Abstract
Chylous leaks, such as chylothorax and chylopericardium, are uncommon effusions resulting from the leakage of intestinal lymphatic fluid from the thoracic duct (TD) and its tributaries, or intestinal lymphatic ducts. The cause can be either traumatic (thoracic surgery) or nontraumatic (idiopathic, malignancy). Treatment has traditionally consisted of dietary modification (nonfat diet) and/or surgery (TD ligation, pleurodesis). Thoracic duct embolization (TDE) has become a viable treatment alternative due to it high success rate and minimal complications. In this article, the authors describe the etiologies of chylothorax, patient population, outcomes, and long-term follow-up of TDE patients. Relevant lymphatic anatomy and physiology are reviewed, with special attention paid to the formation of the duct by tributaries at the cisterna chyli (CC). The technique of TDE is outlined, including bilateral pedal lymphangiography, TD cannulation, and embolic agents used for the procedure.
Keywords: Thoracic duct embolization, chylothorax, chyle leak
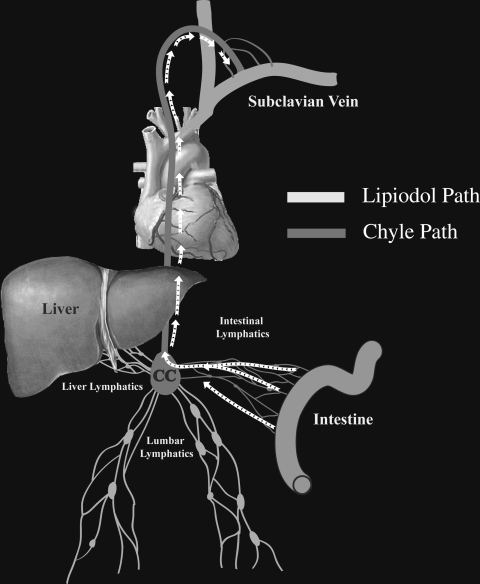
Chylous leaks are uncommon conditions resulting from leakage of intestinal lymph (chyle) outside the lymphatic system. Chylous leaks can occur anywhere along the pathway of chyle that begins in the intestinal lymphatic ducts and continue through the CC and into the TD (Fig. 1). These leaks result in a diversity of clinical presentations such as chylothorax, chylopericardium, postoperative chylous wound leaks, chylous ascites, chyloptysis, and chyluria.
Figure 1.
Schematic representation of the chyle pathway. CC, Cisterna chyli.
Only the leaks that occur above the diaphragm (chylothorax, chylopericardium, and postoperative chylous wound leaks) are discussed in this review.
Generally, there are two reported mechanisms of chyle extravasation. First, direct trauma to the lymphatic vessels in the chyle pathway (see Fig. 7C) can cause leakage of vessel contents. Second, occlusion of the TD can result in development of collateral ducts.1,2 If these collaterals abut the serous surfaces (pleura, pericardium) or skin, small trauma of these collaterals can result in a leak (see Fig. 8B).
The most common cause of chylous leak is iatrogenic, particularly thoracic or abdominal surgery.3 It has been reported to occur at a rate of 0.42% for all general thoracic surgery procedures4 and up to 3.9% after esophagectomy.5
Nontraumatic chylous leaks are rare, and can be caused by any disease process that results in lymphatic occlusion, such as malignancy (lymphoma), lymph vessel disease (Gorham disease, lymphangiomatosis), systemic disease (sarcoidosis, Behcet disease), congenital malformation, and idiopathic causes.6 In our recent series, the most common causes of nontraumatic chylothorax were idiopathic in 14 of 33 (42%) patients and lymphoma in 9 of 33 (27%) patients.7 Traditionally, low-output chylothorax (< 1000 mL/day) has been treated conservatively with diet,8 and high-output chylothorax has been treated with surgical ligation, either open, video- or robot-assisted.9,10 Mortality rates following conservative treatment of chylothorax can reach 50%; those following surgical repair are reported as high as 10%.11,12
HISTORY OF THORACIC DUCT EMBOLIZATION
TDE is the brainchild of one of the fathers of Interventional Radiology, Dr. Constantine Cope. An experienced lymphangiographer and interventional radiologist, Cope envisioned TDE as a minimally invasive alternative to TD ligation.13,14 The treatment consists of pedal lymphangiography followed by transabdominal catheterization of the CC or its tributaries and embolization of the TD/CC proximal to the leak or occlusion.
