Abstract
BACKGROUND
Earlier studies have shown an inverse association between the presence of nausea and vomiting in pregnancy (NVP) and spontaneous abortion (SAB), but no study to date has examined the effects of symptom duration on the risk of SAB.
METHODS
We examined NVP symptom severity and duration in relation to the occurrence of SAB. Data were collected from 2407 pregnant women in three US cities between 2000 and 2004 through interviews, ultrasound assessments and medical records abstractions. Discrete-time continuation ratio logistic survival models were used to examine the association between NVP and pregnancy loss.
RESULTS
Lack of NVP symptoms was associated with increased risk for SAB [adjusted odds ratio (OR) = 3.2, 95% confidence interval (CI): (2.4, 4.3)], compared with having any symptoms. Reduced risks for SAB were found across most maternal age groups for those with NVP for at least half of their pregnancy, but the effects were much stronger in the oldest maternal age group [OR = 0.2, 95% CI: (0.1, 0.8)].
CONCLUSIONS
The absence of NVP symptoms is associated with an increased risk of early pregnancy loss. As symptom duration decreases, the likelihood of early loss increases, especially among women in the oldest maternal age group.
Keywords: spontaneous abortion, miscarriage, nausea and vomiting, pregnancy
Introduction
Nausea and vomiting in early pregnancy (NVP) affects 50–90% of women (Klebanoff et al., 1985; Klebanoff and Mills, 1986; Tierson et al., 1986; Lacroix et al., 2000; Chan et al., 2009) and is commonly the earliest symptom of pregnancy. Severity of symptoms, timing of symptom onset and duration vary both among women and across multiple pregnancies of a woman. Symptoms can begin as early as 2–4 weeks’ gestation (Gadsby et al., 1993; Lacroix et al., 2000) and commonly resolve by 20 weeks’ gestation (Gadsby et al., 1993; Broussard and Richter, 1998), but may continue to affect up to 20% of women beyond 20 weeks’ gestation (Broussard and Richter, 1998).
The pathogenesis of NVP is poorly understood, but has been attributed to rises in the serum levels of human chorionic gonadotrophin (hCG) and estrogen (Jarnfelt-Samsioe et al., 1983; Cunningham et al., 2005). A number of maternal characteristics, including primiparity (Bashiri et al., 1995), younger maternal age (Klebanoff et al., 1985; Bashiri et al., 1995) and lower education (Klebanoff et al., 1985) have been associated with NVP, and higher maternal BMI has been identified as a risk factor for vomiting (Klebanoff et al., 1985).
Spontaneous abortion (SAB) is defined as a pregnancy loss before 20 completed weeks’ gestation. While ∼10–14% of all clinically recognized pregnancies result in SAB (Wilcox et al., 1985; Wilcox, 2010), the actual rate of pregnancy loss, as shown with the use of biochemical assays, is actually two to five times higher (Ellish et al., 1996; Forbes, 1997; Wilcox et al., 1999). Current knowledge on causes of early pregnancy loss is limited, but two consistent risk factors are older maternal age and history of previous pregnancy loss (Andersen et al., 2000; de La Rochebrochard and Thonneau, 2002; George et al., 2006). Many earlier studies that examined the association between NVP and SAB (Jarnfelt-Samsioe et al., 1983; Klebanoff et al., 1985; Tierson et al., 1986; Weigel and Weigel, 1989a,b; Weigel et al., 2006) have methodological limitations. These include aspects of participant selection criteria, sample size, ascertainment of NVP information and classification of NVP symptoms and analytical approach. These differences in study design and analysis make comparison and interpretation of results difficult. Most importantly, no earlier study has examined the association between symptom duration and SAB.
We recruited a large cohort of women early in gestation or before they conceived so early clinically detectable pregnancy losses could be identified. We were able to collect detailed prospective data on the pregnancy, characteristics and timing of nausea symptoms and vomiting episodes, behavioral factors and other maternal characteristics. Using these data, we examined the association between severity and duration of NVP and SAB.
