Abstract
Transthoracic needle biopsy (TTNB) is integral in the diagnosis and treatment of many thoracic diseases, and is an important alternative to more invasive surgical procedures. Both computed tomography and ultrasound may be used as imaging guidance for TTNB, with CT being more commonly utilized. Needle choice depends mostly upon lesion characteristics and location. During the procedure, patients must be able to follow breathing instructions. Common complications of TTNB include pneumothorax and hemoptysis.
Keywords: Interventional, chest, biopsy, computed tomography, cancer
IMAGING GUIDANCE
For transthoracic needle biopsy (TTNB), the choice of imaging guidance depends on lesion character and location, as well as operator preference. Fluoroscopy was once the mainstay of interventional thoracic procedures, but today computed tomography (CT) and ultrasound (US) are used most commonly.1,2
Ultrasound
Ultrasound (US) is most often used for imaging guidance for procedures of the pleura or pleural space. US is commonly used to access pleural fluid collections or for biopsy of peripheral lung and pleural lesions.3,4 Its portability and safety makes it ideal for bedside procedures. US allows the operator to visualize images in real time, allowing for accurate device placement.3
Computed Tomography and Computed Tomographic Fluoroscopy
CT is most commonly used for imaging guidance for TTNB. The spatial resolution of CT allows for accurate needle or instrument placement, even within those lesions smaller than one centimeter in diameter.5 In addition, multiplanar reformatted (MPR) CT images may be used to guide needle placement and improve accuracy in small lesions.6
CT may be used in a traditional format, where spiral or sequential CT scans are intermittently performed by a CT technologist during the procedure, or CT fluoroscopy (CTF) may be used, where the radiologist controls image acquisition during the procedure, typically via a foot pedal near the gantry. In CTF, images are viewed on an adjacent screen; therefore, adjustments to the needle or device can be made in near-real time. Of course, using CTF requires the use of lead aprons and shields for radiation safety purposes. Similar rates of success have been reported for biopsies performed using CT versus CTF, though.7
Radiation risk must also be considered, as the patient-effective dose during CT-guided procedures is often greater than that of a standard chest CT.8 Although the benefits of the procedure generally outweigh the risks, attention should always be paid to patient dose, and techniques to lower patient dose, such as decreasing mAs, should be employed whenever possible.
TRANSTHORACIC NEEDLE BIOPSY
Transthoracic needle biopsy (TTNB) is a commonly performed procedure in thoracic interventional radiology. TTNB can safely and efficiently provide an accurate cytologic or histologic diagnosis.9,10 In addition, a successful TTNB often obviates the need for more costly and invasive surgical procedures, and thus decreases the duration of hospitalization and provides a substantial cost savings.11 Reported accuracy rates for thoracic CT-guided biopsies range from 64–97%.10,12,13 In one large series, CT-guided biopsy was diagnostic in 83% of thoracic biopsies, including 80% of pulmonary lesions, 90% of mediastinal masses, and 83% of pleural lesions.14 A meta-analysis of 19 studies revealed an overall sensitivity of 0.90 (95% CI, 0.88–0.92) for biopsy of pulmonary lesions.9 A trend toward lower diagnostic accuracy was noted for lesions that were less than 1.5 cm in diameter.13 Lesion location has also been shown to influence transthoracic needle biopsy success, as biopsy of small subpleural lesions has been shown to have lower diagnostic rates coupled with higher rates of complication.6,9,10 Sensitivity based on needle type has also been described, with cutting needle biopsy having slightly higher sensitivity for detection of nonmalignant lesions.15 Single and coaxial needle systems have been shown to have similar diagnostic accuracy.16
Indications and Contraindications
Typically, TTNBs are performed for the evaluation of an indeterminate pulmonary nodule or mass. For a solitary pulmonary nodule or mass, the indication for biopsy often depends upon the pretest probability of malignancy, and the presence or absence of metastatic disease. Other indications for TTNB include evaluation of a mediastinal mass, evaluation of pulmonary nodules in a patient with a known extrathoracic malignancy, and evaluation of a perihilar mass after failed or negative bronchoscopy. In cases of lymphoma, TTNBs are often performed for histologic classification of disease. In the postoperative or postradiation patient, TTNB is frequently performed to distinguish treatment changes from recurrent disease. Finally, TTNBs may be performed for the evaluation of infectious processes presenting as nodules or consolidation.
Contraindications to TTNB are not absolute. The most important and first to consider is the presence of a bleeding diathesis. Recent platelet count, prothrombin time (PT), and partial thromboplastin time (PTT) with INR (international normalization ratio) should be obtained. Temporary discontinuation of anticoagulants such as warfarin, heparin, and aspirin is necessary.
Other contraindications include biopsy of deep lesions in patients with pulmonary hypertension, severe emphysematous disease, and large bullae in the biopsy path. Intractable cough and mechanical ventilation are also relative contraindications.
