Abstract
Bronchial artery angiography with embolization has become a mainstay in the treatment of hemoptysis. Major complications are rare and immediate clinical success defined as cessation of hemorrhage ranges in most series from 85% to 100%, although recurrence of hemorrhage ranges from 10% to 33%. Bronchial artery embolization offers a minimally invasive procedure for even the most compromised patient serving as first-line treatment for hemorrhage as well as providing a bridge to more definitive medical or surgical intervention focused upon the etiology of the hemorrhage. The aim of this article is to summarize the etiologies, pathophysiology, and the diagnostic and management strategies of hemoptysis as related to bronchial artery embolization. In addition, the techniques of arteriography and embolization as well as associated procedural outcomes and complications are delineated.
Keywords: Hemoptysis, angiography, bronchial artery, arterial embolization
Despite continued technologic progress, including advances in medical imaging, hemoptysis remains an important clinical and potentially grave condition.1 Given its diverse inflammatory, neoplastic, and vascular etiologies, precise anatomic localization of hemorrhage poses a challenge for all clinicians engaged in its evaluation and management.2 Any hemorrhage resulting in compromise of pulmonary or hemodynamic status should be considered substantial necessitating therapeutic intervention; however, massive hemoptysis comprises only 1.5% of reported cases.3 Although useful guidelines are present in the literature for cases absent clinical compromise, no consensus has yet been reached providing a clear delineation between massive and nonmassive hemoptysis. Reported volumes defining massive hemoptysis range from 200 to 1000 mL over a 24-hour interval, but volume documented as > 300 mL appears to be most frequently accepted.4,5,6,7 In addition, particular attention must be paid to patients with chronic hemoptysis averaging > 100 mL per day for 3 or more days.8
Historically, surgery had been the definitive therapy. Unfortunately, surgical intervention carries a mortality of ~18% when performed electively, rising to 40% when performed emergently.9 Conversely, a more conservative approach to therapy including observation and management with medication has been shown to carry a mortality risk of at least 50%.4 This is understandable given that the patient population predisposed to hemoptysis often additionally possess antecedent medical conditions further compromising the patient's situation. Bronchial artery embolization offers a minimally invasive procedure, which can potentially be offered to even the most compromised patient serving as first-line treatment for hemorrhage as well as providing a bridge to more definitive medical or nonemergent surgical intervention focused upon the etiology of the hemorrhage.
The aim of this article is to outline the etiologies, pathophysiology, diagnostic evaluations, and management strategies of hemoptysis, and outcomes of bronchial artery embolization. In addition, the techniques of arteriography and embolization, pertinent bronchial and systemic anatomy, as well as associated procedural complications are highlighted.
PATHOPHYSIOLOGY OF HEMOPTYSIS
Worldwide, the most common cause of hemoptysis remains active tuberculosis (Table 1).6,10 In developed countries such as the United States, hemoptysis most often occurs in the setting of chronic inflammatory processes including infectious (tuberculosis, aspergillosis) and noninfectious (cystic fibrosis, bronchiectasis) etiologies. The leading causes of noninflammatory hemoptysis in the United States are bronchogenic carcinoma and congenital heart disease. A rare situation arises where the offending agent or process cannot be well elucidated. These are termed cryptogenic, and are most commonly encountered in the smoking population, accounting for up to 42% of hemoptysis complaints.11,12
Table 1.
Etiologies of Hemoptysis
| Pulmonary Causes |
| Airways disease |
| Bronchiectasis |
| Bronchogenic tumor |
| Bronchitis |
| Parenchymal disease |
| Cystic fibrosis |
| Tuberculosis |
| Aspergillosis |
| Histoplasmosis |
| Pneumonia |
| Lung abscess |
| Pneumoconiosis |
| Vasculitides (Behcet's syndrome, Wegener's granulomatosis) |
| Cardiovascular causes |
| Pulmonary arteriovenous malformation |
| Bronchial artery aneurysm |
| Pulmonary embolism |
| Pulmonary artery hypertension |
| Ruptured thoracic artery aneurysm |
| Aortobronchial fistula |
| Other |
| Trauma |
| Coagulopathy |
| Iatrogenic |
| Foreign body |
In the majority of instances, hemoptysis arises from the bronchial arteries due to changes in the terminal vascular bed leading to alteration in normal flow and distribution. In inflammatory conditions, multiple factors including localized hypoxia, contribute to a decrease in pulmonary arterial perfusion. In the setting of chronic inflammation, the liberation of numerous angiogenic growth factors promotes in-growth and the development of neovascularity from the bronchial arteries to compensate for the depressed pulmonary arterial flow.13 Neoplastic cells express similar chemotactic substances to promote extension of arterial supply with their associated proliferation and metabolic demands. Systemic vasculature may also be recruited in a similar fashion, especially where tumor or inflammation is in close approximation to the pleural surfaces and/or reflections. Wherever collateral flow is recruited, the vascular walls are abnormally thin. This fragility in association with increased local blood pressure from exposure to systemic flow, and a tendency for pseudoaneurysm formation predisposes the neovasculature to rupture, resulting in potentially massive hemoptysis.14 Infection arising in the region of proliferated bronchial arteries may also enhance rupture of these friable vessels.
More uncommon underlying disorders can also result in hemoptysis (Table 1). With congenital heart disease, frequently the decrease in overall pulmonary arterial perfusion leads to proliferation of bronchial and systemic arteries. Additionally, the pulmonary artery as well as the aorta (i.e., aortobronchial fistula) may contribute to hemoptysis, but accounts for ~5% of instances of massive hemoptysis.
DIAGNOSTIC EVALUATION AND CLINICAL MANAGEMENT OF HEMOPTYSIS
Localization and treatment of hemoptysis demands a multifaceted evaluation involving medical, radiologic, and surgical disciplines.2 As with any patient, initiation of workup should commence with a thorough history and physical examination. Signs or symptoms of infection, vasculitis, granulomatous disease, or airway disease may assist in narrowing the differential diagnosis. Often however, the required extensive evaluation may have to take a backseat to controlling the hemorrhage.
In the management of moderate and massive hemoptysis, the airways as well as hemodynamic status require prompt assessment and appropriate management. In particular, if a side (right or left) of hemorrhage is localized, every attempt to protect the contralateral lung from exposure to aspiration should be made. Laboratory inquiries should be undertaken and blood typing and cross-matching performed if necessity is anticipated. The assessment of the patient's appropriateness for interventional or even surgical treatment should then follow.
