Abstract
Ligation of the high-affinity IgE receptor (FcεRI) or of c-Kit stimulates cytokine production in mast cells. We show that MEK kinase 2 (MEKK2), a MAPK kinase kinase (MAP3K) that regulates the JNK and ERK5 pathways, is required for cytokine production in embryonic stem (ES) cell-derived mast cells (ESMC). Targeted disruption of the MEKK2 or MEKK1 gene was used to abolish expression of the respective kinases in ESMC. Transcription of specific cytokines in response to IgE or c-Kit ligand was markedly reduced in MEKK2–/– ESMC relative to wild-type ESMC. Cytokine production in MEKK1–/– ESMC was similar to that of wild-type ESMC, demonstrating the specificity of MEKK2 in signaling cytokine gene regulation. MEKK2–/– ESMC also lost receptor-mediated stimulation of JNK. In contrast, JNK activation in response to UV irradiation was normal, showing that MEKK2 is required for receptor signaling but not for cellular stress responses. MEKK2 is the first MAP3K shown to be required for mast cell tyrosine kinase receptor signaling controlling cytokine gene expression.
Keywords: cytokine expression/ES-derived mast cells/MEKK2 knockouts
Introduction
Mast cells are centrally important in inflammatory and immediate allergic reactions (Metcalfe et al., 1997). Ligation of the high-affinity IgE receptor (FcεRI) triggers the release of pre-formed inflammatory mediators including histamine and the synthesis of specific proinflammatory cytokines. Mast cells also express the tyrosine kinase-encoded receptor c-Kit and are responsive to its ligand, c-Kit ligand (KL, also referred to as stem cell factor, SCF). In mast cell lines or bone marrow-derived mast cells, mitogen-activated protein kinase (MAPK) pathways including extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 MAPK are activated by antigen–IgE ligation of FcεRI and KL ligation of c-Kit (Tsai et al., 1993; Ishizuka et al., 1996, 1998, 1999). An important role for these MAPK pathways in mast cell function is predicted based on the ability of ERK1/2, JNK and p38 to regulate the transcriptional activity of genes important in mast cell function, such as cytokine genes (Rooney et al., 1995; Faris et al., 1996; Ishizuka et al., 1997; Nishina et al., 1997).
MAPKs are regulated by a family of proteins known as MAPK kinases (MKKs), which are in turn regulated by a family of MKK kinases (MKKKs or MAP3Ks) (Widmann et al., 1999). Transfection studies in mast cells and Jurkat T cells hint at a role for the MEKK group of MAP3Ks in antigen regulation of cytokine production (Faris et al., 1996; Ishizuka et al., 1997). We used kinase-inactive mutants of different MAP3Ks in initial transfection screens to define the role of specific MAPK pathways in mast cell functions responsive to FcεRI- and c-Kit-mediated stimulation. In MC/9 mouse mast cells, these studies identified MEKK2 as having a role in the regulation of JNK activation and tumor necrosis factor-α (TNF-α) production following FcεRI ligation. MEKK2 is a 70 kDa member of the MEKK group of MAP3Ks that has been shown to regulate the JNK and ERK5 pathways (Blank et al., 1996; Sun et al., 2000). We have shown recently that MEKK2 is recruited to the T-cell receptor (TCR) signaling complex upon presentation of antigen to T cells (Schaefer et al., 1999). MEKK2 was activated in response to antigen presentation and was required for stabilization of conjugates of T cells and antigen-presenting cells. MEKK1 and MEKK3 were not recruited to the TCR signaling complex in response to antigen, showing the selectivity of this response to MEKK2. Also, MEKK2–/– embryonic stem (ES) cells were used for Rag2–/– blastocyst complementation to define the role of MEKK2 in lymphocyte development (B.C. Schaefer, T.P.Garrington, S.Webb, D.M.Russell, E.W.Gelfand, J.W.Kappler, G.L.Johnson and P.Marrack, submitted). It was found that MEKK2 was required for B- and Tαβ+-cell development beyond the pre-BCR and pre-TCR signaling checkpoints, respectively.
To define further the involvement of MEKK2 in antigen-responsive receptor signaling, the technique of in vitro differentiation of ES cells to ES cell-derived mast cells (ESMC) was employed. ESMC provided a powerful system to define the role of MEKK2 in signaling by the mast cell high-affinity IgE receptor, FcεRI. Our results demonstrate that MEKK2 is a critical MAP3K in mast cell receptor signaling and in the control of cytokine production in mast cells.
Results
MEKK2 is stimulated by FcεRI ligation and can stimulate TNF-α promoter activity
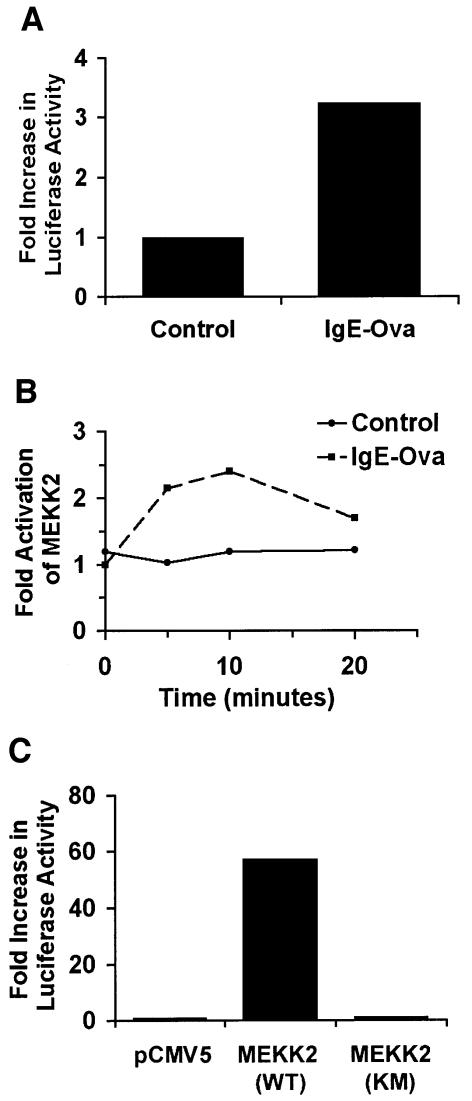
Activation of FcεRI in MC/9 mouse mast cells by sensitization with anti-ovalbumin IgE followed by cross-linking with ovalbumin (IgE-ova) stimulated TNF-α promoter-regulated expression of luciferase (Figure 1A). IgE-ova activation of FcεRI activated MEKK2 in MC/9 cells as measured by immunoprecipitation of MEKK2 from control and stimulated cells followed by an in vitro kinase assay (Figure 1B). MEKK2 expression in MC/9 cells also activated TNF-α promoter-regulated expression of luciferase (Figure 1C). Cumulatively, the findings in MC/9 cells indicate that the mast cell high-affinity IgE receptor, FcεRI, activates MEKK2 in a manner similar to that observed with TCR ligation in D10 T cells (Schaefer et al., 1999). Furthermore, MEKK2 expression is able to stimulate TNF-α promoter activity similarly to IgE-ova activation of FcεRI. These results provide evidence for pathways utilizing MEKK2 to link FcεRI ligation with stimulation of TNF-α promoter activity. In order to define unequivocally the role of MEKK2 in mast cell receptor signaling, the MEKK2 gene was inactivated by targeted gene disruption.
