Abstract
Chest wall and mediastinal wounds may be life-threatening. Although modern reconstruction methods with various muscle flaps have reduced morbidity and mortality, chest wall reconstruction presents unique challenges. Major categories of adverse outcomes include (1) persistent infection; (2) interference with respiratory mechanics; (3) functional deficits of the shoulder; and (4) hernias. Persistent infection may be resolved by providing coverage via muscle or omental flap, performing thorough debridement, filling the “dead space” with adequate volume, buttressing repair of visceral fistulae, and covering exposed prosthetic material with vascularized flaps. Potential deficits in respiratory mechanics and shoulder function may be avoided by stabilizing the chest wall skeleton and decreasing donor muscle functional loss. Hernias may be minimized by maintaining visceral “right of domain” to the chest and abdominal cavities. Complex reconstructive cases represent an intricate interplay of physiology, structural protection, and aesthetic considerations and require integration of several management principles.
Keywords: Chest wall, mediastinum, reconstruction
In this article, we will outline the major categories of adverse outcome after chest wall reconstruction and how these may potentially be avoided. We will conclude with a complex case to illustrate some of these principles. At the outset, however, it is important to acknowledge that the mortality rate for mediastinal infection with wound debridement, wound closure, and antibiotic irrigation prior to the advent of muscle flaps was 14%, and only 80% of patients managed in this way were successfully treated for their infections.1,2 Wide debridement and second-stage muscle flap coverage have eliminated the need for serial debridements and reduced mortality to less than 3% and reinfection rate to less than 5%.3,4,5,6,7,8
Modern reconstruction methods attain their success by allowing wide debridement without fear of inability to reconstruct the wound, enabling sufficient volume to fill the three-dimensional dead space, buttressing repair of hollow visceral leaks, maintaining chest wall stability, restoring aesthetic contour, covering exposed prosthetic materials with vascularized flaps, retaining the “right of domain” of abdominal and chest viscera, and minimizing donor site functional deficits.
PERSISTENT INFECTION
Type of Flap
It has been suggested that omentum controls median sternotomy infection more successfully after debridement than do muscle flaps.8,9 Omentum flap, however, requires a laparotomy with the possible risk of peritoneal cavity infection, late bowel obstruction, and risk of hernia formation. Omentum may also not be readily available if the patient had a previous laparotomy.
Inadequate Debridement
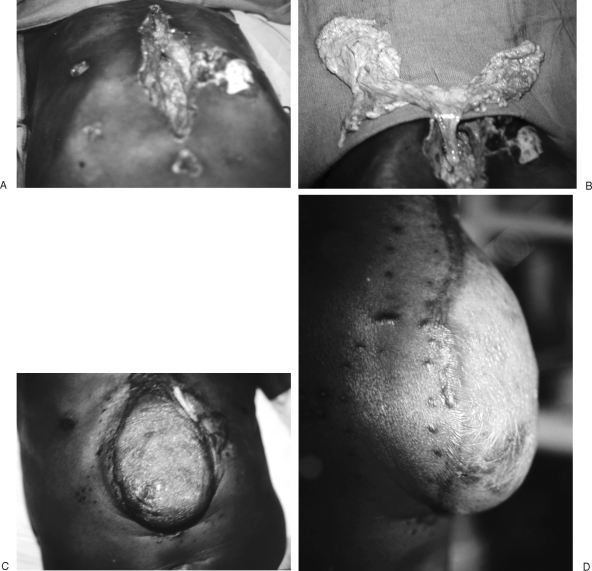
Thorough debridement of necrotic soft tissue and especially infected bone and cartilage is essential. Late infections present from a nidus of residual necrotic bone or cartilage. Costal cartilage, in particular, has an inherently poor blood supply, and infection may persist especially at the costochondral junctions of the lower floating ribs (Fig. 1).
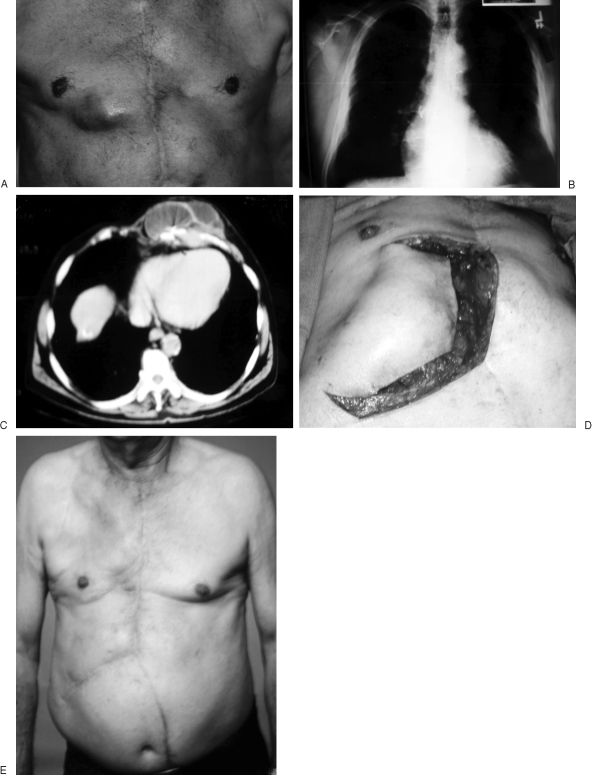
Figure 1.
