Abstract
Defect reconstruction after radical oncologic resection of malignant chest wall tumors requires adequate soft tissue reconstruction with function, stability, integrity, and an aesthetically acceptable result of the chest wall. The purpose of this article is to describe possible reconstructive microsurgical pathways after full-thickness oncologic resections of the chest wall. Several reliable free flaps are described, and morbidity and mortality rates of patients are discussed.
Keywords: Chest wall, free flap, malignant tumor, full-thickness defect, reconstructive microsurgery
Oncologic resection for recurrent breast carcinoma after mastectomy, soft tissue sarcoma, chest wall–penetrating tumors, metastasis from pulmonary carcinoma, and/or radiation necrosis often results in tissue or full-thickness chest wall defects. Full-thickness defects of the thoracic wall require early interdisciplinary cooperation to achieve patient-specific treatment modalities. In these cases, complex plastic-surgical differential therapy is necessary to provide chest wall stability and durability. Local and regional muscle as well as musculocutaneous flaps are often used for reconstruction. However, there is a selected group of patients who require free flap reconstruction when all other options may not lead to a satisfying result (Table 1). Various new developments in the field of plastic and reconstructive surgery during the past decades make it possible to cover very large and complex defects.
Table 1.
Indications for Microsurgical Techniques in Chest Wall Reconstruction
| 1. Salvage procedure after loss/partial loss of local/regional flaps |
| 2. Auxiliary anastomosis for “supercharged” TRAM or distally perforator pedicled latissimus dorsi flap |
| 3. Large defects with radiation fibrosis |
| 4. Intrathoracic dead space and malnutrition |
| 5. Replantation of parts of the upper extremity in forequarter resection |
Patients who undergo chest wall reconstruction have a marginal tolerance for complications. Therefore, the reconstructive surgeon has to aspire to a one-time procedure to minimize frequent operations and to ensure early mobilization of the patient. Sufficiently durable coverage with well-vascularized tissue is necessary to provide a reliable blood supply that allows immediate additional local and systemic treatment such as adjuvant chemotherapy or radiotherapy without delay.
Demographic charts demonstrate an aging population in many countries especially in the Western world. As a consequence of this development, an increasing incidence of tumors including adjunctive procedures such as radiotherapy and invasive cardiac surgical procedures can be observed. Complications such as sternum osteomyelitis, local metastases, and osteo-radionecrosis after radiation are frequently seen in major plastic-surgical centers.
The majority of the resulting defects in these usually seriously ill patients can be reconstructed with local or regional flaps such as the pectoralis major flap or the vertical rectus abdominis. If all local options have already been used or if the resection site is just too large for coverage with a pedicled flap, free flap reconstruction becomes necessary.
PREOPERATIVE SETTINGS
Conventional x-ray of the chest, magnetic resonance imaging (MRI) for soft tissue, and computed tomography (CT) scan for osteologic situation is mandatory. The confirmed histopathologic diagnosis (i.e., using incisional or punch biopsy) and preoperative staging and grading are necessary to decide whether a curative or palliative treatment is indicated.
In case of planned reconstruction using a perforator free flap, a preoperative Doppler ultrasound examination to determine the size and location of perforating blood vessels is necessary.
SURGICAL CONCEPT (PROCEDURE)
Treatment of chest wall tumors is a challenge for the interdisciplinary team of plastic and thoracic surgeons.
The goal is to provide chest wall stability and durable soft tissue coverage to restore the physiologic kinetics of the chest wall and to build a situation where the oncologic patient can undergo early postoperative radiation. In palliative indications, the aim is to relieve serious local symptoms.
Sternal, lateral, and thoracoabdominal are the most common locations for tumor resections.1 A forequarter amputation with resection of the shoulder girdle as well as ribs is performed rarely.2 In a one-stage procedure with tumor resection and primary reconstruction, no compromise regarding adequate radicality is allowed.
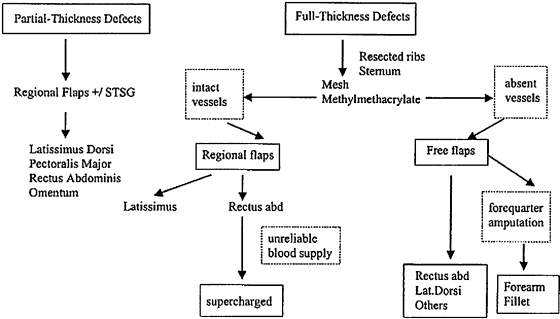
During preoperative planning, the patient's profile and the individual possible operative stress have to be adapted. The general condition, the complexity of the surgical procedure, expectancy of life, and the expected functional and aesthetic outcome have to be considered (Fig. 1).3
Figure 1.