Fig. 1. MEKK2 is stimulated by FcεRI ligation and can stimulate TNF-α promoter activity. (A) A luciferase reporter construct containing a murine 5′-TNF-α was electroporated into MC/9 mast cells. The cells were then treated overnight with anti-ovalbumin IgE, washed and treated with ovalbumin (IgE-ova). Six hours following stimulation with ovalbumin, the cells were lysed and luciferase activity was measured. Cells treated with IgE-ova had 3- to 4-fold higher activity than untreated cells. (B) To determine the effect of IgE-ova stimulation of MC/9 cells on endogenous MEKK2 activity, an MEKK2 kinase assay was performed, using a previously described method (Fanger et al., 1997). Over a 20 min time course, MEKK2 activity with IgE-ova stimulation is shown to be up to twice as high as control MEKK2 activity, with a peak at 10 min. (C) To determine MEKK2’s direct effects on TNF-α-luciferase activity, MC/9 cells were electroporated with wild-type MEKK2 [MEKK2(WT)], kinase-inactive MEKK2 [MEKK2(KM)] or control plasmid (pCMV5) along with TNF-α-luciferase. While kinase-inactive MEKK2 had no effect on luciferase activity, transfection of wild-type MEKK2 resulted in a 60-fold increase in luciferase activity, showing that the kinase activity of MEKK2 is important for positive regulation of TNF-α promoter activity. Cumulatively, these results suggest the involvement of MEKK2 in the regulation of a pathway leading from activation of FcεRI to TNF-α gene expression.
Production of homozygous MEKK2–/– ES cells
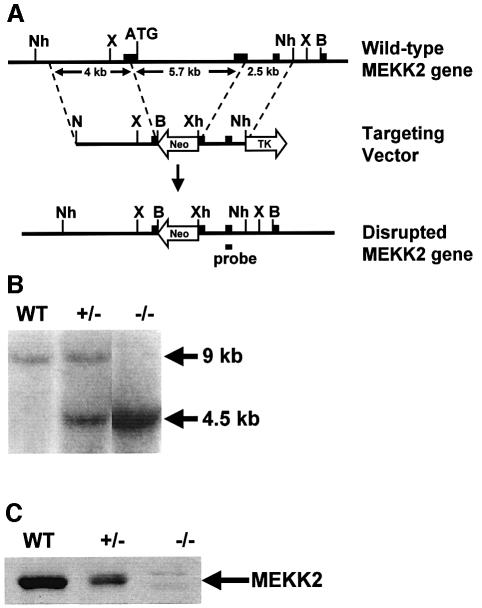
Targeted disruption of the MEKK2 gene in mouse ES cells was accomplished using the gene targeting strategy shown in Figure 2A. The targeting vector was constructed by replacing a 5.7 kb fragment of the MEKK2 gene containing the start site exon, the downstream exon and the intervening intron with a neomycin resistance gene. Both exons were interrupted, the start site was lost and a frameshift mutation was introduced. NotI, BamHI and XhoI restriction sites were inserted by PCR to facilitate placement of the gene fragments into the targeting vector. Outside of the region of homology there was a thymidine kinase gene to allow positive–negative selection. The linearized construct was introduced into R1 ES cells by electroporation. The cells were grown in G418 (400 µg/ml) and ganciclovir (2 µM) for positive–negative selection. G418- and ganciclovir-resistant clones were screened for homologous recombination by Southern (DNA) blotting. The frequency of homologous recombination was 14%. For the generation of MEKK2–/– ES cells, the MEKK2+/– clones were grown in high concentration (10 mg/ml) G418 to select for clones in which replacement of the remaining wild-type MEKK2 gene by the mutant gene had occurred. A Southern blot analysis of wild-type (MEKK2+/+), heterozygous (MEKK2+/–) and homozygous (MEKK2–/–) ES cells is shown in Figure 2B. Two feeder-independent R1 MEKK2–/– clones were developed for further studies, and the clones were screened for MEKK2 protein expression by western blotting. Figure 2C shows that the MEKK2 protein is absent in homozygous –/– ES cell clones.
Fig. 2. Gene targeting of MEKK2. (A) Knockout vector design. Exons are shown as filled boxes, and the position of the DNA segment used as a probe for Southern blot analysis is shown. The start site (ATG) is at the 3′ end of the first exon. The knockout region consists of part of the first exon containing the start site along with the downstream intron and a portion of the following exon. B, BamHI; N, NotI; Nh, NheI; X, XbaI; Xh, XhoI; Neo, neomycin resistance gene; TK, thymidine kinase gene. (B) Southern blot analysis using XbaI-digested genomic DNA. Fragment size with the wild-type DNA is 9 kb. In the knockout cells, fragment size is 4.5 kb. (C) Western blot analysis. Proteins from cell lysates were resolved by SDS–PAGE, transferred to nitrocellulose filters and probed with an antibody recognizing the N-terminus of MEKK2. Antibodies to the C-terminus of MEKK2 also failed to detect MEKK2 in MEKK2–/– cells (not shown).
In vitro differentiation of MEKK2–/– ES cells into mast cells is normal
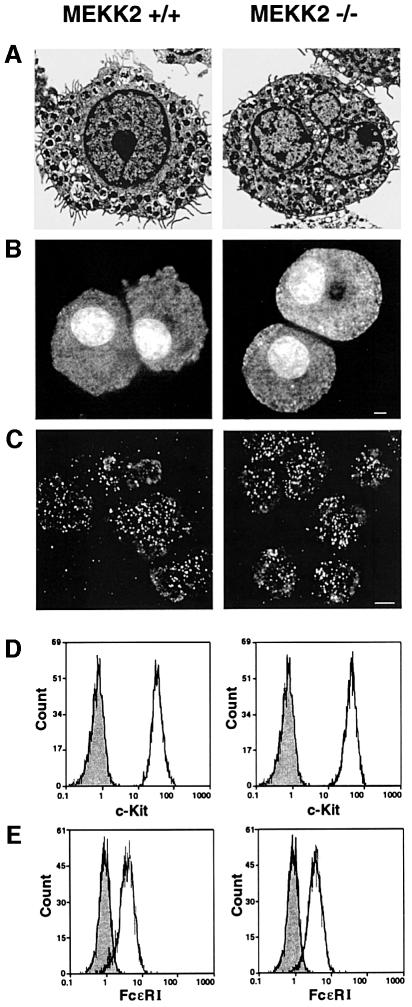
Mast cells were differentiated in vitro from ES cells (see Materials and methods). Wild-type and MEKK2–/– ES cells were first differentiated to embryoid bodies (EBs) for 6 days in culture. The EBs were dissociated with trypsin and the cells were cultured for 4–12 weeks in media containing interleukin-3 (IL-3) and KL. Light microscopy after May Grünwald/Giemsa staining showed similar morphologies of wild-type and MEKK2–/– ESMC (not shown). Electron microscopy showed that wild-type and MEKK2–/– ESMC had microvilli and granules characteristic of mast cells (Figure 3A). These findings indicated that the loss of MEKK2 expression did not alter morphological differentiation of ES cells to mast cells. Granules with the MEKK2–/– and wild-type ESMC contained heparin and chymase (Figure 3B and C). Flow cytometric analysis of cell surface expression of c-Kit and FcεRI also indicated similar receptor expression in MEKK2–/– and wild-type ESMC (Figure 3D and E) (Valent and Bettelheim, 1992). Finally, the growth rate, differentiation potential and degranulation capability of MEKK2–/– and wild-type ESMC were indistinguishable (not shown). Thus, loss of MEKK2 expression had no measurable effect on the growth and differentiation characteristics, morphology, granule content, degranulation or surface receptor expression of ESMC.