Biopsy Planning
After consideration of the patient history and indications for TTNB, a careful review of the patient's imaging studies is necessary. A needle path that avoids traversal of bullae, large vessels, and bronchi should be chosen. Avoidance of interlobar fissures is also desirable, as the more pleural surfaces that are crossed, the higher the risk of pneumothorax.17 If there is more than one lesion, a peripheral lesion should be chosen over a deep lesion because less lung will be traversed, decreasing the risk of complications.17 A lesion in an upper lobe is preferred over one in a lower lobe because of less respiratory excursion in the upper lobes. Lesions that appear hemorrhagic should be avoided. Necrotic portions of lesions should be avoided because the diagnostic value of necrotic tissue is low, and necrotic portions are prone to bleed more than intact tumor.
Biopsy Needle Types
Biopsy needles of varying gauge, lengths, tip configurations, and sampling mechanisms are available. The needle selected depends upon lesion characteristics, type/amount of tissue required, and operator preference. Biopsy needles can be divided into three groups: (1) aspiration needles for retrieval of specimens for cytologic evaluation, (2) cutting needles for retrieval of specimens for histologic evaluation, and (3) automated core biopsy needles for retrieval of specimens for histologic evaluation.
Aspiration Needles
Aspiration needles are thin-walled and flexible, and are used for obtaining specimens for cytologic or microbiologic evaluation. The most commonly used of this group is the Chiba (Cook, Inc. Bloomington, IN), which has a 30-degree bevel, and is available in 18- to 25-gauge sizes. However, because the needles are flexible, they can easily bend or deflect off course; such effects are magnified when using a longer needle. Aspiration needles are useful for making diagnoses of epithelial carcinomas (adenocarcinoma or squamous cell carcinoma) because these diagnoses can be made on cytological analysis alone. Today, though, the diagnosis is not limited to cell type, but includes evaluation of tumor markers and analysis of tumor mutational status, which require procurement of additional tissue.18 In cases where cytologic sampling may have been adequate in the past, histologic sampling may be required to have sufficient tissue for analysis.19
Cutting Needles
For tumors that require a histologic or larger specimen for analysis, a cutting needle may be used. These needles have a variegated tip or cutting notch on the side; two commonly used needles in this class are the Franseen (Cardinal Health, McGaw Park, IL) and Westcott (BD Worldwide Medical, Franklin Lakes, NJ) needles. These are modified aspiration needles, in that they can “cut” tissue in addition to aspirating it. Typical sizes used are 18 to 22 gauge. The Franseen needle has three sharp points at the tip that mimic a “crown,” and cut tissue when the needle is rotated, which yields small pieces or cores of tissue. A Franseen is less flexible and easier to steer than a Chiba of the same length, so may be useful for deeper lesions. The Westcott needle has a notch cut from the side of the needle near its beveled tip. When rotated 360 degrees, it cuts the tissue into a small core specimen.20
Automated Core-Biopsy Needles
Automated core-biopsy needles are generally used to obtain tissue for histologic evaluation. Most of these biopsy devices, such as the Temno® or Achieve® (Cardinal Health, McGaw Park, IL), are double-throw devices that use a spring-activated mechanism to sequentially fire first a thin-notched needle, followed by an outer cutting cannula. The length of the side-notch needle varies between devices, as does the throw-length. Some devices have one set throw-length, whereas others are adjustable, such as the Temno Evolution® (Cardinal Health, McGaw Park, IL). Semi-automated models such as the Biomol® (HS Medical, Rome, Italy) use a tiny battery-powered vacuum device to provide suction to aid tissue sampling.
Use of an automated biopsy device may improve results if a pathologist is not on-site at the time of biopsy because, depending on tumor type, the needle will obtain similar core samples that are free of crush injury sometimes imparted by smaller conventional needles.21,22 With continued refinements in needle design, there appears to be potential for improved sensitivity and specificity for both benign and malignant diagnoses.21,22
Preprocedure Considerations: Positioning and Sedation
Consideration of position should be made during biopsy planning, as patients must maintain the same position throughout the entire procedure. Some patients have difficulty breathing in the prone position, and may not be able to sustain adequate ventilation. Patients with baseline pain may not tolerate lying completely flat. Patients with osteoarthritis or degenerative joint disease often cannot maintain raised arms.
During the procedure, the administration of pain medications or conscious sedation is often desirable, but patients must be alert enough to follow breath-hold instructions, so careful titration of medications is necessary. The pain associated with TTNB is usually limited, and arises from two events: (1) administration of local anesthesia, and (2) violation of the parietal pleura with the needle. In my experience, however, patients complain most of pain and stiffness from lying in one position on the CT table, rather than pain associated with the procedure itself.
Biopsy Technique
After appropriate patient positioning, a radiopaque marker or grid is placed on the patient's skin over the area of interest (Fig. 1A). During suspended respiration, a short spiral CT scan of the region of interest is obtained, and from these images, an appropriate table position and needle trajectory are chosen. The shortest straight pathway from the skin to the lesion is preferred over a longer oblique pathway, and ideally, the needle should cross the pleura at a 90-degree angle rather than at an oblique angle. The depth from the skin entry site to the lesion is then measured. Next, the patient is moved back into the CT gantry to the desired table position. With the use of the gantry laser light to delineate the Z-axis position, and the radiopaque skin marker to reference the X-axis position, the needle entry site is marked with indelible ink on the patient's skin.
