often required for medially based flaps.30
Thoracoepigastric flaps are indicated for defects located in the lower thoracic region, similar to the external oblique flap. Although thoracoepigastric flaps have fallen by the wayside in favor of more reliable muscular and musculocutaneous flaps, two recent series report good outcomes with extended thoracoabdominal flaps. In a series of 18 patients with radical mastectomy defects ranging in size from 15 × 15 cm to 25 × 30 cm, Persichetti et al reported partial- and full-thickness flap loss in 3 and 1 patients, respectively.29 Deo et al demonstrated increased abdominal wall morbidity and blood loss and lengthier hospital stays in patients who underwent musculocutaneous compared with thoracoabdominal flap reconstructions for radical mastectomy defects.31 It is worthwhile to note that in both these series, thoracoepigastric flaps were elevated without the abdominal fascia raising questions about the added value of this layer to flap vascularity.28
The principle disadvantage of thoracoabdominal flaps is the random blood supply to the distal portion of the flap with the potential for delayed wound healing. To ensure adequate vascularity to the tip of larger flaps a delay procedure may be required. Broader based flaps ensure adequate perforators but restrict the arc of rotation. Furthermore, broadly based flaps are complicated by dog-ears that may require future revision. In general, these flaps are advisable for patients with advanced disease who are unlikely to need further procedures including breast reconstruction. Harvest of these flaps precludes future transverse rectus abdominis myocutanous (TRAM) flap elevation but does not rule out use of the contralateral VRAM flap. Compared with musculocutaneous flaps, the lack of bulk in thoracoepigastric flaps is advantageous for contouring in some instances. Color match is superior because the skin comes from the immediately adjacent region. These flaps have minimal donor site morbidity especially when the fascia is spared.
A final alternative to consider in extreme cases of chest wall reconstruction is use of the contralateral breast as a donor flap. Although different variations are described, a large pendulous breast is partially divided, undermined, and advanced as necessary to cover contralateral chest wall defects.15,32 Unfurling of the breast in this manner shifts the nipple areola complex to the midline creating a cyclops appearance.33 Blood supply is based on the lateral thoracic artery. Advantages of this technique are creation of a thick well-padded flap that can be performed quickly. Obvious disadvantages are the poor cosmesis and risk of malignancy in the donor breast.
CONCLUSION
Understanding the intricacies of the omentum, external oblique, and thoracoepigastric flaps is essential for the reconstructive surgeon. Although used uncommonly, unique features of these flaps ensure their place in the reconstructive armamentarium for chest wall defects.
References
- Arnold P G, Pairolero P C. Chest-wall reconstruction: An account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–810. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Chang R R, Mehrara B J, Hu Q Y, Disa J J, Cordeiro P G. Reconstruction of complex oncologic chest wall defects: A 10-year experience. Ann Plast Surg. 2004;52:471–479. discussion 479. doi: 10.1097/01.sap.0000122653.09641.f8. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz M J, Arnold P G. The omentum: An account of its use in the reconstruction of the chest wall. Ann Surg. 1977;185(5):548–554. doi: 10.1097/00000658-197705000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiricuta I. The use of the great omentum in the surgery of breast cancer. Presse Med. 1963;71:15–17. [PubMed] [Google Scholar]
- Kiricuta I, Popescu V. Use of the greater omentum for treating radiation necroses and edema of the upper extremities following Halsted's mastectomy. Khirurgiia (Mosk) 1975;(9):88–93. [PubMed] [Google Scholar]
- Alday E S, Goldsmith H S. Surgical technique for omental lengthening based on arterial anatomy. Surg Gynecol Obstet. 1972;135:103–107. [PubMed] [Google Scholar]
- Saltz R. Endoscopic harvest of the omental and jejunal free flaps. Clin Plast Surg. 1995;22:747–754. [PubMed] [Google Scholar]
- Saltz R, Stowers R, Smith M, Gadacz T R. Laparoscopically harvested omental free flap to cover a large soft tissue defect. Ann Surg. 1993;217:542–546. discussion 546–547. doi: 10.1097/00000658-199305010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes S J, Nahai F. Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast Reconstr Surg. 1981;67:177–187. [PubMed] [Google Scholar]
- Das S K. The size of the human omentum and methods of lengthening it for transplantation. Br J Plast Surg. 1976;29:170–44. doi: 10.1016/0007-1226(76)90045-x. [DOI] [PubMed] [Google Scholar]