Initially, Cope tested the feasibility of TD cannulation in a porcine model.15 In his next animal experiment, the TD and CC were lacerated and embolized with metal and platinum coils.16 In 1998, Cope published TDE results on his first five human patients.17 In two consecutive prospective studies he described his experience with an additional 11 and 42 patients, respectively.13,18 Since then, several case reports and retrospective case series have been published.14,19,20,21
PHYSIOLOGY OF LYMPHATIC FLOW AND ANATOMY OF THE THORACIC DUCT
Physiology of Lymphatic Flow
The TD carries 1–2 L of lymphatic fluid a day; 80% of this fluid comes from intestinal and hepatic lymphatic ducts.22 The amount of flow in the TD fluctuates significantly with a patient's diet; the flow increases significantly immediately after a meal, due to absorption of fluids and nutrients by intestinal lymphatic ducts. Flow also varies in relation to fatty content of a meal. The flow in the TD increases significantly in pathologic conditions that result in the elevation of hepatic sinusoidal pressure, such as cirrhosis and right-sided heart failure23; several postmortem and imaging studies have demonstrated significant distension of the TD in these patients.24,25
Anatomy of the Thoracic Duct
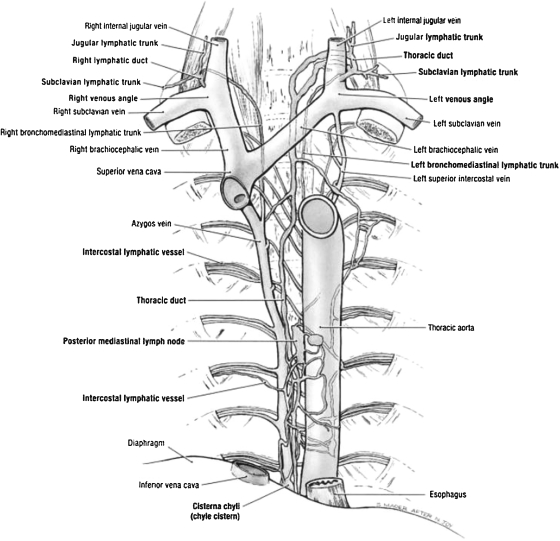
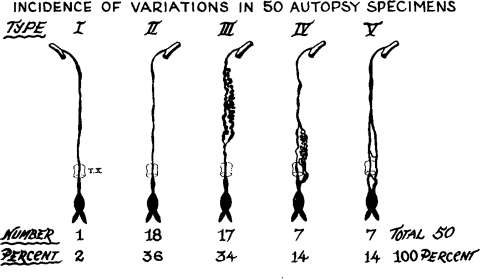
The anatomy of the TD has been well described in multiple anatomic texts (Fig. 2).26 The TD is the largest lymphatic duct in the body, measuring up to 45 cm in length and 2 to 5 mm in diameter. It drains lymph and chyle from the entire body except for the right hemithorax, right head and neck, and right arm.27 The TD originates in the upper abdomen at the CC and enters the thoracic cavity at the aortic hiatus, with the aorta on its left and the azygos vein on its right. In the thorax, the TD ascends along the anterior surface of the vertebrae, and drains into the confluence of the great veins of the left neck. Though the above classical description of the TD is the most common, many variants have been described that are not associated with clinical abnormalities. Variations of the classical TD configuration can be seen at the termination of the TD, its course, the number of ducts, and the location of its tributaries. The course of the TD can be predominantly left-sided, right-sided, or bilateral, in the case of a duplicated TD. Several classification systems have been developed to describe commonly seen anatomic variations. Adachi et al classified the TD into nine “potential” types based on embryologic development of the TD, which originates as two ducts that later fuse.28 However, only five variants of this classification were observed on postmortem and magnetic resonance (MR) ductography studies (Fig. 3).28,29 A postmortem study by Kausel et al divided the TD into five types based on morphology of the TD (single, double, multiple trunks) and the location of its outflow (Fig. 4).30 The fifth type in Kausel's classification schema, a plexiform variation, is of importance to the operator performing TDE because of the difficulty this creates in advancing the wire and catheter distally. In these cases, the embolization must be performed proximally.
Figure 2.
Anatomic rendering of the thoracic duct. (Adapted from Agur AMR, Dalley AF, Grant JCB. Grant's Atlas of Anatomy. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.)
Figure 3.
Schematic representation of the major anatomic variants of the thoracic duct based on embryologic development and the rate of their occurrence observed on magnetic resonance ductography. (Reprinted with permission from Okuda et al. Magnetic resonance-thoracic ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological considerations. Gen Thorac Cardiovasc Surg 2009;57(12):640–646.)
Figure 4.
Schematic representation of the five major anatomic variants of the thoracic duct and the rate of their occurrence observed in postmortem study. (Reprinted with permission from Kausel et al. Anatomic and pathologic studies of the thoracic duct. J Thorac Surg 1957;34(5):631–641.)
