Abstract
Delphinidin is a polyphenolic compound found in many brightly colored fruits and vegetables. Delphinidin is also the major bioactive component found in many dietary supplements that are currently consumed as complementary cancer medicine including pomegranate extract. The purpose of the current study was to determine the in vitro biological effects of delphinidin on established breast cancer cell lines of varying molecular subtypes in comparison to non-transformed breast epithelial cells. We examined cell proliferation, apoptosis, and growth inhibition in response to delphinidin using a tetrazolium salt-based assay, DNA fragmentation assay, and anchorage-independent growth assay. In comparison to vehicle control, delphinidin inhibited proliferation (P < 0.05), blocked anchorage-independent growth (P < 0.05), and induced apoptosis (P < 0.05) of ER-positive, triple negative, and HER2-overexpressing breast cancer cell lines with limited toxicity to non-transformed breast epithelial cells. MAPK signaling was partially reduced in triple negative cells and ER-negative chemically transformed MCF10A cells after treatment with delphinidin. In addition, delphinidin induced a significant level of apoptosis in HER2-overexpressing cells in association with reduced HER2 and MAPK signaling. Since delphinidin is often consumed as a complementary cancer medicine, the effect of delphinidin on response to specific HER2-targeted breast cancer therapies was examined by proliferation assay. Results of these drug combination studies suggested potential antagonism between delphinidin and HER2-directed treatments. In summary, the data presented here suggest that single agent delphinidin exhibits growth inhibitory activity in breast cancer cells of various molecular subtypes, but raise concerns regarding potential drug antagonism when used in combination with existing targeted therapies in HER2-overexpressing breast cancer.
Keywords: breast cancer, delphinidin, HER2, erbB2, triple negative
Introduction
Breast cancer is the most common malignancy amongst women in the United States, and the second leading cause of cancer-related death in women.1 Standard treatments include cytotoxic non-specific chemotherapy and therapies targeted against the estrogen receptor (ER) or HER2. The five year survival rate for patients with early-stage breast cancer who receive treatment is extremely high. However, a subset of patients with advanced stage breast cancer may suffer recurrence within 10 years of treatment. Overall, it is estimated that 11 percent of women with breast cancer will have a recurrence at five years and 20 percent at 10 years post-treatment. Gene expression profiling has been used to separate breast cancers into distinct molecular subtypes, which may have prognostic value.2 Recurrence rates for luminal A breast cancers have been reported to be lower (5-year recurrence rate of approximately 8%) versus subtypes that overexpress HER2 or have basal-like features or triple negative phenotype (15%–20% 5-year recurrence rate).2–5 Despite the availability of effective ER- and HER2-targeted therapies, drug discovery efforts continue to seek additional agents that may inhibit breast cancer cell growth, particularly drugs that may be effective in multiple subtypes of breast cancer.
Recent drug discovery efforts have included examining naturally occurring polyphenolic compounds because of their ability to inhibit proliferation and induce apoptosis of breast cancer cells. The major class of polyphenols consumed by humans, due to their abundance in fruits and vegetables, are the anthocyanidins.6 Delphinidin, one of the major anthocyanidins found in foods commonly consumed in the American diet, is a diphenylpropane-based polyphenolic ring structure that carries a positive charge in its central ring.7 Delphinidin has been shown to inhibit proliferation and induce apoptosis in many different cancer models including colon, uterine, breast, and prostate.8–11 Potential use of delphinidin as a cancer chemoprevention agent has been suggested by studies showing its ability to inhibit transformation of JB6 mouse cells induced by 12-O-tetradecanoylphorbol-13-acetate (TPA), EGF, or H-Ras.12,13 In addition, the cancer prevention and therapeutic actions of delphinidin have been associated with inhibition of EGFR and vascular endothelial growth factor receptor 2 (VEGFR2) tyrosine kinase activity,13 and with inhibition of signaling from MAPK, nuclear factor-kappa B (NF-kB), and activator protein-1 (AP-1).13,14
The purpose of the current study was to determine the in vitro biological effects of delphinidin on breast cancer cell lines of different molecular subtypes, including HER2-overexpressing, ER-positive, and triple negative in comparison to non-transformed breast epithelial cells. We present data indicating that delphinidin blocks proliferation and survival of ER-positive, triple negative, and HER2-overexpressing breast cancer cell lines with limited toxicity to non-transformed breast epithelial cells. MAPK signaling is partially reduced by delphinidin in triple negative HCC1806 cells and ER-negative chemically transformed MCF10A cells. In addition, delphinidin-mediated apoptosis of HER2-overexpressing cells is associated with inhibition of HER2 and MAPK signaling. However, drug combination studies suggest potential antagonism between delphinidin and existing HER2-directed treatments. In summary, the data presented here suggest that single agent delphinidin exhibits growth inhibitory activity in breast cancer cells of various molecular subtypes, but raise concerns regarding potential drug antagonism when used as a complementary cancer medicine in HER2-overexpressing breast cancer.
