Abstract
Upper extremity deep venous thrombosis (UEDVT), though less common than lower extremity DVT, is a significant problem with several possible etiologies. The incidence of UEDVT is on the rise, primarily from the increasing use of central venous access devices. However, there are other causes of UEDVT, including primary venous thrombosis (Paget-Schroetter syndrome) and hypercoagulable states associated with underlying malignancy. The morbidity and mortality associated with UEDVT is largely from pulmonary embolism and the postphlebitic syndrome. Nevertheless, many UEDVTs are asymptomatic or patients may present with nonspecific clinical symptoms; therefore, a high index of suspicion is often necessary to make a correct diagnosis. Currently, there is no standard treatment algorithm for UEDVT. Treatment options may range from systemic anticoagulation to surgical correction depending on the etiology of the thrombus, as well as the patient's associated comorbidities, life expectancy and expected quality of life following treatment.
Keywords: Upper extremity deep venous thrombosis, central venous access devices, Paget-Schroetter syndrome, malignant associated thrombosis, thrombolysis, mechanical thrombectomy, superior vena cava filters
Although still relatively uncommon, with an incidence of ~6 patients per 10,000-hospital admission, the number of patients diagnosed with upper extremity deep venous thrombosis (UEDVT) is on the rise, having more than doubled from the 1960s to the end of the last century.1,2,3 The etiology of UEDVT is overwhelmingly attributed to secondary causes, such as central venous access devices (CVADs) and malignancy, causing either obstruction or a hypercoagulable state. Primary UEDVT, also known as Paget-Schroetter syndrome, accounts for only a small number of cases of upper extremity thrombosis.
CVADs are currently the leading cause of UEDVT. In a review of the literature, Owens et al found that in patients diagnosed with UEDVT, there was an associated CVAD in 1,414 of 3,052 patients (46%).4 Although the exact reason for CVAD-associated thrombosis is not known, there are likely several contributing factors, such as vessel wall trauma during the catheter insertion, vessel trauma from medication infusion through the catheter, and impediment of blood flow from partial luminal obstruction by the catheter.5 Catheter tip position also seems to have a role. In one study, UEDVT developed in only 5 of 87 patients (6%) with catheter tip placed in the right atrium or superior vena cava (SVC), whereas 12 of 26 patients (46%) had catheter-associated thrombosis with the catheter tip placed in the innominate vein or junction of the innominate vein and SVC.6
Underlying malignancy is the second most common risk factor for the development of thrombosis, accounting for 25 to 29% of patients presenting with underlying UEDVT.4,7 The most common malignancies that contribute to thrombus formation are lung cancer and lymphoma. A patient presenting with UEDVT without a history of CVAD may require cross-sectional imaging to evaluate for an occult malignancy given the common association. In cases where no malignancy or history of central venous catheters exists, the patient should be screened for underlying coagulation disorders, and family or personal history of DVT.5
Primary upper extremity venous thrombosis, or Paget-Schroetter syndrome, is typically the result of external venous compression, classically occurring at the thoracic outlet seen in otherwise healthy, typically younger individuals who present with UEDVT. This syndrome has a male predisposition, with a majority of cases (60%) occurring in the dominant arm. It is characteristically seen in patients who are athletes or who have a muscular build whose daily activities include heavy lifting or actions that involve complete abduction of their arms.5,8,9 Obstruction is typically due to compression of the vein between the first rib and a hypertrophied muscle or tendon or compression of the vein by an anomalous cervical rib. Repeated compression causes microtrauma to the intimal wall of the vessel that then initiates the coagulation cascade.5 In young athletic patients, where no malignancy or history of central venous catheters who have a high likelihood for primary upper extremity venous thrombosis, the patient should undergo a full hypercoagulable workup.5 In addition, these patients should have the contralateral limb evaluated because abnormalities that can cause vessel wall injury that lead to obstruction are frequently bilateral.10
PRESENTATION
Many UEDVTs are asymptomatic making detection difficult, if not impossible. The lack of detection may also be causing underestimation of the number of occurrences of thrombosis. Symptomatic UEDVT can present with mild symptoms such as unilateral hand edema, nonspecific shoulder discomfort or pain, and tenderness in the base of the neck due to an inflammatory response incited by the thrombus. Patients with Paget-Schroetter syndrome may present with increased symptoms following vigorous use of the arm. In addition, the patient may experience pain radiating into the fourth and fifth digits from brachial plexus injury, also caused by external compression.5,6,11 Due to the lack of specificity of these symptoms, a high index of clinical suspicion may be needed to diagnose an upper extremity thrombus.6 Patients with catheter-related UEDVT diagnosis may be further complicated by the increased number of comorbidities as compared with the subset of patients with primary UEDVT, who are otherwise healthy.