Anatomy of the Cisterna Chyli
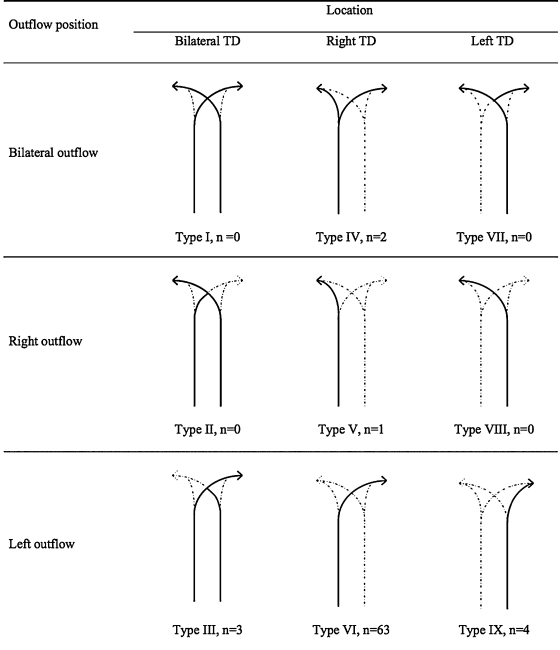
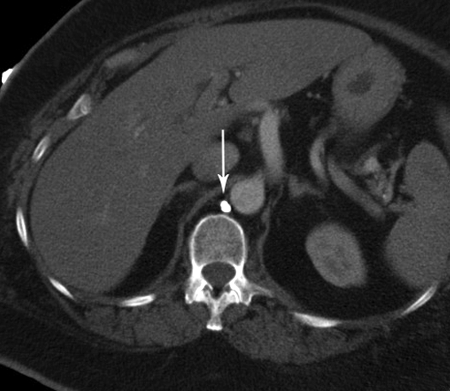
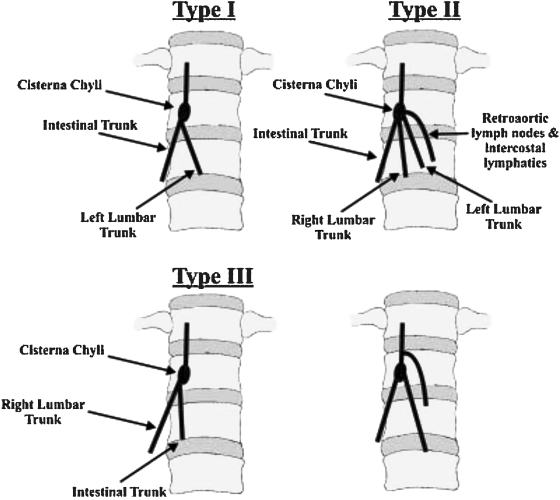
The CC is a triangular or oblong sac ~5 cm in length. On lymphangiography, its dimensions are much smaller, ~1 cm wide and 2 cm long.31 It is typically located at the L1–L2 level, just to the right of the aorta behind the right diaphragmatic crus (Fig. 5), although left-sided CC has been reported.32 The CC is not always visualized, occurring in 35% of patients on postmortem studies,33 30% to 53% on lymphangiographic studies,34 and between 96% and 76% on MR ductography studies.25,35 The CC receives the right and left lumbar trunks, the intestinal trunk, the lowest intercostal trunks, and hepatic lymphatic ducts. The anatomic variations of the tributaries of the CC are of importance for the operator performing TDE. In a recent study by Loukas et al, the most common configuration was a CC formed by the union of the left lumbar trunk and the intestinal trunk, which occurred in 45% of specimens (Fig. 6).36 To prevent potential leakage of chyle from the access point during TDE, we recommend accessing the lymphatic system below the CC through one of its tributaries, ideally the right lumbar trunk (see below).
Figure 5.
Computed tomography image of the retrocrural location of the cisterna chyli following lymphangiogram (arrow).
Figure 6.
Schematic representation of the anatomic variation of the tributaries of the cisterna chyli. (Reprinted with permission from Loukas et al. Cisterna chyli: a detailed anatomic investigation. Clin Anat 2007;20(6):683–688.)
TECHNIQUE OF THORACIC DUCT EMBOLIZATION
TDE is performed under moderate sedation. Preprocedure intravenous antibiotics (cefazolin) are administered.
Bilateral Pedal Lymphangiogram and Opacification of the Thoracic Duct
Due to advances in cross-sectional imaging, lymphangiogram use and expertise has virtually disappeared in most radiology departments. Detailed descriptions of lymphatic duct cannulation technique can be found in recent reviews.37,38 The main objective of lymphangiography in TDE is the imaging of lymphatic vessels. For this reason, over the years we have developed slight modifications of our contrast injection technique that improve imaging of the lymphatic ducts and facilitate embolization of the TD:
We use ~5 mL of Ethiodol (Savage Laboratories, Melville, NY) contrast in each leg, a relatively small amount compared with traditional protocols. Using a large amount of contrast can flood the intestinal lymphatic ducts and CC, thus obscuring the target. Also, a large amount of Ethiodol in the TD can delay glue polymerization and prevent complete embolization of the TD.
To improve visualization of the TD and CC, and to expedite opacification of the CC with intralymphatic contrast, we follow the injection of contrast with a 20 mL “saline push” in each leg. From our experience, this technique significantly decreases the procedure time and improves the success rate of embolization.
Thoracic Duct Cannulation
SELECTION OF THE TARGET VESSEL
Initially, the lymphographic dye progresses slowly through the network of pelvic and retroperitoneal lymphatic vessels to approximately the L1–L2 level. At this level, the lymphatic ducts quickly lose their definition and clarity, an indicator of lymph inflow from intestinal and hepatic lymphatics. To prevent chyle leakage from an access point in the CC, we recommend accessing the TD below this inflow point, through one of the lumbar lymphatic ducts (preferably the right). Even though it is more challenging to cannulate a lumbar lymphatic duct due to its small size, this difficulty is offset by its more constant and defined appearance during lymphangiography.