Materials and Methods
Materials
Delphinidin was purchased from Indofine Chemical (Hillsborough, NJ), and dissolved in DMSO at a final concentration of 10 mg/mL. PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) was purchased from Toronto Research Chemicals and dissolved in DMSO at final concentration 500 μM. Lapatinib was purchased from Santa Cruz, Biotechnology, and dissolved in DMSO at a stock concentration of 10 mM. Herceptin (Trastuzumab) was obtained from the Emory Winship Cancer Institute pharmacy and dissolved in sterile water at a stock concentration of 20 mg/mL.
Cell culture
HCC1806, MDA231, MDA468, SKBR3, MDA453, BT474, and MCF7 breast cancer cell lines and MCF10A non-transformed breast epithelial cell line were purchased from ATCC (Manassas, VA). HCC1806 was maintained in RPMI supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin. MCF10A was maintained in DMEM/F12 with 5% horse serum, 20 ng/mL EGF, 10 μg/mL insulin, and 0.5 μg/mL hydrocortisone. MCF10A. DMSO and MCF10A.PhIP cells were maintained in regular MCF10A cell culture media but were further supplemented with DMSO or 100 nM PhIP, respectively, for more than three months. PhIP was added to media of cells, as it has previously been shown to promote mammary carcinogenesis.15 MDA231, MDA468, SKBR3, MDA453, BT474, and MCF7 were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FCS and 1% penicillin/streptomycin. All cells were incubated at 37 °C with 5% CO2 in humidified incubators.
Cell proliferation assay
Cells were plated at 3000 per well in 96-well format, and treated with delphinidin versus DMSO control corresponding to the volume of DMSO found in the highest delphinidin concentration. For another experiment, cells were treated with delphinidin, trastuzumab, lapatinib, combination trastuzumab plus delphinidin, or lapatinib plus delphinidin. After 6 days of treatment, proliferation was measured using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), as directed by the manufacturer (Promega). All treatments were done in six replicates.
DNA fragmentation assay
Cells were plated at 10,000 cells per well in 6-well format and treated with delphinidin for 48 h. Protein lysates were obtained and analyzed for cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes) representative of apoptosis using the Cell Death Detection ELISA (Roche, Indianapolis, IN) according to manufacturer guidelines. Absorbance was measured at 405 nm. The fold change in absorbance, which was indicative of DNA fragmentation, was determined per treatment group relative to the absorbance of the control DMSO group per cell line. Experiments were done in duplicate.
Anchorage-independent growth
Cells were plated in matrigel (BD Biosciences; Franklin Lakes, NJ) diluted 3:1 (media:matrigel). Media containing delphinidin or DMSO control at the same volume found in the highest dose of delphinidin was added to the matrigel-cell culture. Media plus drug or control was changed twice a week for approximately 3–4 weeks. Photographs were taken with an Olympus IX50 inverted microscope at 4× magnification. Matrigel was then digested using dispase (BD Biosciences), and viable cells were counted by trypan blue exclusion. All treatments were done in duplicate, and experiments were performed on at least two separate occasions.