Due to its convenience and portability in the acutely ill patient, chest radiography remains a basic and useful diagnostic tool in the evaluation of hemoptysis. The ability of chest radiography to accurately localize the disease process is highly variable, and can be normal in up to 30% of patients.15,16,17 Localization can be particularly difficult due to either opacification of both lungs during episodes of massive hemoptysis or in the setting of bilateral disease.
Bronchoscopy is an excellent diagnostic approach to localize and potentially treat the source of hemoptysis.10,18 Traditionally, rigid bronchoscopy is preferred due its superior ability to maintain the airway and provide a robust lumen for suction of large volume hemoptysis; however, it is limited by decreased portability and the requirement for general anesthesia. Flexible, or fiberoptic bronchoscopy is readily available at the bedside, retains the ability to instill medications or perform other therapeutic interventions, and does not necessitate anesthesia making it the preferred bronchoscopic technique as shown in a recent survey of pulmonologists that found 79% preferred the flexible to the rigid bronchoscope.1 Bronchoscopy, however, is plagued by a frequent inability to identify the underlying cause of hemoptysis, and fails to localize the bleeding site in 50% of examinations.17,19 To minimize the number of inconclusive bronchoscopic examinations, early examination (i.e., within the first 24 hours) is advocated, whereas in cases of massive hemoptysis more urgent evaluation is promoted.1 Notably, when the cause and location of hemoptysis can be determined radiographically, Hsiao et al observed no further benefit associated with bronchoscopy in patients considered acceptable for bronchial artery embolization.19
Multidetector computed tomography (CT) is a developing imaging technique for both the localization of hemorrhage and identification of the causative etiology of hemoptysis. The rapid acquisition time possible with solitary breath-hold allows for imaging of a greater volume dataset, with decreased susceptibility to motion artifacts. Isotropic volume acquisitions also provide the ability to construct elegant two-dimensional and three-dimensional (3D) multiplanar reconstructions in addition to maximal intensity projections, which aid in a more thorough characterization of the thoracic vascular system. There are studies suggesting that multidetector CT may be more accurate than arteriography at delineating the origin and course of both the bronchial and nonbronchial systemic arteries, especially when combined with 3D reconstructions.20,21 Although these and several other studies herald its benefits in the evaluation of hemoptysis, its use has not been widely accepted and at present is not a part of most standard evaluation protocols prior to bronchial artery embolization.
BRONCHIAL ARTERY EMBOLOTHERAPY FOR HEMOPTYSIS
Anatomic Considerations
The bronchial and pulmonary arteries comprise a divided blood supply to the lungs. The bronchial arteries course in conjunction with these structures to the level of the respiratory bronchus, where their terminal branches achieve significant overlap with the pulmonary arterial circulation. Although less significant clinically with regards to hemoptysis, the pulmonary artery provides the vast majority of pulmonary perfusion at ~99%, but there is significant overlap between the bronchial arteries and the pulmonary arteries at multiple levels throughout the lung's anatomic structure.22 In addition, nonbronchial systemic arteries are common offenders in the patient with hemoptysis. This obviously necessitates a thorough understanding of the various anatomic permutations and their associated potential clinical significance when considering bronchial artery embolization.
BRONCHIAL ARTERIES
The bronchial arterial distribution not only supplies the bronchi and interstitium of the lung, but also contributes to the visceral pleura, the aortic and pulmonary artery vasa vasorum, mediastinum, and middle one-third of the esophagus. The bronchial arteries vary considerably in their site of origin and subsequent branching pattern (Fig. 1). As seen angiographically, the bronchial arteries arise from the descending thoracic aorta between the upper T5 to the lower T6 vertebral bodies in 70% of the population (Fig. 2).6,23 Another 10% remain a first-order branch of the thoracic aorta or arch, but outside of the T5–T6 confines. The remaining 20% originate from a variety of structures including the thoracic (brachiocephalic, subclavian, internal mammary, pericardiophrenic, or thyrocervical) and abdominal (aorta, inferior phrenic, celiac) branches (Fig. 3).23,24 When originating from the aorta, the branching pattern exhibits several variations including four classic patterns as previously described by Cauldwell et al (Fig. 1).25 The type 1 configuration (40.6%) is comprised of a solitary right bronchial artery arising from an intercostobronchial trunk in conjunction with two left bronchial arteries of separate origin. Type 2 (21.3%) consists of a single right bronchial artery from an intercostobronchial trunk along with a single left bronchial artery. Type 3 has two right bronchial arteries, one of which is in conjunction with an intercostobronchial trunk, and two left bronchial arteries. Type 4 is two right bronchial arteries, one arising from an intercostobronchial trunk with a solitary left bronchial artery. The intercostobronchial trunk is the most frequently encountered vascular structure with a prevalence of at least 80%, and arises similar to solitary right bronchial arteries from the lateral or anterolateral surface of the aorta (Fig. 2).6,8,25,26 Left bronchial arteries tend to be located more anteriorly, and more commonly than the right bronchial arteries they extend onto the lesser curve of the aortic arch.
Figure 1.
The four most prevalent patterns of bronchial artery anatomy. Type I: single right bronchial artery via intercostobronchial trunk (ICBT), paired left bronchial arteries. Type II: single right bronchial artery via ICBT, single left bronchial artery. Type III: paired right bronchial arteries with one from ICBT, paired left bronchial arteries. Type IV: paired right bronchial arteries with one from ICBT, solitary left bronchial artery.
Figure 2.
(A) A 24-year-old man undergoing spinal angiography for hemorrhage. Thoracic aortogram shows single bronchial artery (arrow) supplying left and right sides. (B) Selective angiogram of the single bronchial artery from the aortogram shows normal bronchial artery distribution. A single trunk (arrowhead) gives rise to a right and left bronchial artery (arrows). Note the relatively small caliber of the normal arteries. (C) Normal bronchial angiogram in a 67-year-old man with right lung mass and hemoptysis following bronchoscopy. Angiography demonstrates a common origin (arrowhead) of the left (arrow) and right (arrow) bronchial arteries. Note the small caliber of the normal vessels. Given the patient's history, embolization with 150–250-μm polyvinyl alcohol particles was performed despite normal vessel appearance.
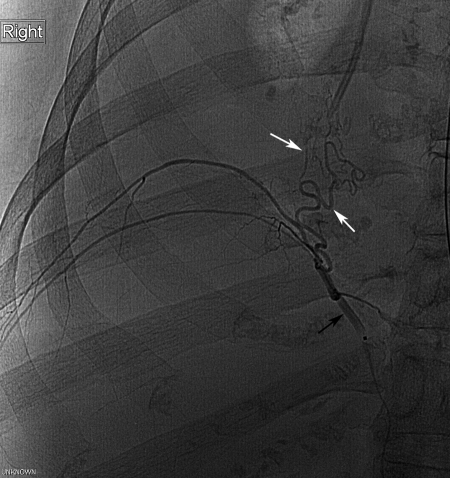
Figure 3.