Though not as commonly seen as with lower extremity DVT, pulmonary embolism (PE) complicates UEDVT in ~12 to 20% of cases.6,12 It is estimated that up to 10% of all cases of venous thromboembolisms are due to upper extremity venous thrombosis.13 Nevertheless, lower extremity deep venous thrombi (LEDVTs) are 14.7 times more likely to occur and are 4.6 times more likely to cause PE than an upper extremity thrombus.4 Evidence suggests that the mortality of patients after the diagnosis of secondary UEDVT is higher (16% and 34% at 1 and 3 months, respectively) than those with primary UEDVT, but the cause of death has usually been related to other underlying illnesses rather than from pulmonary thromboembolic disease.14
When a patient is suspected of having an UEDVT, the next step in the workup is typically imaging. Duplex ultrasonography is often the first test because it is inexpensive, noninvasive and readily available. Noncompressibility of the vessel lumen is the best criterion for positive DVT by ultrasound. Ultrasound does have its limitations, including a relative lack of sensitivity compared with other, more-invasive examinations as well as difficulty visualizing the more central veins. In these situations, cross-sectional imaging or venography may be required when there is a high index of clinical suspicion.6
Computerized tomographic venography (CTV) and magnetic resonance venography (MRV) are noninvasive imaging techniques available for the diagnosis of UEDVT but have been deemed less appropriate than ultrasound by the American College of Radiology.15 The advantages of cross-sectional imaging over ultrasound evaluation include the ability to image the central venous system as well as the ability to fully visualize even thrombosed veins. This improved visualization comes at a cost: both CTV and MRV are more expensive than either ultrasound or subtraction venography. There are other disadvantages as well. MRV may require long examination times and may be limited when imaging the peripheral veins of the arm because the arm is at the periphery of the magnetic field.15 CTV, on the other hand, may require the use of large volumes of nephrotoxic contrast and uses high doses of ionizing radiation. To date, studies have been mixed on the effectiveness of MRV when compared with traditional venography, whereas small studies are available showing similar performance of CTV to traditional venography. However, there are currently no large studies examining the diagnostic accuracy of either test.15
Venography can also be used to diagnose UEDVT. This is more invasive than the aforementioned tests, but has the added benefit of allowing one to treat in the same setting. One potential difficulty in performing venography is challenging intravenous (IV) access in an affected limb with marked edema,16 a situation that can typically be overcome by using ultrasound guidance for IV access. Also, venography can provide information regarding collateral flow and the presence of venous anomalies as well as providing information for surgical planning when necessary.11
TREATMENT
There is currently no established best-treatment algorithm for UEDVT.17 For catheter-related thrombosis, removal of the venous access device may be considered. Rapid improvement in symptoms has been seen with combinations of removal of the thrombogenic stimulus and anticoagulation.18,19,20,21 However, Lee et al argue that in patients who continue to require central venous access in the face of associated thrombus that the catheter should be left in place. It has not been definitively shown that catheter removal is associated with improved outcome. Also, they believe that the catheter should be left in place because of the associated risks and additional costs of new catheter site placement.17 May et al believe that catheter removal may be a risk factor for PE because catheter removal can theoretically dislodge any formed fibrin sheath or thrombus from the vessel wall, resulting in an embolus.22 However, it is unlikely that a fibrin sheath would constitute a significant embolic burden to the lungs, unless it were very large or the patient had extremely poor pulmonary reserve. Provided the catheter is not a medical necessity and removing it would not immediately jeopardize the patient's wellbeing, catheter removal seems to be a logical course of action. Whether or not the catheter is removed, systemic anticoagulation should be instituted provided there are no contraindications.23