Migration assay
Confluent monolayer cultures of SKBR3 cells were scratched down the center with a pipet tip to create a “wound”. Cells were then treated with 50 μg/mL delphinidin or DMSO at the same volume as that found in the dose of delphinidin. Photos were taken with an Olympus IX50 microscope at 4× magnification at 0 hours (h) and 48 h after treatment. All treatments were done in duplicate, and experiments were performed on at least two separate occasions.
Immunoblotting
Cells were lysed in RIPA buffer (Cell Signaling; Danvers, MA), which includes 0.1% NP40, supplemented with protease and phosphatase inhibitor cocktails (Sigma; St. Louis, MO). Total protein extracts (50 μg) were run on SDS-PAGE and immunoblotted using the following antibodies at the indicated dilutions: HER2 monoclonal Ab-3 and p-T180/Y182 p38MAPK were from EMD Chemicals, San Diego, CA and were used at 1:1000 each; from Cell Signaling, p-Ser 473-Akt (monoclonal 587F11), and polyclonal antibodies against Akt, p-Thr202/Tyr204 p42/p44 MAPK, total p42/p44 MAPK at 1:1000, and polyclonal p-Tyr1248 HER2 used at 1:200; from Santa Cruz Biotech, p-JNK (G7) used at 1:200 and survivin monoclonal D8 at 1:500; β-actin monoclonal AC-15 (Sigma-Aldrich) at 1:10,000. Secondary antibodies were chosen according to the species of origin of the primary antibody. Protein bands were detected using the Odyssey Imaging System (Li-Cor Biosciences). Each Western blot was performed twice for reproducibility.
Statistical analysis
Results were analyzed using single factor ANOVA tests to assess statistical significance of differences between multiple breast cancer cell lines or between multiple doses. Student’s t-test was used to determine significance between a single treatment group and control. Values of P < 0.05 were considered statistically significant.
Results
Delphinidin inhibits proliferation and induces apoptosis of breast cancer cells
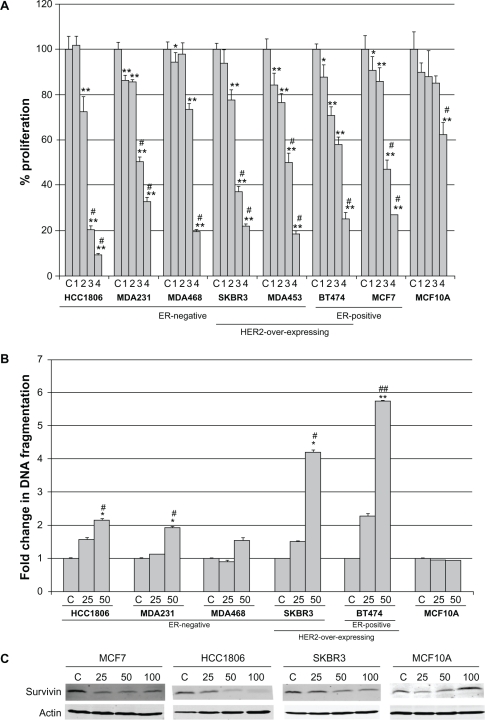
A panel of seven breast cancer cell lines was treated with increasing concentrations of delphinidin ranging from 12.5 μg/mL to 100 μg/mL for 6 d. These concentrations were chosen based on published studies using similar doses. Proliferation was determined by MTS assay, and is expressed as a percentage of control DMSO-treated cells per cell line (Fig. 1A). The majority of cell lines demonstrated 50% inhibition of proliferation at approximately 50 μg/mL, although MDA468 cells were slightly less sensitive with only 20%–30% inhibition at 50 μg/mL. Molecular subtype with respect to ER or HER2 expression status did not appear to predict for response. In contrast to the majority of breast cancer cell lines, proliferation of the non-transformed immortalized breast epithelial cell line MCF10A was not significantly inhibited by delphinidin except at the highest concentration.
Figure 1.