LYMPHATIC DUCT ACCESS
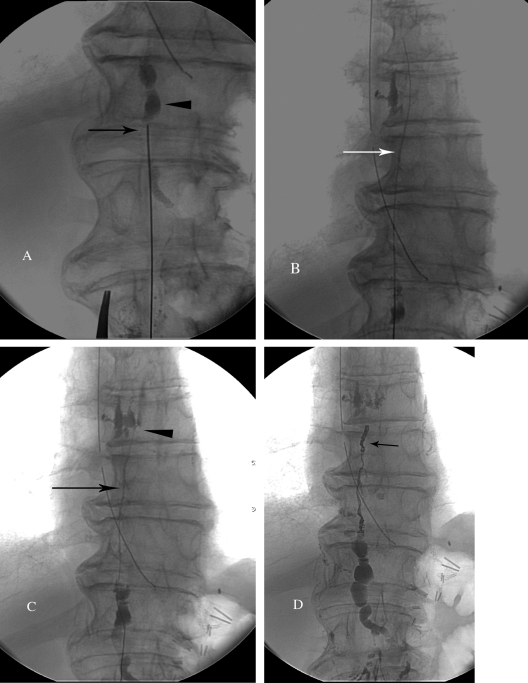
When a target duct is identified, cannulation is performed using 21- or 22-gauge 15- to 20-cm Chiba needle (Cook Inc., Bloomington, IN) and fluoroscopic guidance. We introduce a slight 5–10 degree bend at the distal 2 cm of the needle for directionality. A right transabdominal approach is preferred to avoid the aorta. Quick, deliberate jabs with the Chiba needle are made, such that the needle is not deflected by compliant intervening structures (especially the large and small intestine). Both walls of the duct are penetrated, and a stiff 0.018-inch guidewire (V18 Control, Boston Scientific, Natick, MA) is used to probe for the duct, similar to the biliary drainage technique (Fig. 7A). When the TD is accessed, the wire should pass easily into the upper TD (Fig. 7B). Next, a 65-cm microcatheter (Rapid Transit, Johnson & Johnson, Miami Lakes, FL) is advanced into the TD over the wire. Once the catheter is in the TD, the wire is removed and iodinated contrast is injected through a 1-mL syringe to detect the site of leakage (Fig. 7C).
Figure 7.
Technical steps of thoracic duct (TD) embolization. (A) Fluoroscopic image of the access of the cisterna chyli with a 21-gauge needle (arrowhead). (B) Fluoroscopic imaging of the V-18 wire advanced into the TD (arrow). (C) Injection of the contrast into the TD through the catheter (arrow) to identify the chylous leak (arrowhead). (D) Final fluoroscopy image after embolization of the TD with microcoils (arrow) and Truefill glue.
Thoracic Duct Embolization
After identification of the cause of chyle leakage (extravasation or obstruction), embolization of the TD is performed proximally. First, coils are placed to provide a matrix for glue polymerization. A slight back-and-forth motion during deployment encourages proper coil deployment. Next, a maximum of 0.2 mL of D5W is used to flush the catheter to prevent intracatheter glue polymerization. Injection of larger amounts of D5W can fill the TD and significantly slow the polymerization of glue, resulting in recanalization of the glue cast. n-Butyl cyanoacrylate (n-BCA) (Truefill® Cordis, Johnson & Johnson, Warren, NJ) diluted 1:1 in Ethiodol is used for embolization. The speed of n-BCA polymerization depends on the rate of exposure of the glue to the ionic compounds. Due to relatively stagnant flow in lymphatic vessels, ionic exposure is significantly less than in blood vessels. Therefore, a 1:1 glue/Ethiodol dilution provides ample time for injection. The TD is filled with the glue mixture just proximal to the leak or occlusion (Fig. 7D). If the TD is accessed via a lumbar lymphatic duct, we recommend injecting a small amount of glue at the duct entry site. Immediately after glue injection, the microcatheter is removed. The foot incisions are closed with at least three vertical mattress sutures each, and the percutaneous access site is dressed with a bandage. We do not recommend use of the Onyx® (Micro Therapeutics, Inc., Irvine, CA) embolic system due to the porosity of the cast that is created. We attribute some of our failures to its use.
Thoracic Duct Interruption
Originally described by Cope et al13 as a means of interrupting lymphatic flow in cases of failed TD catheterization, this technique was further explored by Blinkert et al.39 The technique consists of attempts to disrupt the lymphatic ducts by multiple needle punctures at the level of the CC.
OUTCOMES OF TD EMBOLIZATION AND TD INTERRUPTION
In the first large series of 42 patients by Cope et al, the success rate following TDE and TD interruption was reported to be 73.8%.13 This group of patients included patients with both traumatic and nontraumatic causes of chylothorax. Due to significant differences between these two groups in terms of pathophysiology, course, treatment approach, and outcomes, we believe these two groups should be evaluated separately.