Materials and Methods
Some of our methods have been described elsewhere (Promislow et al., 2004; Savitz et al., 2005, 2006; Chan et al., 2009). We identified and recruited women who were newly pregnant and women who were trying to conceive from Raleigh, NC; Memphis, TN and Galveston, TX. Women were recruited from prenatal clinics and community locations (e.g. grocery stores, libraries, coffee shops, churches) between 2000 and 2004 for a prospective cohort epidemiological study (right from the start) that examined the relationship between drinking water disinfection by-products and early pregnancy loss. Women were eligible to be in the study if they were at least 18 years old and pregnant at <12 weeks gestation or had been attempting pregnancy for fewer than 6 months. The recruitment process has been described in detail elsewhere (Promislow et al., 2004; Savitz et al., 2005, 2006). All women completed a screening interview to determine eligibility.
An early pregnancy endovaginal ultrasound was sought around 9 weeks’ gestation (median gestational age at ultrasound = 9.3 weeks) to confirm pregnancy viability and to ascertain the gestational age of the fetus. We found that self-reported last menstrual period (LMP) in this cohort was highly reliable based on the comparison of self-reported LMP and ultrasound dating (Savitz et al., 2005, 2006); therefore, self-reported LMP was primarily used to calculate gestational age. Ultrasound dates were used (18%) only when they differed from self-reported dates by more than 7 days or when LMP was not known.
Detailed interviews took place at baseline (shortly after enrollment but at no later than 16 weeks’ gestation) and at follow-up (between 20 and 25 weeks’ gestation) to collect more detailed information on health behaviors, medical and reproductive history, and current pregnancy history and symptoms such as NVP. All women provided this information regardless of their pregnancy outcome; women who experienced an SAB before a scheduled interview were contacted for an interview as soon as possible, and the language in the interview was amended to take into consideration the pregnancy loss.
Timings of onset (start of NVP symptoms) and ending dates were collected separately via self-report for symptoms of nausea and vomiting episodes, noting the month, day and year. For those who were unable to recall the exact day of onset, we collected timing information with respect to ‘week in the month’ and we imputed the day as the mid-point of the week. We assigned symptom ending date at 20 weeks for women whose symptoms had not subsided at the time of the interview (26% nausea only; 13% both nausea and vomiting).
Absence or presence of NVP symptoms, whether nausea alone or with the addition of vomiting episodes, was used to characterize symptom severity into: (1) no symptoms, (2) nausea only and (3) nausea symptoms with vomiting episodes. Because symptom start and ending dates and the time period in which each individual was considered to be at risk for NVP varied, duration was a time-varying covariate for each individual and was categorized as: (1) no symptoms by week j, (2) having NVP for <half of pregnancy at gestational week j and (3) having NVP for ≥half of pregnancy at gestational week j. Pregnancy outcome data were ascertained via participant self-report, with confirmation through medical record abstraction and/or with the presence or absence of corresponding vital records for the pregnancy. We defined SAB as a loss of a pregnancy before 20 completed weeks’ gestation from LMP.
We used a discrete-time continuation ratio logistic survival model (Cole and Ananth, 2001; Singer and Willett, 2003; Rodriguez, 2008) to examine pregnancy loss in relation to symptoms of NVP. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were generated for the conditional probability of having a pregnancy loss in a given gestational week, conditional on a woman's pregnancy having survived to the beginning of that gestational week. Pregnancies were followed from the time of enrollment into the study and contributed to the risk set until 20 weeks’ gestation, time of SAB or last point of study contact for those loss-to-follow-up, whichever came first.
Two distinct discrete-time survival models were used to estimate the sub-classes (one model for severity and another for duration) of symptoms of NVP in relation to early pregnancy loss. The covariates that we considered were identified using a directed acyclic graph; these were known to be predictors of pregnancy loss, including maternal age (Andersen et al., 2000; de La Rochebrochard and Thonneau, 2002), race and ethnicity (Wen et al., 2001), maternal education, marital status, smoking (Parazzini et al., 1997), alcohol use (Henderson et al., 2007), BMI (Lashen et al., 2004; Metwally et al., 2008), age at menarche (Parazzini et al., 1997), parity (Wen et al., 2001) and pregnancy loss history (George et al., 2006). Effect measure modifiers were evaluated by comparing stratum-specific estimates of each covariate of interest along with examination of the likelihood ratio test using a criterion of P < 0.15. Covariates that were deemed not to be modifiers were then examined next as potential confounders. Covariates were retained as confounders in the final models if they changed the effect estimates for the exposure of interest by >10% when removed from the model. In each model, maternal race and ethnicity, maternal education, marital status, alcohol use, age at menarche, parity and pregnancy loss history were evaluated as potential confounders; maternal age was included as an effect measure modifier. All statistical analyses were conducted using SAS (SAS Institute Inc., Cary, NC, USA). This study was reviewed and approved by The UNC-Chapel Hill Public Health Institutional Review Board (study #06-0285).