Anticoagulation has been proven to decrease the incidence of PE, prevent clot propagation. as well as facilitate the patency of existing venous collaterals.14,24 The length of time that the patient will require anticoagulation will be influenced by the underlying etiology of the thrombosis. Maintaining patients on anticoagulation for at least 6 months is the standard of practice for patients presenting with LEDVT; therefore, provided that there are no contraindications, it seems reasonable to conservatively treat UEDVT in a similar fashion.6 If the catheter is left in place, Lee et al recommend anticoagulation for an additional 3 months.17 Vitamin K antagonists, such as warfarin, have been the primary medication for anticoagulation since the 1950s. Typically, doses are adjusted based on the patient's international normalized ratio (INR) to achieve a value of 2.0–3.0. Standard doses range between 1–10 mg per day for 6 months.17,25
Although there is no agreement of the best therapeutic approach for young patients who develop UEDVT, given their longer life expectancies and fewer comorbidities a more aggressive treatment approach may be justified to prevent future problems, such as postphlebitic syndrome. Using a more aggressive approach may reduce the risk of valvular damage, which is thought to be a primary component to the development of postphlebitic syndrome.11,17 Ideal candidates for this line of therapy are young, otherwise healthy patients with primary UEDVT. Other patients to consider for a more aggressive treatment approach are young patients with catheter-related UEDVT, those with symptomatic SVC syndrome or those whose conditions mandate preservation of their central venous catheter.5
Catheter-Directed Thrombolysis
Indications for catheter-directed thrombolysis (CDTL) are a clot less than 14 days in duration or acute phlegmasia cerulea dolens in patients with no contraindications to thrombolytic therapy.26 A clot present for more than 14 days leads to thrombus organization that limits the effectiveness of thrombolysis.25,27,28 When performing CDTL, an ipsilateral upper extremity venous access is typically used. If this is not possible, a vein from the contralateral arm or a femoral approach may be necessary, but would require retrograde passage of catheters and wires, which can be technically challenging. Once venous access is obtained, an upper extremity venogram is performed to evaluate the extent of thrombosis. Once the lesion is crossed with a guidewire, an infusion catheter is placed as close as possible to the thrombus because collateral circulation can dilute the thrombolytic medication if placed too proximal or distal to the thrombus.27 At our institution, the most common thrombolytic agent used is tissue plasminogen activator (tPA; Genentech, Inc., San Francisco, CA). The tPA is generally administered as a continuous infusion of 0.5–1 mg per hour for at least 8 hours (an initial bolus can also be infused at the physicians discretion) (Fig. 1). The patient should have laboratories drawn every 6–8 hours to monitor fibrinogen levels, which should be kept above 100 mg/dL to avoid depletion. Fibrinogen levels below 100 mg/dL can increase the patient's likelihood of a major hemorrhagic complication.26 During the thrombolytic infusion, the patient may be placed on a sub therapeutic heparin infusion (keeping the thromboplastin time between 40 and 60 seconds) to avoid catheter-related thrombosis.26 Repeat venography is performed at serial intervals, ranging from 8–24 hours, to assess for residual thrombus. During follow-up venography, additional interventions, such as balloon angioplasty, thrombectomy, or percutaneous stent placement, may be necessary to correct underlying venous stenosis and reduce the risk for recurrent thrombosis. Upon termination of the procedure, the patients are systemically anticoagulated with warfarin for 6 months.26
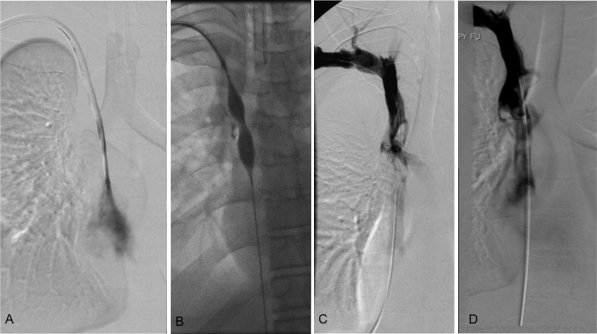
Figure 1.