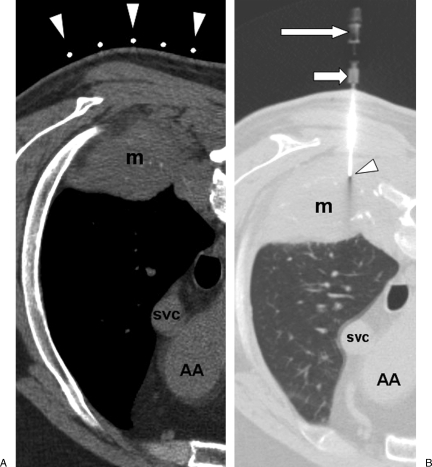
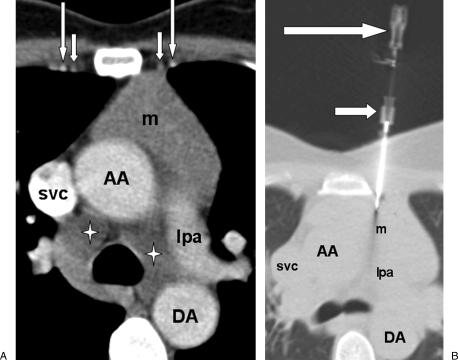
Figure 1.
(A) Computed tomography (CT) image with soft tissue windows shows patient in prone position, with radiopaque markers on patient's skin overlying a right lower lobe mass (m). Ascending aorta (AA), superior vena cava (svc). The arrows represent the radioopaque markers on the biopsy grid. (B) CT image with lung windows shows biopsy needle (arrowhead) inserted through right posterior chest wall and needle tip within the mass (m). The middle arrow is the guiding trocar and the upper arrow is the biosy needle hub.
The skin site is prepped and draped using sterile technique. Palpation of underlying structures to determine the contour of the ribs or the location of adjacent skeletal structures is helpful. For local anesthesia, a 27-gauge or similar needle is used to inject 1% or 2% lidocaine into the skin and subcutaneous tissues, followed by deeper infiltration of the intercostal muscles.
Discomfort from lidocaine injection may be decreased by addition of sodium bicarbonate, which increases its pH.23 A small dermatotomy is made to facilitate needle entry through the dermis.
The biopsy needle is inserted through the dermatotomy into the subcutaneous tissues. During needle insertion, one hand should hold the needle at skin level to stabilize and steer the needle. The other hand should hold the hub, and provide downward force to advance the needle. All needle movements should be performed with patient's respiration suspended. When advancing the needle, it is important to maintain the same trajectory with each movement, as even slight deviations of the needle at the skin or within the subcutaneous tissues will produce marked deviation at a deeper level. If an automated core-biopsy needle is utilized, the hub weight of the device should be considered because at times the apparatus may fall or list to one side if the needle is not seated deeply enough within the tissues to support its weight.
The needle is advanced to the level of the pleura, and the needle position and angle are verified with a short segment CT (typically obtained using a sequential technique). Next, the needle is advanced in one motion through the pleura to the prescribed depth. Afterwards, the patient may breathe quietly, and the needle should be allowed to sway to-and-fro with respiratory motion; the needle should not be held or fixed during respiration, as this will cause a lacerating effect on the pleura with each breath.
Confirmation of needle tip position must be performed before aspirating or cutting (Fig. 1B). If the entirety of the needle is not within the scan plane, additional images above or below the entry site must be obtained (Fig. 2). The key to recognizing the true tip of the needle is the identification of an abrupt square tip with a black shadowing artifact arising from it (Fig. 3).24 The use of a spiral technique instead of a sequential technique may decrease the time required to locate the needle tip because spiral CT can image a larger volume of tissue faster and therefore decrease respiratory misregistration.25
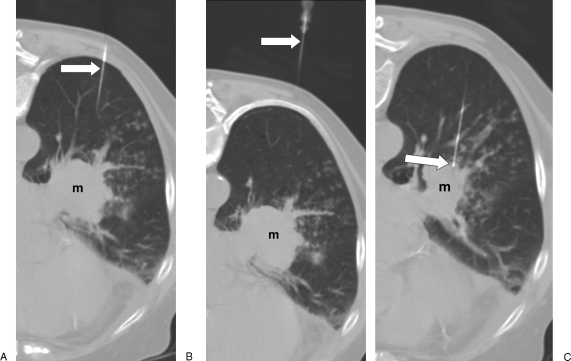
Figure 2.
Needle localization. (A) Computed tomography (CT) image with patient in prone position shows proximal portion of 22-gauge biopsy needle (arrow); left perihilar mass (m). (B) CT image cephalad to A shows needle (arrow) traversing the left posterior chest wall and a portion of the left lower lobe. (C) CT image caudal to A shows needle (arrow) entering the mass (m).