Outcomes of Thoracic Duct Embolization in Traumatic Chylothorax
In a recent study, Itkin et al reported outcomes of TDE and TD disruption in 109 patients with chylous leaks.14 The causes of chylous leak are listed in Table 1. In 71 patients the TD was embolized, and in 18 patients the TD was disrupted. Embolization with coils only was performed in 13 (18.3%) patients; embolization with liquid embolic agents only was performed in 18 (25.4%) patients; embolization with a combination of coils and liquid embolic agents was performed in 40 (56.3%) patients. Follow-up was available for 107 patients. The overall success rate of the entire series on an intent-to-treat basis was 71% (77/109). The overall success rate in the subgroup of patients with attempted interventions (excluding patients in whom intervention was deemed inappropriate and was not attempted) was 88% (77/88). Clinical success after TDE was 90% (64/71). In cases where liquid embolic agents were used, the success rate of embolization was 91% (53/58). The success rate for coil embolization was 84% (11/13). In the subgroup with failed TD ligation, TDE was successful in 15 of 17 (88%) patients. The cure rate of chyle leak is directly related to the ability to catheterize the CC/TD; in cases where catheterization was successful, the cure rate was 90%. Surprisingly, TD interruption resulted in a cure in 72% of our series' cases. However, it is well known that lymphangiography alone can result in cessation of chylothorax.40 We strongly feel that the use of a combination of coils and a liquid embolic agent is necessary to achieve optimal TDE. Coils serve as a matrix for glue polymerization and provide an additional embolic mechanism in case of glue failure. Embolization with coils alone results in lower success rates when compared with coils combined with liquid embolic agents (84% vs 91%).
Table 1.
Causes of Traumatic Chylous Leak
| Esophagectomy | 31 |
| Lung resection for cancer | 29 |
| Coronary bypass | 9 |
| Thoracic aneurysm repair | 6 |
| Heart transplant | 4 |
| Trauma | 4 |
| Lung transplant | 3 |
| Aortic valve replacement | 2 |
| Mediastinal biopsy | 2 |
| Mediastinal mass resection | 2 |
| Cardiac tumor resection | 2 |
| Thyroidectomy | 2 |
| Abdominal aortic aneurysm repair | 1 |
| Aortic dissection repair | 1 |
| Aortic transection repair | 1 |
| Back surgery | 1 |
| Diaphragmatic hernia repair | 1 |
| Mitral valve replacement | 1 |
| Neck dissection for cancer | 1 |
| Neck dissection for lipoma | 1 |
| Patent ductus arteriosus ligations | 1 |
| Pericardial surgery | 1 |
| Radiation | 1 |
| Rib resection for thoracic outlet syndrome | 1 |
| Thymomectomy | 1 |
| Total | 109 |
Outcomes of Thoracic Duct Embolization in Nontraumatic Chylothorax
In our group's recent analysis, we reported 33 cases of TDE following nontraumatic chylous leak.7 The patient population was predominantly female (24 women and 7 men). The causes of chylothorax are listed in the Table 2. In 23 of 33 (70%) patients, catheterization and embolization of the TD were successful. In 4 of 23 of these cases, TDE was repeated due to clinical failure. In 4 of 33 (12%) patients, catheterization failed, and in 6 (18%) patients, embolization was not attempted due to nonvisualization of the CC and TD.
Table 2.
Causes of Nontraumatic Chyle Leak
| Idiopathic | 14 |
| Lymphoma | 9 |
| Subclavian vein occlusion | 2 |
| Waldenstrom macroglobulinemia | 2 |
| Lymphangiomatosis | 2 |
| Idiopathic chylopericardium | 2 |
| Gorham disease | 1 |
| Behcet disease | 1 |
Four lymphangiography patterns of TD were identified:
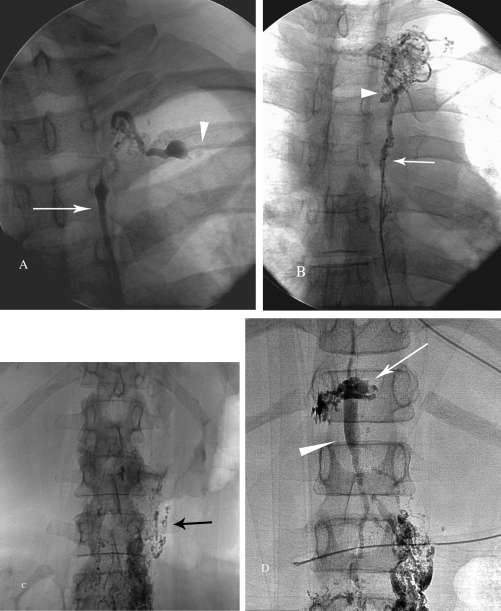
Normal thoracic duct (6/33) was observed on the lymphangiogram in its entirety, with a normal transition to the subclavian vein and free passage of the contrast into the subclavian vein (Fig. 8A).
Occlusion of the thoracic duct (19/33) was diagnosed when no contrast passed into the subclavian vein and multiple mediastinal collateral ducts were observed (Fig. 8B).
Failure to opacify the thoracic duct (6/33) was defined as either blockage of the lymphatic flow at the level of the groin or retroperitoneal lymphatic ducts (Fig. 8C).
Extravasation of contrast (2/33) was diagnosed when leakage of contrast from the TD or its tributaries could be clearly identified (Fig. 8D).
Figure 8.
Fluoroscopic images of four lymphangiography patterns of the thoracic duct (TD) nontraumatic chylous effusion population. (A) Distal part of normal TD (arrow) and the termination of the TD at the subclavian vein (arrowhead). (B) Injection of the contrast agent into TD via microcatheter (arrow) demonstrated complete occlusion of the TD with development of multiple collaterals (arrowhead). (C) Complete occlusion of the caudal lymphatic flow at the level of the midabdomen (black arrow). Patient with the history of previously treated lymphoma. (D) Leakage of the contrast from the cisterna chyli (white arrow) in patient post unsuccessful TD ligation. Note distended cisterna chyli (arrowhead).
