Abstract
The goals of chest wall stabilization include maintenance of a rigid airtight cavity, protection of the thoracic and abdominal contents, optimization of respiration, and, whenever possible, an aesthetic reconstruction. Evidence suggests that bony fixation results in reduced ventilator dependence, a shorter overall hospital stay, and improved upper extremity function. We prefer to accomplish this with autologous tissue alone (such as the pectoralis major, latissimus dorsi, or rectus abdominus muscle flaps) for small to moderate defects. En bloc resection of defects larger than 5 cm or containing four or more ribs will likely benefit from chest wall stabilization. For patients previously treated with radiation, even larger defects may be tolerated owing to fibrosis. For these larger defects, methyl methacrylate composite meshes are used and covered with vascularized tissue. Contaminated wounds are generally reconstructed with bioprosthetic mesh rather than synthetic mesh. Using these principles, the reconstructive plastic surgeon can devise a comprehensive and safe plan to repair tremendous defects of the chest wall.
Keywords: Chest wall, reconstruction, stabilization, autologous, alloplastic
In the late 19th century, Parham reported resecting a chest wall tumor en bloc with three ribs. He described 48 cases that had been reported in the literature up to that date.1 Parham also gave credit to Fell and O'Dwyer for their work with intubation, upper airway control, and positive-pressure ventilation, before which his case would not have been possible.2,3 During the next 50 years, several advances in chest wall reconstruction were made, including the first successful pneumonectomy, advances in endotracheal intubation, the use of closed pleural drainage, and the advent of the use of antibiotics. Despite these advances, repair of full-thickness defects of the chest wall remained complex and controversial.
As is true for much of medicine, the Second World War and its ravages brought innovation and advances to our understanding and treatment of chest wall defects. During this era, the techniques of fascia lata grafts, cutaneous flaps, rib grafts, and muscle flaps were pioneered. On the shoulders of these developments, surgeons in the 1960s continued to push the limits of reconstructing large chest wall defects. After the 1960s, the literature exploded with developments in complex reconstruction, including more precise descriptions of anatomy and physiology, tissue expansion muscle/myocutaneous/fasciocutaneous/osteocutaneous/perforator flaps, and microsurgery. The most significant advances of the past two decades were the introduction of plating systems for osteosynthesis and synthetic and bioprosthetic meshes.
CHEST WALL FUNCTIONS
Composite defects of either the anterior or posterior chest wall can result from ablative procedures (oncologic or infectious), trauma, radiation, congenital defects, pressure sores, or iatrogenic causes (surgical access, instrumentation, or hardware). The insult to the chest wall can subsequently interfere with its primary functions:
Protection of both the intrathoracic and upper abdominal contents (stomach, intestines, liver, spleen, and kidney).
Respiration: The chest wall facilitates the function of the diaphragm and accessory muscles of respiration to generate the negative pressure necessary for inspiration and the positive pressure necessary for exhalation.
Support: Both shoulder and upper extremity function are largely dependent on the stable platform created by the chest wall.
Aesthetic: Although often the last priority, restoration of the normal body contours of both male and female anatomy nonetheless deserves consideration.
THE NEED FOR RECONSTRUCTION
A variety of philosophies about the amount of chest wall stabilization needed exist, and more recently there has been a focus on stability across the midline. When plating systems were first introduced, they were met with a great deal of enthusiasm. Sternal wounds in particular were thought to increase the dependence on abdominal breathing, prolong the need for respiratory support, and result in long-term chronic pain. However, as researchers looked for respiratory functional benefits and were unable to identify them clearly, enthusiasm for skeletal fixation waned. Eventually, Kroll and co-workers were able to demonstrate conclusively a reduction in ventilator dependence and overall hospital stay with bony fixation.4 More recent literature has focused on the function of the upper extremities, and surgeons have shown significant improvement in function with skeletal stabilization.5,6,7 Researchers have found that the activities of daily living were not affected by compromised latissimus, pectoralis major, or rectus muscles8 and therefore have postulated that reduced shoulder girdle function could be attributed to not having a stable base on which to function. These findings may generate a renewed interest in chest wall stabilization.