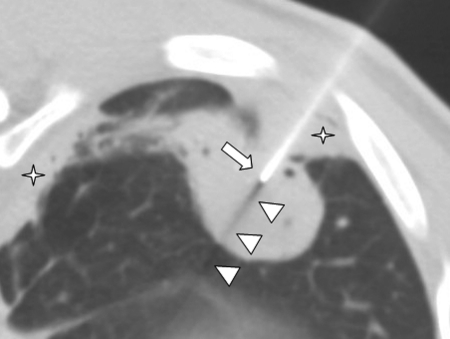
Figure 3.
Needle tip localization. Computed tomography image with patient in decubitus position shows blunt square needle tip (arrow) and black shadowing artifact (arrowheads) emanating from needle tip within the mass. Adjacent pleural disease is present (stars).
After needle tip position within the lesion is confirmed, a tissue sample may be obtained. If an aspiration or cutting needle is used, the inner stylet is removed from the needle and the needle hub quickly covered to block entry of air into the needle. Next, a syringe is attached to the needle either directly or via short connection tubing. With the patient's respiration suspended, the needle is moved to-and-fro and rotated within the lesion for ~5–7 seconds, while continuous suction is applied. This motion dislodges material near the tip, and the suction aids in retrieval. To obtain an optimal tissue sample, 10 mL of suction is utilized in combination with needle excursions of ~4 mm, angled into different portions of the lesion.26 If the syringe is applied directly to the needle, the operator must at the same time apply suction and deftly move the needle within the lesion, which can be mechanically challenging, especially if the lesion is small. If the syringe is attached to the needle via short connection tubing, an assistant may apply suction, while the operator uses two hands to precisely control the needle within the lesion.
If an automated core biopsy needle is used, the tip location must be documented before the device is “fired” into the lesion during suspended respiration. As described earlier, when using a double-throw device, the inner side-notch needle fires first, followed rapidly by the outer cutting cannula, both of which occur in less than one second; the needle cannot be imaged as it fires into the lesion. At times, the inner side-notch needle, which is thinner and more flexible than the outer cutting cannula, can unexpectedly deflect from the presumed trajectory, and the outer cutting cannula will follow the same path. This can be problematic, especially if the lesion is adjacent to a vital structure, such as the aorta. Some core biopsy needles, such as the Temno®, allow for advancement of the inner side-notch needle without firing the outer cutting cannula. Documentation of the location of the inner side-notch needle within the lesion before firing the outer cutting cannula allows for definitive knowledge of needle trajectory and accurate sampling.
Coaxial Technique
For a coaxial technique, a larger entry needle, such as an 18-gauge spinal needle, is used as a guidance cannula, and is seated in the subcutaneous tissues or even into the lesion depending on location and size. A smaller biopsy needle, which may be any needle type, is passed through the lumen of the larger needle and into the lesion (Fig. 4A,B). The main advantage of the coaxial technique is that multiple needle passes may be made into a lesion without the time-consuming process of repositioning the needle within the subcutaneous tissues for each pass. Diagnostic accuracy, however, has not been shown to be significantly different between coaxial and single needle techniques.16 Rates of pneumothorax between the two techniques are also similar.27
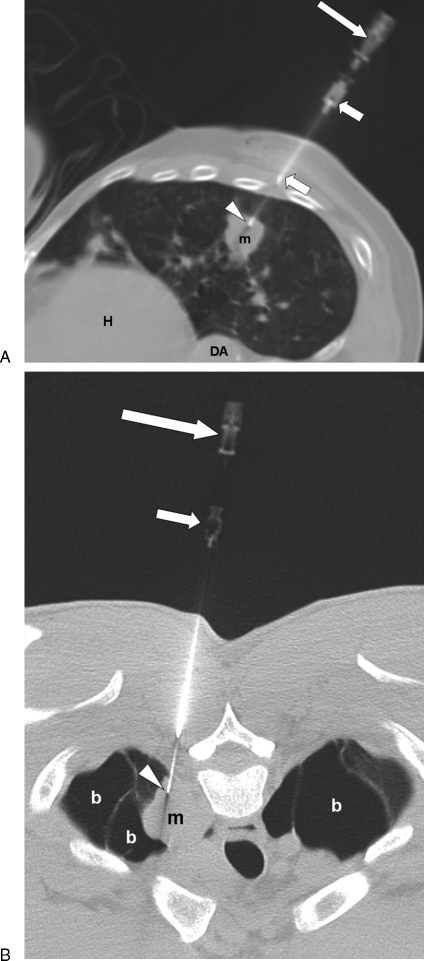
Figure 4.
COAXIAL technique: (A) Computed tomography (CT) image shows shorter entry needle (short arrows) seated in the chest wall. Longer aspiration needle (long arrow) passes through entry needle, with tip (arrowhead) in mass (m). Heart (H), descending aorta (DA). (B) CT image of different patient in prone position shows long aspiration needle (long arrow) passing through entry needle (short arrow). Tip of aspiration needle (arrowhead) is within mass (m), which is surrounded by bullae (b).