All but two patients were available for follow-up. The overall cure rate was 52% (17/33). This compares favorably with a study by Maldonado et al reporting a 27% success rate of combined surgical and conservative management of nontraumatic chylothorax.41 In the subgroup of patients who had undergone successful embolization, the cure rate was 65% (15/23). The highest clinical success rate (73%) was seen in cases of TD occlusion. When a normal TD was seen, only ⅙ (16%) patients was cured. In cases where the TD could not be opacified and no attempt to access or embolize the duct was made, ⅙ (16%) patients was cured. In the two cases where a leak could be identified, one patient was cured and the other was lost to follow-up. The main difficulty in treating nontraumatic chylothorax was the identification of the cause of the chylous leak. A pedal lymphangiogram was able to identify the cause in only 21 of 33 (63%) cases. Knowing that pedal lymphangiography fails to detect leaks below the diaphragm, we assume that the source of the leak in cases of normal TD were intestinal lymphatic ducts. Pedal lymphangiography is crucial to identify the cause of chyle leak or, more importantly, to exclude normal supradiaphragmatic lymphatic ducts.
Immediate Complications Post TDE and TD Interruption
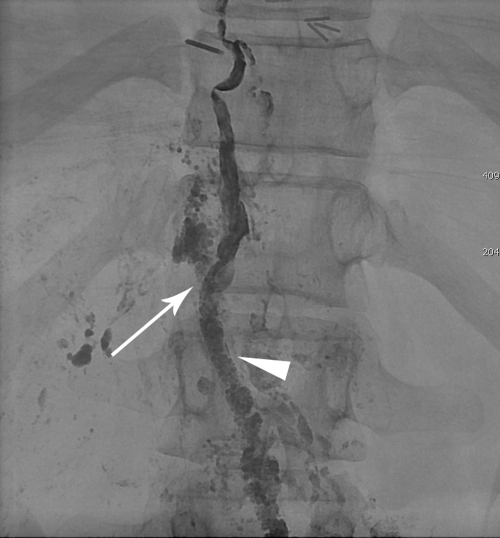
Transgression of the needle through visceral organs, including intestines and vessels, can potentially result in complications such as peritonitis or bleeding. However, these complications have never been reported. In the prospective study by Cope et al where 42 patients were followed until cure or death, no complications related to the procedure were observed. In our recent report of TDE for traumatic chylothorax, three complications were identified: one case of asymptomatic embolization of the pulmonary artery with glue, and two cases of leg edema and pedal suture dehiscence that resulted in wound infections.14 The leg edema eventually subsided, and the infections were cured with local wound care and antibiotics. In our report of TDE for nontraumatic chylothorax, we observed two major complications: one symptomatic pulmonary artery embolization with glue and one infection at the pedal lymphangiogram incision7 site. TDE occludes the main outflow of chyle into the venous system. However, logical questions remain: What happens to the chyle after TDE? Can TDE convert chylothorax to chylous ascites? Does this occlusion result in the subsequent development of long-term complications? The presence of lymphovenous communication has been well explored and described in the literature,42,43 and it is believed that after occlusion of the TD, these “dormant” anastomoses reopen. In our practice, we have never observed chylous ascites following TDE. However, we have observed a few cases of the development of contralateral chylothorax. This has an anatomic explanation, as the CC and proximal TD are located in the retrocrural space (Fig. 5). A lymphangiogram performed in one of these cases demonstrated leakage from the CC at the point of the previous CC access (Fig. 9). To avoid this complication, we access the TD at the level of lumbar lymphatic ducts, and inject glue at this access point after TDE. Since adopting these technique modifications, we have not seen this complication.
Figure 9.
Lymphangiographic image demonstrating contrast extravasation (arrow) from the hole created by previous access of the cisterna chyli (CC). Note the contrast in the CC from the first thoracic duct embolization (arrowhead).
Long-Term Complications
Recently, we performed a retrospective analysis for long-term complications following successful TDE (Laslet, Itkin, manuscript submitted for publication44). We hypothesize that potential complications of TDE include chronic lower extremity edema, chronic diarrhea, and abdominal ascites. We contacted the patients and health care providers and surveyed for development of these or other new symptoms. Between the 1995 and 2010, 169 patients underwent TDE for symptomatic chylous effusion. In 115 of 169 (68%) patients TDE embolization was technically successful. Follow-up information was available for 91 of 115 (79%) patients. Mean length of follow-up was 39 months. Thirty-four of the 91 (37%) patients died of causes unrelated to TDE. Fifty-seven (63%) patients were alive at the time of follow-up; 44 patients did not develop new symptoms related to TDE. The complications and their relationship to the procedure are summarized in Table 3. Four of 57 (7%) patients complained of chronic leg swelling that was likely related to the procedure. Three of 57 (5%) patients complained of abdominal swelling not confirmed to be ascites, and seven (12%) patients suffered from chronic diarrhea. In five of these patients, the diarrhea was considered procedure related. Two of these patients were evaluated for, but found not to have protein-losing enteropathy. One patient (2%) complained of bilateral breast swelling. Though it is difficult to establish the relationship between symptoms and the procedure due to the complexity of the patients' conditions, we feel that some of the observed complications may well be related to TDE and should be part of informed consent for the procedure.
Table 3.