The impact of skeletal chest wall resection on respiratory function remains controversial. Chest wall reconstruction has an acute impact on breathing mechanics.9 In the first 2 weeks after resection, reconstruction may reduce the length of time when ventilator support is needed. In long-term follow-up, chest wall resection does not appear to impact significantly standard pulmonary function testing.5 That is, in previously healthy individuals, these minor pulmonary function changes are well tolerated. However, patients requiring skeletal chest wall reconstruction are often medically compromised, and minor changes in pulmonary function may be significant, such as in patients with chronic pulmonary disease.10 In addition, when midline sternal continuity is not restored, patients suffer from acute discomfort caused by shifting of the two halves of the chest wall.6 This discomfort may be minimized with the use of negative-pressure wound therapy (NPWT). Using NPWT as a bridge to wound closure may also increase the stability of the chest in the long term, decrease the wound size, minimize contamination, and allow time until the patient is hemodynamically and physiologically ready for definitive reconstructive surgery.6,11
Posterior chest wall and axillary defects are less common but can be as challenging as anterior or lateral defects. Congenital defects, surgical instrumentation, trauma, and resection defects can leave the surgeon facing incredibly large defects with dead space, exposed hardware and vital neurovascular structures, and compromised vascularity. These defects largely involve soft tissue but can occasionally require reconstruction of rib defects.
A complete understanding of the functions of the chest wall leads naturally to the goals of reconstruction, which include a rigid and airtight cavity, protection of the thoracic and abdominal contents, optimization of respiration, and, whenever possible, an aesthetic reconstruction.
SKELETAL DEFECTS
Sternal Defects
The sternum consists of the manubrium, body (often referred to as the sternum), and xiphoid. The manubrium, and its articulations with the clavicles and first and second ribs, is thought to be critical to the arch contour and ring structure of the chest wall. Defects of the sternum are associated with varying functional consequences and are classified as follows12:
Partial sternal and adjacent rib defects: In these defects, intact upper arches maintain a stable chest wall with minimal to no physiologic deficit.
Entire sternum and adjacent rib defects: Here, the physiologic effect is initially moderate. Over time the deficit diminishes, and only minor impairment of respiratory function is seen.
Loss of the manubrium and sternum: Although the diaphragmatic component of respiration is not impaired, the physiologic deficit can be severe. Ventilatory support may be necessary for extended periods of time, and stabilization of the chest wall is recommended in this case.
Rib Defects
The necessity of reconstructing rib defects and the size/extent of the defect necessitating reconstruction remain controversial. Proponents insist on protecting against a flail segment based on the Pendelluft principle (also known as paradoxical respiration; this is a phenomenon in which there is air flow back and forth between the lungs, resulting in increased dead-space ventilation). However, the benefits of this approach are not clearly defined,13 as no hard data exist regarding the critical size of the defect for reconstruction. Most surgeons, the current authors included, now seem to agree that en bloc resection resulting in a defect larger than 5 cm or four or more ribs will benefit from some form of reconstruction/stabilization. Anterior and posterior defects are typically better tolerated than lateral defects. In patients who have had radiation treatment, even larger defects may be tolerated owing to fibrosis. With defects larger than 5 cm, most surgeons agree that reconstruction and skeletal stabilization benefit the patient.
AUTOLOGOUS RECONSTRUCTION
Fifty years ago, autogenous reconstruction was the standard procedure. These reconstructions, when successful, proved to be very durable. The most common technique used for skeletal reconstruction was bone grafts or vascularized bone. Donor sites included other ribs (split ribs or anterior ribs for lateral defects), the iliac crest, and the fibula. Ribs were used most frequently and could be harvested as a whole segment or split longitudinally. Pain and possible instability at the donor site limited the amount of bone that could be harvested. Ideally, the bone grafts were either vascularized or placed in a well-vascularized bed to facilitate graft survival and osteoconduction. If the graft failed to take and the bone was not revascularized, it would slowly be resorbed over time, leaving behind a firm capsule. Occasionally, this capsule was sufficiently rigid to provide stabilization and protection.
Another option used in the past was fascial grafts. The most common graft was the tensor fascia lata, but the dura was also used. Many surgeons found that tensor fascia lata, initially rigid when stretched, became flaccid over time, leading to instability. As with all nonvascularized reconstructions, the fascia was easily contaminated/infected. Vascularized fascial flaps have been found to have a lower risk of infection than that of synthetic mesh.14,15 Tension on the fascial component of a fasciocutaneous flap, however, may compromise the flap's vascularity, resulting in loss of the flap and evisceration or hernia.