Algorithm for reconstruction of chest wall defects. (Data from Cordeiro PG, Santamaria E, Hidalgo D. The role of microsurgery in reconstruction of oncologic chest wall defects. Plast Reconstr Surg 2001;108:1924–1930.)
STABILIZATION OF THE THORACIC WALL
A loss of four rib segments or large defects at the upper part of the sternum influence the pulmonary function and may cause paradoxical movement of the chest wall while breathing or herniation. Free dermis flaps, fascia lata, fibula, or free rib grafts may be used to stabilize the thoracic wall. The rib grafts are fixed with cerclage wires to the bony chest wall. Alternatively, nylon or Gore-Tex material mesh may be used as alloplastic graft. Further inert materials are Vicryl mesh (Ethicon Johnson & Johnson Medical, Norderstedt, Germany) polypropylene and methyl methacrylate.
FREE AND SUPERCHARGED FLAPS
Tensor Fascia Lata Flap
The thickness of the fascia lata over the tensor muscle makes it a strong facial donor site suitable for reconstruction of the chest wall.
The tensor fascia lata (TFL) flap is easily harvested with or without a skin paddle simultaneously while a second team of surgeons is preparing the recipient vessels. The pedicle of the flap, the ascending branch of the lateral circumflex artery, has its origin on the deep femoral artery. Disadvantages can be the short pedicle (4 to 6 cm) and the significant aesthetic donor deformity, especially if skin grafting is required to close the defect.
Latissimus Dorsi Flap
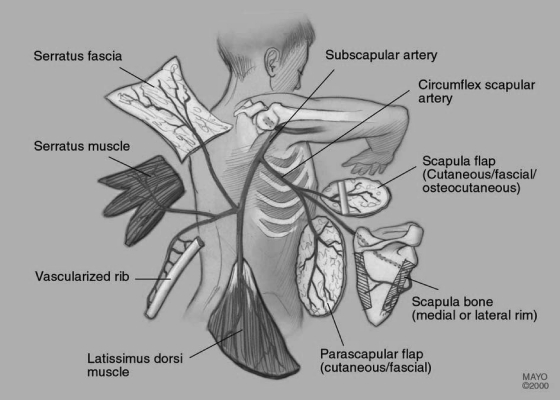
Wide full-thickness resections are covered most effectively with pedicled axial musculocutaneous flaps. Among the pedicled flaps, the musculus latissimus dorsi flap (Fig. 2) is one of the most common used. As a free flap raised from the contralateral side, it is likely used because of the large diameter of the supplying thoracodorsal vessels (2 to 4 mm). Good reliability, large volume, and marginal donor site morbidity are further advantages. It may be raised as a myocutaneous or simply as a muscle flap with or without simultaneous split-thickness skin graft. A combination with the serratus anterior muscle flap increases the indications for even larger defects.
Figure 2.
The subscapular system. (Reprinted with permission of the Mayo Foundation.)
Transverse or Vertical Rectus Abdominis Muscle Flaps
The rectus abdominis musculocutaneous flaps are the most commonly used flaps for chest wall reconstruction.4 In particular, the transverse rectus abdominis muscle (TRAM) flap is the workhorse to cover extensive soft tissue defects. TRAM and vertical rectus abdominis muscle (VRAM) flaps are usually used as pedicled flaps receiving their blood supply from the superior epigastric vessels. The blood supply can be augmented by supercharging using the inferior epigastric vessels. Microsurgical anastomosis in the area of the defect (e.g., A./V. thoracica interna, A./V. thoracoacromialis, A./V. carotis, A./V. thoracodorsalis) increases blood supply and venous return (drainage) significantly.
The disadvantages are donor site morbidity: The unilateral TRAM flap leaves a defect in the rectus muscle and anterior rectus sheath. This defect may lead to weakness, bulge, hernia, and abdominal discomfort, however the abdominal wall can be stabilized using the Mayo procedure or a synthetic mesh.