Fig. 3. MEKK2–/– ESMC have normal mast cell morphology, granule content and receptor expression for FcεRI and c-Kit in comparison with wild-type ESMC. (A) Electron micrographs of wild-type and MEKK2–/– ESMC. The cells were grown in culture for 4 weeks. Mast cell granules are present. Short, villous processes, typical of mast cells (Dvorak et al., 1983), are also seen on the cell surface. (B) Berberine staining of wild-type and MEKK2–/– mast cells. Berberine stains heparin, a component of mast cell granules (Enerback, 1974; Metcalfe et al., 1997). Staining of neutrophils was used as a negative control (not shown). (C) Cy3-labeled antibody staining of wild-type and MEKK2–/– mast cells for chymase, an enzyme specific to mast cell granules (Metcalfe et al., 1997). (D and E) Flow cytometry. For c-Kit staining (D), the cells were treated with FITC–anti c-Kit antibody. Negative controls (shaded) were untreated. For FcεRI staining (E), cells were treated with anti-DNP IgE, washed, then treated with FITC–anti IgE. The negative control (shaded) was treated with FITC–anti-IgE alone. Wild-type and MEKK2–/– cells expressed both receptors in equal amounts. The ES-derived mast cell cultures were 50–60 days old at the time of testing.
Cytokine mRNA biosynthesis is reduced markedly in MEKK2–/– ESMC responding to stimulation through c-Kit and FcεRI
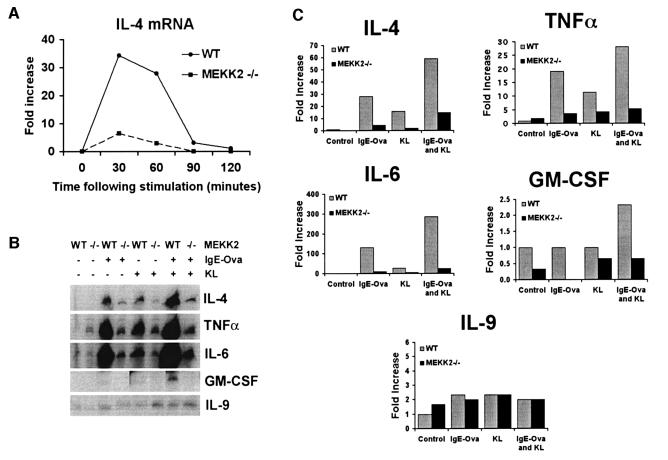
Transfection studies in MC/9 mast cells indicated that MEKK2 can regulate TNF-α promoter activity (Figure 1C). Several cytokines, including IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-13, granulocyte–macrophage colony-stimulating factor (GM-CSF) and TNF-α, are synthesized and released by mature mast cells and are believed to be important in the responses initiated by mast cells and in their recruitment of other immune cells to sites of inflammation (Plaut et al., 1989; Paul et al., 1993; Jaffe et al., 1995, 1996; Okayama et al., 1995a,b; Gagari et al., 1997; Moller et al., 1998). The targeted disruption of MEKK2 expression was exploited to test directly the role of MEKK2 in regulating cytokine gene expression in response to receptor activation of mast cells. RNase protection assays (RPAs) were performed to measure the expression of specific cytokine mRNAs in ESMC. Wild-type and MEKK2–/– ESMC were challenged with KL to stimulate c-Kit, IgE-ova to stimulate FcεRI, or the combination of KL and IgE-ova, and then analyzed for cytokine mRNA expression. A panel of cytokines including IL-2, IL-4, IL-5, IL-6, IL-9, IL-15, GM-CSF, TNF-α and interferon-γ (IFN-γ) was screened in control and stimulated ESMC. Figure 4A shows a time course for fold increase in IL-4 mRNA production in response to KL stimulation in wild-type and MEKK2–/– ESMC. IL-4 mRNA expression peaked at 30–60 min following stimulation, and mRNA levels were markedly reduced in the MEKK2–/– ESMC compared with wild-type ESMC. Figure 4B shows RPA results for five different cytokines 1 h after stimulation with KL, IgE-ova or the combination of KL and IgE-ova. The induction of cytokine mRNA expression was altered significantly in stimulated MEKK2–/– ESMC compared with MEKK2+/+ ESMC. In several independent experiments, the ability of FcεRI or c-Kit ligation, alone or in combination, to stimulate the expression of the mRNAs for IL-4, IL-6, GM-CSF and TNF-α was diminished markedly in MEKK2–/– ESMC compared with wild-type ESMC. Quantitation of cytokine mRNA expression relative to control GAPDH and L32 mRNA expression is shown in Figure 4C. Interestingly, stimulation of IL-9 expression was not affected, indicating that the regulation of cytokine expression by MEKK2 is selective for specific cytokine genes. When FcεRI and c-Kit were co-stimulated, there was synergy in the ability to stimulate cytokine mRNA expression. The mRNA levels for IL-4, IL-6, TNF-α and GM-CSF were nonetheless reduced significantly in MEKK2–/– relative to wild-type ESMC co-stimulated with IgE-ova and KL. The results clearly show that MEKK2 expression is required for normal receptor-stimulated induction of specific cytokine gene expression in mast cells.
Fig. 4. (A) Time course for IL-4 mRNA expression in ESMC following c-Kit ligand (KL) stimulation. Mast cells were deprived of KL for 16 h. For c-Kit ligation, cells were exposed to recombinant KL (250 ng/ml), followed by lysis and RNA extraction at each of the indicated time points. RNase protection assays were then performed. Results showing IL-4 mRNA production at 0, 30, 60, 90 and 120 min following stimulation in wild-type versus MEKK2–/– ESMC are shown. Results were quantified by PhosphorImager analysis and normalized to L32 and GAPDH mRNA expression levels for each sample. Expression of IL-4 mRNA in wild-type ESMC was 5- to 10-fold higher than in MEKK2–/– ESMC. (B) RNase protection assay analysis of mRNA expression for five different cytokines in ESMC following stimulation with IgE-ova and/or KL. Mast cells were deprived of KL for 16 h. For c-Kit ligation, cells were exposed to recombinant KL (250 ng/ml) for 1 h. For FcεRI ligation, cells were incubated with anti-ovalbumin IgE (0.8 µg/ml) overnight, washed and incubated at 37°C for an additional 2 h. They were then treated with ovalbumin, 10 µg/ml, for 1 h and lysed. For co-stimulation, the cells were treated with both stimuli for 1 h and lysed. (C) Quantitation was by PhosphorImager analysis, and results were normalized to L32 and GAPDH mRNA expression levels for each sample. Results show decreased mRNA production for IL-4, TNF-α, IL-6 and GM-CSF 1 h after stimulation of MEKK2–/– ESMC versus wild-type ESMC, while IL-9 mRNA production remained unchanged.