For those defects that do not require stabilization, pedicled muscle, myocutaneous, fasciocutaneous, perforator, and omental flaps are commonly used. Although most chest wall reconstructions can be accomplished with pedicled flaps, free-tissue transfer may be required in areas that are difficult to reach with pedicled flaps and/or when local flaps are compromised or unavailable and when a single regional flap is inadequate to cover the defect. These forms of reconstruction are covered in a different article of this issue of Seminars in Plastic Surgery.
The main advantage of autologous tissue is to avoid alloplastic implantable materials. The disadvantages are donor site morbidity and the limited amount of tissue available for repairing larger defects. Advances in the care of the surgical patient have led to the performance of larger and larger resections, which has created a larger challenge for plastic surgeons.
ALLOPLASTIC RECONSTRUCTION
A major advance in chest wall reconstruction was the introduction of prosthetic implantable materials. Developments and refinements of these materials continue to drive reconstructive surgeons to attempt to reconstruct tremendous defects of both the sternum and rib cage that were previously unfeasible. Advantages to using these materials include no donor site morbidity, limitless (but often costly) sources, and no operative time for harvesting the graft (although there are preparation times with some of the materials). The ideal alloplast would be inexpensive, easy to use, incorporated by the body, durable, physically and chemically inert, resistant to infection and strain, radiolucent, elicit no inflammatory or foreign body reaction, noncarcinogenic, hypoallergenic, malleable, and sterilizable. However, there is no such ideal material currently available, and surgeons continue to investigate the use of several different materials.
Mesh and Composite Implants
As the need for new materials has increased, so too has the ingenuity of the surgeon and the list of mesh options. There are now several synthetic meshes available with varying thicknesses and properties. Polypropylene knitted meshes (PPKMs) are available as both single- and double-knit fabrics. Marlex (CR Bard, Murray Hill, NJ) is a single-knit PPKM that is stretchable in one direction and rigid in the other. This material was preferred by Boyd et al16 and McCormack17 but has been found to be subject to fraying. Prolene (Ethicon Inc., Somerville, NJ) is a double-knitted PPKM that is flexible in two dimensions yet resists fraying. Proponents of this material included Arnold and Pairolero18 and Morgan et al19 (Fig. 1). Both materials need to be sutured under tension, are permanent, and induce intense fibrovascular infiltration, resulting in incorporation into the surrounding tissues. If these materials become infected or exposed, they can occasionally be managed conservatively with wound care, avoiding the need for surgical explantation. More commonly, however, these materials will require surgical removal, resulting in a contaminated chest wall defect.
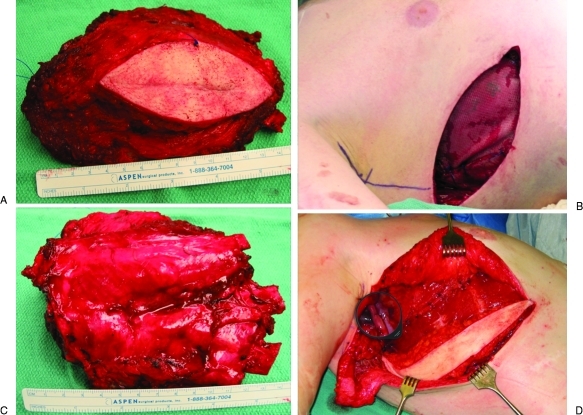
Figure 1.
A 28-year-old man with a chondrosarcoma of the lateral chest wall. (A) En bloc resection included the overlying previous biopsy site and three ribs. (B) Deep surface of the resection specimen. (C) The resultant resection defect was stabilized using inlay polypropylene mesh. (D) The mesh was covered with a free vertical rectus abdominus myocutaneous flap anastomosed to the thoracodorsal pedicle that had been transected during the resection.
Several other mesh materials have also been described: polyester (Mersilene [Ethicon Inc.] or Dacron [Invista, Wichita, KS]), polyglycolic acid (Dexon; Covidien, Mansfield, MA), polylactic acid, expanded polytetrafluoroethylene (e-PTFE/Gore-Tex; Gore, Flagstaff, AZ), polydioxanone (PDS; Ethicon Inc.), polyglactin (Vicryl; Ethicon Inc.), PTFE + carbon (Proplast; Vitek, Houston, TX), and stainless steel. PTFE has the benefit of being an airtight/watertight material, which can be advantageous for intrathoracic closures. However, there is negligible fibrovascular incorporation of the mesh into the surrounding tissue, and it has very little resistance to infection. When PTFE becomes infected or exposed, it generally requires explantation. Similarly, Proplast20 and silicone21 were plagued by problems of extrusion, capsule formation, slippage, and infection requiring removal.