Anterolateral Thigh Flap
The perforator flap of the anterolateral thigh is the optimal flap in thin and normal up to moderately thick patients. In the right patient, the skin and subcutaneous fat of the anterolateral thigh can be quite thin, making this flap a potentially large donor site of supple and sometimes sensate fasciocutaneous tissue. The skin paddle can be as large as 8 × 25 cm with primary closure attainable. Wider flaps can be harvested if the surgeon is prepared to skin graft the donor area. The flap has a large-caliber pedicle, but the anatomy can be variable. Most flaps in our experience require dissection of musculocutaneous perforators and are infrequently supplied solely by the septocutaneous branches. Musculocutaneous perforators nourish more than 80% of flaps making this perforator flap dissection potentially difficult and tedious. The lateral circumflex femoral arterial system also provides composite free tissue transfer for a variety of flaps. While harvesting the anterolateral thigh (ALT) flap, one can include the tensor fascia lata or the rectus femoris muscle as well as other muscles of the thigh (chimeric flap).
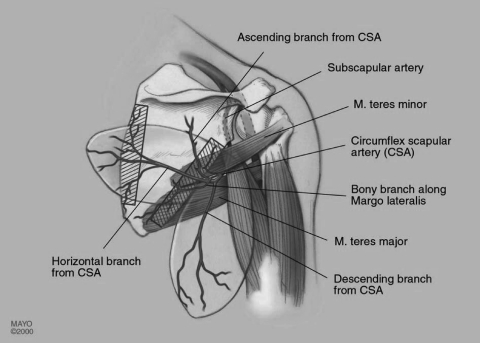
Scapula and Parascapular Flaps
The system of the subscapular vessels arising from the axillary artery and vein supplies numerous flaps possibly composed of different types of tissue (Fig. 2, Fig. 3). The long pedicle provides variable combinations. Most commonly used besides the latissimus dorsi flap are the scapula and parascapular flaps. The circumflex scapular artery is a branch of the subscapular artery which arises from the axillary artery. After the circumflex scapular artery pierces the space surrounded by the muscle borders of the long head of triceps, teres minor, and teres major (triangular space), it sprouts a transverse cutaneous scapular branch and a vertical parascapular branch. The parascapular branch forms the basis of the parascapular flap.
Figure 3.
The scapula and parascapular flaps. (Reprinted with permission of the Mayo Foundation.)
The subscapular artery pedicle can be from 3 to 7 cm in length with vessel circumference at this level up to 4 mm in size. Although the circumflex scapular artery is usually accompanied by two venae comitantes, the subscapular artery is typically accompanied by one vein.
Flap sizes up to 12 × 25 cm are possible. Aesthetic result of the donor site may sometimes be unsatisfying because the scar tends to spread.
Deep Inferior Epigastric Perforator Flap
The deep inferior epigastric perforator (DIEP) flap is a method to transfer abdominal wall skin, subcutaneous fat, and Scarpás fascia pedicled on the deep inferior epigastric vessels. Compared with the TRAM flap, the weakening of the abdominal wall is reduced. Intraoperatively, the largest periumbilical perforator is determined to assure maximum perfusion of zones III and IV. However, the DIEP flap requires a longer operative time and technical ability in microsurgery due to the subtle dissection of the pedicle within the rectus muscle.
Forearm Fillet Flap
After forequarter amputation, the concept of “spare part surgery” can be an option for reconstruction using the amputated limb as a donor site.5,6 If oncologically safe, a fillet of forearm free flap from the amputated arm can provide excellent coverage of the shoulder defect. The stump of the subclavian vessels or neck vessels are the best donor vessels in this location.
Omentum Majus Free Flap
The formerly often used omentum majus flap is currently a salvage procedure. It consists of fat, connective tissue, and lymphatics and can be used as a pedicled or free flap. Because of the fact that two cavities are involved, the flap is used infrequently.
Dead-Space Obliteration with Intrathoracic and Extrathoracic Free Flap
In cases of penetrating lung tumors, curative resections accompanied by a full-thickness chest wall defect and partial lung lobule resection result in an intrathoracic dead space with the risk of bronchopleural fistula. A combined intrathoracic and extrathoracic free flap may obliterate dead space and cover the defect. Therefore, a musculocutaneous flap is needed.
Building Recipient Vessels via AV-Loops
Most commonly used donor vessels are the internal mammary artery, the external carotid artery, and the transverse cervical artery for arterial donor vessels. The external and internal jugular veins are used most frequently for venous anastomosis (Table 2).