Mediastinal, Pleural, and Chest Wall Lesions
During mediastinal biopsy, the risk of pneumothorax is generally less because often the lung parenchyma is not crossed.28,29 If an anterior mediastinal lesion is to be biopsied, care should be taken to locate the internal mammary vessels (Fig. 5A). It is prudent to place the needle either medial or lateral to the internal mammary vessels, although placement of the needle medial to the vessels in a parasternal approach may be technically easier because the sternum provides a plane along which the needle can pass (Fig. 5B).
Figure 5.
Anterior mediastinal mass biopsy. (A) Prebiopsy contrast-enhanced computed tomography (CT) image shows homogeneous anterior mediastinal mass (m). Note internal mammary arteries (long arrows) and veins medially (short arrows). Mediastinal lymphadenopathy (stars) is present. Ascending aorta (AA), descending aorta (DA), superior vena cava (svc), left pulmonary artery (lpa). (B) CT image with lung window settings shows 19-gauge entry needle (short arrow) inserted along the lateral sternal border, medial to the internal mammary vessels. A 21-gauge biopsy needle (long arrow) is passed through entry needle into the mass (m).
Posterior mediastinal lesions may be biopsied with the patient in the prone or decubitus positions. To avoid traversing the lung parenchyma, the extrapleural space may be widened by injection of saline or lidocaine into the tissues medial to the pleura, which will cause lateral displacement of the pleura (Fig. 6).30 This creates a window through which a larger gauge biopsy needle may be passed.
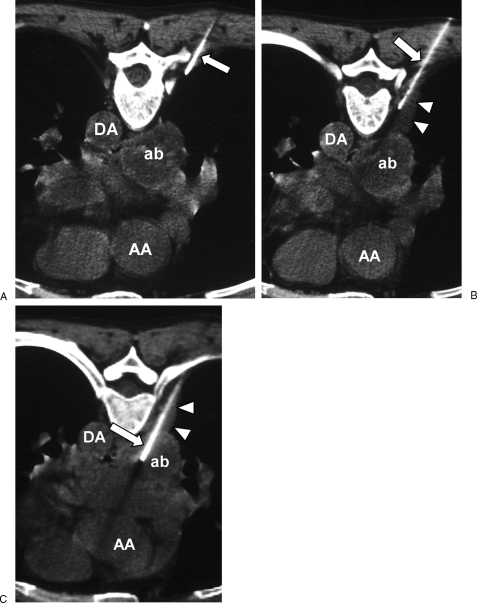
Figure 6.
Pleural displacement with saline. (A) Computed tomography (CT) image with patient in prone position shows abscess (ab) in subcarinal space. A 25-gauge needle (arrow) is inserted extrapleurally through a right paravertebral approach. Ascending aorta (AA), descending aorta (DA). (B) Needle (arrow) is advanced and saline injected to widen the extrapleural space (arrowheads). (C) A larger 18-gauge needle (arrow) is inserted via the widened extrapleural space and into the abscess (ab).
Pleural or chest wall lesions may be biopsies using any needle type, with a single-needle or a coaxial-needle technique. When appropriate, US guidance for peripheral lesions may be used. As with CT, needle position within the lesion must be confirmed before a sample is obtained. Note that very thin needles may be difficult to locate with US, so larger or needles with an echogenic coating (Echo-Coat®, STS Biopolymers, Henrietta, NY) should be considered.
Postbiopsy
After the biopsy is complete, a short spiral CT is performed to evaluate for complications including pneumothorax, hemothorax, and soft tissue hematoma. If the scan is normal, the patient is monitored in a recovery area by appropriate personnel, and a chest film is obtained at 2 hours postprocedure to assess for a delayed pneumothorax. If no pneumothorax present, subsequent development of a pneumothorax is unlikely to occur.31,32 If the film is normal and the patient is in good condition, he or she may be discharged in accordance with local institutional policies.
Complications of Transthoracic Needle Biopsies
PNEUMOTHORAX
Complications of TTNB include pneumothorax, hemoptysis, hemothorax, infection, and air embolism, with the most common complication as pneumothorax. Factors increasing the risk of pneumothorax include increased patient age, obstructive lung disease, increased depth of lesion, multiple pleural passes, increased time of needle across the pleura, and traversal of a fissure.17,31,32 Pneumothoraces can occur during or immediately after the procedure, which is why it is important to perform a CT scan of the region following removal of the needle (Fig. 7). The incidence of pneumothorax in patients undergoing transthoracic needle biopsy has been reported to be from 9–54%, with an average of around 20%.31 It is thought that the risk of pneumothorax increases with increasing needle diameter,32 but some studies suggest no difference in pneumothorax rate between larger and smaller needles.10 When comparing needle biopsy techniques, however, the incidence of pneumothorax is essentially the same for single-needle fine needle aspiration (FNA), coaxial-needle FNA, and core biopsy using automated biopsy devices.12,17,33,34 Of those patients that develop a pneumothorax, 5–18% require chest tube placement.14
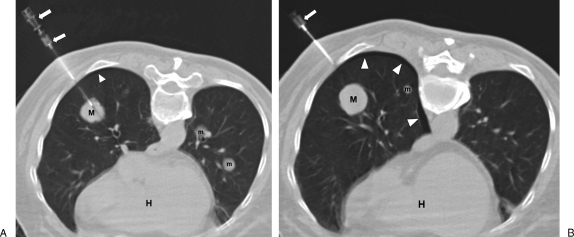
Figure 7.