A 66-year-old woman with sarcoidosis and hemoptysis. Only a very small right bronchial artery found on selective catheterization and aortography. Celiac artery arteriography showed mildly enlarged right phrenic artery. Subselective angiogram of the right phrenic artery (black arrow) shows arterial flow (white arrows) to the poorly aerated right lung base. This was successfully embolized using 250–350-μm polyvinyl alcohol particles.
Venous return from the bronchial arterial circulation is most often via the pulmonary veins, with smaller contributions from the superior vena cava, azygos, and hemiazygos systems (Fig. 4). This venous system is well visualized during bronchial angiography and the interventionist must determine if direct arteriovenous shunting is present.
Figure 4.
A 54-year-old man with known bronchiectasis and prior right upper lobe lung resection now presenting with aspergillosis and hemoptysis. (A) Early-phase arteriogram of the right bronchial artery (black arrow) showing abnormal hypervascularity (white arrows) of the diseased partially collapsed right lower lobe. (B) Late-phase arteriogram of the right bronchial artery showing venous drainage via the pulmonary veins (arrows). This bronchial artery was successfully embolized with 355–500-μm polyvinyl alcohol particles.
NONBRONCHIAL SYSTEMIC ARTERIES
Hemoptysis recalcitrant to percutaneous embolic therapy despite apparent adequate technique should raise the possibility of accessory, nonbronchial arterial involvement. This is often the case when bronchial artery embolization has been previously performed. As mentioned earlier, this arterial supply may originate from thoracic or abdominal vascular distributions (Fig. 3). Systemic nonbronchial collaterals can pose an angiographic dilemma, and must be differentiated from true aberrant bronchial arteries. The most reliable method to distinguish bronchial from systemic collaterals is through careful observation of the congruence of the vascular course with that of the associated bronchi. It is important to note that both ectopic and orthotopic bronchial arteries assume a more vertical or horizontal course prior to joining the bronchial tree. Systemic nonbronchial collateral arteries do not adhere to this pattern, instead following a transpleural course or potentially ascend via the inferior pulmonary ligament, never joining the bronchial tree. Aberrant anatomy may be more common in patients with cystic fibrosis, near 35% in one case series; it is as of yet unclear whether this is true of other chronic inflammatory conditions.27,29 Nonbronchial systemic collaterals should be investigated and treated concurrently with the hypertrophied bronchial arteries at the time of initial arteriogram when possible.29 Early recurrence of hemoptysis following an apparently, at least technically, successful bronchial artery embolization of typically configured bronchial arteries should prompt investigation of suspected systemic contribution.
The anterior spinal artery courses along the ventral surface of the spinal cord receiving collaterals from up to eight anterior segmental medullary arteries throughout its course (Fig. 5).22 Angiographically, these assume the classic “hairpin” configuration. The most prominent of these, the artery of Adamkiewicz, arises in the majority of cases from an intercostal artery at T8–L1 (Fig. 5). Contribution to one or more of these medullary arteries in the thorax is documented in 5–10% of cases involving the intercostal branch of an intercostobronchial trunk. The true incidence is unknown, however, and may in fact be over reported.6 Nontarget embolization of the medullary artery has been associated with transverse myelitis; therefore, meticulous technique with coaxial microcatheter approach distal to the origin of the artery should be undertaken.30
Figure 5.
(A) A 24-year-old man undergoing spinal angiography for hemorrhage, same patient as Fig. 2A. Injection of the left T12 intercostal artery demonstrates a prominent normal anterior spinal artery (artery of Adamkiewicz) (arrows). (B) A 24-year-old woman with cystic fibrosis and hemoptysis. Injection of the right supreme intercostal artery (black arrowhead) demonstrates a large, abnormal bronchial artery (white arrow) designating this as an intercostobronchial trunk. Note supply to the anterior spinal artery from the supreme intercostal arterial supply (black arrows). Embolization was performed in this patient beyond the origin of the supreme intercostal artery with the microcatheter placed at the level of the white arrow (see Fig. 8). Care was taken not to reflux particles into the supreme intercostal artery distribution (white arrowheads).
Embolotherapy Technique for Hemoptysis
Since its introduction in 1974, bronchial artery embolization is now considered by many to be first-line therapy.31 A recent survey of clinicians revealed 50% prefer an interventional radiology approach over observation or surgery when treating massive hemoptysis.1
ANGIOGRAPHY IN THE DIAGNOSIS OF HEMOPTYSIS
Digital subtraction arteriography prior to undergoing bronchial artery embolization is optimally undertaken utilizing radiographic units capable of high frame-rate acquisition. This allows for excellent delineation of both bronchial and non-bronchial systemic arteries. Angiography and intervention are performed under either moderate sedation or general anesthesia, as dictated by the clinical presentation and status of the patient.
Standard common femoral arterial access predominates although brachial artery access may be necessary to address extraordinarily difficult nonbronchial systemic arterial contributions. It is, however, felt to be associated with higher morbidity and complication rates.32,33,34,35 At our institution, all imaging and interventions are preferentially performed via a 5 French vascular access. All arteriography should be performed with either low-osmolar or iso-osmolar nonionic contrast material, as high-osmolar contrast has been implicated in transverse myelitis.30,36 Many advocate initial thoracic aortography to delineate the number, size, and position of the bronchial arteries (Fig. 5).6,7,31 This is particularly helpful in cases of aberrant or ectopic bronchial arteries.
Both normal and enlarged diameter bronchial arteries discovered via thoracic aortography should be investigated for signs of abnormality in the terminal vascular bed.6 Active extravasation, while extremely helpful and specific, occurs in up to only 10.7% of examinations.37 Absent identifying a bleeding site, findings sensitive for localization of hemoptysis are vascular hypertrophy and tortuosity, neovascularity, hypervascularity, aneurysm formation, and shunting (bronchial artery to pulmonary vein or bronchial artery to pulmonary artery) (Fig. 6). Generally accepted guidelines for abnormal bronchial artery diameter is > 3 mm, with normal vascular diameter typically 1.5 mm (Fig. 2).38 Combining chest CT findings with angiographic findings may further increase the sensitivity and specificity of localization of hemoptysis at angiography. Of particular importance is the presence of pleural thickening measuring 3 mm or greater adjacent to a parenchymal abnormality (Fig. 7). Extrapleural fat hypertrophy may also be present with enlarged vessels visualized in this expanded space.39,40
Figure 6.