(A, B) Patient with recurrent lower right sternocostal infection after prior muscle flap reconstruction of a sternal wound (A) associated with residual sternal wires seen on routine chest radiograph (B). (C) CT scan is helpful to delineate the extent of osteomyelitis and also to confirm the absence of mediastinal abscess. (D, E) A prior rectus abdominis flap had been used, but an external oblique myocutaneous flap was still available for soft tissue reconstruction.
Persistent sternal wires may be a source of late infection. Plain chest radiographs are important in evaluating acute sternotomy wounds. They not only reveal the condition of the lung fields but also the presence of remaining sternal wires, which if not removed may be a source of subsequent recurrent or persistent infection. In contrast, magnetic resonance and computed tomography imaging have greater utility in evaluation of chronic sternal wounds and are of little benefit in preoperative evaluation of acute median sternotomy wounds.7
Failure to Obliterate or Adequately Decontaminate Dead Space
Kanavel stressed the value of muscle to obliterate cavities with noncollapsible walls. He used the latissimus muscle to fill an empyema chest cavity.10 Liberal use of closed suction drains also helps maintain obliteration of these spaces until flaps become adherent. Failure to obliterate spaces completely will allow bacteria to proliferate. This is of particular importance when treating an empyema cavity after pneumonectomy and a persistent bronchopleural fistula.
There are several therapeutic options for surgical treatment of the post-pneumonectomy empyema cavity: (i) Eloesser flap to establish dependent drainage11; (ii) collapse the chest wall by a disfiguring and scapulothoracic shoulder function–impairing thoracoplasty; (iii) stuff the space with multiple regional pedicle flaps12; (iv) buttress any potential bronchopleural fistula with a muscle flap and then close the chest wall and fill the pleural space with an antibiotic solution.13,14 All of these options seem to work equally effectively in controlling infection in these huge pneumonectomy spaces. A combination of these procedures may sometimes be the most suitable. Aesthetic and functional morbidity of a thoracoplasty is reduced by resecting rib in the upper chest only, and the remainder of the pneumonectomy cavity is then filled with one or more pedicle flaps, as well as buttressing any bronchopleural fistula repair15,16 (Fig. 2). Problems with the Eloesser flap and drainage include continued air escape through the persistent bronchial fistula, excoriation and troublesome bleeding, and the need for constant wound packing. One of the authors (D.T.N.) has revised four of these patients by upper thoracoplasty, free flap obliteration of the fistula and pleural dead space, and turning out of the original Eloesser flap
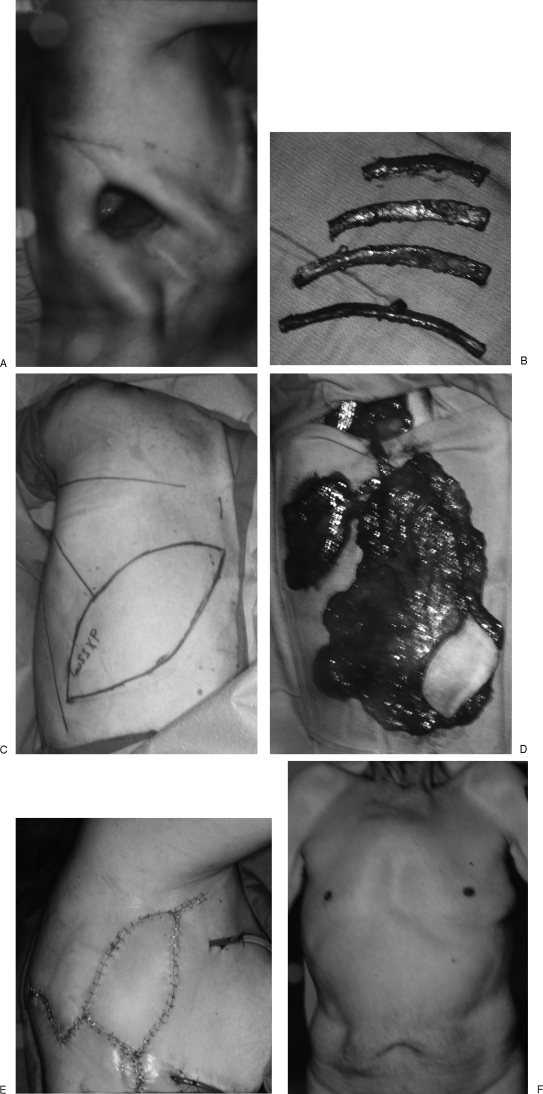
Figure 2.
(A) Patient was unhappy with Eloesser flap for drainage of empyema cavity. (B) Upper four rib thoracoplasty was done to help close down the apex of the pleural space. (C, D) Contralateral composite extended latissimus dorsi and serratus anterior musculocutaneous flap was elevated on a single thoracodorsal vascular pedicle. (E) Remaining dead space obliterated with latissimus muscle, and bronchial repair buttressed with serratus anterior. Skin island is external. (F) Patient has no external chest wall deformity, and his ability to climb stairs is greatly improved with no further “escape” of inspired air through the fistula.