Receptor stimulation of cytokine mRNA biosynthesis is normal in MEKK1–/–ESMC
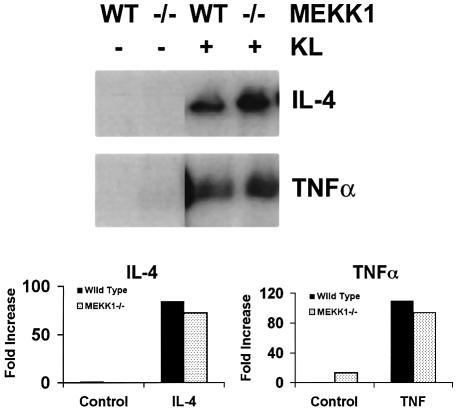
To demonstrate the specificity of MEKK2 in mast cell receptor regulation of cytokine production, MEKK1–/– ESMC were analyzed for their response to ligation of c-Kit by KL. We have shown previously that MEKK1 is required for JNK activation in response to perturbation of the microtubule cytoskeleton (Yujiri et al., 1998). As with MEKK2–/– ES cells, MEKK1–/– ES cells differentiate normally to mast cells (not shown). Figure 5 shows expression of mRNA for IL-4 and TNF-α in MEKK1–/– ESMC compared with wild-type ESMC 1 h after stimulation with KL. IL-4 and TNF-α mRNA expression is similar in wild-type and MEKK1–/– ESMC, indicating that MEKK2, but not MEKK1, is required for c-Kit receptor-mediated regulation of mast cell cytokine production.
Fig. 5. RPA results showing mRNA production for IL-4 and TNF-α 1 h after stimulation with KL in wild-type and MEKK1–/– ESMC. Mast cells were deprived of c-Kit ligand (KL) for 16 h. For c-Kit ligation, cells were exposed to recombinant KL (250 ng/ml) for 1 h and lysed. Quantitation was by PhosphorImager analysis, and results were normalized to L32 and GAPDH mRNA expression levels for each sample. Results show no significant difference in cytokine mRNA production in wild-type versus MEKK1–/– ESMC.
JNK activation is lost in response to c-Kit and FcεRI stimulation of ESMC
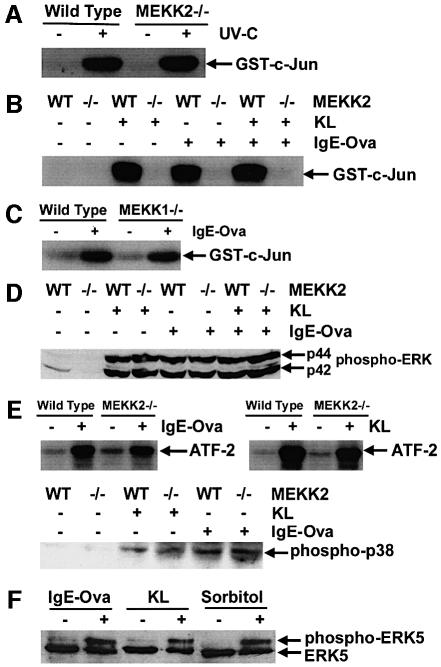
Targeted disruption of different MAP3Ks results in the selective loss of specific signaling functions in response to different upstream stimuli, demonstrating a unique role for each of the different MAP3Ks in the regulation of MAPK signaling pathways. Figure 6 defines the regulation of MAPK signaling pathways in wild-type and MEKK2–/– ESMC. We have shown previously that JNK activation in response to stress stimuli, including UV irradiation, heat shock, cold shock, hyperosmolarity, anisomycin, cytochalasin D and nocodazole, is normal in MEKK2–/– ES cells (Yujiri et al., 1999; unpublished observations). MEKK2–/– ESMC also have a normal JNK activation in response to exposure to UV irradiation (Figure 6A). Thus, the signal response for stress activation is intact in MEKK2–/– ESMC. Dramatically, the ability of both FcεRI and c-Kit to activate the JNK pathway is lost in MEKK2–/– ESMC (Figure 6B). This result was observed in two independently generated MEKK2–/– clones differentiated to ESMC. In contrast, ligation of FcεRI (Figure 6C) or c-Kit (not shown) in MEKK1–/– ESMC produces a JNK response similar to that in wild-type ESMC. Thus, loss of JNK activation in response to FcεRI ligation is specific to the loss of MEKK2 expression. In contrast to the loss of JNK activation in MEKK2–/– cells following stimulation of FcεRI or c-Kit, the activation of ERK1/2 and p38 is normal (Figure 6D and E). Therefore, MEKK2 is required for FcεRI and c-Kit activation of the JNK pathway but not for ERK1/2 or p38 activation. Importantly, the findings show that the knockout of MEKK1 or MEKK2 expression does not disrupt the overall integrity of MAPK pathway regulation. Rather, selectivity in loss of signaling to a specific stimulus is observed.
Fig. 6. Stimulation of ERK, JNK, p38 and BMK1/ERK5 in ESMC or MC/9 mast cells in response to c-Kit and/or FcεRI ligation. Mast cells were deprived of c-Kit ligand (KL) for 16 h. For c-Kit ligation, cells were exposed to recombinant KL (250 ng/ml) for 30 min and lysed. For FcεRI ligation, cells were incubated with anti-ovalbumin IgE (0.8 µg/ml) overnight, washed and incubated at 37°C for an additional 2 h. They were then treated with ovalbumin, 10 µg/ml, for 15 min and lysed. For co-stimulation with both KL and IgE-ova, cells were treated with KL 15 min prior to treatment with ovalbumin, so that stimulation with KL was for 30 min, and stimulation with ova was for 15 min. For UV light stimulation (UV-C), cells were exposed for 32 s at a 2 cm distance (100 J/m2) and lysed following an additional hour of incubation at 37°C. JNK activity was measured using a kinase assay with GST–c-Jun(1–79) bound to glutathione–Sepharose beads as a substrate. (A) JNK activity in response to UV-C was normal in MEKK2–/– ESMC in comparison with wild-type ESMC. (B) JNK activity in MEKK2–/– ESMC was markedly reduced in response to treatment with KL, IgE-ova or the combination of KL and IgE-ova. (C) Change in JNK activity in response to FcεRI ligation was also measured in MEKK1–/– ESMC. No difference in JNK activity was seen, indicating that the effect on JNK activity was MEKK2 specific. (D) The degree of ERK phosphorylation was assessed by western blot using anti-phospho-p44/p42 MAPK antibody. In contrast to JNK, no significant difference in phosphorylation between wild-type and MEKK2–/– ESMC was seen. (E) For measurement of p38 activity, a kinase assay using ATF-2 as a substrate was used (Abdel-Hafiz et al., 1992; Han et al., 1994). Similarly to ERK, no difference in p38-MAPK between wild-type and MEKK2–/– ESMC was seen. These results were confirmed by western blotting using anti-phospho-p38 antibody. (F) BMK1/ERK5 activity in MC/9 mast cells was determined using western blot analysis to show a gel shift of the BMK1/ERK5 protein in stimulated cells, indicative of BMK1/ERK5 phophorylation. A gel shift of BMK1/ERK5 has been shown previously to correlate with kinase activity (Kato et al., 1998). MC/9 cells were stimulated with IgE-ova and KL as described above. As a positive control (Abe et al., 1996), cells were treated with 0.4 M sorbitol for 30 min. Results show that both IgE-ova and KL can increase BMK1/ERK5 activity in stimulated mast cells.