Table 2.
Recipient Vessels (Artery and Vein)
| Artery |
| Internal mammary |
| External carotid |
| Transverse cervical |
| Subclavian |
| Thoracodorsal |
| Axillary |
| Thoracoacromial |
| Lingual |
| Subscapular |
| Brachial |
| Vein |
| External jugular |
| Innominate |
| Internal jugular |
| Internal mammary |
| External carotid |
| Axillary |
| Thoracodorsal |
| Brachial |
However, there are situations in which easily accessible vessels are no longer available, already used in previous procedures, damaged by previous therapy (e.g., radiation), or are of inadequate size.
In these cases, an arterio-venous fistula (AV-loop) allows good flap positioning with easily accessible end-to-end anastomosis of the flap vessels, which shortens operation time. There are different vessels of the neck and arms that may be used as loop vessels. One example is the cephalic-thoraco-acromial loop.7 For this loop, the cephalic vein is dissected in the calculated length from the upper arm toward the supraclavicular area, leaving the drainage into the subclavian vein intact. The thoraco-acromial pedicle is then prepared for the temporal arterio-venous anastomosis.
RESULTS
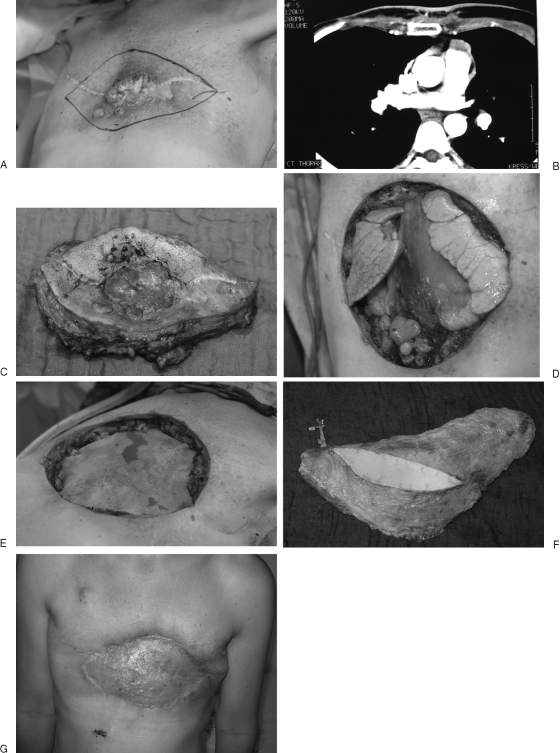
Between January 1999 and May 2007, 69 patients were treated in the former hospital of the senior author, M.S., (Department for Hand, Plastic and Reconstructive Surgery, BG Trauma Center Ludwigshafen, University of Heidelberg, Germany; Chair: Günter Germann, M.D., Ph.D.) with chest wall defects. Fifty-six patients had a tumor resection of the chest wall because of malignant infiltration. In 21 cases, the soft tissue reconstruction required microvascular techniques (Fig. 4, Fig. 5 and Fig. 6). Seven TFL flaps, three latissimus dorsi flaps, two rectus abdominis flaps, one ALT flap, one gracilis flap, and one forearm fillet flap were performed. Five rectus abdominis flaps were “supercharged” by using microvascular anastomosis of the inferior epigastric vessels with neck vessels as well as a flap combination of latissimus dorsi and rectus abdominis flaps.
Figure 4.