(A) Digital subtraction venography through a dialysis catheter from a 19-year-old man with bilateral upper extremity as well as facial edema shows the presence of a fibrin sheath. (B) The fibrin sheath was macerated using a 12 mm × 4 cm balloon. (C) Repeat venogram following balloon venoplasty shows a large, acute thrombus in the lower superior vena cava. An infusion catheter was placed across the thrombus and the patient was admitted to the intensive care unit for catheter-directed thrombolysis. (D) Follow-up venogram after a 12-hour infusion of tissue plasminogen activator shows persistent, but decreased clot burden in the superior vena cava. The patient had some immediate postprocedural relief and was started on anticoagulation. Swelling resolved completely upon catheter removal.
The two major complications associated with CDTL are bleeding and PE, which are reported to occur in ~0 to 17% and 0.9% patients, respectively. It appears that there is a direct correlation between infusion time and the risk of bleeding due to the higher cumulative dose of thrombolytic delivered over time.26 Mewissen et al, in a large multicenter venous registry study, outlined several drawbacks with CDTL when used in the lower extremities.29 In this registry, up to 11% of patients being treated had complications and the average time for the procedure was 53.4 hours. These long infusion times are often difficult for patients to tolerate and may result in higher complication rates. In addition, the associated costs of CDTL must be considered, including the cost of a monitoring bed, thrombolytic infusions, and numerous laboratory studies.
For patients with Paget-Schroetter syndrome, thrombolysis and clot resolution alone are generally not adequate as a stand-alone treatment because there is a source of external compression that must be further managed. For patients suspected of having thoracic outlet compression, CDTL is often the first step in identifying any causative lesion. Once the vein patency has been restored, venography of the upper extremity can be performed with the patient in a neutral position and a provocative position to demonstrate venous compression (Fig. 2). Venography of the contralateral arm may be considered as there is a high association of bilateral disease.
Figure 2.
Right upper extremity venogram in a 31-year-old woman with chronic swelling with exercise. Top image is the venogram with the arm in a neutral position that shows stenosis of the subclavian vein at the thoracic outlet. Bottom image shows the venogram with the arm abducted (provocative maneuver), in this image, there is complete occlusion of the subclavian vein at the thoracic outlet. Patient underwent thoracic outlet decompression; repeat venogram showed resolution of the compression without residual stenosis.
Percutaneous Mechanical Thrombectomy
To date, there has yet to be a large multicenter trial studying the effectiveness of percutaneous mechanical thrombectomy for the treatment of acute UEDVT. However, multiple case reports and small retrospective studies have shown pharmacomechanical thrombectomy to be effective in both the upper and the lower extremities.30,31 In a study by O'Sullivan et al using the Trellis™ isolated thrombolysis catheter (Bacchus Vascular, Santa Clara, CA), all 22 patients studied had restoration of flow in the lower extremities; only one patient had less than 50% of their clot removed by the device. In the majority of the patients, 82% had between 50 to 95% of the clot burden removed; three patients had > 95% of their clot burden removed. Advantages to this device include markedly reduced treatment time with an average treatment time of only 91 minutes per affected limb compared with multiple hours for CDTL. In addition, O'Sullivan et al reported no major complications.30 Similar results were reported for the Trellis™ device by Hilleman et al, who found a greater than 50% clot resolution in 93% of patients treated with mechanical thrombectomy compared with only 79% in those treated with CDTL. They also reported a cost savings of approximately $1775 with mechanical thrombectomy compared with the overall cost of CDTL.32
SUPERIOR VENA CAVA FILTERS