Pneumothorax. (A) Computed tomography (CT) image with patient in prone position shows coaxial needle apparatus (arrows) inserted through right posterior chest wall and into mass (M). Note additional smaller parenchymal masses (m). There is a suggestion of a small pneumothorax (arrowhead). Heart (H). (B) After the longer biopsy needle is removed, the pneumothorax (arrowheads) is more evident. The entry needle (arrow) is still in place.
Pneumothorax Management
If the patient develops a pneumothorax during or after TTNB, the patient should immediately be placed biopsy-side down, and oxygen via nasal cannula or facemask should be administered at 2–4 L/min. If the biopsy needle is still within the thorax, manual aspiration of the pneumothorax may be performed.35 If the biopsy needle has been removed and the pneumothorax is large, there is mediastinal shift, or the patient is symptomatic, emergent percutaneous needle decompression using a Yueh (Cook Inc, Bloomington, IN) or similar needle is necessary. If the pneumothorax is moderate in size without shift, and the patient is asymptomatic, emergent percutaneous decompression may not be necessary, but a chest tube is often needed. Depending on pneumothorax size, a small-bore or large bore tube may be used. Supplies for pneumothorax treatment or pre-packaged kits such as the Arrow Pneumothorax Evacuation Kit® (Teleflex Medical, Limerick, PA) should be kept in stock with other TTNB supplies.
If the pneumothorax is small (< 20% lung volume), and the patient is asymptomatic, conservative management is appropriate. An upright chest film should be obtained immediately after biopsy as a baseline. The patient should be monitored closely, remain on supplemental oxygen, and positioned biopsy side-down. If films at 2 and 4 hours postprocedure show a stable small or decreasing pneumothorax and the patient is asymptomatic, discharge can be considered in accordance with institutional policy. An unchanged small pneumothorax at 4 hours postbiopsy is unlikely to become larger.31,36 If during the recovery period the pneumothorax increases in size, or if the patient becomes symptomatic, chest tube placement and/or hospital admission may be required. Management specifics vary by institution, but good communication with the referring clinician or appropriate inpatient service regarding patient status and disposition is vital.
Hemorrhage and Hemoptysis
Incidence of parenchymal hemorrhage and hemoptysis during TTNB is ~11% and 7%, respectively.14,37 Focal hemorrhage may occur around the biopsied lesion or along the needle tract (Fig. 8), and typically requires no intervention. In more severe cases of hemorrhage, the patient should be placed biopsy-side down to prevent transbronchial aspiration of blood. Data suggests that the risk of hemoptysis is the same to slightly higher with use of an automated cutting needle versus a standard aspiration or cutting needle.12 Hemoptysis is, however, almost always self-limited.4
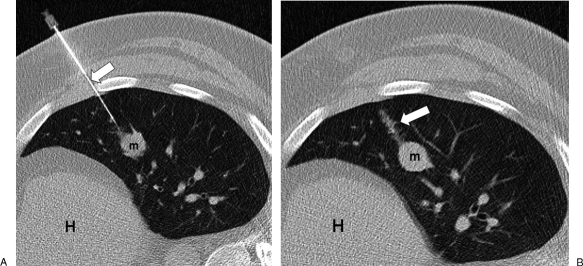
Figure 8.
Parenchymal hemorrhage. (A) Computed tomography (CT) image in a different patient shows the biopsy needle (arrow) inserted through left lateral chest wall and into mass (m). (B) After removal of the needle, a linear tract of hemorrhage (arrow) is seen along the needle path. Heart (H).