Our finding of loss of JNK activation in response to receptor stimulation in MEKK2–/– ESMC prompted us to investigate whether or not the JNK pathway was acting as the primary mediator of IgE-ova- and KL-stimulated cytokine production in mast cells. We were unsuccessful in transfecting ESMC using a variety of methods, including lipofectamine, calcium phosphate and electroporation. For this reason, we used MC/9 mast cells to express a constitutively active MKK7–JNK fusion protein along with the same TNF-α promoter-regulated luciferase reporter plasmid as used in Figure 1. Activated JNK failed to stimulate luciferase expression (data not shown), indicating that activated JNK alone is insufficient to stimulate cytokine gene expression in mast cells. We have shown recently that MEKK2 activates the BMK1/ERK5 pathway as well as the protein kinase C-related kinase, PRK2 (Sun et al., 2000; W.Sun, K.Kesavan, B.Schaefer, N.L.Johnson, M.Ware, E.W.Gelfand and G.L.Johnson, submitted). Figure 6F shows that in MC/9 mast cells, IgE-ova and KL are capable of activating BMK1/ERK5. Thus, it is likely that in addition to JNK, IgE-ova and KL are activating the BMK1/ERK5 pathway in ESMC. The expression of BMK1/ERK5 in ESMC is extremely low, and, although we can measure BMK1/ERK5 activation in MC/9 cells, we have been unable to measure BMK1/ERK5 activity in ESMC. Nonetheless, our cumulative work with MEKK2 indicates that it regulates more than one MAPK pathway and that the sum of its downstream effects, not limited to JNK activation and possibly including BMK1/ERK5 activation, is involved with controlling cytokine gene expression. While further work is required to determine the specific downstream pathways affected in addition to loss of JNK activation in MEKK2–/– ESMC, our targeted gene disruption studies demonstrate that MEKK2 is the pivotal MAP3K that regulates the receptor stimulation of cytokine gene expression in mast cells.
Discussion
Our findings provide direct evidence that MEKK2-dependent signaling pathways regulate production of several cytokines in mast cells. The regulation of IL-4, IL-6, TNF-α and GM-CSF is highly dependent on MEKK2, while the regulation of IL-9 shows no dependence on MEKK2 signaling. The promoters for these cytokine genes contain binding sites for several transcription factors, including AP-1, NF-AT, NF-IL6, STAT family members and GATA family members (Luo et al., 1996; Zhu et al., 1996; Shannon et al., 1997; Szabo et al., 1997; Grassl et al., 1999). TNF-α, IL-6, IL-9 and GM-CSF gene transcription is also regulated in part by NF-κB (Zhu et al., 1996; Blackwell and Christman, 1997). We have been unable to transfect ESMC successfully. For this reason, we have been unable to dissect promoter elements responsible for the differential regulation of IL-9 mRNA expression, which is MEKK2 independent, versus other cytokines such as IL-4 and TNF-α, whose mRNA expression in response to receptor activation is dependent on MEKK2 expression.
More recently, we have shown that MEKK2 is critical for both B-cell and Tαβ+-cell development (B.C.Schaefer, T.P.Garrington, S.Webb, D.M.Russell, E.W.Gelfand, J.W.Kappler, G.L.Johnson and P.Marrack, submitted), a response that is dependent, respectively, on pre-BCR and pre-TCR antigen receptor signaling. Thus, MEKK2 is involved in the signaling of antigen receptors in both lymphocytes and mast cells. Interestingly, MEKK3, the MAP3K that is most closely related in homology to MEKK2, is not required for these responses. We have shown recently that MEKK2, but not MEKK3, binds to adaptor proteins that are recruited to activated protein tyrosine kinases such as Lck that associate with antigen receptors (W.Sun, K.Kesavan, B.Schaefer, N.L.Johnson, M.Ware, E.W.Gelfand and G.L.Johnson, submitted). The specificity for MEKK2 in antigen receptor signaling relative to MEKK1 or MEKK3 is predicted to be a function of MEKK2’s interaction with these adaptor proteins.
Our results also demonstrate that specific MEKKs selectively control specific MAPK pathways. MEKK2 is required for JNK activation in response to FcεRI and c-Kit ligation in mast cells. In MEKK1–/– ES cells, JNK activation is diminished in response to cold shock, hyperosmolarity and treatment of the ES cells with lysophosphatidic acid, taxol or nocodazole. MEKK2–/– cells have a normal JNK response to these stimuli (Yujiri et al., 1999). Thus, targeted disruption of MEKK1 or MEKK2 expression results in the selective loss of JNK activation in response to different stimuli. MEKK2 gene disruption has little if any effect on receptor-mediated or stress stimulation of ERK or p38 activity in mast cells. In activated T cells, however, MEKK2 has been found to regulate the activity of the ERK and p38 pathways (Schaefer et al., 1999), suggesting that protein scaffolding and subcellular localization processes unique to different cell types may be playing a role in determining MEKK2’s downstream targets (Garrington and Johnson, 1999).
In addition to the JNK pathway, MEKK2 regulates BMK1/ERK5 and PRK2, a protein kinase C-related kinase (Sun et al., 2000; W.Sun, K.Kesavan, B.Schaefer, N.L.Johnson, M.Ware, E.W.Gelfand and G.L.Johnson, submitted). Thus, MEKK2 regulates several kinase relay pathways that are capable of regulating specific gene transcription. Regarding cytokine production, the phenotype of MEKK2–/– ESMC appears different from that of the JNK1/JNK2 double knockout in T cells (Dong et al., 2000). MEKK2–/– ESMC lose cytokine production in response to receptor activation, whereas the JNK1/JNK2 double knockouts are hyper-responsive with regard to TCR stimulation of IL-2 production. This difference is probably related in part to the fact that JNK is downstream of MEKK2 and represents only one arm of MEKK2-regulated signaling pathways. An important concept that emerges from these studies is that the phenotype of a MAP3K knockout, such as that for MEKK2, can include the very specific loss of a cellular function, such as receptor-stimulated cytokine production, even though the downstream MAPKs are responsive to alternative stimuli. Thus, while several MAP3Ks capable of stimulating a downstream target such as JNK may be present in a given cell, activation of a given pathway in response to a specific stimulus is dependent on the presence of a specific MAP3K member, giving greater selectivity and specificity of response to the system. The targeted disruption of MAP3K expression will define the selective role of different MAP3Ks in regulating kinase relay pathways in response to different cellular stimuli.
Materials and methods
Luciferase assay
For luciferase assays, MC/9 mast cells were first transfected by electroporation with 2.5 µg of a pGL3 Basic vector (Promega) containing a 5′ TNF-α promoter sequence, along with 7.5 µg of other experimental plasmids, including wild-type MEKK2, kinase-inactive MEKK2 (K385M) and pCMV5 empty vector. For electroporation, 1 × 107 MC/9 cells were suspended in 0.5 ml of RPMI with 10% fetal calf serum (FCS) and kept at room temperature for 10 min. Cells were electroporated using a Gene Pulser II® (Bio-Rad) with pulse conditions of 800 µF and 320 V, kept an additional 10 min at room temperature and then placed overnight in mast cell medium. For FcεRI stimulation, cells were then incubated overnight at 37°C with 0.8 µg/ml anti-ova IgE, washed and treated with 10 µg/ml ovalbumin for 6 h. The MC/9 mast cells were then washed twice with phosphate-buffered saline (PBS) and lysed using Reporter Lysis Buffer (Promega). Luciferase assays were done using a Luciferase Assay System Kit (Promega).