A 52-year-old female with sarcoidosis, bronchiectasis, and cavitation with mycetoma. She presents with recurrent hemoptysis. (A) Aortogram (frontal view) shows enlarged left T6 intercostal artery (arrow) providing a tortuous hypervascular supply to abnormal tissue. In addition, a previously embolized left bronchial artery (black arrowhead) that has partially recanalized is also seen. Supply is also noted from a left supreme intercostal artery (white arrowhead). The patient has undergone previous successful coil embolization on the right (white arrow). Aortography nicely provides a working roadmap as it is useful to visualize bronchial arteries and collateral supply including supply from the intercostal arteries as shown here. (B) Left sixth intercostal arteriogram in the early phase shows collateral supply (black arrows) to abnormal left lung tissue. Note prior embolization coils (white arrows). (C) Left sixth intercostal arteriogram in a later phase shows branches of the pulmonary artery (arrows) consistent with shunting. As the shunting is through abnormal tissue with small vessels, this intercostal artery was successfully and safely embolized using larger (355–500 μm) polyvinyl alcohol particles.
Figure 7.
(A) 45-year-old man with sarcoidosis and aspergilloma presenting with hemoptysis. Single image from axial computed tomography shows pleural thickening (black arrow) and aspergilloma (white arrowhead), which was the etiology of this patient's hemoptysis. (B) Right third intercostal artery (arrow) gives rise to collaterals (arrowheads) supplying the aspergilloma. (C) Right third intercostal following successful embolization with 150–250-μm polyvinyl alcohol particles via a microcatheter (arrow). Note the lack of blood flow to the area of the mycetoma (arrowhead).
The use of microcatheters in a coaxial technique is now widespread, and its utility is well documented both for superselective angiography as well as for the administration of embolic agents (Fig. 8).6,41,42 This can be of benefit when the 5F catheter is unable to maintain secure access for diagnostic angiography, and of course for the delivery of embolic materials. When negotiating an intercostobronchial trunk with the microcatheter, special attention is paid to manipulation of the catheter beyond the intercostal moiety that may give rise to the aforementioned anterior spinal artery (Fig. 5). The injection method and rate should be selected based also on intraprocedural assessment of individual bronchial artery diameter and rate of blood flow. Hand injection of contrast through microcatheters is best executed with small-volume syringes capable of generating adequate pressures to achieve the flow rates necessary for satisfactory vascular opacification. Alternatively, power injection may be performed with attention to the maximal pressure tolerable by the individual microcatheter.
Figure 8.
(A) A 24-year-old woman with cystic fibrosis and hemoptysis, same patient as Fig. 5B. Chest radiograph shows bilateral opacities in this patient with cystic fibrosis. (B) Injection of the left supreme intercostal artery shows the enlarged bronchial artery (arrow). (C) A microcatheter (arrowhead) was placed beyond the intercostal branch, which contributes arterial supply to the anterior spinal artery (see Fig. 5B), and embolization was successfully performed using large (1000–1180 μm) polyvinyl alcohol particles. Larger particles were used to prevent migration into spinal artery supply should accidental reflux transpire, although care was taken not to reflux into the intercostal artery. (D) Postembolization angiogram of the right supreme intercostobronchial trunk. Note the very slow flow in the bronchial artery (arrow) and its distal branches (black arrowheads). Microcatheter tip is in the intercostobronchial trunk (white arrowhead). Note the excellent filling of the distal supreme intercostal artery, which supplied the anterior spinal artery in the lower cervical/upper thoracic region (Fig. 5B). Patient was neurologically intact following the procedure.
Transpleural angiogenesis occurs in the setting of chronic inflammatory or neoplastic conditions as well (Fig. 7). As previously noted, these can arise from thoracic and/or abdominal sources. Many have documented the benefit of performing a thorough investigation of these vessels at initial presentation, as the greatest impact of their presence is the potential to result in recurrent hemoptysis.6,7,43,44 Interrogation of the subclavian artery and its distribution or the abdominal vasculature should be made with selective end-hole catheters (Fig. 3).
It is well known that bronchial arteries comprise the vast majority of instances of hemoptysis. However, it has been reported that up to 5% of patients presenting with hemoptysis have the pulmonary artery as the offending vascular bed.45 In patients with disease known to result in direct pulmonary arterial injury such as tuberculosis, lung abscess, iatrogenic trauma, or malignancy, bronchial artery embolization may not achieve adequate clinical resolution.6,46 These subsets may present with hemoptysis classified as “early” recurrence, and when this arises both nonbronchial systemic as well as pulmonary arterial investigation should be performed. It is not uncommon that patients with hemoptysis of pulmonary arterial origin may require multiple interventions in the angiographic suite prior to definitive diagnosis and treatment.
Aneurysmal disease and pseudoaneurysm contribute to pulmonary arterial hemorrhage and hemoptysis. The classic situation is the finding of enhancing nodules along the periphery of cavitary lesions of a patient with known tuberculosis where hemoptysis should suggest the possibility of Rasmussen aneurysm.47 Aneurysmal rupture is possible and carries a high mortality rate, but is fortunately rare in developed countries due to the rarity of tuberculosis.46,47 Rarely, in a patient with hereditary hemorrhagic telangiectasia rupture of a congenital pulmonary arteriovenous malformation may result in hemoptysis.48
MATERIALS AND TECHNIQUES OF THE EMBOLIZATION OF HEMOPTYSIS
The interventional radiologist has at his or her disposal a variety of materials capable of achieving vascular occlusion. Considerations when choosing an embolic agent should include ease of delivery, durability of occlusion, propensity for recanalization, and size. Size depends clinically upon the site of desired vessel occlusion (proximal vs distal) as well as the catheter lumen used for delivery. The latter of course regards the use of microcatheters, which are available in a variety of lumen sizes. Regarding the former, utilization of materials of diminutive size results in very distal embolization occluding at the end-arteriolar level, which conceivably may result in ischemic complications to the bronchi, esophagus, or vascular structures.6 Alternatively, shunting of small embolic agents into the pulmonary venous system in effect places the embolic agent into the left heart with subsequent systemic arterial embolization. Alternatively, however, embolization with agents that occlude proximally may produce a suboptimal result due to the propensity to form collaterals around the occlusion site. As with all embolotherapy, the choice of agent is critical to the success and safety of the procedure.