Delphinidin inhibits proliferation of breast cancer cells with limited toxicity to non-transformed cells. (A) Breast cancer cells and non-transformed mammary epithelial cell line MCF10A were treated with 2-fold serial dilutions of delphinidin ranging from 12.5 μg/mL to 100 μg/mL. Control (C) cells were treated with DMSO at the volume found in the highest dose of delphinidin. After 6 days, proliferation was measured by MTS assay (Promega; Madison, WI). Treatments were done in 6 replicates, and experiments were performed on at least two separate occasions. Proliferation is shown as a percentage of DMSO control per cell line; error bars represent standard deviation between replicates. Statistical significance was determined across doses per cell line by single factor ANOVA; #P < 0.005. Statistical significance of each treatment versus control was determined per cell line by student’s t-test, *P < 0.05, **P < 0.005. (B) Breast cancer cells and MCF10A were treated with DMSO control or delphinidin at 25 μg/mL or 50 μg/mL. After 48 h, DNA fragmentation was measured using the Cell Death Detection ELISA kit (Roche; Indianapolis, IN). Each treatment group was done in duplicate. Fold change in DNA fragmentation is shown as a percentage of the control per cell line; error bars represent standard deviation between duplicates. Statistical significance of differences in DNA fragmentation was determined across doses per cell line by single factor ANOVA; #P < 0.05, ##P < 0.005. Statistical significance of each treatment versus control was determined per cell line by student’s t-test, *P < 0.05, **P < 0.005. (C) Cells were treated with 25, 50, or 100 μg/mL delphinidin for 48 h, or with DMSO at a volume equivalent to that in 100 μg/mL delphinidin as a control. Lysates were blotted for survivin anti-apoptotic protein; actin served as a loading control.
To determine if delphinidin-mediated inhibition of proliferation was a reflection of apoptosis, cells were treated with 25 μg/mL or 50 μg/mL delphinidin for 48 h, since these are the lowest doses at which delphinidin showed anti-proliferative effects. Colorimetric ELISA-based detection of histone-complexed DNA fragments (mono- and oligonucleosomes) demonstrated that 50 μg/mL delphinidin induced significant DNA fragmentation in breast cancer cell lines (Fig. 1B), confirming induction of apoptosis. Similar to the proliferation assay, MDA468 cells showed lower sensitivity to delphinidin versus other cell lines. However, in contrast to delphinidin-mediated inhibition of proliferation which showed no dependence on molecular subtype, delphinidin induced the highest level of apoptosis (4- to 6-fold) in HER2-overexpressing lines SKBR3 and BT474 versus 2-fold in triple negative lines HCC1806 and MDA231. Delphinidin did not promote apoptosis in non-transformed MCF10A cells, consistent with results from proliferation assays. In addition, delphinidin suppressed expression of the anti-apoptotic protein survivin in breast cancer cell lines but not in MCF10A cells (Fig. 1C). These results indicate that delphinidin inhibits proliferation and induces apoptosis in breast cancer cell lines of varying molecular subtype, with limited toxicity to non-cancerous breast epithelial cells.
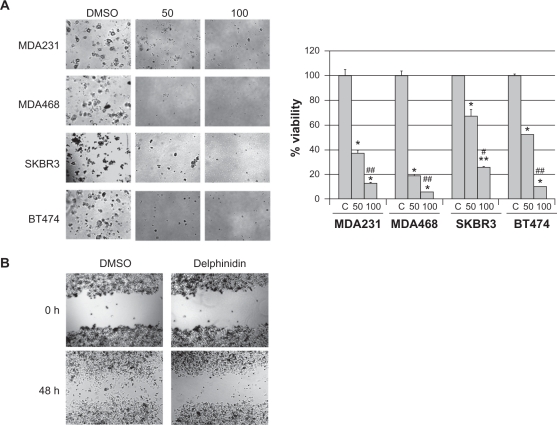
Delphinidin blocks anchorage-independent growth and migration of breast cancer cells
Anchorage-independent growth is considered to be an in vitro indicator of the invasive potential of cancer cells. Triple negative MDA231 and MDA468, and HER2-overexpressing SKBR3 and BT474 cells were plated in matrigel and treated with 50 μg/mL or 100 μg/mL of delphinidin for 3 to 4 weeks. All cell lines showed statistically significant reduction in anchorage-independent growth in response to delphinidin versus vehicle control and across all doses (Fig. 2A). Although delphinidin showed limited effects on proliferation and apoptosis of MDA468 cells, anchorage-independent growth was inhibited in this line as significantly as in other breast cancer lines, suggesting that delphinidin may inhibit invasiveness of breast cancer cells. In addition to anchorage-independent growth, cell migration in response to delphinidin was assessed. SKBR3 HER2-overexpressing breast cancer cells were plated as confluent cultures, and scratched down the middle to create an open area. Cells were then treated with DMSO control or 50 μg/mL delphinidin for 48 h (Fig. 2B). Delphinidin inhibited migration of SKBR3 cells. Collectively, these assays indicate that delphinidin blocks anchorage-independent growth and migration, suggesting that delphinidin may suppress the highly invasive potential of breast cancer cells of the triple negative or HER2-overexpressing molecular subtype.