Failure to Control Contaminating Visceral Fistulae
Muscle and omentum are used to buttress intrathoracic esophageal repair and also bronchopleural fistulae.16,17,18 Persistent leak from a fistula will result in recurrent closed space infection. If the original thoracotomy did not spare latissimus and serratus muscles, available regional muscle flaps to buttress visceral fistulae may be scarce. However, omentum is available, and rhomboid major muscle may be reached posteriorly.19
Persistent Periprosthetic Infection
Orthopedic hardware may be exposed in back wounds, and vascular prosthetic grafts may be exposed in either the anterior or posterior mediastinum or in the axilla. Frequently, the prosthetic material cannot be removed.
The mortality of infected thoracic vascular prostheses was as high as 75%; fortunately, the infection rate is low for thoracic aortic prostheses (~1.9%).20 Now, frequently one can maintain the graft in place while performing debridement, antibiotic irrigation, muscle flap rotation, and probable lifetime use of suppressant systemic antibiotics. This carries an 88% chance of salvage.20,21,22,23 If significant graft infection involves the anastomotic suture line and extends along the length of the graft, rerouting blood through an extra-anatomic clean operative field may be prudent.21
If an aortic vascular graft is exposed in the anterior and superior mediastinum, a pectoralis muscle flap can be made an island flap on the thoracoacromial vessels, mobilizing it sufficiently to wrap around the vascular graft.16 The remaining mediastinal dead space must then be filled with additional muscle flaps (such as opposite pectoralis, rectus abdominis) or omentum. If a vascular graft is exposed in the posterior mediastinum through a large thoracoabdominal incision, then omentum brought through the lateral diaphragm may serve well to wrap around the descending aortic vascular graft
Rigidity of Sternal Fixation
Some have reported favorable outcomes with rigid plate and screw fixation and pectoralis muscle flap reconstruction for separated sternal wounds.24 Rigid primary sternal plate fixation at initial surgery may reduce the risk of sternal separation and infected mediastinitis,25 although further studies and outcomes are necessary.
Untreated Microorganisms
It is possible that the bacteria on a recurrent chest wall infection may be resistant to the antibiotics previously used. The microorganisms accounting for a recurrent infection may not have been treated. Thus, on a recurrent infection one should submit material to the microbiology laboratory not only for routine aerobic and anaerobic bacterial cultures but also for fungal culture as well as for mycobacterial cultures.
EFFECT ON RESPIRATORY MECHANICS
Failure to Stabilize the Chest Wall
There is very little published literature on changes in breathing mechanics when chest wall integrity is violated and when accessory muscles of respiration are used for reconstruction. Resting inspiration is a function of the diaphragm and intercostal muscles, and expiration is the result of passive chest wall recoil.
ANTERIOR MIDLINE CHEST WOUNDS
Chronic midline sternal wounds after open heart surgery increase stiffness in the chest wall. In contrast, acute midline chest wounds occur after skeletal tumor resection. If the chest wall ring is intact in at least one location, large sternal wounds are well tolerated if a muscle flap is done immediately. Sternal wounds, however, often involve increased dependence on abdominal breathing and prolonged need for ventilator assistance.
Earlier descriptions of sternal wound reconstruction did recommend rib grafting for skeletal stability,26 but it was eventually realized that the effects of sternal wound debridement on pulmonary mechanics were not as severe as originally thought. Enthusiasm for restoring rigid skeletal stability waned, especially with donor site morbidity incurred from bone grafting. There is, however, renewed interest in restoring skeletal stability across the midline with rigid plate and screw fixation.24
There are a few studies that evaluate pulmonary function for reconstruction of anterior chest wall oncologic defects. One such study compared preoperative and postoperative pulmonary function tests in patients who had anterior hemithorax and major sternal defects after oncologic resections. Reconstruction with musculocutaneous flaps alone seemed sufficient to provide adequate chest wall rigidity to maintain pulmonary mechanics. Forced expiratory volume in 1 second and vital capacity were measured, but the degree of postoperative ventilator dependence was not defined.27 Two other studies have likewise found minimal influence on late pulmonary function tests and exercise tolerance and concluded that dependence on abdominal muscles for effective pulmonary mechanics may increase after chest wall reconstruction for anterior midline defects and that restoration of rigid skeletal stability was not essential.28,29
LATERAL CHEST WALL
There is a lack of consensus but general agreement that lateral chest wall skeletal reconstruction is done if four or more ribs are resected or if the defect is larger than 5 cm.30 Chest wall rigidity and avoidance of adverse effects of a flail segment can be restored by polytetrafluoroethylene patch, polypropylene mesh, or composite mesh and methyl methacrylate sandwich.31 These alloplastic materials must then be covered with healthy, well-vascularized soft tissue (Fig. 3).