Perhaps the most economical agent is absorbable gelatin sponge (Gelfoam®, Pharmacia & Upjohn, Kalamazoo, MI). However, the delivery of this material via a microcatheter is somewhat challenging and gelatin-sponge embolization results in temporary arterial occlusion, with a high rate of recanalization, potentially necessitating reembolization for recurrent hemoptysis.49
Polyvinyl alcohol (PVA) particles (e.g., Contour® PVA Embolization Particles, Boston Scientific, Natick, MA) are also readily available and relatively inexpensive (Fig. 8). Unlike the gelatin sponge, PVA particles do not undergo absorption and therefore theoretically provide a more durable vascular occlusion. The most common particle size for bronchial artery embolization ranges from 250–500 μm.42 Maintaining particulate size above a threshold of 325 μm theoretically ensures that no significant bronchopulmonary shunting will occur.22 Nonspherical PVA particles are, however, prone to clumping resulting in a more proximal occlusion than anticipated based solely on particle size.
Microspheres (e.g., Embosphere® Microspheres, BioSphere Medical, Rockland, MA) are composed of cross-linked gelatin, and have been utilized successfully, in particular for the embolization of uterine fibroids. Due to their smoothly spherical shape and hydrophilic nature, they are less prone to clumping and are more uniform in size than their PVA counterpart. In a recent study, bronchial artery embolization with 500–700 μm microspheres achieved short-term clinical success comparable to PVA particles.50
The use of liquid embolic agents such as n-butyl-2-cyanoacrylate (NBCA; e.g., TruFill® n-BCA Liquid Embolic System, Johnson & Johnson/DePuy, Raynham, MA) and ethylene vinyl alcohol polymer (Onyx® Liquid Embolic System, eV3 Neurovascular, Irvine, CA) for bronchial artery embolization have been infrequently reported. Utilization of NBCA requires expertise and knowledge in the art of varying the concentration to alter the rate of polymerization and the depth of vascular penetration. This, in conjunction with the risk of distal embolization with tissue necrosis and propensity for nontarget embolization, has relegated NBCA to a very peripheral role in bronchial artery embolization to date. In a recent study examining 25 patients who underwent bronchial artery embolization with NBCA, technical and clinical success was similar to standard particulate embolic agents. No major complications were noted, but 16% had prolonged chest pain or dysphagia perhaps due to distal embolization.51 Likewise, a recent case series of patients with hemoptysis undergoing embolization with ethylene vinyl alcohol polymer demonstrated a high rate of clinical success as both a first-line agent and for recalcitrant disease.52 Chest pain was the only documented complication in this series. Although these data are promising, the future utilization of these agents in the setting of hemoptysis remains unclear.
Metallic coils achieve a relatively proximal occlusion in the vascular bed. In this patient population with a high rate of rebleeding, this position within the vascular tree may jeopardize further embolic attempts. In addition, as with the gelatin sponge, proximal occlusion permits collateral flow resulting in poor control of hemoptysis (Fig. 9). Both pushable and detachable coils have been utilized. In a study comparing mechanically detachable coils to conventional coils, a lower rate of recurrence was noted with the detachable group.53 Data on the efficacy of coil embolization is scarce and dated, probably signifying that most do not employ the use of these agents for bronchial artery embolization today. Although not first-line therapy for hemoptysis per se, the presence of pseudoaneurysm in the bronchial arteries may represent an ideal situation to be managed by application of metallic coils.
Figure 9.
(A) A 12-year-old woman with Lennox-Gastaut syndrome and history of recurrent hemoptysis with multiple previous embolization procedures. As this patient had undergone multiple prior bronchial embolization procedures, pulmonary angiogram was performed to exclude this arterial circulation as a source. It is normal with no evidence for a bleeding site. (B) Angiogram via a microcatheter (white arrowhead) of an enlarged collateral branch of the left thyrocervical artery shows collateral filling (black arrows) around and through the coils placed from a previous embolization. Proximal embolization such as with coils can often lead to this situation. (C) Embolization successfully performed via the microcatheter (white arrowhead) using 355–500 μm polyvinyl alcohol particles resulting in slow flow in the main trunk (black arrow) and no flow distally (black arrowheads).
Outcomes for Bronchial Artery Embolization for Hemoptysis
Multiple studies have established transcatheter embolization as an effective treatment for massive hemoptysis arising from both the bronchial and nonbronchial systemic circulation (Table 2). As technology has evolved, a tendency toward increased immediate clinical success has been realized. Technical success occurs in greater than 90% of interventions, with associated clinical success immediately postembolization attainable in 73–99% of patients.9,26,37,40,54,55,56,57,58,59,60,61 Unfortunately, recurrence remains frequent ranging from 10–55% for follow-up as long as 46 months.
Table 2.
Outcomes of Bronchial Artery Embolization for Hemoptysis
| Authors | Year | N | Embolic Material | Immediate Clinical Success % | Clinical Recurrence % | Complication Rates % |
|---|---|---|---|---|---|---|
| Swanson et al58 | 2002 | 54 | Coils; PVA; gelatin sponge | 94 | 24.1 | 7 |
| Mal et al57 | 1999 | 56 | Gelatin sponge; tris-acryl microspheres; dura mater; PVA; bucrylate | 77 | 55.3 | 12 |
| Ramakantan et al37 | 1996 | 140 | Gelatin sponge | 73 | 27.1 | 27.8 |
| Katoh et al43 | 1990 | 33 | PVA with gelatin sponge | - | 21.2 | 6 |
| Rabkin et al55 | 1987 | 306 | polyurethane particles; albumin macroaggregates; penicillin or 10% NaCl | 90.8 | 33.7 | 0.3 |
| Uflacker et al52 | 1983 | 33 | Gelatin sponge | 100 | 18.2 | 3 |
| Uflacker et al26 | 1985 | 64 | Gelatin sponge; e-amino-caproic acid; cellulose sponge; EtOH | 76.6 | 21.4 | 10.9 |
| Remy et al54 | 1977 | 104 | Gelatin sponge | 84 | 28.6 | 11.5 |
| Baltacioglu et al51 | 2010 | 25 | n-BCA | 100 | 16 | 16 |
| Corr et al50 | 2005 | 70 | tris-acryl microspheres | 87 | 13 | 8.6 |
| Chun et al59 | 2010 | 50 | PVA | 86 | 28.0 | 14 |
| Kato et al60 | 2000 | 101 | PVA; gelatin sponge; coils | 94 | 37.5 | 5.9 |
| Poyanli et al70 | 2007 | 140 | PVA; coils | 98.5 | 10 | 0 |
| Hayakawa et al56 | 1992 | 58 | Gelatin sponge | 86.2 | 28 | 6.9 |
N, number of patients; PVA, polyvinyl alcohol; NaCl, sodium chloride; NBCA, n-butyl cyanoacrylate; EtOH, ethanol.
Inadequate immediate clinical results are most often thought to be due to technically inadequate occlusion. Alternatively, incomplete characterization of all arteries responsible for hemorrhage at initial arteriography may result in poor immediate control.