Composite implant techniques were developed to allow contouring of the construct to the defect while maintaining rigidity and the ability to inset securely the construct into the defect. The most common composite is the combination of polypropylene mesh and poly(methyl methacrylate), or PMMA. The mesh is cut to fit the defect, leaving sufficient extra material to secure to the surrounding edges. The PMMA is then added to the pattern of the defect only. A second layer of polypropylene mesh is placed on top of this construct, creating a “sandwich.” The construct is then placed over the area to be reconstructed to conform to it, taking caution to protect vital structures, as the hardening is an exothermic reaction that can reach 140°F. This technique allows for molding of the construct to the original contours of the chest wall/defect (Fig. 2). Other composites include various combinations of the meshes described above combined with PTFE, silicone, rubber, or carbon fiber.
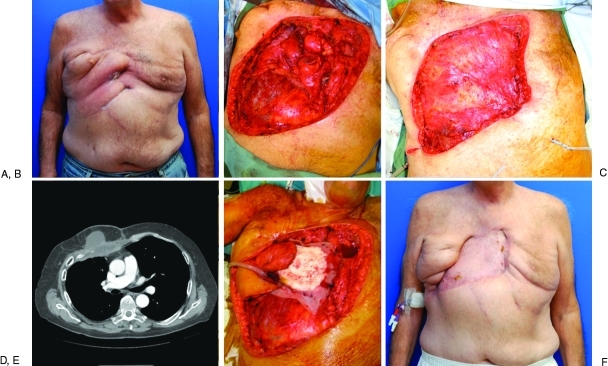
Figure 2.
(A) A 64-year-old man with recurrent squamous cell carcinoma of the chest wall previously treated with composite resection and pectoralis flap reconstruction with postoperative radiotherapy and chemotherapy. (B) Preoperative CT scan demonstrating involvement of the anterior and right chest wall. (C) Resection defect included the sternum, ribs 2 to 6, and the right upper lobe. (D) The defect was reconstructed with a composite polypropylene mesh/poly(methyl methacrylate) sandwich. (E) An ipsilateral pedicled latissimus dorsi muscle flap was harvested for soft tissue coverage and covered with a split-thickness skin graft from the thigh. (F) Appearance of chest wall reconstruction 2 months postoperatively. (Photographs courtesy of Donald P. Baumann, M.D.)
Each mesh and composite has its own relative advantages and disadvantages, as well as proponents and antagonists. Composite meshes with PTFE, silicone, or carbon fiber have a high rate of infection often necessitating removal.22 The remainder of the materials for the most part are reliable and provide reasonable stability, and we could fill this article with arguments and counterarguments for all of them. The decision about which mesh to use depends on the characteristics of the defect and the surgeon's preference and experience, as there is currently no level III or better evidence for one over the other.
Regardless of the mesh selected, use of mesh to repair a previously irradiated or contaminated defect significantly increases the chance of infection and exposure. This limitation and its devastating consequences have pushed reconstructive surgeons to begin exploring the use of bioprosthetic material.
Bioprosthetic Materials
Bioprosthetic meshes, such as AlloDerm (LifeCell Corporation, Branchburg, NJ), were popularized in 1995,23 and based on early promising results, these products have undergone rapid and widespread acceptance. There are currently 12 U.S. Food and Drug Administration (FDA)-approved bioprosthetic mesh products. Bioprosthetic meshes are classified by source material (xenograft or allograft), postharvesting processing technique, and handling characteristics. Most products are based on decellularized, processed dermis. Of the 12 available products, only four have been evaluated in a published peer-reviewed paper,24 and some portions of these data are from industry-sponsored trials.25 The advantage of decellularized dermis (xenograft or allograft) is that it is gradually revascularized and remodeled into autologous tissue while maintaining its structural integrity. These products have been shown to be more resistant to infection and to incorporate into irradiated tissues.26
ALLOGRAFTS
Human acellular dermal matrixes (HADMs) are derived from cadaveric dermal allografts and are classified by the FDA as “minimally processed human tissue” and as such are not subject to the same level of scrutiny as xenografts, which are classified as medical devices. Cadaveric allografts are regulated by tissue banks and include AlloDerm, AlloMax (Tutogen Medical Inc., Alachua, FL), and Flex HD (Ethicon Inc.). There are currently no published human data on the use of the last two products for chest wall reconstruction.