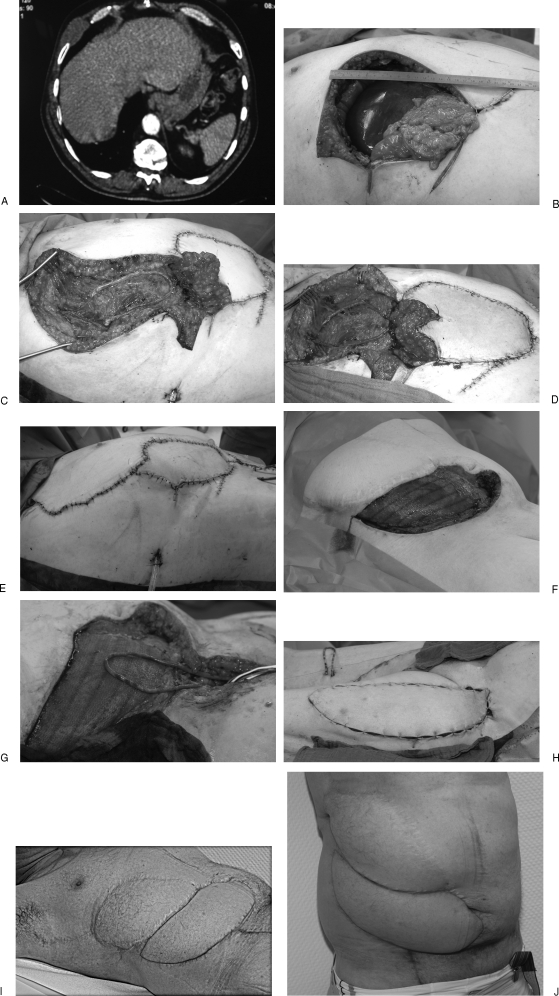
Case 1: A 40-year-old woman with local recurrent breast cancer infiltrating the thoracic wall after bilateral mastectomy. (A) Clinical aspect. (B) CT scan of the patient with infiltration of the sternum and ribs. (C) Resected tumor specimen including parts of the sternum and five ribs. (D) Defect size 28 × 23 cm with exposed thoracic organs. (E) Stabilization of the thorax by the thoracic surgeons using synthetic mesh (Marlex). (F) Harvested free latissimus dorsi flap. (G) Postoperative result 5 months after coverage of the defect; the latissimus muscle was hooked up with the internal mammary vessels.
Figure 5.
Case 2: A 54-year-old patient who already underwent sarcoma resection elsewhere. (A) The MRI scan shows recurrence of the tumor (malignant fibrous histiocytoma) at the right lower thorax. (B) After resection of the recurrent tumor, the defect size was 18 × 20 cm. For coverage, a pedicled VRAM flap was planned. (C) The perfusion of the VRAM flap was not sufficient; for salvage of this problem, a free greater saphenous vein graft was anastomosed with the thoracodorsal vessels. (D) The flap is well perfused after anastomosis of the inferior epigastric vessels with the loop. (E) Final result in the operating room. (F) Despite resection with clear margins, another recurrence of the tumor appeared, and the ablative surgeons created a defect of 30 × 15 cm at the lower abdominal wall. Stabilization of the abdominal wall was performed with synthetic mesh. For microsurgical reconstruction, an AV-loop with ipsilateral greater saphenous vein hooked up with the femoral artery was performed. (G) This photograph shows the well-perfused loop. (H) For defect coverage, a combined ALT-TFL flap was harvested. (I) One and one-half years after flap reconstruction, no recurrence of the tumor was seen, and the two free flaps provided a stable thoracic and abdominal wall. (J) The patient is satisfied with the result and is able to perform activities of daily living.
Figure 6.
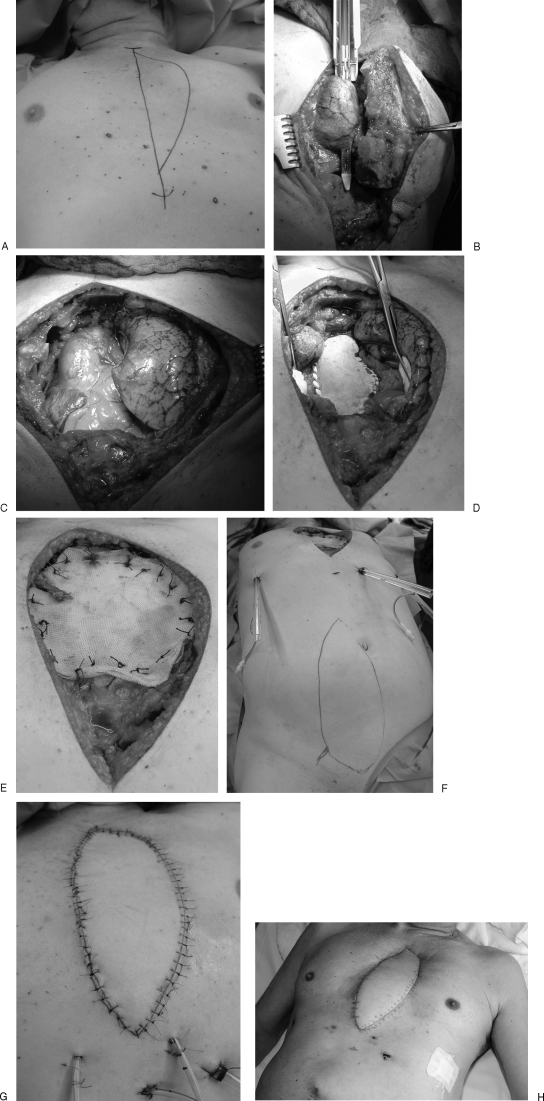
Case 3: (A) A 69-year-old man suffering from a Pancoast tumor. (B, C) Tumor resection with parts of sternum and ribs including the internal mammary artery, lung, and pericardium. (D, E) Reconstruction of the pericardium and thoracic wall with alloplastic material and Marlex mesh. (F–H) Coverage of the sternal defect with free VRAM flap.