Indications for SVC filter placement are failure or contraindication to therapeutic anticoagulation or for presurgical prophylaxis in the setting of substantial thromboembolic risk factors. Placement of SVC filters remains controversial because there has not been a well-documented study with routine imaging and surveillance of patients with PE and UEDVT that excludes the lower extremity as the source of the presenting PE.4 When possible, the procedure should be performed from a common femoral vein approach. Similar to inferior vena cava (IVC) filter placement, SVC filter placement should begin with superior vena cavograms to exclude underlying venous stenosis and to document SVC patency.33 Theoretically, though yet unproven, it is believed that patients with some degree of SVC stenosis on venogram are at a decreased risk for PE because the stenosis could potentially halt the progress of a large embolism through the stenotic vessel lumen.34 However, any vascular stenosis can impede blood return and eventually be the cause of thrombus formation. Ideal filter placement has been described with the filter hooks within the SVC at the confluence of the right and left brachiocephalic veins with the apex of the filter directed toward the right atrium. Placement in this location helps prevent the azygous vein from associated thromboembolic complications.12,33 The two SVC filters that have been most extensively studied are the Greenfield filter (Boston Scientific, Watertown, MA) and the TrapEase® filter (Cordis, Miami Lakes, FL).4 Because of the large numbers of each of these filters used when compared with a very limited number of other filter types studied in the SVC, it is difficult to determine the superiority of one filter over another. One stated advantage of the Greenfield filter is its smaller “caval footprint,” which is the surface area over which the hooks of the filter make contact with the caval wall in a craniocaudal dimension. This is important given the shorter “safe landing” zone for filter deployment in the SVC relative to the much larger IVC. However, in a literature review by Owens et al, there were no reported recurrent pulmonary emboli after filter placement in 209 patients.4
Complications of SVC filter placement are rare, but potentially more severe compared with IVC filter placement. Of the 209 patients in the review by Owens et al, only 8 of 209 patients (3%) had reported major filter-related complications. These complications included SVC perforation (n = 6), cardiac tamponade (n = 4), aortic perforation (n = 2), SVC thrombosis (n = 2), and pneumothorax (n = 1). In the case of strut perforation, there is little to no structure in the mediastinum equivalent to the retroperitoneal cavity to provide any form of barrier to protect surrounding organs. Whereas strut perforation in the IVC occurs often without consequence, perforation through the wall of the SVC may result in injury to the lung, aorta, pulmonary artery, heart, or pericardium.
Filter migration is also a concern, given the close proximity to the heart. However, this appears to be unwarranted. Spence et al reported no cases of filter migrations in 41 patients.12 Because SVC filters may result in SVC occlusion and do not prevent thrombus propagation, anticoagulation should be instituted or reinstituted in all possible cases. Also, SVC filter placement should not preclude central line placement, but it is recommended that straight-tipped wires be used preferentially over j-tipped wires to avoid inadvertent dislodgement of the filter when placing the catheter (Fig. 3).35,36
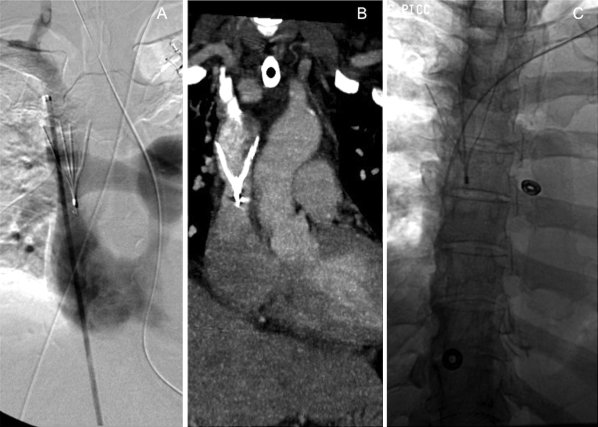
Figure 3.
A 55-year-old woman with a bilateral upper extremity deep vein thrombosis and intracranial hemorrhage. (A) A Celect™ filter (Cook, Bloomington, IN) was deployed from a right femoral vein approach. Venogram immediately following superior vena cava (SVC) filter placement shows the filter legs at the junction of the right and left brachiocephalic veins and the tip near the SVC/right atrial junction. (B) Coronal reconstruction from computed tomography of the chest shows the filter in place in the SVC.(C) Later in the patient's hospital course, a PICC line was successfully placed through the SVC filter without complication.
CONCLUSION
Although still uncommon, the incidence of UEDVT will undoubtedly continue to rise as the use of CVAD becomes increasingly common. Detection of UEDVT requires a high index of clinical suspicion as symptoms tend to be nonspecific. Even though no gold-standard treatment exists, multiple treatment strategies are available and are similar to established strategies for LE-DVT. Factors determining what treatment should be used depend on multiple factors, including underlying etiology of the UEDVT, the patient's age, comorbidities, quality of life, and potential contraindications to certain treatment regimens.