MEKK2 kinase assay
MEKK2 kinase activity was assayed using a previously described method (Fanger et al., 1997). MC/9 cells were lysed in buffer containing 20 mM Tris–HCl pH 7.6, 0.5% NP-40, 0.25 M NaCl, 3 mM EDTA, 3 mM EGTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM sodium vanadate, 10 µg/ml aprotinin, 5 µg/ml leupeptin and 1 mM dithiothreitol (DTT). Endogenously expressed MEKK2 was immunoprecipitated with rabbit anti-C-terminal MEKK2 antibody (1:100). The immunoprecipitate was incubated at 30°C for 20 min in kinase buffer (20 mM HEPES pH 7.5, 10 mM MgCl2, 10 mM β-glycerophosphate, 10 mM p-nitrophenyl phosphate, 1 mM DTT, 50 µM sodium vanadate) containing wild-type JNK (polyhistidine-human JNK1), wild-type SEK1 (GST–mouse SEK1) and 50 µM ATP. This reaction was then incubated with GST–c-Jun substrate conjugated to Sepharose beads for 20 min at 4°C, before being washed and incubated for 20 min at 30°C in kinase buffer containing 10 µCi of [γ-32P]ATP. The kinase reaction was stopped by addition of SDS sample buffer, separated by SDS–PAGE and visualized with autoradiography. Phosphorylation of GST–c-Jun was quantified by PhosphorImager analysis (Molecular Dynamics).
Screening of a genomic DNA library and MEKK2 knockout vector construction
Targeted gene disruption of MEKK1 has been described previously (Yujiri et al., 1998). For targeted disruption of MEKK2, an MEKK2 genomic clone was isolated by screening a λ FixII phage library prepared from mouse strain 129/sv (Stratagene) using an MEKK2 cDNA fragment that included bp 593–1032 of the MEKK2 gene. The targeting vector was constructed by inserting a 4 kb fragment from the 5′ end of the genomic clone in which NotI and BamHI restriction sites were engineered by PCR for ligation into the vector, called Osdupdel (a gift from Dr O.Smithies). A 2.5 kb fragment from the 3′ end of the genomic clone was inserted, using an NheI site and an XhoI site inserted by PCR. This construct deleted 40 codons, including the ATG start site of the MEKK2 gene, and inserted the neomycin resistance gene with a polyadenylation signal in the antisense orientation. Outside of the region of homology, there was a thymidine kinase gene for negative selection of randomly inserted vectors.
ES cell electroporation, and selection of +/– and –/– clones
The targeting vector was linearized using NotI and electroporated into an R1 ES cell line using a Bio-Rad Gene Pulser™. A total of 1 × 107 cells suspended in 0.8 ml of PBS were electroporated using pulse conditions of 250 V and 500 µF. Cells were then placed on ice for 5 min and plated onto 100 mm dishes with feeder cells. The cells were then grown in medium containing G418 (400 µg/ml) and ganciclovir (2 mM) for 8 days. Surviving clones were picked and grown in medium lacking G418 and ganciclovir. A portion of the cells was collected for DNA analysis by Southern blotting, and the remaining cells were frozen in liquid N2. For selection of MEKK2–/– clones, MEKK2+/– clones were grown in medium containing G418 (10 mg/ml). After 7 days, surviving clones were picked, grown and used for DNA analysis or for freezing.
Mast cell differentiation
For differentiation of ES cells into mast cells, feeder cell-independent ES cells were first maintained on gelatinized dishes in medium containing Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) with 15% FCS, 1.5 × 10–4 M monothioglycerol (MTG) (Sigma) and10 ng/ml leukemia inhibitory factor (LIF) (R&D Systems). At 24–48 h prior to differentiation, the cells were placed in medium containing Iscove’s modified Dulbecco’s medium (IMDM) (Gibco) with15% FCS, 1.5 × 10–4 M MTG and 10 ng/ml LIF. The ES cells were then trypsinized and washed in IMDM with 15% FCS and suspended at a concentration of 7.5 × 103–12.5 × 103 cells/ml in differentiation medium [15% FCS, 2 mM l-glutamine (Gibco), 300 µg/ml transferrin (Boehringer Mannheim), 4 × 10–4 M MTG, 50 µg/ml ascorbic acid (Sigma), 5% protein-free hybridoma medium (PFHM-II) (Gibco) and IMDM (to 100%)] to a volume of 5 ml in 60× 15 mm Petri grade dishes (Fisher).
The ES cells were then maintained in the differentiation medium for 5–6 days, forming embryoid bodies (EBs). The EBs were transferred to 50 ml conical tubes and allowed to settle by gravity for 10 min. The supernatant was removed and the EBs were dissociated by addition of 3 ml of trypsin-EDTA, incubation for 5 min at 37°C, and addition of 1 ml of FCS, followed by passage twice through a syringe with a 20 gauge needle. The dissociated cells were then centrifuged at 1200 r.p.m. for 5 min, and the pellet was resuspended in mast cell medium [10% FCS, 1.5 × 10–4 M MTG, 1 ng/ml IL-3 (R&D Systems), 100 ng/ml c-Kit ligand (R&D Systems) and IMDM (to 100%)] and placed in 6-well dishes at a concentration of 1.0 × 106–2.0 × 106 cells/ml in a final volume of 3–4 ml. Non-adherent cells were transferred to new plates with fresh mast cell medium 24 h later, and again transferred 48–72 h later to remove any remaining adherent cells. Half of the medium was then replaced every 2–3 days, and the mast cells were grown and expanded over the following 4–12 weeks.
DNA extracts and Southern blotting
For DNA analysis, cells were harvested, washed twice in PBS and lysed in buffer containing 0.35 M NaCl, 15 mM sodium citrate pH 7.0, 0.5% SDS, 1 mM DTT and 80 µg/ml proteinase K, and incubated overnight at 37°C. DNA was then extracted twice in 1:1 phenol:chloroform and once in chloroform, followed by ethanol precipitation. The DNA was then digested overnight with XbaI, resolved on a 0.8% agarose gel, and transferred by Southern blot to Hybond™-N nylon membrane (Amersham). The blots were hybridized with [γ-32P]CTP-labeled DNA probe overnight, washed and visualized by autoradiography.
Protein extracts and western blotting
Cells were harvested, washed twice in PBS and lysed in buffer containing 20 mM Tris–HCl pH 7.5, 0.5% NP-40, 0.25 M NaCl, 3 mM EDTA, 3 mM EGTA, 1 mM PMSF, 2 mM sodium vanadate, 0.2 mg/ml aprotinin, 1 mg/ml leupeptin and 1 mM DTT. Nuclei were removed by centrifugation at 15 000 g for 10 min. A total of 200 µg of protein per sample was resolved on SDS–10% polyacrylamide gels. Protein was then electroblotted to nitrocellulose membrane. MEKK2 protein was detected using rabbit antiserum (1:500 dilution) against the N-terminus of MEKK2, followed by protein A conjugated to horseradish peroxidase (protein A–HRP) (Zymed). Western blots were then visualized by chemiluminescence using ECL (Amersham). Western blots were also done using rabbit antiserum (1:500 dilution) against the C-terminus of MEKK2. Western blots for ERK1/2 activity used a phospho-p44/42 MAPK (T202/Y204) rabbit polyclonal antibody (New England Biolabs) followed by an HRP-linked donkey anti-rabbit IgG antibody (Jackson Immunoresearch Laboratories). Western blots for p38 activity used a phospho-p38 MAPK (Thr180/Tyr182) rabbit polyclonal antibody (Cell Signaling Technology) followed by an HRP-linked donkey anti-rabbit IgG antibody.