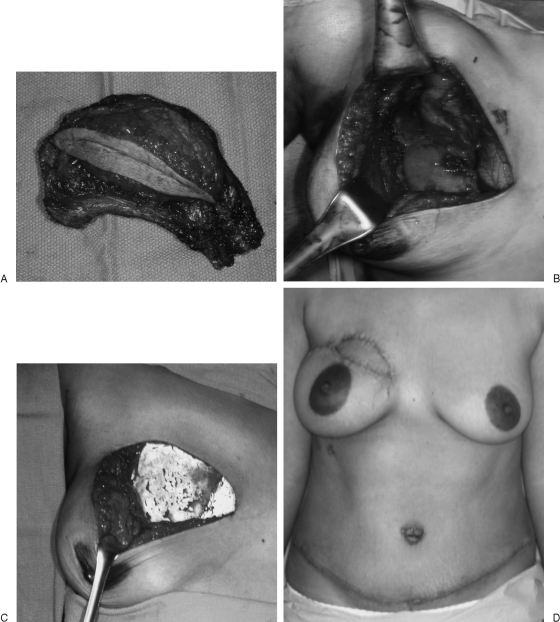
Figure 3.
(A, B) Excision of full-thickness chest wall right upper chondrosarcoma. (C) Segmental rib resections are patched with Gore-Tex to restore chest wall stability and prevent lung herniation. (D) Pedicle transverse rectus abdominis musculotaneous (TRAM) flap helps restore chest wall and upper breast aesthetic contour.
Alloplastic materials may increase complication rates when placed directly over exposed viscera or when the recipient area has been irradiated or contaminated with bacteria.32 AlloDerm (Lifecell, KCI Co., Branchburg, NJ) (decellularized human cadaveric dermis) may be an alternative as it becomes vascularized and remodeled into autogenous tissue after implantation. Although initial studies imply that AlloDerm appears to form a strong repair in fascial reconstruction, resists infection, and causes minimal visceral adhesions, long-term clinical studies are still awaited.32 In these situations, we should perhaps resort back to the advice of the early reconstructive general surgeons and use fascia lata either as a flap or a graft, the latter of which can yield a large surface area graft with little clinical morbidity.33,34,35
Diaphragm
Diaphragmatic wounds must be closed to maintain diaphragmatic integrity and prevent visceral herniation. If direct suture repair of a diaphragmatic wound is not possible, then prosthetic materials are used. Vascularized tissue has an advantage over prosthetic materials in contaminated or radiated diaphragmatic wounds and also to enable diaphragmatic growth potential in children with either acquired or congenital diaphragmatic wounds. Regional flaps used for this purpose include de-epithelialized transverse rectus abdominis muscle flap,36 reverse or upper latissimus muscle flaps,37,38 oblique abdominal wall muscles,39 or omentum.40
Accessory Respiratory Muscles
The pectorals, scalenes, and sternocleidomastoid are accessory muscles of inspiration, and accessory muscles of expiration include abdominal oblique muscles and rectus abdominis. However, potential adverse effects on pulmonary mechanics of using these muscles for reconstruction is more theoretical than real. This hypothesis is supported anecdotally by routine use of the rectus abdominis muscle in breast and other reconstructions without noticeable deficit.
EFFECT ON SHOULDER FUNCTION
There is some published literature on the functional outcome to the shoulder girdle when regional muscles are used for chest wall reconstruction. Most pertain to the latissimus dorsi. Activities of daily living are generally unaffected by compromise of latissimus function because other shoulder girdle muscles substitute for it.41,42,43 However, these muscles (such as teres major) take 6 to 12 months to fully assume latissimus functions. Patients in whom the latissimus dorsi has been used are more likely to notice rapid onset of fatigue rather than loss of power during prolonged activities such as ladder climbing, swimming, and overhead painting.44,45 Sacrifice of pectoralis major and unilateral rectus abdominis leaves few functional deficits, either in isolated muscle testing or in activities of daily living.46
Perhaps more important than the muscle used is the role of the rigid support provided by the chest wall. The anterior chest wall, aside from its role in pulmonary mechanics, provides a rigid base for upper limb movement. Loss of chest wall skeletal stability may reduce shoulder girdle function.46 For this reason as well, recent publications promote rigid fixation and restoration of midline support for sternal wounds.24 If midline sternal continuity is not restored after sternal debridement and muscle flap reconstruction, patient discomfort from shifting chest wall halves may be significant.47
Potential functional loss from donor muscles should, however, not be underestimated. Avoiding donor muscle functional deficits may be accomplished by segmentally splitting muscles and understanding the intramuscular neurovascular anatomy that has been clearly elucidated for pectoralis major, serratus anterior, latissimus dorsi, and trapezius especially with regard to avoidance of scapular winging48,49,50,51 (Fig. 4). Perforator flaps have also been designed not only to minimize impairment of upper and lower limb function and morbidity but also to enable greater pedicle length and flap reach.52,53,54,55,56
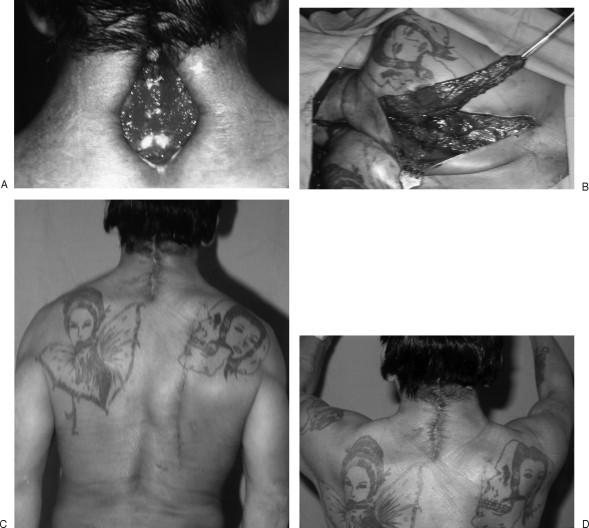
Figure 4.