Morbidity and Mortality
EARLY POSTOPERATIVE COMPLICATIONS
One patient who had a thoracic wall recurrence of a mammary carcinoma with reconstruction using a pedicled TRAM flap with supercharged venous anastomosis of the inferior epigastric vein with the external jugular vein showed a flap malperfusion on day 1 postoperatively. During the operative revision, the necessity of an additional arterial anastomosis was obvious. A greater saphenous vein graft that was connected to the external carotid artery solved the problem.
One free TFL flap was totally lost. The patient received another TFL free flap from the contralateral side.
Another total flap loss of the free ALT flap was temporarily repaired with a free TFL flap. A sternal bleeding with hematoma led to another flap loss. After all, a free latissimus dorsi flap healed without further complication.
The complications mentioned above affected 25 patients of our total sample. This means we found an overall morbidity rate of the whole cohort of 44.64%. Thirty-one (55.36%) patients healed without complications .
One patient died because of a fulminant lung embolia. The perioperative mortality rate was 1.79%.
Long-Term Follow-Up
Concerning long-term follow-up data, we obtained information on 34 of our patients. The 5-year overall survival rate was 36%.
DISCUSSION
The survival of patients who undergo chest wall resection depends on variable factors. First of all, the histologic findings and the anatomic extent of the tumor at the beginning of treatment of the disease are the main prognostic factors. Essential is a complete resection of the tumorous tissue with negative surgical margins if possible. Patients who need a chest wall resection of that extent with simultaneous reconstruction are usually in a progressive oncologic state of the primary malignancy, and the prognosis is a priori poor. In the literature, a 5-year overall survival rate of 18 up to 60% is described.2,8,9 This is confirmed by our data with 36% general 5-year survival rate.
The perioperative mortality rate after chest wall resection is between 3.5 and 4.5% in the literature.10,11,12,13 In our cohort, the mortality rate was 1.79%. The results of Henderson et al14 and Friedel et al15 were similar to ours. Morbidity rates between 20 and 50% were seen in several studies.15,16 We found a morbidity rate of 44.64% in our study. Thus patients with reasonable preoperative risk who have to undergo chest wall tumor resections are likely to have acceptable morbidity and mortality.
CONCLUSION
In the selected group of patients with extensive resections of the chest wall for tumor, use of microsurgical techniques helps to transfer tissue from distant sites with good blood supply. After all local options are exhausted, these techniques provide reliable flaps to cover even the largest full-thickness chest wall defects. Such coverage can be safely and successfully accomplished in a single stage with relatively low morbidity. The cooperation of the reconstructive plastic surgeon and thoracic surgeon is useful to achieve control of the underlying disease and to reconstruct a mechanically stable tissue cover and a pain-free unrestricted breathing function.17 The distinct preoperative analysis of the disease and the extent of the expected defect is essential. A stabilization of the chest wall using an alloplastic mesh should be done if paradox breathing is to be expected due to the size of the defect. Even in palliative situations, a reconstructive surgical procedure may be indicated with the goal to achieve an acceptable quality of life for the patient.
Acknowledgments
The authors thank Katrin Riedel, M.D. for the support during the thesis of Sina Dittler, M.D.