Figure 2.
Delphinidin blocks anchorage-independent growth and migration of breast cancer cells. (A) Cells were plated in matrigel and treated with 50 μg/mL or 100 μg/mL delphinidin; control cells were treated with DMSO at the same volume as that found in the highest dose of delphinidin. Cells were maintained for 3–4 weeks, at which point photos were taken with an Olympus IX50 microscope at 4× magnification. Matrigel was then dissolved with dispase (5 mg/mL), and viable cells were counted by trypan blue exclusion. Cell survival is shown as a percentage of DMSO control cultures; error bars represent standard deviation between replicates. Statistical significance between doses including control was determined per cell line by single factor ANOVA; #P < 0.05, ##P < 0.005. Statistical significance between cell lines was determined by single factor ANOVA. Statistical significance of each treatment versus control was determined per cell line by student’s t-test, *P ≤ 0.05, **P < 0.005. (B) Confluent cultures of SKBR3 cells were scratched down the center with a pipet tip. Cells were then treated with 50 μg/mL delphinidin or DMSO control. Photos were taken with an Olympus IX50 microscope at 4× magnification at 0 h and 48 h after treatment.
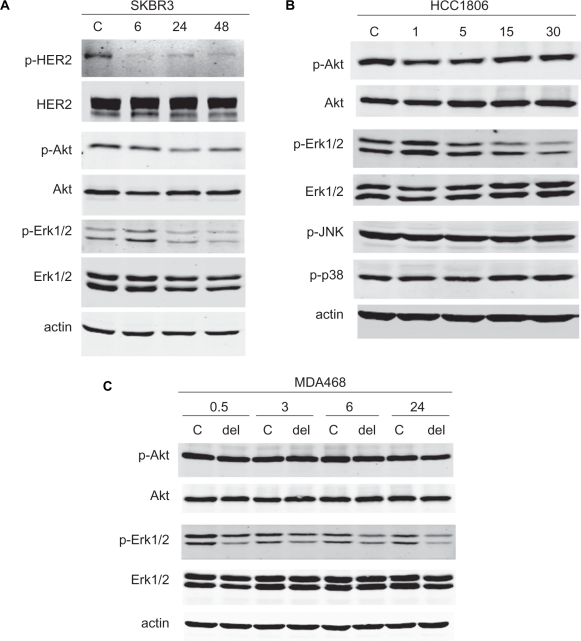
Delphinidin reduces HER2 and MAPK signaling in breast cancer cells
SKBR3 HER2-overexpressing breast cancer cells were treated with 50 μg/mL delphinidin for 6, 24, or 48 h, since this is the dose at which approximately 50% inhibition of proliferation was achieved. Protein lysates were examined for phosphorylated and total HER2, Akt, and Erk1/2 (Fig. 3 A). Delphinidin showed dramatic inhibition of HER2 signaling with reduced phosphorylation of HER2, Akt, and Erk1/2. Signaling through MAPK appeared to be more significantly affected than PI3K signaling, suggesting possible selectivity of delphinidin against the HER2-MAPK signaling axis versus HER2-PI3K signaling. Similarly, a short time course of delphinidin treatment (50 μg/mL) in the HCC1806 triple negative breast cancer line showed that phosphorylation of Erk1/2 was inhibited without any effect on Akt phosphorylation (Fig. 3B). Phosphorylation of MAPK proteins p38 and JNK were also unaffected by delphinidin, suggesting that delphinidin may specifically block downstream signaling through Erk1/2. Treatment of triple negative MDA468 breast cancer cells with 50 μg/mL delphinidin also inhibited phosphorylation of Erk1/2 without effect on Akt (Fig. 3C). Inhibition of Erk1/2 phosphorylation occurred as early as 30 min post-treatment and was sustained over a 24 h time course. Thus, delphinidin suppressed HER2 signaling in HER2-overexpressing breast cancer cells, and inhibited Erk1/2 phosphorylation in HER2-overexpressing and triple negative breast cancer cells.