References
- Monreal M, Lafoz E, Ruiz J, Valls R, Alastrue A. Upper-extremity deep venous thrombosis and pulmonary embolism. A prospective study. Chest. 1991;99(2):280–283. doi: 10.1378/chest.99.2.280. [DOI] [PubMed] [Google Scholar]
- Coon W W, Willis P W., III Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657–663. doi: 10.1001/archsurg.1967.01330110073010. [DOI] [PubMed] [Google Scholar]
- Otten T R, Stein P D, Patel K C, Mustafa S, Silbergleit A. Thromboembolic disease involving the superior vena cava and brachiocephalic veins. Chest. 2003;123(3):809–812. doi: 10.1378/chest.123.3.809. [DOI] [PubMed] [Google Scholar]
- Owens C A, Bui J T, Knuttinen M G, Gaba R C, Carrillo T C. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21(6):779–787. doi: 10.1016/j.jvir.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Joffe H V, Goldhaber S Z. Upper-extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880. doi: 10.1161/01.cir.0000031705.57473.1c. [DOI] [PubMed] [Google Scholar]
- Haire W D. Available at: Catheter-induced upper extremity venous thrombosis. http://www.udtol.org. Accessed July 19, 2010. http://www.udtol.org
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455–457. doi: 10.1097/00001721-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Becker D M, Philbrick J T, Walker F B., IV Axillary and subclavian venous thrombosis. Prognosis and treatment. Arch Intern Med. 1991;151(10):1934–1943. [PubMed] [Google Scholar]
- Prandoni P, Bernardi E. Upper extremity deep vein thrombosis. Curr Opin Pulm Med. 1999;5(4):222–226. doi: 10.1097/00063198-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Machleder H I. The role of thrombolytic agents for acute subclavian vein thrombosis. Semin Vasc Surg. 1992;5:82. [Google Scholar]
- Haire W D. Available at: Spontaneous upper extremity venous thrombosis (Paget-Schroetter syndrome) http://www.udtol.org. Accessed July 19, 2010. http://www.udtol.org
- Spence L D, Gironta M G, Malde H M, Mickolick C T, Geisinger M A, Dolmatch B L. Acute upper extremity deep venous thrombosis: safety and effectiveness of superior vena caval filters. Radiology. 1999;210(1):53–58. doi: 10.1148/radiology.210.1.r99ja1353. [DOI] [PubMed] [Google Scholar]
- Joffe H V, Kucher N, Tapson V F, Goldhaber S Z, Deep Vein Thrombosis (DVT) FREE Steering Committee Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26(5):853–860. doi: 10.1016/s0741-5214(97)70100-9. [DOI] [PubMed] [Google Scholar]
- Yucel E K, Oldan J D, Rybicki F J, et al. Available at: ACR Appropriateness Criteria suspected upper-extremity deep vein thrombosis. [Online publication]. Reston, VA: American College of Radiology (ACR); 2008. http://www.acr.org/secondarymainmenucategories/quality_safety/app_criteria/pdf/vascular/suspectedupperextremitydeepveinthrombosisdoc20.aspx. Accessed October 17, 2010. http://www.acr.org/secondarymainmenucategories/quality_safety/app_criteria/pdf/vascular/suspectedupperextremitydeepveinthrombosisdoc20.aspx
- Martin E C, Koser M, Gordon D H. Venography in axillary-subclavian vein thrombosis. Cardiovasc Radiol. 1979;2(4):261–266. doi: 10.1007/BF02552073. [DOI] [PubMed] [Google Scholar]
- Lee A Y, Ginsberg J S. Venous thrombosis of the upper extremities. Curr Treat Options Cardiovasc Med. 2001;3(3):207–214. doi: 10.1007/s11936-001-0039-0. [DOI] [PubMed] [Google Scholar]
- Donayre C E, White G H, Mehringer S M, Wilson S E. Pathogenesis determines late morbidity of axillosubclavian vein thrombosis. Am J Surg. 1986;152(2):179–184. doi: 10.1016/0002-9610(86)90238-2. [DOI] [PubMed] [Google Scholar]