References
- Sullivan S R, Truxillo T M, Mann G N, Isik F F. Utility of the free deep inferior epigastric perforator flap in chest wall reconstruction. Breast J. 2007;13:50–54. doi: 10.1111/j.1524-4741.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- Liapakis I E, Korkolis D, Papadopoulos O, et al. Reconstruction of the chest wall. J BUON. 2008;13:185–191. [PubMed] [Google Scholar]
- Cordeiro P G, Santamaria E, Hidalgo D. The role of microsurgery in reconstruction of oncologic chest wall defects. Plast Reconstr Surg. 2001;108:1924–1930. doi: 10.1097/00006534-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Erdmann D, Küntscher M, Petracic A, et al. Plastic surgery coverage of osteocutaneous defects of the sternum area with the vertical and transversal rectus abdominis muscle (VRAM/TRAM) flap. Chirurg. 2000;71:1156–1160. doi: 10.1007/s001040051194. [DOI] [PubMed] [Google Scholar]
- Küntscher M V, Erdmann D, Homann H H, Steinau H U, Levin S L, Germann G. The concept of fillet flaps: classification, indications, and analysis of their clinical value. Plast Reconstr Surg. 2001;108:885–896. doi: 10.1097/00006534-200109150-00011. [DOI] [PubMed] [Google Scholar]
- Küntscher M V, Erdmann D, Strametz S, Sauerbier M, Germann G, Levin L S. The use of fillet flaps in upper extremity and shoulder reconstruction. Chirurg. 2002;73:1019–1024. doi: 10.1007/s00104-002-0529-y. [DOI] [PubMed] [Google Scholar]
- Engel H, Pelzer M, Sauerbier M, Germann G, Heitmann C. An innovative treatment concept for free flap reconstruction of complex central chest wall defects—the cephalic-thoraco-acromial (CTA) loop. Microsurgery. 2007;27:481–486. doi: 10.1002/micr.20391. [DOI] [PubMed] [Google Scholar]
- Daigeler A, Druecke D, Hakimi M, et al. Reconstruction of the thoracic wall—long-term follow-up including pulmonary function tests. Langenbecks Arch Surg. 2009;394:705–715. doi: 10.1007/s00423-008-0400-9. [DOI] [PubMed] [Google Scholar]
- Santillan A A, Kiluk J V, Cox J M, et al. Outcomes of locoregional recurrence after surgical chest wall resection and reconstruction for breast cancer. Ann Surg Oncol. 2008;15:1322–1329. doi: 10.1245/s10434-007-9793-x. [DOI] [PubMed] [Google Scholar]
- al-Kattan K M, Breach N M, Kaplan D K, Goldstraw P. Soft-tissue reconstruction in thoracic surgery. Ann Thorac Surg. 1995;60:1372–1375. doi: 10.1016/0003-4975(95)00644-Z. [DOI] [PubMed] [Google Scholar]
- Anderson B O, Burt M E. Chest wall neoplasms and their management. Ann Thorac Surg. 1994;58:1774–1781. doi: 10.1016/0003-4975(94)91691-8. [DOI] [PubMed] [Google Scholar]
- Pameijer C R, Smith D, McCahill L E, Bimston D N, Wagman L D, Ellenhorn J D. Full-thickness chest wall resection for recurrent breast carcinoma: an institutional review and meta-analysis. Am Surg. 2005;71:711–715. [PubMed] [Google Scholar]
- Warzelhan J, Stoelben E, Imdahl A, Hasse J. Results in surgery for primary and metastatic chest wall tumors. Eur J Cardiothorac Surg. 2001;19:584–588. doi: 10.1016/s1010-7940(01)00638-8. [DOI] [PubMed] [Google Scholar]
- Henderson M A, Burt J D, Jenner D, Crookes P, Bennett R C. Radical surgery with omental flap for uncontrolled locally recurrent breast cancer. ANZ J Surg. 2001;71:675–679. doi: 10.1046/j.0004-8682.2001.02234.x. [DOI] [PubMed] [Google Scholar]
- Friedel G, Kuipers T, Dippon J, et al. Full-thickness resection with myocutaneous flap reconstruction for locally recurrent breast cancer. Ann Thorac Surg. 2008;85:1894–1900. doi: 10.1016/j.athoracsur.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Faneyte I F, Rutgers E J, Zoetmulder F A. Chest wall resection in the treatment of locally recurrent breast carcinoma: indications and outcome for 44 patients. Cancer. 1997;80:886–891. [PubMed] [Google Scholar]
- Riedel K, Kremer T, Hoffmann H, et al. Plastic surgical reconstruction of extensive thoracic wall defects after oncologic resection. Chirurg. 2008;79:164–174. doi: 10.1007/s00104-007-1382-9. [DOI] [PubMed] [Google Scholar]