Long-Term Complications Postthoracic Duct Embolization and Their Relationship to the Procedure
| Leg Swelling | Abdominal Swelling | Diarrhea | Other | |
|---|---|---|---|---|
| Related | 4 | 0 | 5 | Breast swelling-1 |
| Probably unrelated | 0 | 3 | 2 |
CONCLUSION
TDE is an important alternative and first line of treatment to patients with traumatic and nontraumatic chylous leaks with no associated mortality, minimal morbidity, and a high success rate.
More research is needed in the subgroup of patients with nontraumatic chylous leaks, with attention paid to better identifying the cause and location of chyle leaks. This could greatly improve both planning and outcomes of TDE.
References
- Dunn R P. Primary chylopericardium: a review of the literature and an illustrated case. Am Heart J. 1975;89(3):369–377. doi: 10.1016/0002-8703(75)90088-5. [DOI] [PubMed] [Google Scholar]
- Itkin M, Swe N M, Shapiro S E, Shrager J B. Spontaneous chylopericardium: delineation of the underlying anatomic pathology by CT lymphangiography. Ann Thorac Surg. 2009;87(5):1595–1597. doi: 10.1016/j.athoracsur.2008.09.054. [DOI] [PubMed] [Google Scholar]
- Doerr C H, Allen M S, Nichols F C, III, Ryu J H. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80(7):867–870. doi: 10.4065/80.7.867. [DOI] [PubMed] [Google Scholar]
- Cerfolio R J, Allen M S, Deschamps C, Trastek V F, Pairolero P C. Postoperative chylothorax. J Thorac Cardiovasc Surg. 1996;112(5):1361–1365. discussion 1365–1366. doi: 10.1016/S0022-5223(96)70152-6. [DOI] [PubMed] [Google Scholar]
- Dougenis D, Walker W S, Cameron E W, Walbaum P R. Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet. 1992;174(6):501–506. [PubMed] [Google Scholar]
- Romero S. Nontraumatic chylothorax. Curr Opin Pulm Med. 2000;6(4):287–291. doi: 10.1097/00063198-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Itkin M, Kucharczuk J C. Thoracic duct embolization (TDE) for non-traumatic chylous effusion: Experience in 31 patients. Chest. 2010;138(4, Suppl):654A. [Google Scholar]
- Marts B C, Naunheim K S, Fiore A C, Pennington D G. Conservative versus surgical management of chylothorax. Am J Surg. 1992;164(5):532–534. discussion 534–535. doi: 10.1016/s0002-9610(05)81195-x. [DOI] [PubMed] [Google Scholar]
- Christodoulou M, Ris H B, Pezzetta E. Video-assisted right supradiaphragmatic thoracic duct ligation for non-traumatic recurrent chylothorax. Eur J Cardiothorac Surg. 2006;29(5):810–814. doi: 10.1016/j.ejcts.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Thompson K J, Kernstine K H, Grannis F W, Jr, Mojica P, Falabella A. Treatment of chylothorax by robotic thoracic duct ligation. Ann Thorac Surg. 2008;85(1):334–336. doi: 10.1016/j.athoracsur.2007.04.109. [DOI] [PubMed] [Google Scholar]
- Bolger C, Walsh T N, Tanner W A, Keeling P, Hennessy T P. Chylothorax after oesophagectomy. Br J Surg. 1991;78(5):587–588. doi: 10.1002/bjs.1800780521. [DOI] [PubMed] [Google Scholar]
- Orringer M B, Bluett M, Deeb G M. Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery. 1988;104(4):720–726. [PubMed] [Google Scholar]
- Cope C, Kaiser L R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- Itkin M, Kucharczuk J C, Kwak A, Trerotola S O, Kaiser L R. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139(3):584–589. discussion 589–590. doi: 10.1016/j.jtcvs.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Cope C. Percutaneous thoracic duct cannulation: feasibility study in swine. J Vasc Interv Radiol. 1995;6(4):559–564. doi: 10.1016/s1051-0443(95)71134-4. [DOI] [PubMed] [Google Scholar]
- Cope C. Percutaneous transabdominal embolization of thoracic duct lacerations in animals. J Vasc Interv Radiol. 1996;7(5):725–731. doi: 10.1016/s1051-0443(96)70840-0. [DOI] [PubMed] [Google Scholar]
- Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998;9(5):727–734. doi: 10.1016/s1051-0443(98)70382-3. [DOI] [PubMed] [Google Scholar]
- Cope C, Salem R, Kaiser L R. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10(9):1248–1254. doi: 10.1016/s1051-0443(99)70227-7. [DOI] [PubMed] [Google Scholar]
- Bonn J, Sperling D, Walinsky P, Mannion J. Percutaneous embolization of thoracic duct injury. Circulation. 2000;102(2):268–269. doi: 10.1161/01.cir.102.2.268. [DOI] [PubMed] [Google Scholar]
- Schild H, Hirner A. Percutaneous translymphatic thoracic duct embolization for treatment of chylothorax. Rofo. 2001;173(7):580–582. doi: 10.1055/s-2001-15838. [DOI] [PubMed] [Google Scholar]
- Choo J C, Foley P T, Lyon S M. Percutaneous management of high-output chylothorax: case reviews. Cardiovasc Intervent Radiol. 2009;32(4):828–832. doi: 10.1007/s00270-009-9545-3. [DOI] [PubMed] [Google Scholar]