Results
A total of 2766 women were enrolled into the study, with ∼10% (n = 252) pre-enrolled into the study before becoming pregnant. Of these, 32 women withdrew from the study. We excluded 227 women for ineligibility due to being >12 weeks’ gestation at the time of enrollment, being lost to follow-up (unreachable by study staff for >7 weeks) or relocating outside the study areas. We further excluded 69 women with second or third study pregnancies, 8 with invalid key data elements for pregnancy dating and 23 with multiple gestation pregnancies. These exclusions resulted in a total of 2407 women in the final analysis (Chan et al., 2009).
The majority of the study participants (82.5%) were recruited from the Raleigh or Memphis study areas. Mean age at enrollment was 27.8 years (SD = 5.5) and mean gestational age at enrollment was 54.8 days (SD = 14.0). Although non-Hispanic White women made up over 50% of the cohort, a substantial proportion (31.7%) was non-Hispanic Blacks. Very few women reported being smokers or consumers of alcohol during pregnancy (Chan et al., 2009). Approximately 45% were considered overweight or obese prior to pregnancy based on the World Health Organization cutpoints (World Health Organization). Almost half were nulliparious and 21% reported having had a prior pregnancy loss (Table I). There were 258 (10.7%) SABs in this study population, and percent SAB did not vary by study site.
Table I.
Sociodemographic characteristics, selected maternal behavior, and reproductive histories of women in analysis: Right from the Start (2000–2004), n = 2407.
| n | % | |
|---|---|---|
| Study site | ||
| Raleigh | 1088 | 45.2 |
| Memphis | 897 | 37.3 |
| Galveston | 422 | 17.5 |
| Maternal age | ||
| <25 years old | 725 | 30.1 |
| 25–29 years old | 746 | 31.0 |
| 30–34 years old | 654 | 27.2 |
| ≥35 years old | 282 | 11.7 |
| Race/ethnicity | ||
| Non-Hispanic White | 1346 | 56.0 |
| Non-Hispanic Black | 762 | 31.7 |
| Hispanic | 208 | 8.7 |
| Asian/Other | 89 | 3.6 |
| Education | ||
| <12 years | 712 | 29.5 |
| ≥12 to <16 years | 516 | 21.5 |
| ≥16 years | 1178 | 49.0 |
| Marital status | ||
| Married | 1587 | 66.0 |
| Other | 819 | 34.0 |
| Income | ||
| ≤$40 000/year | 1003 | 43.4 |
| $40 001–$80 000/year | 765 | 33.2 |
| >$80 000/year | 540 | 23.4 |
| Smoking | ||
| None | 2276 | 94.5 |
| <10 cigarettes/day | 86 | 3.6 |
| ≥10 cigarettes/day | 45 | 1.9 |
| Alcohol use | ||
| Any | 58 | 2.4 |
| None | 2347 | 97.6 |
| Prepregnancy BMI (kg/m2)a | ||
| Underweight (<18.5) | 87 | 3.7 |
| Normal weight (18.5–24.9) | 1191 | 50.8 |
| Overweight (25.0–29.9) | 564 | 24.1 |
| Obese (≥30.0) | 502 | 21.4 |
| Age at menarche | ||
| ≤11 years old | 531 | 22.2 |
| 12–13 years old | 1258 | 52.7 |
| ≥14 years old | 599 | 25.1 |
| Parity | ||
| Nulliparous | 1193 | 49.6 |
| 1 | 759 | 31.5 |
| 2+ | 455 | 18.9 |
| Pregnancy loss history | ||
| No prior loss | 720 | 29.9 |
| ≥1 pregnancy with no loss | 1178 | 48.9 |
| ≥1 pregnancy with ≥1 loss | 509 | 21.2 |
| NVPb symptom severity | ||
| No symptom | 279 | 11.6 |
| Nausea symptoms only | 852 | 35.4 |
| Nausea and vomiting | 1274 | 53.0 |
aBMI, body mass index, based on World Health Organization classifications.
bNVP, nausea and vomiting during pregnancy.