Little impact has been made on the recurrence rates of hemoptysis despite the development of new embolic materials, and the emergence of larger inner-diameter and more flexible microcatheters capable of achieving greater selectivity (Table 2). Processes contributing to recurrence include recanalization, especially when absorbable agents are utilized, as well as further angiogenesis and vascular recruitment.49 Tuberculosis and aspergillus have been identified as independent risk factors for the recurrence of hemoptysis.43,62 Patients with lung cancer carry a 10–30% risk of developing hemoptysis, and are also at risk for recurrence following embolization.57,63 Repeat embolization is an appropriate treatment approach for recurrence of hemoptysis from all etiologies. In a recent study, Lee et al showed that repeat bronchial artery embolization achieves comparable immediate clinical success and similar recurrence rates when compared with the initial embolization procedure.62
In the hands of a skilled interventionalist, technical success rates of 90% can be achieved (Table 2). However, attaining control of hemoptysis does not alleviate the underlying cause of hemorrhage. Dependent upon the etiology, recurrence rates can be highly variable, and in the setting of infectious (e.g., tuberculosis, aspergillus) or neoplastic (e.g., bronchogenic carcinoma) offenders, one can expect nearly all patients to eventually rehemorrhage. Although the embolization technique may be entirely adequate, clinical remission is not always achieved. Generally accepted rates of cessation of hemoptysis following bronchial artery embolization approach 90%.7,23 Reembolization is an accepted approach to recurrent hemoptysis; however, surgery remains as the definitive treatment of hemoptysis recalcitrant to multiple embolizations and maximum medical therapy.
Complications of Bronchial Artery Embolization for Hemoptysis
Aside from the typical complications associated with angiography, adverse events most frequently arise from unintentional, nontarget embolization (Table 2). As previously discussed, the vascular distribution of the bronchial arteries includes mediastinal structures, pleura, bronchi, esophagus, and the walls of the thoracic and pulmonary vasculature. Chest pain represents the most common adverse event following bronchial artery embolization occurring in 24–91%, and is self-limiting in the vast majority of cases.28,37,64 Esophageal nontarget embolization resulting in transient dysphagia occurs in up to 18% of interventions, also usually self-limiting.37,64
Transverse myelitis due to spinal cord ischemia is the most serious complication associated with angiography and embolization of the bronchial circulation. Its rate of occurrence has been reported to vary from 1.4–6.5%, though many feel its prevalence is overstated.37,49,57,65 Superselective microcatheter techniques with special attention to position distal to the anterior medullary arteries has reportedly reduced the number of cases.49 Identification of a spinal artery on angiography with today's procedural techniques, including distal placement of the microcatheter, does not represent an absolute contraindication to embolotherapy, but one must be sure that transcatheter therapy is the single best option and other therapeutic modalities have been considered and rejected (Fig. 5). Although some believe spinal ischemia may in fact be due to toxicity related to the contrast media, low and iso-osmolar contrast agents have for the most part eliminated this line of thinking.
Cortical blindness has been reported and represents an extraordinarily rare neurologic complication. The predominant proposed pathway is from unintentional embolization of the occipital cortex in the setting of fistula formation arising from the bronchial artery to either the pulmonary veins or the vertebral arterial distribution.66 Pain in the orbit or temporal region ipsilateral to the side of embolization may occur, but is thought to be referred pain rather than nontarget embolization in these territories.67 Other rare complications include bronchial stenosis, necrosis, and bronchoesophageal fistula presumably due to bronchial wall ischemia as well as ischemic necrosis of the aorta with or without associated dissection.66,68,69 Pulmonary infarction and ischemic colitis have also been described, all of these consisting of isolated case reports.70
CONCLUSIONS
The management of life-threatening hemoptysis demands a well-integrated, multidisciplinary approach. Bronchial artery embolization serves as both first-line therapy for massive hemoptysis, and as a bridge to more definitive therapies targeted to the underlying etiology. Bronchial artery embolization possesses high rates of immediate clinical success coupled with low complication rates. It can be performed repeatedly for hemorrhage recurrence and associated angiography can elucidate alternative sources of hemoptysis including nonbronchial systemic and pulmonary arteries.
References
- Haponik E F, Fein A, Chin R. Managing life-threatening hemoptysis: has anything really changed? Chest. 2000;118(5):1431–1435. doi: 10.1378/chest.118.5.1431. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Wan I Y, Yu S C, et al. Multidisciplinary management of life-threatening massive hemoptysis: a 10-year experience. Ann Thorac Surg. 2009;87(3):849–853. doi: 10.1016/j.athoracsur.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Wyngaarden J B, Smith L H, Bennett J C. Cecil Textbook of Medicine. 19th ed. Philadelphia: WB Saunders; 1992. p. 370. [Google Scholar]
- Crocco J A, Rooney J J, Fankushen D S, DiBenedetto R J, Lyons H A. Massive hemoptysis. Arch Intern Med. 1968;121(6):495–498. [PubMed] [Google Scholar]
- Ferris E J. Pulmonary hemorrhage. Vascular evaluation and interventional therapy. Chest. 1981;80(6):710–714. doi: 10.1378/chest.80.6.710. [DOI] [PubMed] [Google Scholar]
- Marshall T J, Jackson J E. Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol. 1997;7(8):1221–1227. doi: 10.1007/s003300050279. [DOI] [PubMed] [Google Scholar]