AlloDerm is cadaveric human dermis from tissue banks that is chemically and physically processed to remove cellular components while preserving the extracellular matrix structure and basement membrane. It is the most extensively researched of the bioprosthetic materials. The material is non-cross-linked, freeze dried, and requires 15 to 20 minutes to rehydrate prior to use. There is minimal inflammatory response to this material,27,28 rapid cellular infiltration and vascularization,29 a low infection rate in contaminated wounds,30,31,32 tolerance of cutaneous exposure without the need for surgical removal,27 and a strong implant-fascia interface,33,34 and it becomes remodeled with native host tissue.35 The long- and short-term use of AlloDerm for chest wall stabilization after major resections has been studied and found to be safe and reliable28,36 (Fig. 3).
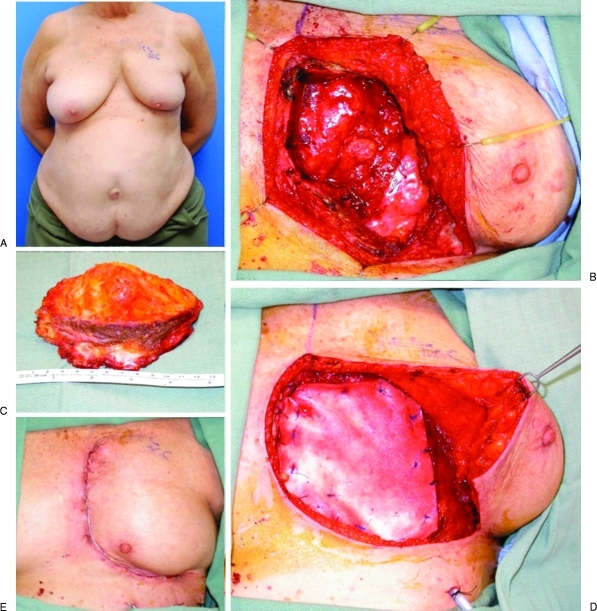
Figure 3.
(A) Preoperative photograph of a 76-year-old woman with left chest wall radiation-associated sarcoma after segmental mastectomy and postoperative radiotherapy 12 years prior. (B) The left chest wall resection included the medial sternum and anterolateral segments of ribs 3, 4, and 5. (C) Composite chest wall resection specimen. (D) The defect was reconstructed with an onlay bioprosthetic mesh and a left pectoralis majors myocutaneous rotation advancement flap. (E) Appearance of the reconstructed composite defect postoperatively. (Photographs courtesy of Donald P. Baumann, M.D.)
Techniques that we have used that may have contributed to the success include the following: applying appropriate physiologic tension, bioprosthetic/fascia overlap, securing the allografts by drilling holes in the surrounding bone, and efforts at seroma prevention (using a suction-drainage catheter, quilting sutures, and compression garments when possible). Seroma formation is the most common short-term complication. “Bulges” or material laxity can occur months later. Whereas bulges may be caused by technical error, such as inadequate rehydration and insufficient inset tension, we have seen this problem even in technically sound repairs. Material bulge appears to be more likely to occur in cases where there is infection or prolonged cutaneous exposure of the HADM and when using the HADM to bridge fascial gaps (as opposed to reinforce direct fascial closure). Material bulge may also be caused by ongoing host remodeling. Paradoxical breathing has not been reported, despite large chest wall reconstructions with HADM,37 but long-term data are not available.
Using these techniques, large composite trunk defects can be repaired successfully in patients at increased risk for prosthetic mesh complications. When compared with synthetic mesh, HADM is more favorable for irradiated and/or contaminated wounds with potential for cutaneous exposure, but it has the disadvantage of higher cost.
XENOGRAFTS
There are currently nine xenografts available for use.24 They are derived from either porcine products, such as Surgisis (small intestine submucosa; Cook Biomedical, Bloomington, IN), Permacol (dermis; Covidien, Norwalk, CT), CollaMend/XenMtrix (dermis; Davol Inc., Warwick, RI), and Strattice (dermis; Life Cell Inc., Branchburg, NJ), or bovine products, such as Tutopatch (pericardium; Tutogen, Alachua, FL), Veritas/Peri-guard (pericardium; Synovis Surgical Inc., St. Paul, MN), and Surgimend (dermis; TEI Biosciences Inc., Boston, MA). Other than for Surgisis, Permacol, and Tutopatch, there are no peer-reviewed human data available for these xenografts.24 The proposed advantages to these products are that they are ready to use (no rehydration needed), are available in large sheets, and do not require prestretching. However, both short- and long-term data on the use of these materials are needed. Our anecdotal experience is that the grafts seem to perform well in clean and clean-contaminated wounds. In contaminated wounds, there is still a potential for clinical infection, although likely less than with a synthetic mesh. In infected cases, it is often beneficial to stage the definitive reconstruction after several debridements and dressing changes. We have successfully used serial debridements followed by NPWT as a bridge to definitive reconstruction.