As stated in an earlier report (Chan et al., 2009), 89% of the women in this cohort experienced some form of NVP, and more than half (53.0%) report experiencing symptoms of nausea with vomiting episodes (Table I). Further, onset for NVP was most likely to begin in early pregnancy, with 99% of initial nausea symptoms and 95.3% of initial vomiting episodes taking place in the first trimester (Chan et al., 2009). When vomiting was at its worst, 51% reported vomiting once a day and 13% reported having more than three vomiting episodes daily. On average, women with both nausea and vomiting reported having vomiting episodes 3.3 days each week when symptoms were considered ‘the worst’ by the women. The median onset time for nausea symptoms was 5.7 weeks and that for vomiting episodes was 7.0 weeks from LMP (data not shown) (Chan et al., 2009). The overall mean symptom duration was eight gestational weeks.
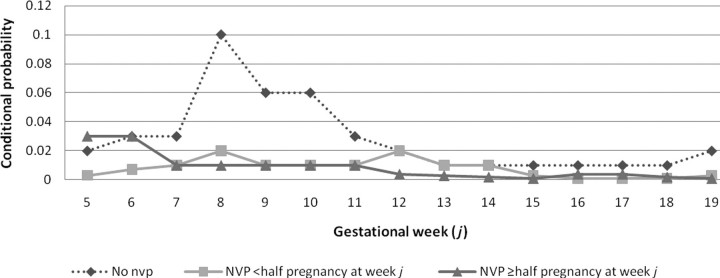
Week-specific conditional probability of SAB by NVP status and symptom duration is shown in Fig. 1. There was a 3- to 10-fold increase in risk of first trimester pregnancy loss for women without NVP symptoms, compared with women who experienced some NVP symptoms; however, the risk of SAB for women without NVP symptoms reverts to the baseline risk after the first trimester. Risk of SAB by week in the first trimester did not differ greatly for women with a shorter duration (NVP <half pregnancy at given week j) than those with a longer duration; no difference in risk was seen between groups beyond the first trimester based on duration of NVP symptoms.
Figure 1.
Week-specific conditional probability of pregnancy loss by NVP status and symptom duration, Right from the Start (2000–2004).
Overall, women with no symptoms had three times the odds of having an SAB [OR = 3.2, 95% CI: (2.4, 4.3)] compared with women with any form of NVP symptoms. Table II shows the results of modeling the week-specific odds of a woman having an early pregnancy loss for the different characteristics of NVP symptoms, stratified by maternal age. Adjustment for the potentially confounding factors did not materially alter the OR estimates. Compared with women with nausea and vomiting, the OR for no NVP symptom and SAB increased with increasing maternal age, from an OR of 4.0 [95% CI: (2.1, 7.6)] for women <25 years old to an OR of 11.7 [95% CI: (4.5, 26.7)] for women 35 years and older (likelihood ratio test P < 0.0001). An unvarying and more moderate pattern of association was found across maternal age groups for having nausea symptoms only, compared with nausea and vomiting. Maternal age also modified the association between duration and SAB. Compared with no symptoms, longer duration (NVP ≥half pregnancy) was found to have a much reduced odds for SAB among the oldest maternal age group [OR = 0.2, 95% CI: (0.1, 0.8) for ≥35 years old]. However, this pattern of association was not observed in the younger age groups. Fixed effects by study site did not alter the magnitude of association.
Table II.
Unadjusted and adjusted OR on the association between NVPa symptom severity and duration and risk of pregnancy loss and adjusted ORs stratified by maternal age: Right from the Start (2000–2004), n = 2407.