- Rangel-Moreno J, Moyron-Quiroz J E, Carragher D M, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen E W, Gerber S A, Sedlacek A L, Rybalko V Y, Chan W M, Lord E M. Omental immune aggregates and tumor metastasis within the peritoneal cavity. [Epub ahead of print] Immunol Res. 2009;(February):28. doi: 10.1007/s12026-009-8100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acarturk T O, Swartz W M, Luketich J, Quinlin R F, Edington H. Laparoscopically harvested omental flap for chest wall and intrathoracic reconstruction. Ann Plast Surg. 2004;53:210–216. doi: 10.1097/01.sap.0000116285.98328.f7. [DOI] [PubMed] [Google Scholar]
- Hultman C S, Carlson G W, Losken A, et al. Utility of the omentum in the reconstruction of complex extraperitoneal wounds and defects: donor-site complications in 135 patients from 1975 to 2000. Ann Surg. 2002;235:782–795. doi: 10.1097/00000658-200206000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W P. Coverage of thoracic wall defects by a split breast flap. Plast Reconstr Surg (1946) 1953;12:64–73. doi: 10.1097/00006534-195307000-00007. [DOI] [PubMed] [Google Scholar]
- Ferron G, Garrido I, Martel P, et al. Combined laparoscopically harvested omental flap with meshed skin grafts and vacuum-assisted closure for reconstruction of complex chest wall defects. Ann Plast Surg. 2007;58:150–155. doi: 10.1097/01.sap.0000237644.29878.0f. [DOI] [PubMed] [Google Scholar]
- Tansley P, Kakar S, Withey S. A novel modification of omental transposition to reduce the risk of gastrointestinal herniation into the chest. Plast Reconstr Surg. 2006;118:676–680. doi: 10.1097/01.prs.0000233042.09732.7d. [DOI] [PubMed] [Google Scholar]
- Kuzbari R, Worseg A, Burggasser G, et al. The external oblique muscle free flap. Plast Reconstr Surg. 1997;99:1338–1345. doi: 10.1097/00006534-199704001-00021. [DOI] [PubMed] [Google Scholar]
- Brown R G, Vasconez L O, Jurkiewicz M J. Transverse abdominal flaps and the deep epigastric arcade. Plast Reconstr Surg. 1975;55:416–421. [PubMed] [Google Scholar]
- Moschella F, Cordova A. A new extended external oblique musculocutaneous flap for reconstruction of large chest-wall defects. Plast Reconstr Surg. 1999;103:1378–1385. doi: 10.1097/00006534-199904050-00006. [DOI] [PubMed] [Google Scholar]
- Bogossian N, Chaglassian T, Rosenberg P H, Moore M P. External oblique myocutaneous flap coverage of large chest-wall defects following resection of breast tumors. Plast Reconstr Surg. 1996;97:97–103. doi: 10.1097/00006534-199601000-00016. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar B, Sloan G M, Beatty J D. The external oblique myocutaneous flap for extended hemipelvectomy reconstruction. Cancer. 1988;62:1022–1025. doi: 10.1002/1097-0142(19880901)62:5<1022::aid-cncr2820620531>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Dumanian G A, Heckler F R, Bernard S L. The external oblique turnover muscle flap. Plast Reconstr Surg. 2003;111:2344–2348. doi: 10.1097/01.PRS.0000061012.42444.23. [DOI] [PubMed] [Google Scholar]
- Meland N B, Ivy E J, Woods J E. Coverage of chest wall and pelvic defects with the external oblique musculofasciocutaneous flap. Ann Plast Surg. 1988;21:297–302. doi: 10.1097/00000637-198810000-00001. [DOI] [PubMed] [Google Scholar]
- Marshall D R, Anstee E J, Stapleton M J. Soft tissue reconstruction of the breast using an external oblique myocutaneous abdominal flap. Br J Plast Surg. 1982;35:443–451. doi: 10.1016/0007-1226(82)90043-1. [DOI] [PubMed] [Google Scholar]
- Bakamjian V YA. A two-stage method for pharyngoesophageal reconstruction with a primary pectoral skin flap. Plast Reconstr Surg. 1965;36:173–184. doi: 10.1097/00006534-196508000-00004. [DOI] [PubMed] [Google Scholar]
- Baroudi R, Pinotti J A, Keppke E M. A transverse thoracoabdominal skin flap for closure after radical mastectomy. Plast Reconstr Surg. 1978;61:547–554. doi: 10.1097/00006534-197804000-00008. [DOI] [PubMed] [Google Scholar]
- Davis W M, McCraw J B, Carraway J H. Use of a direct, transverse, thoracoabdominal flap to close difficult wounds of the thorax and upper extremity. Plast Reconstr Surg. 1977;60:526–533. doi: 10.1097/00006534-197710000-00005. [DOI] [PubMed] [Google Scholar]
- Persichetti P, Di Lella F, Delfino S, Scuderi N. Adipose compartments of the upper eyelid: anatomy applied to blepharoplasty. Plast Reconstr Surg. 2004;113:373–378. discussion 379–380. doi: 10.1097/01.PRS.0000097290.64125.C3. [DOI] [PubMed] [Google Scholar]
- Skoracki R J, Chang D W. Reconstruction of the chestwall and thorax. J Surg Oncol. 2006;94:455–465. doi: 10.1002/jso.20482. [DOI] [PubMed] [Google Scholar]
- Deo S V, Purkayastha J, Shukla N K, Asthana S. Myocutaneous versus thoraco-abdominal flap cover for soft tissue defects following surgery for locally advanced and recurrent breast cancer. J Surg Oncol. 2003;83:31–35. doi: 10.1002/jso.10236. [DOI] [PubMed] [Google Scholar]
- Marshall D R. The contralateral breast flap in reconstruction of the breast and chest wall. Ann Plast Surg. 1993;31:508–513. doi: 10.1097/00000637-199312000-00006. [DOI] [PubMed] [Google Scholar]
- Hughes K C, Henry M J, Turner J, Manders E K. Design of the cyclops flap for chest-wall reconstruction. Plast Reconstr Surg. 1997;100:1146–1151. discussion 1152. doi: 10.1097/00006534-199710000-00011. [DOI] [PubMed] [Google Scholar]