For BMK1/ERK5 western blots, cells were lysed in buffer containing 25 mM Tris–HCl pH 7.4, 50 mM NaCl, 0.5% sodium deoxycholate, 2% NP-40, 0.2% SDS, 1 mM PMSF, 50 µg/ml aprotinin and 50 µM leupeptin at 4°C for 15 min. Samples were boiled and resolved on SDS–7% polyacrylamide, and the protein was electroblotted to nitrocellulose membrane. ERK5 protein was detected using a rabbit polyclonal antibody (1:2000 dilution) to a synthetic peptide corresponding to the C-terminus of ERK5 (Sigma), followed by a protein G–HRP conjugate (Bio-Rad) at a 1:2000 dilution.
Flow cytometry
For analysis of c-Kit expression, mast cells were treated with 1 µg/ml fluorescein isothiocyanate (FITC)-conjugated anti-c-Kit antibody (PharMingen) for 30 min at 4°C, washed with PBS and analyzed by flow cytometry. Negative controls were untreated. For analysis of FcεRI expression, mast cells were treated with 1 µg/ml anti-DNP IgE for 30 min at 4°C, washed with culture medium then treated with 1 µg/ml FITC–anti-IgE (PharMingen) for 30 min at 4°C and analyzed. Negative controls were treated with FITC–anti-IgE alone.
Assay of JNK activity
JNK activity was measured using GST–c-Jun(1–79) coupled to glutathione–Sepharose 4B (Hibi et al., 1993). Cells were lysed in buffer containing 20 mM Tris–HCl pH 7.6, 0.5% NP-40, 0.25 M NaCl, 3 mM EDTA, 3 mM EGTA, 1 mM PMSF, 2 mM sodium vanadate, 0.20 µg/ml aprotinin, 5 µg/ml leupeptin and 1 mM DTT. Nuclei were removed by centrifugation at 15 000 g for 10 min, and the supernatants (100 µg of protein) were mixed with 10 µl of a slurry of GST–c-Jun(1–79)–Sepharose [3–5 µg of GST–c-Jun(1–79)]. The mixture was rotated at 4°C for 1 h, and washed twice in lysis buffer and once in kinase buffer (20 mM HEPES pH 7.5, 10 mM MgCl2, 20 mM β-glycerophosphate, 10 mM p-nitrophenyl phosphate, 1 mM DTT, 50 µM sodium vanadate). Beads were suspended in 40 µl of kinase assay buffer containing 10 µCi of [γ-32P]ATP and incubated at 30°C for 20 min. The kinase reaction was stopped by addition of SDS sample buffer, and phosphorylated proteins were resolved on SDS–10% polyacrylamide gels. Results were visualized by autoradiography and quantified by PhosphorImaging (Molecular Dynamics).
Assay of p38 kinase activity
p38 activity was measured using an in vitro kinase assay (Abdel-Hafiz et al., 1992; Han et al., 1994). Cells were lysed in buffer containing 20 mM Tris–HCl pH 7.6, 0.5% NP-40, 1% Triton X-100, 150 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 5 mM PMSF and 0.2 mM sodium vanadate. p38 was immunoprecipitated from cell lysates (500 µg of protein) by mixture with rabbit antiserum (1:100 dilution) raised against the C-terminal peptide sequence of p38 to a final volume of 500 µl and rotation at 4°C for 1 h. Immune complexes were captured by adding 10 µl of a 1:1 slurry of protein A–Sepharose (Sigma). The mixture was rotated at 4°C for an additional hour and washed twice in lysis buffer and once in kinase buffer (25 mM HEPES, 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM DTT, 0.1 mM sodium vanadate). Beads were suspended in 40 µl of kinase assay buffer containing 3 µCi of [γ-32P]ATP and 2 µg of ATF-2 as a substrate, and incubated at 30°C for 30 min. Reaction mixtures were added to Laemmli sample buffer, boiled, and phosphorylated proteins were resolved on SDS–10% polyacrylamide gels. Results were visualized by autoradiography.
RPA analysis
For RPA analysis, a RiboQuant® Multi-Probe RNase Protection Assay System (PharMingen) was used. An mCK-1 custom probe set (PharMingen) was designed, containing DNA templates for IL-2, IL-4, IL-5, IL-6, IL-9, IL-15, GM-CSF, TNF-α, IFN-γ, L32 and GAPDH. The DNA template set was used for T7 RNA-polymerase-directed synthesis of [α-32P]UTP-labeled antisense RNA probes. The probes were hybridized with 20 ng of RNA isolated from mast cells using RNAzol™B (Tel-Test, Inc). Samples were then digested with RNases to remove single-stranded (non-hybridized) RNA. The remaining probes were resolved on denaturing polyacrylamide gels. Quantitation was done by PhosphorImaging (Molecular Dynamics).
Acknowledgments
Acknowledgements
We thank Dr Oliver Smithies for the Osdupdel gene targeting vector. Supported in part by the Leukemia Research Foundation, the Cancer League of Colorado and NIH grants CA85276, DK37871, GM30324 and AI42246.
References
- Abdel-Hafiz H.A., Heasley,L.E., Kyriakis,J.M., Avruch,J., Kroll,D.J., Johnson,G.L. and Hoeffler,J.P. (1992) Activating transcription factor-2 DNA-binding activity is stimulated by phosphorylation catalyzed by p42 and p54 microtubule-associated protein kinases. Mol. Endocrinol., 6, 2079–2089. [DOI] [PubMed] [Google Scholar]
- Abe J., Kusuhara,M., Ulevitch,R.J., Berk,B.C. and Lee,J.D. (1996) Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem., 271, 16586–16590. [DOI] [PubMed] [Google Scholar]
- Blackwell T.S. and Christman,J.W. (1997) The role of nuclear factor-κB in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol., 17, 3–9. [DOI] [PubMed] [Google Scholar]
- Blank J.L., Gerwins,P., Elliott,E.M., Sather,S. and Johnson,G.L. (1996) Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J. Biol. Chem., 271, 5361–5368. [DOI] [PubMed] [Google Scholar]
- Dong C., Yang,D.D., Tournier,C., Whitmarsh,A.J., Xu,J., Davis,R.J. and Flavell,R.A. (2000) JNK is required for effector T-cell function but not for T-cell activation. Nature, 405, 91–94. [DOI] [PubMed] [Google Scholar]
- Dvorak A.M., Dvorak,H.F. and Galli,S.J. (1983) Ultrastructural criteria for identification of mast cells and basophils in humans, guinea pigs and mice. Am. Rev. Respir. Dis., 128, S49–S52. [DOI] [PubMed] [Google Scholar]
- Enerback L. (1974) Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry, 42, 301–313. [DOI] [PubMed] [Google Scholar]
- Fanger G.R., Johnson,N.L. and Johnson,G.L. (1997) MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J., 16, 4961–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris M., Kokot,N., Lee,L. and Nel,A.E. (1996) Regulation of interleukin-2 transcription by inducible stable expression of dominant negative and dominant active mitogen-activated protein kinase kinase kinase in jurkat T cells. Evidence for the importance of Ras in a pathway that is controlled by dual receptor stimulation. J. Biol. Chem., 271, 27366–27373. [DOI] [PubMed] [Google Scholar]
- Gagari E., Tsai,M., Lantz,C.S., Fox,L.G. and Galli,S.J. (1997) Differential release of mast cell interleukin-6 via c-kit. Blood, 89, 2654–2663. [PubMed] [Google Scholar]
- Garrington T.P. and Johnson,G.L. (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol., 11, 211–218. [DOI] [PubMed] [Google Scholar]