| NVP characteristics | Main effects model |
Models with an interaction term (maternal age) |
||||
|---|---|---|---|---|---|---|
| <25 years old |
25–29 years old |
30–34 years old |
≥35 years old |
|||
| Unadj. ORb (95% CI)c | Adj. OR (95% CI) | Adj. OR (95% CI) | Adj. OR (95% CI) | Adj. OR (95% CI) | Adj. OR (95% CI) | |
| Symptom severityd | ||||||
| No symptoms | 5.7 (4.0, 8.0) | 5.1 (3.6, 7.3) | 4.0 (2.1, 7.6) | 3.9 (1.9, 8.0) | 6.5 (3.2, 13.2) | 11.7 (4.5, 26.7) |
| Nausea only | 2.4 (1.8, 3.3) | 2.5 (1.8, 3.4) | 2.0 (1.5, 2.8) | 2.0 (1.4, 2.9) | 2.5 (1.8, 3.6) | 3.4 (2.2, 5.4) |
| Symptom duratione | ||||||
| NVP <half pregnancy | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.6) | 1.0 (0.2, 1.7) | 1.1 (0.6, 2.2) | 0.7 (0.4, 1.4) | 0.4 (0.2, 0.9) |
| NVP ≥half pregnancy | 0.2 (0.2, 0.3) | 0.3 (0.2, 0.4) | 1.0 (0.3, 3.4) | 1.3 (0.4, 4.6) | 0.5 (0.1, 2.1) | 0.2 (0.1, 0.8) |
aNVP, nausea and vomiting during pregnancy.
bOR, odds ratio.
cCI, confidence interval.
dCompared with symptoms of both nausea and vomiting; models stratified by maternal age and adjusted for maternal race and ethnicity, education, marital status, alcohol use, age at menarche, parity and pregnancy loss history.
eCompared with no symptoms; models stratified by maternal age and adjusted for maternal race and ethnicity, education, marital status, alcohol use, age at menarche, parity and pregnancy loss history.
Discussion
In a prospective cohort study, we examined the associations between the subgroups of NVP and SAB in a population of women recruited early in their pregnancy or women who were planning a pregnancy. The prevalence of NVP (35.4% for nausea symptoms only, 53.0% for both symptoms) was within the range for nausea symptoms only (20–50%) and for both symptoms (48–80%) reported by previous cohort studies (Kallen et al., 2003; Lagiou et al., 2003; Weigel et al., 2006); the prevalences for nausea only and both nausea symptoms and vomiting episodes are higher than those reported in other cohort studies in which recruitment took place exclusively in prenatal clinics (Gadsby et al., 1993; Weigel et al., 2006). Symptom duration was found to be within the range for mean duration (8–12 gestational weeks) as seen in the literature (Zhou et al., 1999; Kallen et al., 2003), but a sizable proportion of our cohort reported their symptoms lasting for 6 weeks or less. This may be linked to our study design that collected symptom data early in pregnancy, increasing the completeness of reporting and thus may have included more short-duration symptom reports. We observed an increase in risk for SAB that was limited to mid-first trimester among women without NVP, and no observable difference in risk was seen after the first trimester in this group compared with women with NVP (regardless of duration). The gestational weeks during which heightened weekly probability of SAB was seen, corresponded to a critical window in embryonic and early fetal development for viable pregnancies.
In addition to confirming earlier findings that absence of NVP symptoms is associated with pregnancy loss, we also were able to provide evidence that longer duration of symptoms reduced risk of pregnancy loss. The mechanisms by which NVP predicts favorable pregnancy outcome are not known; nevertheless, several different mechanisms have been postulated to explain the reported feto-protective effect of NVP. These include the role of NVP in reducing fetal exposure to potential teratogens present in the maternal diet (Weigel and Weigel, 1989a), improving the quality of maternal diets to favor the consumption of certain nutrients, increasing energy expenditure that alters hormonal balance in favor of maternal and fetal tissue growth (Weigel and Weigel, 1989a; Coad et al., 2002), along with other dietary pathways. Alternatively, one theory suggests NVP simply is a reflection of maternal sensitivity to hormones (e.g. hCG) that themselves are related to pregnancy outcome (Stein and Susser, 1991; Forbes, 2002).
Our findings also provided insights into the contribution of maternal age in the etiology of SAB. The effects of symptom severity and duration were found to be different across maternal age groups. The risk for SAB related to no NVP symptoms increased with increasing maternal age. Shorter symptom duration was associated with decreasing risk for SAB as maternal age increases; the same pattern was seen in longer symptom duration but the decrease in SAB in older women was more marked compared with younger women. Some of the ORs were imprecise within maternal age categories, and caution in interpretation is warranted. It is known that the rate of pregnancy loss begins to increase for women between ages 30 and 35, resulting from chromosomally aberrant or chromosomally normal losses (Kline et al., 1989), with each having a different underlying biological process. While losses from chromosomal abnormalities are related to the quality of the ovum and the viability of the conceptus, chromosomally normal losses are likely related to anatomical or physiological changes that occur with age. Our finding suggested that, among women of ages 30 and over, the presence of NVP may reflect increased oocyte and embryo quality relative to women without NVP. The extent to which this explains the effect of NVP on age-specific outcome is not known. The use of assisted reproduction techniques registries suggested strongly that the effect of increasing age on early pregnancy loss is related to decreases in oocyte and embryo quality (Schieve et al., 2003).