Figure 3.
Delphinidin inhibits HER2 and MAPK signaling in breast cancer cells. (A) SKBR3 cells were treated with 50 μg/mL delphinidin for 6, 24, or 48 h. (B) HCC1806 cells were treated with 50 μg/mL delphinidin for 1, 5, 15, or 30 min. Control cells were treated with DMSO at the volume found in this concentration of delphinidin for the longest time point (48 h in (A) or 30 min in (B)). Total protein lysates were immunoblotted for (A) phosphorylated and total HER2, Akt, and Erk1/2, or (B) p-Akt, total Akt, p-Erk1/2, total Erk1/2, p-JNK MAPK, and p-p38 MAPK; actin served as a loading control. (C) MDA468 cells were treated with 50 μg/mL delphinidin for 0.5, 3, 6, or 24 h. Control cells were treated with DMSO at the volume equivalent to that in 50 μg/mL delphinidin for 24 h. Lysates were blotted for phosphorylated and total Akt and Erk1/2; actin served as a loading control.
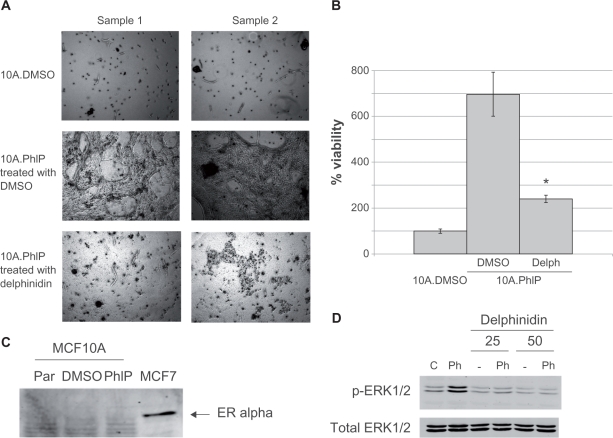
Delphinidin inhibits survival of chemically transformed ER-negative MCF10A cells
To better examine the anti-cancer effects of delphinidin, we chemically transformed the MCF10A breast epithelial cell line by treating cells chronically with 100 nM of the dietary carcinogen PhIP, which has been shown to induce mammary carcinoma in rats.15 After 3 months of PhIP exposure, duplicate cultures of MCF10A.PhIP cells and corresponding control DMSO-maintained MCF10A cells were grown in matrigel for 4–6 weeks. MCF10A.PhIP cells were either treated with 50 μg/mL delphinidin or DMSO. MCF10A.DMSO cells formed very few colonies (Fig. 4A), consistent with the fact that this cell line is not transformed. In contrast, MCF10A.PhIP cells showed significant anchorage-independent colony formation, indicating that PhIP had transformed MCF10A cells, with 6-fold higher colony counts versus MCF10A.DMSO (Fig. 4B). Importantly, delphinidin treatment suppressed growth of MCF10A.PhIP cells. Thus, delphinidin inhibited anchorage-independent growth of the chemically transformed MCF10A.PhIP cell line. To determine if ER status of MCF10A cells had changed after PhIP exposure, MCF10A parental, DMSO control, and PhIP-transformed cells were examined by Western blot for ER in comparison to ER-positive MCF-7 cells (Fig. 4C). PhIP exposure did not alter ER expression. Finally, since PhIP was previously shown to mediate transformation by activating Erk1/2 signaling,16 MCF10A cells were stimulated with 100 nM PhIP in the absence or presence of delphinidin, and examined by Western blotting for Erk1/2 phosphorylation (Fig. 4D). While PhIP induced phosphorylation of Erk1/2, co-treatment with delphinidin prevented activation of Erk1/2 signaling. Thus, while delphinidin showed limited activity in non-transformed parental MCF10A and control MCF10A.DMSO cells, anchorage-independent growth and Erk1/2 signaling were significantly inhibited by delphinidin in PhIP-transformed MCF10A cells.