With no donor site morbidity and a relatively unlimited supply (except for the expense), bioprosthetic materials hold the promise of playing an important role in complex reconstructions. Thus, it is paramount that surgeons continue to publish their experiences with these products, as the literature remains sparse. More research is needed to elucidate the indications and contraindications, performance, and outcomes of these materials. These cases remain uncommon, and a multicenter effort will likely be required to complete a prospective comparative trial.
OSTEOSYNTHESIS
Sternum
The use of traditional cerclage wires, which are employed by cardiac and thoracic surgeons for closure of the sternum, has been challenged. Sternal instability contributes to increased rates of postoperative mediastinitis, especially in high-risk patients.37,38,39 Studies have demonstrated both the biomechanical40 and clinical superiority37,41,42 of rigid plate fixation over wire cerclage in healing sternotomies. When direct approximation of the sternal edges is not possible, as is often the case in large resections/debridements, both plating systems and cerclage wires become less than ideal. We prefer to bridge these gaps with autologous tissue alone (such as the pectoralis major, greater omentum, or rectus abdominus flaps) for small to moderate defects. For larger defects, methyl methacrylate composite meshes are used and covered with vascularized tissue. Contaminated wounds are generally not reconstructed with synthetic mesh techniques but rather with bioprosthetic mesh.
Ribs
Despite concerns, paradoxical movements of the chest wall during respiration and disturbances of ventilation and perfusion are rare with most chest wall reconstructions. Stabilization with an autologous and/or alloplastic material seems to prevent effectively or limit the clinical impact of this complication. Open reduction and osteosynthesis of acute traumatic rib fractures has never become widely accepted. Likewise, for the reconstruction of rib defects, plating systems have not been widely used. The underlying vital structures would necessitate a layer between the plate and viscera that would only complicate an already complex reconstruction. Erosion, extrusion, and migration of the plates and screws have also been seen, which limits their usefulness. We have rarely had the occasion to use plating systems in the reconstruction of rib defects.
CONCLUSION
Chest wall stabilization begins with a fundamental understanding of the defect—what is missing, what remains, what needs to be replaced, and what resources are available for the reconstruction. In addition to reestablishing the normal functions of the chest wall (protection, respiration, support, and aesthetics), evidence suggests that bony fixation results in reduced ventilator dependence, shorter overall hospital stay, and improved upper extremity function. Small to moderate defects (en bloc resection of defects smaller than 5 cm or involving three or fewer ribs) can be reconstructed with autologous tissue alone. Other than in the case of previously irradiated defects, larger defects are reconstructed with methyl methacrylate composite meshes and covered with vascularized tissue. Contaminated wounds are generally not reconstructed with synthetic mesh techniques but rather with bioprosthetic mesh. Using these principles, the reconstructive plastic surgeon can devise a comprehensive and safe repair plan for tremendous defects of the chest wall.