(A, B) Open lower neck wound with exposed wires is closed with a function-preserving lower trapezius muscle flap. (C, D) Healed wound without any winging of the scapula.
HERNIAS
Use of rectus abdominis musculocutaneous and muscle flaps may result in abdominal bulges and hernias. In addition, passing omentum subcutaneously to the chest results in obligatory epigastric hernia, although the risk of hernia formation is reduced by splitting the diaphragm and passing the omentum through it (Fig. 5). The obligatory tunnel through which the omentum passes can be reduced by skeletonizing the omentum pedicle on either the left or right gastro-epiploic vessels.
Figure 5.
(A, B) Omentum exposed through a small upper laparotomy incision and passed subcutaneously. Fascia was closed around the narrow vascular pedicle on the left gastro-epiploic vessels. (C, D) In spite of all these precautions, the patient developed a large epigastric hernia.
COMPLEX RECONSTRUCTIVE CASE
A complex reconstructive case that involves the posterior lateral chest wall, diaphragm, and upper lumbar region may illustrate some of the principles to which we have alluded in this article (Fig. 6). These principles pertain especially to restoration of chest wall stability, maintenance of respiratory mechanics, use of prosthetic materials, maintenance of visceral “right of domain” to the chest and abdominal cavities, and obliteration of dead space.
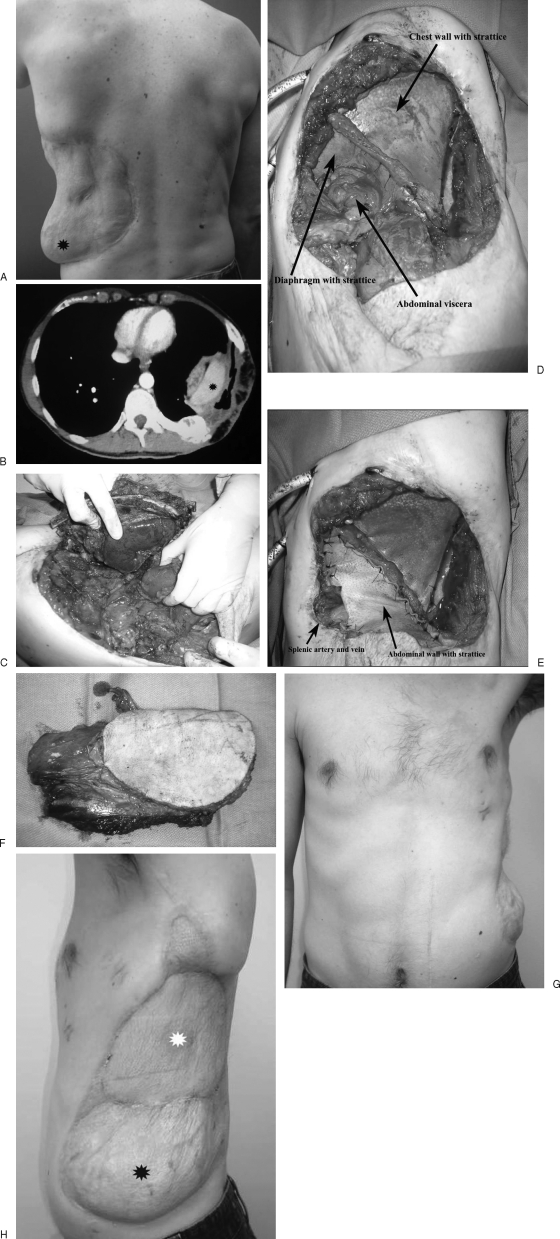
Figure 6.
(A, B) Patient had prior reconstruction with omentum and presents with recurrent lower rib chondrosarcoma seen both clinically and on CT scan. (asterisks). (C) Tumor being resected in continuity with splenectomy. (D) Diaphragm and chest wall have been reconstructed with porcine acellular dermal matrix. (E) Abdominal wall has now been reconstructed with porcine acellular dermal matrix as well, and splenic artery and vein are exteriorized through a window in the dermal matrix. (F) Lateral thigh flap including large skin island and entire vastus lateralis muscle. (G, H) Final reconstruction has retained chest wall and abdominal contour without hernias (white asterisk, lateral thigh skin island; black asterisk, original omentum flap).
A 33-year-old man with no comorbidities required chest wall and diaphragm reconstruction after recurrent chondrosarcoma of the 11th and 12th ribs. Wide local excision involved en bloc resection of the 9th to 12th ribs, left diaphragm, and abdominal wall and spleen. All components of the resection were reconstructed. The diaphragm and abdominal wall were reconstructed with porcine acellular dermal matrix (Strattice; LifeCell, KCI Co., Branchburg, NJ). This provided separation of the thoracic and abdominal cavities as well as a rigid thoracic wall, allowing creation of negative pressure within the thoracic cavity with respiration. Anteriorly, the splenic artery and vein were mobilized and anchored to the superior abdominal wall superficial to the acellular dermal matrix.