- Yoon W, Kim J K, Kim Y H, Chung T W, Kang H K. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395–1409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- Kalva S P. Bronchial artery embolization. Tech Vasc Interv Radiol. 2009;12(2):130–138. doi: 10.1053/j.tvir.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Fernando H C, Stein M, Benfield J R, Link D P. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg. 1998;133(8):862–866. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28(5):1642–1647. doi: 10.1097/00003246-200005000-00066. [DOI] [PubMed] [Google Scholar]
- Hirshberg B, Biran I, Glazer M, Kramer M R. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112(2):440–444. doi: 10.1378/chest.112.2.440. [DOI] [PubMed] [Google Scholar]
- Hiyama J, Horita N, Shiota Y, Ono T, Yamakido M. Cryptogenic hemoptysis and smoking. Chest. 2002;121(4):1375–1376. author reply 1376. doi: 10.1378/chest.121.4.1375. [DOI] [PubMed] [Google Scholar]
- McDonald D M. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- Liebow A A, Hales M R, Lindskog G E. Enlargement of the bronchial arteries, and their anastomoses with the pulmonary arteries in bronchiectasis. Am J Pathol. 1949;25(2):211–231. [PMC free article] [PubMed] [Google Scholar]
- Abal A T, Nair P C, Cherian J. Haemoptysis: aetiology, evaluation and outcome—a prospective study in a third-world country. Respir Med. 2001;95(7):548–552. doi: 10.1053/rmed.2001.1053. [DOI] [PubMed] [Google Scholar]
- Hirshberg B, Biran I, Glazer M, Kramer M R. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112(2):440–444. doi: 10.1378/chest.112.2.440. [DOI] [PubMed] [Google Scholar]
- Marshall T J, Flower C DR, Jackson J E. The role of radiology in the investigation and management of patients with haemoptysis. Clin Radiol. 1996;51(6):391–400. doi: 10.1016/s0009-9260(96)80156-5. [DOI] [PubMed] [Google Scholar]
- Dweik R A, Stoller J K. Role of bronchoscopy in massive hemoptysis. Clin Chest Med. 1999;20(1):89–105. doi: 10.1016/s0272-5231(05)70129-5. [DOI] [PubMed] [Google Scholar]
- Hsiao E I, Kirsch C M, Kagawa F T, Wehner J H, Jensen W A, Baxter R B. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol. 2001;177(4):861–867. doi: 10.2214/ajr.177.4.1770861. [DOI] [PubMed] [Google Scholar]
- Hartmann I J, Remy-Jardin M, Menchini L, Teisseire A, Khalil C, Remy J. Ectopic origin of bronchial arteries: assessment with multidetector helical CT angiography. Eur Radiol. 2007;17(8):1943–1953. doi: 10.1007/s00330-006-0576-8. [DOI] [PubMed] [Google Scholar]
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet P Y, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology. 2004;233(3):741–749. doi: 10.1148/radiol.2333040031. [DOI] [PubMed] [Google Scholar]
- Pump K K. Distribution of bronchial arteries in the human lung. Chest. 1972;62(4):447–451. doi: 10.1378/chest.62.4.447. [DOI] [PubMed] [Google Scholar]
- Stoll J F, Bettmann M A. Bronchial artery embolization to control hemoptysis: a review. Cardiovasc Intervent Radiol. 1988;11(5):263–269. doi: 10.1007/BF02577032. [DOI] [PubMed] [Google Scholar]
- Botenga A SJ. Selective Bronchial and Intercostal Arteriography. Baltimore: Williams and Wilkins; 1970. pp. 7–82. [Google Scholar]
- Cauldwell E W, Siekert R G, Lininger R E, et al. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet. 1948;86(4):395–412. [PubMed] [Google Scholar]
- Uflacker R, Kaemmerer A, Picon P D, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157(3):637–644. doi: 10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- Sancho C, Escalante E, Domínguez J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol. 1998;21(4):300–304. doi: 10.1007/s002709900265. [DOI] [PubMed] [Google Scholar]
- Cohen A M, Doershuk C F, Stern R C. Bronchial artery embolization to control hemoptysis in cystic fibrosis. Radiology. 1990;175(2):401–405. doi: 10.1148/radiology.175.2.2326467. [DOI] [PubMed] [Google Scholar]
- Keller F S, Rosch J, Loflin T G, Nath P H, McElvein R B. Nonbronchial systemic collateral arteries: significance in percutaneous embolotherapy for hemoptysis. Radiology. 1987;164(3):687–692. doi: 10.1148/radiology.164.3.3615866. [DOI] [PubMed] [Google Scholar]
- Feigelson H H, Ravin H A. Transverse myelitis following selective bronchial arteriography. Radiology. 1965;85(4):663–665. doi: 10.1148/85.4.663. [DOI] [PubMed] [Google Scholar]
- Phillips S, Ruttley M S. Bronchial artery embolization: the importance of preliminary thoracic aortography. Clin Radiol. 2000;55(4):317–319. doi: 10.1053/crad.1999.0084. [DOI] [PubMed] [Google Scholar]
- Watkinson A F, Hartnell G G. Complications of direct brachial artery puncture for arteriography: a comparison of techniques. Clin Radiol. 1991;44(3):189–191. doi: 10.1016/s0009-9260(05)80868-2. [DOI] [PubMed] [Google Scholar]
- Heenan S D, Grubnic S, Buckenham T M, Belli A M. Transbrachial arteriography: indications and complications. Clin Radiol. 1996;51(3):205–209. doi: 10.1016/s0009-9260(96)80324-2. [DOI] [PubMed] [Google Scholar]
- Armstrong P J, Han D C, Baxter J A, Elmore J R, Franklin D P. Complication rates of percutaneous brachial artery access in peripheral vascular angiography. Ann Vasc Surg. 2003;17(1):107–110. doi: 10.1007/s10016-001-0339-6. [DOI] [PubMed] [Google Scholar]
- Di Chiro G. Unintentional spinal cord arteriography: a warning. Radiology. 1974;112(1):231–233. doi: 10.1148/112.1.231. [DOI] [PubMed] [Google Scholar]
- Khalil A, Fartoukh M, Bazot M, Parrot A, Marsault C, Carette M F. Systemic arterial embolization in patients with hemoptysis: initial experience with ethylene vinyl alcohol copolymer in 15 cases. AJR Am J Roentgenol. 2010;194(1):W104–W110. doi: 10.2214/AJR.09.2379. [DOI] [PubMed] [Google Scholar]
- Ramakantan R, Bandekar V G, Gandhi M S, Aulakh B G, Deshmukh H L. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200(3):691–694. doi: 10.1148/radiology.200.3.8756916. [DOI] [PubMed] [Google Scholar]
- Deffebach M E, Charan N B, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis. 1987;135(2):463–481. doi: 10.1164/arrd.1987.135.2.463. [DOI] [PubMed] [Google Scholar]
- Yoon W, Kim Y H, Kim J K, Kim Y C, Park J G, Kang H K. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology. 2003;227(1):232–238. doi: 10.1148/radiol.2271020324. [DOI] [PubMed] [Google Scholar]
- Yoon Y C, Lee K S, Jeong Y J, Shin S W, Chung M J, Kwon O J. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology. 2005;234(1):292–298. doi: 10.1148/radiol.2341032079. [DOI] [PubMed] [Google Scholar]