- Haire W D, Lieberman R P, Edney J A, et al. Hickman catheter-induced thoracic vein thrombosis. Frequency and long-term sequelae in patients receiving high-dose chemotherapy and marrow transplantation. Cancer. 1990;66(5):900–908. doi: 10.1002/1097-0142(19900901)66:5<900::aid-cncr2820660515>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Moss J F, Wagman L D, Riihimaki D U, Terz J J. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. JPEN J Parenter Enteral Nutr. 1989;13(4):397–400. doi: 10.1177/0148607189013004397. [DOI] [PubMed] [Google Scholar]
- Smith V C, Hallett J W., Jr Subclavian vein thrombosis during prolonged catheterization for parenteral nutrition: early management and long-term follow-up. South Med J. 1983;76(5):603–606. doi: 10.1097/00007611-198305000-00018. [DOI] [PubMed] [Google Scholar]
- Mayo D J. Catheter-related thrombosis. J Intraven Nurs. 2001;24:S13–S22. [Google Scholar]
- Lokich J J, Bothe A, Jr,Benotti P, Moore C. Complications and management of implanted venous access catheters. J Clin Oncol. 1985;3(5):710–717. doi: 10.1200/JCO.1985.3.5.710. [DOI] [PubMed] [Google Scholar]
- Swinton N W, Jr, Edgett J W, Jr, Hall R J. Primary subclavian-axillary vein thrombosis. Circulation. 1968;38(4):737–745. doi: 10.1161/01.cir.38.4.737. [DOI] [PubMed] [Google Scholar]
- Hicken G J, Ameli F M. Management of subclavian-axillary vein thrombosis: a review. Can J Surg. 1998;41(1):13–25. [PMC free article] [PubMed] [Google Scholar]
- Kandarpa K, Aruny J. Handbook of Interventional Radiologic Procedures. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2002.
- Fraschini G, Jadeja J, Lawson M, Holmes F A, Carrasco H C, Wallace S. Local infusion of urokinase for the lysis of thrombosis associated with permanent central venous catheters in cancer patients. J Clin Oncol. 1987;5(4):672–678. doi: 10.1200/JCO.1987.5.4.672. [DOI] [PubMed] [Google Scholar]
- Chang R, Horne M K, III, Mayo D J, Doppman J L. Pulse-spray treatment of subclavian and jugular venous thrombi with recombinant tissue plasminogen activator. J Vasc Interv Radiol. 1996;7(6):845–851. doi: 10.1016/s1051-0443(96)70858-8. [DOI] [PubMed] [Google Scholar]
- Mewissen M W, Seabrook G R, Meissner M H, Cynamon J, Labropoulos N, Haughton S H. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- O'Sullivan G J, Lohan D G, Gough N, Cronin C G, Kee S T. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol. 2007;18(6):715–724. doi: 10.1016/j.jvir.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Arko F R, Cipriano P, Lee E, Filis K A, Zarins C K, Fogarty T J. Treatment of axillosubclavian vein thrombosis: a novel technique for rapid removal of clot using low-dose thrombolysis. J Endovasc Ther. 2003;10(4):733–738. doi: 10.1177/152660280301000408. [DOI] [PubMed] [Google Scholar]
- Hilleman D E, Razavi M K. Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J Vasc Interv Radiol. 2008;19(3):377–383. doi: 10.1016/j.jvir.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Pais S O, De Orchis D F, Mirvis S E. Superior vena caval placement of a Kimray-Greenfield filter. Radiology. 1987;165(2):385–386. doi: 10.1148/radiology.165.2.3659362. [DOI] [PubMed] [Google Scholar]
- Usoh F, Hingorani A, Ascher E, et al. Long-term follow-up for superior vena cava filter placement. Ann Vasc Surg. 2009;23(3):350–354. doi: 10.1016/j.avsg.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Ascher E, Hingorani A, Tsemekhin B, Yorkovich W, Gunduz Y. Lessons learned from a 6-year clinical experience with superior vena cava Greenfield filters. J Vasc Surg. 2000;32(5):881–887. doi: 10.1067/mva.2000.110883. [DOI] [PubMed] [Google Scholar]
- Kaufman J A, Thomas J W, Geller S C, Rivitz S M, Waltman A C. Guide-wire entrapment by inferior vena caval filters: in vitro evaluation. Radiology. 1996;198(1):71–76. doi: 10.1148/radiology.198.1.8539409. [DOI] [PubMed] [Google Scholar]