A subtotal thigh flap (including skin and vastus lateralis muscle) based on the descending branch of the lateral circumflex artery was used to provide vascularized soft tissue coverage for the reconstructed chest and abdominal wall. The large amount of muscle obliterated the dead space created with resection of the thoracic cavity, which was reconstructed without the normal curvature. The flap was inset over the acellular dermal matrix, and vascular anastomoses were performed to the splenic artery and vein. The patient was monitored in the intensive care unit and ultimately was discharged on postoperative day 16. This reconstruction provided functional reconstruction of the thoracic and abdominal cavity as well as soft tissue coverage.
CONCLUSION
This article provides an overview of the major categories of adverse outcome and how these may potentially be avoided. Any attempt at chest wall reconstruction begins with a fundamental understanding of the defect itself—what is missing (does it require bulk, structural support, or covering of vital structures), what remains, what tissues are available for reconstruction—and integrates the priorities of function, stability, contour, prior treatment, and general health of the patient. Modern reconstruction methods have increased successful treatment of these complex wounds.
References
- Bryant L R, Spencer F C, Trinkle J K. Treatment of median sternotomy infection by mediastinal irrigation with an antibiotic solution. Ann Surg. 1969;169:914–920. doi: 10.1097/00000658-196906000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi E A, Culliford A T, Krieger K H, et al. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg. 1985;40:214–223. doi: 10.1016/s0003-4975(10)60030-6. [DOI] [PubMed] [Google Scholar]
- Ringelman P R, Vander Kolk C A, Cameron D, Baumgartner W A, Manson P N. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208–1214. discussion 1215–1216. [PubMed] [Google Scholar]
- Jones G, Jurkiewicz M J, Bostwick J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg. 1997;225:766–776. discussion 776–778. doi: 10.1097/00000658-199706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahai F, Rand R P, Hester T R, Bostwick J, III, Jurkiewicz M J. Primary treatment of the infected sternotomy wound with muscle flaps: a review of 211 consecutive cases. Plast Reconstr Surg. 1989;84:434–441. doi: 10.1097/00006534-198909000-00009. [DOI] [PubMed] [Google Scholar]
- Pairolero P C, Arnold P G, Harris J B. Long-term results of pectoralis major muscle transposition for infected sternotomy wounds. Ann Surg. 1991;213:583–589. discussion 589–590. doi: 10.1097/00000658-199106000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman N H, Subramanian V. Sternal wound reconstruction: 252 consecutive cases. The Lenox Hill experience. Plast Reconstr Surg. 2004;114:44–48. doi: 10.1097/01.prs.0000127793.77267.da. [DOI] [PubMed] [Google Scholar]
- López-Monjardin H, de-la-Peña-Salcedo A, Mendoza-Muñoz M, López-Yáñez-de-la-Peña A, Palacio-López E, López-García A. Omentum flap versus pectoralis major flap in the treatment of mediastinitis. Plast Reconstr Surg. 1998;101:1481–1485. doi: 10.1097/00006534-199805000-00008. [DOI] [PubMed] [Google Scholar]
- Milano C A, Georgiade G, Muhlbaier L H, Smith P K, Wolfe W G. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg. 1999;67:377–380. discussion 380–381. doi: 10.1016/s0003-4975(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Kanavel A B. Plastic procedures for the obliteration of cavities with non-collapsible walls. Surg Gynecol Obstet. 1921;32:453. [Google Scholar]
- Thourani V H, Lancaster R T, Mansour K A, Miller J I., Jr Twenty-six years of experience with the modified eloesser flap. Ann Thorac Surg. 2003;76:401–405. discussion 405–406. doi: 10.1016/s0003-4975(03)00470-3. [DOI] [PubMed] [Google Scholar]
- Miller J I, Mansour K A, Nahai F, Jurkiewicz M J, Hatcher C R., Jr Single-stage complete muscle flap closure of the postpneumonectomy empyema space: a new method and possible solution to a disturbing complication. Ann Thorac Surg. 1984;38:227–231. doi: 10.1016/s0003-4975(10)62243-6. [DOI] [PubMed] [Google Scholar]
- Wangensteen O H. The pedicle muscle flap in the closure of persistent bronchopleural fistulas. J Thorac Surg. 1935;5:27. [Google Scholar]
- Clagett O T, Geraci J E. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg. 1963;45:141–145. [PubMed] [Google Scholar]