References
- Parham D W. Thoracic resection for tumors growing from the bony chest wall. Trans South Surg Assoc. 1899;2:223–363. [Google Scholar]
- Fell G E. Forced respiration. JAMA. 1891;16:325–330. [Google Scholar]
- O'Dwyer J. Fifty cases of croup in private practice treated by intubation of the larynx with a description of the method and of the dangers incident thereto. Med Rec. 1887;32:557–561. [Google Scholar]
- Kroll S S, Walsh G, Ryan B, King R C. Risks and benefits of using Marlex mesh in chest wall reconstruction. Ann Plast Surg. 1993;31:303–306. doi: 10.1097/00000637-199310000-00003. [DOI] [PubMed] [Google Scholar]
- Netscher D T, Eladoumikdachi F, McHugh P M, Thornby J, Soltero E. Sternal wound debridement and muscle flap reconstruction: functional implications. Ann Plast Surg. 2003;51:115–122. discussion 123–125. doi: 10.1097/01.SAP.0000058497.92264.E2. [DOI] [PubMed] [Google Scholar]
- Ringelman P R, Vander Kolk C A, Cameron D, Baumgartner W A, Manson P N. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208–1214. discussion 1215–1216. [PubMed] [Google Scholar]
- Cicilioni O J, Jr, Stieg F H, III, Papanicolaou G. Sternal wound reconstruction with transverse plate fixation. Plast Reconstr Surg. 2005;115:1297–1303. doi: 10.1097/01.prs.0000156918.15595.85. [DOI] [PubMed] [Google Scholar]
- Spear S L, Hess C L. A review of the biomechanical and functional changes in the shoulder following transfer of the latissimus dorsi muscles. Plast Reconstr Surg. 2005;115:2070–2073. doi: 10.1097/01.prs.0000163329.96736.6a. [DOI] [PubMed] [Google Scholar]
- Castelló J R, Centella T, Garro L, et al. Muscle flap reconstruction for the treatment of major sternal wound infections after cardiac surgery: a 10-year analysis. Scand J Plast Reconstr Surg Hand Surg. 1999;33:17–24. doi: 10.1080/02844319950159587. [DOI] [PubMed] [Google Scholar]
- Meadows J A, III, Staats B A, Pairolero P C, Rodarte J R, Arnold P G. Effect of resection of the sternum and manubrium in conjunction with muscle transposition on pulmonary function. Mayo Clin Proc. 1985;60:604–609. doi: 10.1016/s0025-6196(12)60984-7. [DOI] [PubMed] [Google Scholar]
- Song D H, Wu L C, Lohman R F, Gottlieb L J, Franczyk M. Vacuum assisted closure for the treatment of sternal wounds: the bridge between débridement and definitive closure. Plast Reconstr Surg. 2003;111:92–97. doi: 10.1097/01.PRS.0000037686.14278.6A. [DOI] [PubMed] [Google Scholar]
- Pairolero P C, Arnold P G. Thoracic wall defects: surgical management of 205 consecutive patients. Mayo Clin Proc. 1986;61:557–563. doi: 10.1016/s0025-6196(12)62004-7. [DOI] [PubMed] [Google Scholar]
- Wall M J, Huh J. Chest wall, mediastinum, trachea: Chest wall trauma. In: Cameron J L, editor. Current Surgical Therapy. Philadelphia, PA: Elsevier, Mosby; 2001. pp. 663–664. [Google Scholar]
- Disa J J, Klein M H, Goldberg N H. Advantages of autologous fascia versus synthetic patch abdominal reconstruction in experimental animal defects. Plast Reconstr Surg. 1996;97:801–806. doi: 10.1097/00006534-199604000-00017. [DOI] [PubMed] [Google Scholar]
- Disa J J, Goldberg N H, Carlton J M, Robertson B C, Slezak S. Restoring abdominal wall integrity in contaminated tissue-deficient wounds using autologous fascia grafts. Plast Reconstr Surg. 1998;101:979–986. doi: 10.1097/00006534-199804040-00014. [DOI] [PubMed] [Google Scholar]
- Boyd A D, Shaw W W, McCarthy J G, et al. Immediate reconstruction of full-thickness chest wall defects. Ann Thorac Surg. 1981;32:337–346. doi: 10.1016/s0003-4975(10)61754-7. [DOI] [PubMed] [Google Scholar]
- McCormack P M. Use of prosthetic materials in chest-wall reconstruction. Assets and liabilities. Surg Clin North Am. 1989;69:965–976. doi: 10.1016/s0039-6109(16)44932-7. [DOI] [PubMed] [Google Scholar]
- Arnold P G, Pairolero P C. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–810. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Morgan R F, Edgerton M T, Wanebo H J, Daniel T M, Spotnitz W D, Kron I L. Reconstruction of full thickness chest wall defects. Ann Surg. 1988;207:707–716. doi: 10.1097/00000658-198806000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M L. Proplast reconstruction of the lower sternum. Plast Reconstr Surg. 1981;68:795–797. doi: 10.1097/00006534-198111000-00029. [DOI] [PubMed] [Google Scholar]