- Bierman H R, Byron R L, Jr, Kelly K H, et al. The characteristics of thoracic duct lymph in man. J Clin Invest. 1953;32(7):637–649. doi: 10.1172/JCI102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A E, Mulholland J H. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med. 1960;263:471–474. doi: 10.1056/NEJM196009082631001. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Linhart P, Baggenstoss A H. Hepatic lymph drainage in cirrhosis and congestive heart failure. A postmortem lymphangiographic study. Arch Pathol. 1968;86(5):551–562. [PubMed] [Google Scholar]
- Verma S K, Mitchell D G, Bergin D, et al. Dilated cisternae chyli: a sign of uncompensated cirrhosis at MR imaging. Abdom Imaging. 2009;34(2):211–216. doi: 10.1007/s00261-008-9369-7. [DOI] [PubMed] [Google Scholar]
- Agur A MR, Dalley A F, Grant J CB. Grant's Atlas of Anatomy. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Skandalakis J E, Skandalakis L J, Skandalakis P N. Anatomy of the lymphatics. Surg Oncol Clin N Am. 2007;16(1):1–16. doi: 10.1016/j.soc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Adachi B. Der Ductus Thoracicus der Japane. In Das Lymphgefässsystem der Japaner. Tokyo: Kenkyusha; 1953. [Google Scholar]
- Okuda I, Udagawa H, Takahashi J, Yamase H, Kohno T, Nakajima Y. Magnetic resonance-thoracic ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological considerations. Gen Thorac Cardiovasc Surg. 2009;57(12):640–646. doi: 10.1007/s11748-009-0483-4. [DOI] [PubMed] [Google Scholar]
- Kausel H W, Reeve T S, Stein A A, Alley R D, Stranahan A. Anatomic and pathologic studies of the thoracic duct. J Thorac Surg. 1957;34(5):631–641. [PubMed] [Google Scholar]
- Browse N L, Burnand K G, Mortimer P. Diseases of the Lymphatics. London/New York: Arnold/Oxford University Press; 2003. [Google Scholar]
- Kurosaki Y, Fujikawa A. Left-sided cisterna chyli. AJR Am J Roentgenol. 2000;175(5):1462. doi: 10.2214/ajr.175.5.1751462. [DOI] [PubMed] [Google Scholar]
- Pomerantz M, Herdt J R, Rockoff S D, Ketcham A S. Evaluation of the functional anatomy of the thoracic duct by lymphangiography. J Thorac Cardiovasc Surg. 1963;46:568–575. [PubMed] [Google Scholar]
- Rosenberger A, Adler O, Abrams H L. The thoracic duct: structural, functional, and radiologic aspects. CRC Crit Rev Radiol Sci. 1972;3(4):523–541. [PubMed] [Google Scholar]
- Erden A, Fitoz S, Yagmurlu B, Erden I. Abdominal confluence of lymph trunks: detectability and morphology on heavily T2-weighted images. Abstracts of the 105th Annual Meeting of the American Roentgen Ray Society. May 15-20, 2005, New Orleans, Louisiana, USA. AJR Am J Roentgenol. 2005;184(4, Suppl):1. doi: 10.2214/ajr.184.1.01840035. [DOI] [PubMed] [Google Scholar]
- Loukas M, Wartmann C T, Louis R G, Jr, et al. Cisterna chyli: a detailed anatomic investigation. Clin Anat. 2007;20(6):683–688. doi: 10.1002/ca.20485. [DOI] [PubMed] [Google Scholar]
- Plotnik A N, Foley P T, Koukounaras J, Lyon S M. How I do it: Lymphangiography. J Med Imaging Radiat Oncol. 2010;54(1):43–46. doi: 10.1111/j.1754-9485.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- Guermazi A, Brice P, Hennequin C, Sarfati E. Lymphography: an old technique retains its usefulness. Radiographics. 2003;23(6):1541–1558. discussion 1559–1560. doi: 10.1148/rg.236035704. [DOI] [PubMed] [Google Scholar]
- Binkert C A, Yucel E K, Davison B D, Sugarbaker D J, Baum R A. Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol. 2005;16(9):1257–1262. doi: 10.1097/01.rvi.0000167869.36093.43. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamagami T, Kato T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009;82(976):286–290. doi: 10.1259/bjr/64849421. [DOI] [PubMed] [Google Scholar]
- Maldonado F, Cartin-Ceba R, Hawkins F J, Ryu J H. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci. 2010;339(4):314–318. doi: 10.1097/MAJ.0b013e3181cdcd6c. [DOI] [PubMed] [Google Scholar]
- Abbes M, Juillard G. Study of 27 lymphovenous communications observed by lymphography in man. Ann Radiol (Paris) 1969;12(1):107–115. [PubMed] [Google Scholar]
- Edwards J M, Kinmonth J B. Lymphovenous shunts in man. BMJ. 1969;4(5683):579–581. doi: 10.1136/bmj.4.5683.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslett D, Itkin M. Delayed complications following technically successful thoracic duct embolization (TDE). Paper presented at: Society of Interventional Radiology. 2011. Chicago. doi: 10.1016/j.jvir.2011.10.008. [DOI] [PubMed] [Google Scholar]