- Netscher D T, Valkov P L. Reconstruction of oncologic torso defects: Emphasis on microvascular reconstruction. Semin Surg Oncol. 2000;19:255–263. doi: 10.1002/1098-2388(200010/11)19:3<255::aid-ssu7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Netscher D T, Baumholtz M A. Chest reconstruction: I. Anterior and anterolateral chest wall and wounds affecting respiratory function. Plast Reconstr Surg. 2009;124:240e–252e. doi: 10.1097/PRS.0b013e3181b98c9c. [DOI] [PubMed] [Google Scholar]
- Arnold P G, Pairolero P C. Intrathoracic muscle flaps. An account of their use in the management of 100 consecutive patients. Ann Surg. 1990;211:656–660. discussion 660–662. [PMC free article] [PubMed] [Google Scholar]
- Arnold P G, Pairolero P C, Waldorf J C. The serratus anterior muscle: Intrathoracic and extrathoracic utilization. Plast Reconstr Surg. 1984;73:240–248. doi: 10.1097/00006534-198402000-00015. [DOI] [PubMed] [Google Scholar]
- Lucas A E, Snow N, Tobin G R, Flint L M., Jr Use of the rhomboid major muscle flap for esophageal repair. Ann Thorac Surg. 1982;33:619–623. doi: 10.1016/s0003-4975(10)60823-5. [DOI] [PubMed] [Google Scholar]
- Hargrove W C, III, Edmunds L H., Jr Management of infected thoracic aortic prosthetic grafts. Ann Thorac Surg. 1984;37:72–77. doi: 10.1016/s0003-4975(10)60714-x. [DOI] [PubMed] [Google Scholar]
- Mathes D W, Yaremchuk M J, Isselbacher E M, Madsen J C. Successful in situ treatment of an infected ascending aortic graft. Ann Thorac Surg. 2000;70:1410–1412. doi: 10.1016/s0003-4975(00)01964-0. [DOI] [PubMed] [Google Scholar]
- Perler B A, Kolk C A, Manson P M, Williams G M. Rotational muscle flaps to treat localized prosthetic graft infection: long-term follow-up. J Vasc Surg. 1993;18:358–364. discussion 364–365. [PubMed] [Google Scholar]
- Zeltsman D, Tzarnas C D, Kerstein M D. Management of vascular prosthetic infections: Results of long-term follow-up. Am Surg. 1999;65:331–333. [PubMed] [Google Scholar]
- Cicilioni O J, Jr, Stieg F H, III, Papanicolaou G. Sternal wound reconstruction with transverse plate fixation. Plast Reconstr Surg. 2005;115:1297–1303. doi: 10.1097/01.prs.0000156918.15595.85. [DOI] [PubMed] [Google Scholar]
- Lee J C, Raman J, Song D H. Primary sternal closure with titanium plate fixation: Plastic surgery effecting a paradigm shift. Plast Reconstr Surg. 2010;125:1720–1724. doi: 10.1097/PRS.0b013e3181d51292. [DOI] [PubMed] [Google Scholar]
- Arnold P G, Pairolero P C. Use of pectoralis major muscle flaps to repair defects of anterior chest wall. Plast Reconstr Surg. 1979;63:205–213. doi: 10.1097/00006534-197902000-00008. [DOI] [PubMed] [Google Scholar]
- Larson D L, McMurtrey M J. Musculocutaneous flap reconstruction of chest-wall defects: An experience with 50 patients. Plast Reconstr Surg. 1984;73:734–740. doi: 10.1097/00006534-198405000-00003. [DOI] [PubMed] [Google Scholar]
- Kohman L J, Auchincloss J H, Gilbert R, Beshara M. Functional results of muscle flap closure for sternal infection. Ann Thorac Surg. 1991;52:102–106. doi: 10.1016/0003-4975(91)91428-x. [DOI] [PubMed] [Google Scholar]
- Meadows J A, III, Staats B A, Pairolero P C, Rodarte J R, Arnold P G. Effect of resection of the sternum and manubrium in conjunction with muscle transposition on pulmonary function. Mayo Clin Proc. 1985;60:604–609. doi: 10.1016/s0025-6196(12)60984-7. [DOI] [PubMed] [Google Scholar]
- Din A M, Evans G RD. Chest wall reconstruction. In: McCarthy J B, Galiano R D, Boutros S, editors. Current Therapy in Plastic Surgery. Philadelphia, PA: Saunders; 2006. pp. –362. [Google Scholar]
- Bellón J M, Buján J, Contreras L A, Carrera-San Martín A, Jurado F. Comparison of a new type of polytetrafluoroethylene patch (Mycro Mesh) and polypropylene prosthesis (Marlex) for repair of abdominal wall defects. J Am Coll Surg. 1996;183:11–18. [PubMed] [Google Scholar]
- Butler C E, Langstein H N, Kronowitz S J. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg. 2005;116:1263–1275. discussion 1276–1277. doi: 10.1097/01.prs.0000181692.71901.bd. [DOI] [PubMed] [Google Scholar]
- Wangensteen O H. Repair of recurrent and difficult hernias and other large defects of the abdominal wall employing the ilio-tibial tract of fascia lata as a pedicled flap. Surg Gynecol Obstet. 1934;59:766. [Google Scholar]
- Watson W L, James A G. Fascia lata grafts for chest wall defects. J Thorac Surg. 1947;16:399–406. [PubMed] [Google Scholar]
- Campbell D A. Reconstruction of the anterior thoracic wall. J Thorac Surg. 1950;19:456–461. [PubMed] [Google Scholar]
- Hallock G G, Lutz D A. Turnover TRAM flap as a diaphragmatic patch. Ann Plast Surg. 2004;52:93–96. doi: 10.1097/01.SAP.0000070682.67456.6E. [DOI] [PubMed] [Google Scholar]