The strengths of this study include our ability to enroll from the community (37%) and prospectively collect data from newly pregnant women early in pregnancy, thus allowing for a more complete assessment and minimizing misclassification from recall bias. Moreover, studies of early pregnancy loss are subject to left truncation because a proportion of the source population is excluded due to pregnancy losses prior to recruitment (Hertz-Picciotto et al., 1989; Howards et al., 2007); our analytical methods account for left truncation in minimizing biased effect estimates due to participation differential by outcome.
Our analysis builds upon past research on the association between NVP symptoms and SAB; nevertheless, limitations still exist in our study regarding the information collected on NVP symptoms. First, misclassification of exposure reporting and of the timing of NVP from self-reported data could have occurred; however, because NVP assessment took place prior to recognition of 71% of pregnancy losses (the remaining 29% interviewed after loss), the potential for recall error would be limited. Second, we imputed symptom ending dates for 18% of women with any NVP symptoms who reported that their symptoms had not subsided at the time of the interview; even if symptom duration for them was calculated from an actual ending date, the effect estimates would have been impacted minimally because all women were censored by week 20, an end-point in the gestational period that was reflective of a time when most symptoms are expected to subside (Lacroix et al., 2000). Third, we did not collect information daily on NVP symptoms; hence, we made assumptions that a woman's symptom was always “nausea only” or always “nausea and vomiting”. The variability in patterns of the NVP cycle and the direct influences from such characteristics on SAB cannot be directly addressed. Lastly, although we utilized community recruitment and targeted recruitment at women who were early in their gestation period or those who were not yet pregnant, we still could have missed capturing women with subclinical pregnancy losses and a fraction of clinically recognized losses because of missed enrollment, potentially producing more imprecise estimates and underestimating the magnitude of the associations.
In conclusion, we found, as have others, the presence of NVP decreases the risk of SAB, with this decrease higher in women 30 years and older. We also found that the effect of symptom duration is greatest among that group. With a more complete assessment of the timing and occurrence of NVP and the use of biochemical assays, we may achieve a better understanding of the biological and physiological mechanisms of NVP and in turn, elucidate the possible interplay between this common pregnancy phenomenon and modifiable risk factors (e.g. smoking, alcohol, environmental toxicants) as well as non-modifiable risk factors (e.g. age, chromosomal karyotype) associated with pregnancy loss.
Funding
This study was supported jointly by the American Water Works Association Research Foundation (AwwaRF) and the US Environmental Protection Agency (USEPA) under Cooperative Agreement Nos CR825625-01, CR827268-01 and CR828216-01.
Acknowledgements
The authors gratefully acknowledge Dr J. Chris Slaughter for useful statistical suggestions.