Air Embolism
Air embolism is an extremely rare but potentially fatal complication of TTNB, with an estimated occurrence of 0.02–1.8%.38,39 Air may enter the pulmonary venous system by (1) inadvertent placement of the needle tip within a pulmonary vein, followed by entry of air into the needle; or (2) inadvertent placement of the needle through both a bronchus and an adjacent pulmonary vein, creating a fistula between the two structures, allowing passage of air into the pulmonary vein. Both mechanisms may create an air embolus, which travels via the left heart into the systemic circulation. Air emboli may cause cerebral infarction, myocardial infarction, and occasionally death. Historically, immediate placement of patients in the Trendelenburg position is recommended if air embolus is suspected,40 although a review by Muth et al suggests a supine horizontal position may be effective to minimize propagation of air emboli to the brain.41
Other
Infection is an uncommon complication and is usually the result of introduction of skin flora into the lung. Proper sterile preparation of the skin is always necessary. Malignant seeding of the biopsy tract is a rare complication. Most cases of tumor seeding have been reported with malignant mesothelioma and other malignant pleural tumors.42
References
- House A J, Thomson K R. Evaluation of a new transthoracic needle for biopsy of benign and malignant lung lesions. AJR Am J Roentgenol. 1977;129(2):215–220. doi: 10.2214/ajr.129.2.215. [DOI] [PubMed] [Google Scholar]
- Westcott J L. Percutaneous catheter drainage of pleural effusion and empyema. AJR Am J Roentgenol. 1985;144(6):1189–1193. doi: 10.2214/ajr.144.6.1189. [DOI] [PubMed] [Google Scholar]
- McGahan J P, Anderson M W, Walter J P. Portable real-time sonographic and needle guidance systems for aspiration and drainage. AJR Am J Roentgenol. 1986;147(6):1241–1246. doi: 10.2214/ajr.147.6.1241. [DOI] [PubMed] [Google Scholar]
- Klein J S, Schultz S, Heffner J E. Interventional radiology of the chest: image-guided percutaneous drainage of pleural effusions, lung abscess, and pneumothorax. AJR Am J Roentgenol. 1995;164(3):581–588. doi: 10.2214/ajr.164.3.7863875. [DOI] [PubMed] [Google Scholar]
- Mueller P R, vanSonnenberg E. Interventional radiology in the chest and abdomen. N Engl J Med. 1990;322(19):1364–1374. doi: 10.1056/NEJM199005103221906. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Hatabu H, Takenaka D, Imai M, Ohbayashi C, Sugimura K. Transthoracic CT-guided biopsy with multiplanar reconstruction image improves diagnostic accuracy of solitary pulmonary nodules. Eur J Radiol. 2004;51(2):160–168. doi: 10.1016/S0720-048X(03)00216-X. [DOI] [PubMed] [Google Scholar]
- Kirchner J, Kickuth R, Laufer U, Schilling E M, Adams S, Liermann D. CT fluoroscopy-assisted puncture of thoracic and abdominal masses: a randomized trial. Clin Radiol. 2002;57(3):188–192. doi: 10.1053/crad.2001.0716. [DOI] [PubMed] [Google Scholar]
- Tsalafoutas I A, Tsapaki V, Triantopoulou C, Gorantonaki A, Papailiou J. CT-guided interventional procedures without CT fluoroscopy assistance: patient effective dose and absorbed dose considerations. AJR Am J Roentgenol. 2007;188(6):1479–1484. doi: 10.2214/AJR.06.0705. [DOI] [PubMed] [Google Scholar]
- Schreiber G, McCrory D C. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123(1, Suppl):115S–128S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- Swischuk J L, Castaneda F, Patel J C, et al. Percutaneous transthoracic needle biopsy of the lung: review of 612 lesions. J Vasc Interv Radiol. 1998;9(2):347–352. doi: 10.1016/s1051-0443(98)70279-9. [DOI] [PubMed] [Google Scholar]
- Klein J S, Zarka M A. Transthoracic needle biopsy. Radiol Clin North Am. 2000;38(2):235–266. vii. doi: 10.1016/s0033-8389(05)70161-5. [DOI] [PubMed] [Google Scholar]
- Connor S, Dyer J, Guest P. Image-guided automated needle biopsy of 106 thoracic lesions: a retrospective review of diagnostic accuracy and complication rates. Eur Radiol. 2000;10(3):490–494. doi: 10.1007/s003300050082. [DOI] [PubMed] [Google Scholar]
- Li H, Boiselle P M, Shepard J O, Trotman-Dickenson B, McLoud T C. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol. 1996;167(1):105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- vanSonnenberg E, Casola G, Ho M, et al. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology. 1988;167(2):457–461. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- Klein J S, Salomon G, Stewart E A. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198(3):715–720. doi: 10.1148/radiology.198.3.8628859. [DOI] [PubMed] [Google Scholar]
- Kucuk C U, Yilmaz A, Yilmaz A, Akkaya E. CT-guided transthoracic fine-needle aspiration in diagnosis of lung cancer: a comparison of single-pass needle and multiple pass coaxial needle systems and the value of immediate cytological assessment. Respirology. 2004;9:392–396. doi: 10.1111/j.1440-1843.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- Kazerooni E A, Lim F T, Mikhail A, Martinez F J. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198(2):371–375. doi: 10.1148/radiology.198.2.8596834. [DOI] [PubMed] [Google Scholar]