- Whetzel T P, Stokes R B, Greenholz S K, Saunders C J. Reconstruction of the toddler diaphragm in severe anterolateral congenital diaphragmatic hernia with the reverse latissimus dorsi flap. Ann Plast Surg. 1997;39:615–619. doi: 10.1097/00000637-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Bedini A V, Valente M, Andreani S, Ravasi G. Reverse flap of distal latissimus dorsi for diaphragm reconstruction in the adult: specification of the technical procedure and report on six cases. J Thorac Cardiovasc Surg. 1997;114:846–848. doi: 10.1016/S0022-5223(97)70091-6. [DOI] [PubMed] [Google Scholar]
- Simpson J S, Gossage J D. Use of abdominal wall muscle flap in repair of large congenital diaphragmatic hernia. J Pediatr Surg. 1971;6:42–44. doi: 10.1016/0022-3468(71)90666-x. [DOI] [PubMed] [Google Scholar]
- Edington H D, Evans S, Sindelar W F. Reconstruction of a functional hemidiaphragm with use of omentum and latissimus dorsi flaps. Surgery. 1989;105:442–445. [PubMed] [Google Scholar]
- Brumback R J, McBride M S, Ortolani N C. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74:377–382. [PubMed] [Google Scholar]
- Laitung J K, Peck F. Shoulder function following the loss of the latissimus dorsi muscle. Br J Plast Surg. 1985;38:375–379. doi: 10.1016/0007-1226(85)90245-0. [DOI] [PubMed] [Google Scholar]
- Russell R C, Pribaz J, Zook E G, Leighton W D, Eriksson E, Smith C J. Functional evaluation of latissimus dorsi donor site. Plast Reconstr Surg. 1986;78:336–344. doi: 10.1097/00006534-198609000-00009. [DOI] [PubMed] [Google Scholar]
- Fraulin F O, Louie G, Zorrilla L, Tilley W. Functional evaluation of the shoulder following latissimus dorsi muscle transfer. Ann Plast Surg. 1995;35:349–355. doi: 10.1097/00000637-199510000-00003. [DOI] [PubMed] [Google Scholar]
- Spear S L, Hess C L. A review of the biomechanical and functional changes in the shoulder following transfer of the latissimus dorsi muscles. Plast Reconstr Surg. 2005;115:2070–2073. doi: 10.1097/01.prs.0000163329.96736.6a. [DOI] [PubMed] [Google Scholar]
- Netscher D T, Eladoumikdachi F, McHugh P M, Thornby J, Soltero E. Sternal wound debridement and muscle flap reconstruction: functional implications. Ann Plast Surg. 2003;51:115–122. discussion 123–125. doi: 10.1097/01.SAP.0000058497.92264.E2. [DOI] [PubMed] [Google Scholar]
- Ringelman P R, Vander Kolk C A, Cameron D, Baumgartner W A, Manson P N. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208–1214. discussion 1215–1216. [PubMed] [Google Scholar]
- Tobin G R. Pectoralis major segmental anatomy and segmentally split pectoralis major flaps. Plast Reconstr Surg. 1985;75:814–824. doi: 10.1097/00006534-198506000-00009. [DOI] [PubMed] [Google Scholar]
- Tobin G R, Moberg A W, DuBou R H, Weiner L J, Bland K I. The split latissimus dorsi myocutaneous flap. Ann Plast Surg. 1981;7:272–280. doi: 10.1097/00000637-198110000-00004. [DOI] [PubMed] [Google Scholar]
- Tobin G R, Moberg A, Ringberg A, Netscher D. Mandibular-facial reconstruction with segmentally split serratus anterior composite flaps. Clin Plast Surg. 1990;17:663–672. [PubMed] [Google Scholar]
- Seyfer A E. The lower trapezius flap for recalcitrant wounds of the posterior skull and spine. Ann Plast Surg. 1988;20:414–418. doi: 10.1097/00000637-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Koshima I, Moriguchi T, Soeda S, Kawata S, Ohta S, Ikeda A. The gluteal perforator-based flap for repair of sacral pressure sores. Plast Reconstr Surg. 1993;91:678–683. doi: 10.1097/00006534-199304000-00017. [DOI] [PubMed] [Google Scholar]
- de Weerd L, Weum S. The sensate medial dorsal intercostal artery perforator flap for closure of cervicothoracic midline defects after spinal surgery: an anatomic study and case reports. Ann Plast Surg. 2009;63:418–421. doi: 10.1097/SAP.0b013e31819537b4. [DOI] [PubMed] [Google Scholar]
- Chandra R, Kumar P, Abdi S H. The subaxillary pedicled flap. Br J Plast Surg. 1988;41:169–173. doi: 10.1016/0007-1226(88)90046-x. [DOI] [PubMed] [Google Scholar]
- Kim D Y, Cho S Y, Kim K S, Lee S Y, Cho B H. Correction of axillary burn scar contracture with the thoracodorsal perforator-based cutaneous island flap. Ann Plast Surg. 2000;44:181–187. doi: 10.1097/00000637-200044020-00010. [DOI] [PubMed] [Google Scholar]
- Angrigiani C, Grilli D, Karanas Y L, Longaker M T, Sharma S. The dorsal scapular island flap: an alternative for head, neck, and chest reconstruction. Plast Reconstr Surg. 2003;111:67–78. doi: 10.1097/01.PRS.0000037682.59058.6B. [DOI] [PubMed] [Google Scholar]