- Allen R G, Douglas M. Cosmetic improvement of thoracic wall defects using a rapid setting silastic mold: a special technique. J Pediatr Surg. 1979;14:745–749. doi: 10.1016/s0022-3468(79)80258-4. [DOI] [PubMed] [Google Scholar]
- Cobb W S, Harris J B, Lokey J S, McGill E S, Klove K L. Incisional herniorrhaphy with intraperitoneal composite mesh: a report of 95 cases. Am Surg. 2003;69:784–787. [PubMed] [Google Scholar]
- Wainwright D J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–248. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- Rosen M J. Biologic mesh for abdominal wall reconstruction: a critical appraisal. Am Surg. 2010;76:1–6. [PubMed] [Google Scholar]
- Bellows C F, Alder A, Helton W S. Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Devices. 2006;3:657–675. doi: 10.1586/17434440.3.5.657. [DOI] [PubMed] [Google Scholar]
- Dubin M G, Feldman M, Ibrahim H Z, et al. Allograft dermal implant (AlloDerm) in a previously irradiated field. Laryngoscope. 2000;110:934–937. doi: 10.1097/00005537-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg. 2004;52:188–194. doi: 10.1097/01.sap.0000100895.41198.27. [DOI] [PubMed] [Google Scholar]
- Lattari V, Jones L M, Varcelotti J R, Latenser B A, Sherman H F, Barrette R R. The use of a permanent dermal allograft in full-thickness burns of the hand and foot: a report of three cases. J Burn Care Rehabil. 1997;18:147–155. doi: 10.1097/00004630-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Eppley B L. Experimental assessment of the revascularization of acellular human dermis for soft-tissue augmentation. Plast Reconstr Surg. 2001;107:757–762. doi: 10.1097/00006534-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Butler C E, Langstein H N, Kronowitz S J. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg. 2005;116:1263–1275. discussion 1276–1277. doi: 10.1097/01.prs.0000181692.71901.bd. [DOI] [PubMed] [Google Scholar]
- Diaz J J, Jr, Guy J, Berkes M B, Guillamondegui O, Miller R S. Acellular dermal allograft for ventral hernia repair in the compromised surgical field. Am Surg. 2006;72:1181–1187. discussion 1187–1188. [PubMed] [Google Scholar]
- Kim H, Bruen K, Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg. 2006;192:705–709. doi: 10.1016/j.amjsurg.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Gobin A S, Butler C E, Mathur A B. Repair and regeneration of the abdominal wall musculofascial defect using silk fibroin-chitosan blend. Tissue Eng. 2006;12:3383–3394. doi: 10.1089/ten.2006.12.3383. [DOI] [PubMed] [Google Scholar]
- Holton L H, III, Chung T, Silverman R P, et al. Comparison of acellular dermal matrix and synthetic mesh for lateral chest wall reconstruction in a rabbit model. Plast Reconstr Surg. 2007;119:1238–1246. doi: 10.1097/01.prs.0000254347.36092.9c. [DOI] [PubMed] [Google Scholar]
- Livesey S A, Herndon D N, Hollyoak M A, Atkinson Y H, Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60:1–9. [PubMed] [Google Scholar]
- Butler C E, Langstein H N, Kronowitz S J. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg. 2005;116:1263–1275. discussion 1276–1277. doi: 10.1097/01.prs.0000181692.71901.bd. [DOI] [PubMed] [Google Scholar]
- Song D H, Lohman R F, Renucci J D, Jeevanandam V, Raman J. Primary sternal plating in high-risk patients prevents mediastinitis. Eur J Cardiothorac Surg. 2004;26:367–372. doi: 10.1016/j.ejcts.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Molina J E, Lew R S, Hyland K J. Postoperative sternal dehiscence in obese patients: incidence and prevention. Ann Thorac Surg. 2004;78:912–917. discussion 912–917. doi: 10.1016/j.athoracsur.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Losanoff J E, Jones J W, Richman B W. Primary closure of median sternotomy: techniques and principles. Cardiovasc Surg. 2002;10:102–110. doi: 10.1016/s0967-2109(01)00128-4. [DOI] [PubMed] [Google Scholar]
- Pai S, Gunja N J, Dupak E L, et al. In vitro comparison of wire and plate fixation for midline sternotomies. Ann Thorac Surg. 2005;80:962–968. doi: 10.1016/j.athoracsur.2005.03.089. [DOI] [PubMed] [Google Scholar]
- Wu L C, Renucci J D, Song D H. Sternal nonunion: a review of current treatments and a new method of rigid fixation. Ann Plast Surg. 2005;54:55–58. doi: 10.1097/01.sap.0000139564.37314.1f. [DOI] [PubMed] [Google Scholar]
- Raman J, Straus D, Song D H. Rigid plate fixation of the sternum. Ann Thorac Surg. 2007;84:1056–1058. doi: 10.1016/j.athoracsur.2006.11.045. [DOI] [PubMed] [Google Scholar]