- Najarian K E, Morris C S. Arterial embolization in the chest. J Thorac Imaging. 1998;13(2):93–104. doi: 10.1097/00005382-199804000-00004. [DOI] [PubMed] [Google Scholar]
- White R I., Jr Bronchial artery embolotherapy for control of acute hemoptysis: analysis of outcome. Chest. 1999;115(4):912–915. doi: 10.1378/chest.115.4.912. [DOI] [PubMed] [Google Scholar]
- Katoh O, Kishikawa T, Yamada H, Matsumoto S, Kudo S. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest. 1990;97(3):541–546. doi: 10.1378/chest.97.3.541. [DOI] [PubMed] [Google Scholar]
- Keller F S, Rosch J, Loflin T G, Nath P H, McElvein R B. Nonbronchial systemic collateral arteries: significance in percutaneous embolotherapy for hemoptysis. Radiology. 1987;164(3):687–692. doi: 10.1148/radiology.164.3.3615866. [DOI] [PubMed] [Google Scholar]
- Remy J, Lemaitre L, Lafitte J J, Vilain M O, Saint Michel J, Steenhouwer F. Massive hemoptysis of pulmonary arterial origin: diagnosis and treatment. AJR Am J Roentgenol. 1984;143(5):963–969. doi: 10.2214/ajr.143.5.963. [DOI] [PubMed] [Google Scholar]
- Sbano H, Mitchell A W, Ind P W, Jackson J E. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol. 2005;184(4):1253–1259. doi: 10.2214/ajr.184.4.01841253. [DOI] [PubMed] [Google Scholar]
- Picard C, Parrot A, Boussaud V, et al. Massive hemoptysis due to Rasmussen aneurysm: detection with helicoidal CT angiography and successful steel coil embolization. Intensive Care Med. 2003;29(10):1837–1839. doi: 10.1007/s00134-003-1912-y. [DOI] [PubMed] [Google Scholar]
- Ference B A, Shannon T M, White R I, Jr, Zawin M, Burdge C M. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest. 1994;106(5):1387–1390. doi: 10.1378/chest.106.5.1387. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol. 1997;8(1 Pt 1):65–70. doi: 10.1016/s1051-0443(97)70517-7. [DOI] [PubMed] [Google Scholar]
- Corr P D. Bronchial artery embolization for life-threatening hemoptysis using tris-acryl microspheres: short-term result. Cardiovasc Intervent Radiol. 2005;28(4):439–441. doi: 10.1007/s00270-004-0227-x. [DOI] [PubMed] [Google Scholar]
- Baltacioğlu F, Cimşit N C, Bostanci K, Yüksel M, Kodalli N. Transarterial microcatheter glue embolization of the bronchial artery for life-threatening hemoptysis: technical and clinical results. Eur J Radiol. 2010;73(2):380–384. doi: 10.1016/j.ejrad.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Uflacker R, Kaemmerer A, Neves C, Picon P D. Management of massive hemoptysis by bronchial artery embolization. Radiology. 1983;146(3):627–634. doi: 10.1148/radiology.146.3.6828674. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Kimura T, Oya A, et al. Application of interlocking detachable coil (IDC) in superselective bronchial artery embolization. Nihon Kokyuki Gakkai Zasshi. 2004;42(8):730–736. [PubMed] [Google Scholar]
- Rémy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122(1):33–37. doi: 10.1148/122.1.33. [DOI] [PubMed] [Google Scholar]
- Rabkin J E, Astafjev V I, Gothman L N, Grigorjev Y G. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987;163(2):361–365. doi: 10.1148/radiology.163.2.3562815. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Tanaka F, Torizuka T, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992;15(3):154–158. discussion 158–159. doi: 10.1007/BF02735578. [DOI] [PubMed] [Google Scholar]
- Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115(4):996–1001. doi: 10.1378/chest.115.4.996. [DOI] [PubMed] [Google Scholar]
- Swanson K L, Johnson C M, Prakash U B, McKusick M A, Andrews J C, Stanson A W. Bronchial artery embolization : experience with 54 patients. Chest. 2002;121(3):789–795. doi: 10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- Chun J Y, Belli A M. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol. 2010;20(3):558–565. doi: 10.1007/s00330-009-1591-3. [DOI] [PubMed] [Google Scholar]
- Kato A, Kudo S, Matsumoto K, et al. Bronchial artery embolization for hemoptysis due to benign diseases: immediate and long-term results. Cardiovasc Intervent Radiol. 2000;23(5):351–357. doi: 10.1007/s002700010062. [DOI] [PubMed] [Google Scholar]
- Poyanli A, Acunas B, Rozanes I, et al. Endovascular therapy in the management of moderate and massive haemoptysis. Br J Radiol. 2007;80(953):331–336. doi: 10.1259/bjr/34204483. [DOI] [PubMed] [Google Scholar]
- Lee S, Chan J W, Chan S C, et al. Bronchial artery embolisation can be equally safe and effective in the management of chronic recurrent haemoptysis. Hong Kong Med J. 2008;14(1):14–20. [PubMed] [Google Scholar]
- Winter S M, Ingbar D H. Massive hemoptysis: pathogenesis and management. J Intensive Care Med. 1988;3:171–188. [Google Scholar]
- Tonkin I L, Hanissian A S, Boulden T F, et al. Bronchial arteriography and embolotherapy for hemoptysis in patients with cystic fibrosis. Cardiovasc Intervent Radiol. 1991;14(4):241–246. doi: 10.1007/BF02578470. [DOI] [PubMed] [Google Scholar]
- Wong M L, Szkup P, Hopley M J. Percutaneous embolotherapy for life-threatening hemoptysis. Chest. 2002;121(1):95–102. doi: 10.1378/chest.121.1.95. [DOI] [PubMed] [Google Scholar]
- Liu S F, Lee T Y, Wong S L, Lai Y F, Lin A S. Transient cortical blindness: a complication of bronchial artery embolization. Respir Med. 1998;92(7):983–986. doi: 10.1016/s0954-6111(98)90205-0. [DOI] [PubMed] [Google Scholar]
- Ramakantan R, Ketkar M, Maddali K, Deshmukh H. Referred pain to the ipsilateral forehead and orbit: An unusual phenomenon during bronchial artery embolization. Cardiovasc Intervent Radiol. 1999;22(4):275–277. doi: 10.1007/s002709900387. [DOI] [PubMed] [Google Scholar]
- Girard P, Baldeyrou P, Lemoine G, Grunewald D. Left main-stem bronchial stenosis complicating bronchial artery embolization. Chest. 1990;97(5):1246–1248. doi: 10.1378/chest.97.5.1246. [DOI] [PubMed] [Google Scholar]
- Ivanick M J, Thorwarth W, Donohue J, Mandell V, Delany D, Jaques P F. Infarction of the left main-stem bronchus: a complication of bronchial artery embolization. AJR Am J Roentgenol. 1983;141(3):535–537. doi: 10.2214/ajr.141.3.535. [DOI] [PubMed] [Google Scholar]
- Lemoigne F, Rampal P, Petersen R. Fatal ischemic colitis after bronchial artery embolization. Presse Med. 1983;12(33):2056–2057. [PubMed] [Google Scholar]