- Grassl C., Luckow,B., Schlondorff,D. and Dendorfer,U. (1999) Transcriptional regulation of the interleukin-6 gene in mesangial cells. J. Am. Soc. Nephrol., 10, 1466–1477. [DOI] [PubMed] [Google Scholar]
- Han J., Lee,J.D., Bibbs,L. and Ulevitch,R.J. (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science, 265, 808–811. [DOI] [PubMed] [Google Scholar]
- Hibi M., Lin,A., Smeal,T., Minden,A. and Karin,M. (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev., 7, 2135–2148. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Oshiba,A., Sakata,N., Terada,N., Johnson,G.L. and Gelfand,E.W. (1996) Aggregation of the FcεRI on mast cells stimulates c-Jun amino-terminal kinase activity. A response inhibited by wortmannin. J. Biol. Chem., 271, 12762–12766. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Terada,N., Gerwins,P., Hamelmann,E., Oshiba,A., Fanger,G.R., Johnson,G.L. and Gelfand,E.W. (1997) Mast cell tumor necrosis factor α production is regulated by MEK kinases. Proc. Natl Acad. Sci. USA, 94, 6358–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T., Kawasome,H., Terada,N., Takeda,K., Gerwins,P., Keller,G.M., Johnson,G.L. and Gelfand,E.W. (1998) Stem cell factor augments FcεRI-mediated TNF-α production and stimulates MAP kinases via a different pathway in MC/9 mast cells. J. Immunol., 161, 3624–3630. [PubMed] [Google Scholar]
- Ishizuka T., Chayama,K., Takeda,K., Hamelmann,E., Terada,N., Keller,G.M., Johnson,G.L. and Gelfand,E.W. (1999) Mitogen-activated protein kinase activation through Fcε receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells. J. Immunol., 162, 2087–2094. [PubMed] [Google Scholar]
- Jaffe J.S., Glaum,M.C., Raible,D.G., Post,T.J., Dimitry,E., Govindarao,D., Wang,Y. and Schulman,E.S. (1995) Human lung mast cell IL-5 gene and protein expression: temporal analysis of upregulation following IgE-mediated activation. Am. J. Respir. Cell Mol. Biol., 13, 665–675. [DOI] [PubMed] [Google Scholar]
- Jaffe J.S., Raible,D.G., Post,T.J., Wang,Y., Glaum,M.C., Butterfield,J.H. and Schulman,E.S. (1996) Human lung mast cell activation leads to IL-13 mRNA expression and protein release. Am. J. Respir. Cell Mol. Biol., 15, 473–481. [DOI] [PubMed] [Google Scholar]
- Kato Y., Tapping,R.I., Huang,S., Watson,M.H., Ulevitch,R.J. and Lee,J.D. (1998) Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature, 395, 713–716. [DOI] [PubMed] [Google Scholar]
- Luo C., Burgeon,E., Carew,J.A., McCaffrey,P.G., Badalian,T.M., Lane,W.S., Hogan,P.G. and Rao,A. (1996) Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol. Cell. Biol., 16, 3955–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe D.D., Baram,D. and Mekori,Y.A. (1997) Mast cells. Physiol. Rev., 77, 1033–1079. [DOI] [PubMed] [Google Scholar]
- Moller A., Henz,B.M., Grutzkau,A., Lippert,U., Aragane,Y., Schwarz,T. and Kruger-Krasagakes,S. (1998) Comparative cytokine gene expression: regulation and release by human mast cells. Immunology, 93, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina H. et al. (1997) Impaired CD28-mediated interleukin 2 production and proliferation in stress kinase SAPK/ERK1 kinase (SEK1)/mitogen-activated protein kinase kinase 4 (MKK4)-deficient T lymphocytes. J. Exp. Med., 186, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y., Petit-Frere,C., Kassel,O., Semper,A., Quint,D., Tunon-de-Lara,M.J., Bradding,P., Holgate,S.T. and Church,M.K. (1995a) IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J. Immunol., 155, 1796–1808. [PubMed] [Google Scholar]
- Okayama Y., Semper,A., Holgate,S.T. and Church,M.K. (1995b) Multiple cytokine mRNA expression in human mast cells stimulated via FcεRI. Int. Arch. Allergy Immunol., 107, 158–159. [DOI] [PubMed] [Google Scholar]
- Paul W.E., Seder,R.A. and Plaut,M. (1993) Lymphokine and cytokine production by FcεRI+ cells. Adv. Immunol., 53, 1–29. [PubMed] [Google Scholar]
- Plaut M., Pierce,J.H., Watson,C.J., Hanley-Hyde,J., Nordan,R.P. and Paul,W.E. (1989) Mast cell lines produce lymphokines in response to cross-linkage of FcεRI or to calcium ionophores. Nature, 339, 64–67. [DOI] [PubMed] [Google Scholar]
- Rooney J.W., Hoey,T. and Glimcher,L.H. (1995) Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity, 2, 473–483. [DOI] [PubMed] [Google Scholar]
- Schaefer B.C., Ware,M.F., Marrack,P., Fanger,G.R., Kappler,J.W., Johnson,G.L. and Monks,C.R. (1999) Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity, 11, 411–421. [DOI] [PubMed] [Google Scholar]
- Shannon M.F., Coles,L.S., Vadas,M.A. and Cockerill,P.N. (1997) Signals for activation of the GM-CSF promoter and enhancer in T cells. Crit. Rev. Immunol., 17, 301–323. [DOI] [PubMed] [Google Scholar]
- Sun W., Vincent,S., Settleman,J. and Johnson,G.L. (2000) MEK kinase 2 binds and activates protein kinase C-related kinase 2. Bifurcation of kinase regulatory pathways at the level of a MAPK kinase kinase. J. Biol. Chem., 275, 24421–24428. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., Glimcher,L.H. and Ho,I.C. (1997) Genes that regulate interleukin-4 expression in T cells. Curr. Opin. Immunol., 9, 776–781. [DOI] [PubMed] [Google Scholar]
- Tsai M., Chen,R.H., Tam,S.Y., Blenis,J. and Galli,S.J. (1993) Activation of MAP kinases, pp90rsk and pp70-S6 kinases in mouse mast cells by signaling through the c-kit receptor tyrosine kinase or FcεRI: rapamycin inhibits activation of pp70-S6 kinase and proliferation in mouse mast cells. Eur. J. Immunol., 23, 3286–3291. [DOI] [PubMed] [Google Scholar]
- Valent P. and Bettelheim,P. (1992) Cell surface structures on human basophils and mast cells: biochemical and functional characterization. Adv. Immunol., 52, 333–423. [DOI] [PubMed] [Google Scholar]
- Widmann C., Gibson,S., Jarpe,M.B. and Johnson,G.L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev., 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Yujiri T., Sather,S., Fanger,G.R. and Johnson,G.L. (1998) Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science, 282, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Yujiri T., Fanger,G.R., Garrington,T.P., Schlesinger,T.K., Gibson,S. and Johnson,G.L. (1999) MEK kinase 1 (MEKK1) transduces c-Jun NH2-terminal kinase activation in response to changes in the microtubule cytoskeleton. J. Biol. Chem., 274, 12605–12610. [DOI] [PubMed] [Google Scholar]
- Zhu Y.X., Kang,L.Y., Luo,W., Li,C.C.H., Yang,L. and Yang,Y.C. (1996) Multiple transcription factors are required for activation of human interleukin 9 gene in T cells. J. Biol. Chem., 271, 15815–15822. [DOI] [PubMed] [Google Scholar]