References
- Andersen A-MN, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. doi:10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiri A, Neumann L, Maymon E, Katz M. Hyperemesis gravidarum: epidemiologic features, complications and outcome. Eur J Obstet Gynecol Reprod Biol. 1995;63:135–138. doi: 10.1016/0301-2115(95)02238-4. doi:10.1016/0301-2115(95)02238-4. [DOI] [PubMed] [Google Scholar]
- Broussard CN, Richter JE. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 1998;27:123–151. doi: 10.1016/s0889-8553(05)70350-2. doi:10.1016/S0889-8553(05)70350-2. [DOI] [PubMed] [Google Scholar]
- Chan RL, Olshan AF, Savitz DA, Herring AH, Daniels JL, Peterson HB, Martin SL. Maternal influences on nausea and vomiting in early pregnancy. Matern Child Health J. 2009 doi: 10.1007/s10995-009-0548-0. DOI: 10.1007/s10995-009-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coad J, Al-Rasasi B, Morgan J. Nutrient insult in early pregnancy. Proc Nutr Soc. 2002;61:51–59. doi: 10.1079/pns2001136. [DOI] [PubMed] [Google Scholar]
- Cole SR, Ananth CV. Regression models for unconstrained, partially or fully constrained continuation odds ratios. Int J Epidemiol. 2001;30:1379–1382. doi: 10.1093/ije/30.6.1379. doi:10.1093/ije/30.6.1379. [DOI] [PubMed] [Google Scholar]
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap L, Wenstrom KD. Williams Obstetrics, 22nd edn. New york, NY: McGraw-Hill Medical Publishing Division; 2005. [Google Scholar]
- de La Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17:1649–1656. doi: 10.1093/humrep/17.6.1649. doi:10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- Ellish NJ, Saboda K, O'Connor J, Nasca PC, Stanek EJ, Boyle C. A prospective study of early pregnancy loss. Hum Reprod. 1996;11:406–412. doi: 10.1093/humrep/11.2.406. [DOI] [PubMed] [Google Scholar]
- Forbes LS. The evolutionary biology of spontaneous abortion in humans. Trends Ecol Evol. 1997;12:446–450. doi: 10.1016/s0169-5347(97)01179-8. doi:10.1016/S0169-5347(97)01179-8. [DOI] [PubMed] [Google Scholar]
- Forbes S. Pregnancy sickness and embryo quality. Trends Ecol Evol. 2002;17:115–120. doi:10.1016/S0169-5347(01)02428-4. [Google Scholar]
- Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Pract. 1993;43:245–248. [PMC free article] [PubMed] [Google Scholar]
- George L, Granath F, Johansson AL, Olander B, Cnattingius S. Risks of repeated miscarriage. Paediatr Perinat Epidemiol. 2006;20:119–126. doi: 10.1111/j.1365-3016.2006.00703.x. doi:10.1111/j.1365-3016.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- Henderson J, Kesmodel U, Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J Epidemiol Community Health. 2007;61:1069–1073. doi: 10.1136/jech.2006.054213. doi:10.1136/jech.2006.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Swan SH, Neutra RR, Samuels SJ. Spontaneous abortions in relation to consumption of tap water: an application of methods from survival analysis to a pregnancy follow-up study. Am J Epidemiol. 1989;130:79–93. doi: 10.1093/oxfordjournals.aje.a115325. [DOI] [PubMed] [Google Scholar]
- Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165:444–452. doi: 10.1093/aje/kwk027. doi:10.1093/aje/kwk027. [DOI] [PubMed] [Google Scholar]
- Jarnfelt-Samsioe A, Samsioe G, Velinder GM. Nausea and vomiting in pregnancy—a contribution to its epidemiology. Gynecol Obstet Invest. 1983;16:221–229. doi: 10.1159/000299262. doi:10.1159/000299262. [DOI] [PubMed] [Google Scholar]
- Kallen B, Lundberg G, Aberg A. Relationship between vitamin use, smoking, and nausea and vomiting of pregnancy. Acta Obstet Gynecol Scand. 2003;82:916–920. [PubMed] [Google Scholar]
- Klebanoff MA, Mills JL. Is vomiting during pregnancy teratogenic. Br Med J (Clin Res Ed) 1986;292:724–726. doi: 10.1136/bmj.292.6522.724. doi:10.1136/bmj.292.6522.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff MA, Koslowe PA, Kaslow R, Rhoads GG. Epidemiology of vomiting in early pregnancy. Obstet Gynecol. 1985;66:612–616. [PubMed] [Google Scholar]
- Kline J, Stein Z, Susser M. Conception to Birth: Epidemiology of Prenatal Development. New York: Oxford University Press; 1989. [Google Scholar]
- Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182:931–937. doi: 10.1016/s0002-9378(00)70349-8. doi:10.1016/S0002-9378(00)70349-8. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Tamimi R, Mucci LA, Trichopoulos D, Adami HO, Hsieh CC. Nausea and vomiting in pregnancy in relation to prolactin, estrogens, and progesterone: a prospective study. Obstet Gynecol. 2003;101:639–644. doi: 10.1016/s0029-7844(02)02730-8. doi:10.1016/S0029-7844(02)02730-8. [DOI] [PubMed] [Google Scholar]
- Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:1644–1646. doi: 10.1093/humrep/deh277. doi:10.1093/humrep/deh277. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. doi:10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Chatenoud L, Tozzi L, Benzi G, Dal Pino D, Fedele L. Determinants of risk of spontaneous abortions in the first trimester of pregnancy. Epidemiology. 1997;8:681–683. doi: 10.1097/00001648-199710000-00012. doi:10.1097/00001648-199710000-00012. [DOI] [PubMed] [Google Scholar]
- Promislow JH, Makarushka CM, Gorman JR, Howards PP, Savitz DA, Hartmann KE. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol. 2004;18:143–152. doi: 10.1111/j.1365-3016.2003.00546.x. doi:10.1111/j.1365-3016.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez G. Multilevel Modeling, Generalized Linear Model: WWS509. Princeston University; 2007. [Google Scholar]
- Savitz DA, Singer PC, Hartmann KE, Herring AH, Weinberg HS, Makarushka C, Hoffman C, Chan R, MacLehose R. Drinking Water Disinfection By-products and Pregnancy Outcome. Denver, CO: Awwa Research Foundation; 2005. [Google Scholar]
- Savitz DA, Singer PC, Herring AH, Hartmann KE, Weinberg HS, Makarushka C. Exposure to drinking water disinfection by-products and pregnancy loss. Am J Epidemiol. 2006;164:1043–1051. doi: 10.1093/aje/kwj300. doi:10.1093/aje/kwj300. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Tatham L, Peterson HB, Toner J, Jeng G. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstet Gynecol. 2003;101:959–967. doi: 10.1016/s0029-7844(03)00121-2. doi:10.1016/S0029-7844(03)00121-2. [DOI] [PubMed] [Google Scholar]
- Singer J, Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. New York: Oxford University Press; 2003. [Google Scholar]
- Stein Z, Susser M. Miscarriage, caffeine, and the epiphenomena of pregnancy: the causal model. Epidemiology. 1991;2:163–167. doi:10.1097/00001648-199105000-00001. [PubMed] [Google Scholar]
- Tierson FD, Olsen CL, Hook EB. Nausea and vomiting of pregnancy and association with pregnancy outcome. Am J Obstet Gynecol. 1986;155:1017–1022. doi: 10.1016/0002-9378(86)90337-6. [DOI] [PubMed] [Google Scholar]
- Weigel MM, Weigel RM. Nausea and vomiting of early pregnancy and pregnancy outcome. An epidemiological study. Br J Obstet Gynaecol. 1989a;96:1304–1311. doi: 10.1111/j.1471-0528.1989.tb03228.x. [DOI] [PubMed] [Google Scholar]
- Weigel RM, Weigel MM. Nausea and vomiting of early pregnancy and pregnancy outcome. A meta-analytical review. Br J Obstet Gynaecol. 1989b;96:1312–1318. doi: 10.1111/j.1471-0528.1989.tb03229.x. [DOI] [PubMed] [Google Scholar]
- Weigel MM, Reyes M, Caiza ME, Tello N, Castro NP, Cespedes S, Duchicela S, Betancourt M. Is the nausea and vomiting of early pregnancy really feto-protective? J Perinat Med. 2006;34:115–122. doi: 10.1515/JPM.2006.021. doi:10.1515/JPM.2006.021. [DOI] [PubMed] [Google Scholar]
- Wen W, Shu XO, Jacobs DR, Jr, Brown JE. The associations of maternal caffeine consumption and nausea with spontaneous abortion. Epidemiology. 2001;12:38–42. doi: 10.1097/00001648-200101000-00008. doi:10.1097/00001648-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ. Fertility and Pregnancy. 1st edn . New York, NY: Oxford University Press; 2010. [Google Scholar]
- Wilcox AJ, Weinberg CR, Wehmann RE, Armstrong EG, Canfield RE, Nisula BC. Measuring early pregnancy loss: laboratory and field methods. Fertil Steril. 1985;44:366–374. [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. doi:10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- World Health Organization. BMI Classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. (17 December 2009, date last accessed)
- Zhou Q, O'Brien B, Relyea J. Severity of nausea and vomiting during pregnancy: what does it predict. Birth. 1999;26:108–114. doi: 10.1046/j.1523-536x.1999.00108.x. doi:10.1046/j.1523-536x.1999.00108.x. [DOI] [PubMed] [Google Scholar]