- Solomon S B, Zakowski M F, Pao W, et al. Core needle lung biopsy specimens: adequacy for EGFR and KRAS mutational analysis. AJR Am J Roentgenol. 2010;194(1):266–269. doi: 10.2214/AJR.09.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Sneige N, Guo M, Hicks M E, Moran C A. Transthoracic fine-needle aspiration vs concurrent core needle biopsy in diagnosis of intrathoracic lesions: a retrospective comparison of diagnostic accuracy. Am J Clin Pathol. 2006;125(3):438–444. [PubMed] [Google Scholar]
- Andriole J G, Haaga J R, Adams R B, Nunez C. Biopsy needle characteristics assessed in the laboratory. Radiology. 1983;148(3):659–662. doi: 10.1148/radiology.148.3.6878680. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Nakajima Y, Kurihara Y, Niimi H, Ishikawa T. CT-guided transthoracic needle biopsy: a comparison between automated biopsy gun and fine needle aspiration. Clin Radiol. 1996;51(7):503–506. doi: 10.1016/s0009-9260(96)80191-7. [DOI] [PubMed] [Google Scholar]
- Bernardino M E. Automated biopsy devices: significance and safety. Radiology. 1990;176(3):615–616. doi: 10.1148/radiology.176.3.2389013. [DOI] [PubMed] [Google Scholar]
- Xia Y, Chen E, Tibbits D L, Reilley T E, McSweeney T D. Comparison of effects of lidocaine hydrochloride, buffered lidocaine, diphenhydramine, and normal saline after intradermal injection. J Clin Anesth. 2002;14(5):339–343. doi: 10.1016/s0952-8180(02)00369-0. [DOI] [PubMed] [Google Scholar]
- Haaga J R. New techniques for CT-guided biopsies. AJR Am J Roentgenol. 1979;133(4):633–641. doi: 10.2214/ajr.133.4.633. [DOI] [PubMed] [Google Scholar]
- Silverman S G, Bloom D A, Seltzer S E, Tempany C M, Adams D F. Needle-tip localization during CT-guided abdominal biopsy: comparison of conventional and spiral CT. AJR Am J Roentgenol. 1992;159(5):1095–1097. doi: 10.2214/ajr.159.5.1414782. [DOI] [PubMed] [Google Scholar]
- Hueftle M G, Haaga J R. Effect of suction on biopsy sample size. AJR Am J Roentgenol. 1986;147(5):1014–1016. doi: 10.2214/ajr.147.5.1014. [DOI] [PubMed] [Google Scholar]
- Mullan C P, Kelly B E, Ellis P K, Hughes S, Anderson N, McCluggage W G. CT-guided fine-needle aspiration of lung nodules: effect on outcome of using coaxial technique and immediate cytological evaluation. Ulster Med J. 2004;73(1):32–36. [PMC free article] [PubMed] [Google Scholar]
- Morrissey B, Adams H, Gibbs A R, Crane M D. Percutaneous needle biopsy of the mediastinum: review of 94 procedures. Thorax. 1993;48(6):632–637. doi: 10.1136/thx.48.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Seaberg K, Wallace M J, et al. Imaging-guided percutaneous biopsy of mediastinal lesions: different approaches and anatomic considerations. Radiographics. 2005;25(3):763–786. discussion 786–788. doi: 10.1148/rg.253045030. [DOI] [PubMed] [Google Scholar]
- Langen H J, Klose K C, Keulers P, Adam G, Jochims M, Günther R W. Artificial widening of the mediastinum to gain access for extrapleural biopsy: clinical results. Radiology. 1995;196(3):703–706. doi: 10.1148/radiology.196.3.7644632. [DOI] [PubMed] [Google Scholar]
- Choi C M, Um S W, Yoo C G, et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521. doi: 10.1378/chest.126.5.1516. [DOI] [PubMed] [Google Scholar]
- Geraghty P R, Kee S T, McFarlane G, Razavi M K, Sze D Y, Dake M D. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 2003;229(2):475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol. 1999;172(4):1049–1053. doi: 10.2214/ajr.172.4.10587145. [DOI] [PubMed] [Google Scholar]
- Cox J E, Chiles C, McManus C M, Aquino S L, Choplin R H. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212(1):165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- Yamagami T, Nakamura T, Iida S, Kato T, Nishimura T. Management of pneumothorax after percutaneous CT-guided lung biopsy. Chest. 2002;121:1013–1015. doi: 10.1378/chest.121.4.1159. [DOI] [PubMed] [Google Scholar]
- Byrd R P, Jr, Fields-Ossorio C, Roy T M. Delayed chest radiographs and the diagnosis of pneumothorax following CT-guided fine needle aspiration of pulmonary lesions. Respir Med. 1999;93(6):379–381. doi: 10.1053/rmed.1999.0573. [DOI] [PubMed] [Google Scholar]
- Berquist T H, Bailey P B, Cortese D A, Miller W E. Transthoracic needle biopsy: accuracy and complications in relation to location and type of lesion. Mayo Clin Proc. 1980;55(8):475–481. [PubMed] [Google Scholar]
- Sinner W N. Complications of percutaneous transthoracic needle aspiration biopsy. Acta Radiol Diagn (Stockh) 1976;17(6):813–828. doi: 10.1177/028418517601700609. [DOI] [PubMed] [Google Scholar]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59(1):60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Arnold B W, Zwiebel W J. Percutaneous transthoracic needle biopsy complicated by air embolism. AJR Am J Roentgenol. 2002;178(6):1400–1402. doi: 10.2214/ajr.178.6.1781400. [DOI] [PubMed] [Google Scholar]
- Muth C M, Shank E S. Gas embolism. N Engl J Med. 2000;342(7):476–482. doi: 10.1056/NEJM200002173420706. [DOI] [PubMed] [Google Scholar]
- Agarwal P P, Seely J M, Matzinger F R, et al. Pleural mesothelioma: sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology. 2006;241(2):589–594. doi: 10.1148/radiol.2412051020. [DOI] [PubMed] [Google Scholar]