Figure 4.
Delphinidin blocks survival of chemically transformed ER-negative MCF10A cells. MCF10A cells were maintained in DMSO or the carcinogen PhIP for 2 months, at which point cells were plated in matrigel. MCF10A.DMSO cells in matrigel were maintained in DMSO; MCF10A.PhIP cells in matrigel were either maintained in DMSO or 50 μg/mL delphinidin. Cells were maintained for 3–4 weeks, at which point (A) photos of duplicate cultures were taken for each treatment condition using an Olympus IX50 microscope at 4× magnification, and (B) matrigel was dissolved with dispase (5 mg/mL), and viable cells were counted by trypan blue exclusion. Cell survival is shown as a percentage of MCF10A.DMSO cells; error bars represent standard deviation between replicates. Statistical significance between delphinidin-treated MCF10A.PhIP cells versus control DMSO-treated MCF10A.PhIP cells was determined by student’s t-test; *P < 0.05. (C) MCF10A parental, DMSO control, and PhIP-transformed cells were Western blotted for expression of estrogen receptor using MCF-7 cell lysate as a positive control. (D) MCF10A cells were treated for 60 min with DMSO or 100 nM PhIP in the absence or presence of 25 μg/mL or 50 μg/mL delphinidin. Total protein lysates were immunoblotted for phosphorylated and total Erk1/2.
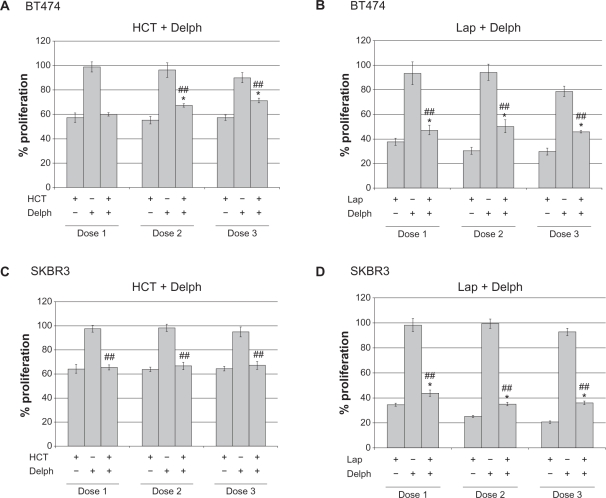
Combination delphinidin plus HER2-targeted therapy show potential antagonistic effects
Our initial data suggested that delphinidin inhibits HER2 signaling. Since delphinidin is a major bioactive component of some complementary cancer medicines, we examined the effect of combining delphinidin with the currently approved HER2-targeted drugs Herceptin and lapatinib in BT474 and SKBR3 HER2-overexpressing breast cancer cells. Low doses of delphinidin (6, 12, or 24 μg/mL) and clinically relevant concentrations of Herceptin (5, 10, or 20 μg/mL) and lapatinib (100, 200, or 400 nM) were used in these studies. MTS proliferation assays showed that BT474 and SKBR3 cells were sensitive to Herceptin and lapatinib, but that addition of delphinidin did not further inhibit proliferation (Fig. 5). Instead, addition of delphinidin significantly reduced the anti-proliferative effect of Herceptin in BT474 (Fig. 5A) and SKBR3 (Fig. 5C) cell lines. Similarly, delphinidin reduced the anti-proliferative activity of lapatinib in BT474 (Fig. 5B) and SKBR3 (Fig. 5D). Thus, there was not any apparent benefit to adding delphinidin to HER2-targeted therapy; rather, delphinidin appeared to actually reduce efficacy of these approved breast cancer drugs. Thus, while single agent delphinidin showed activity in breast cancer cell lines, combination with currently approved targeted therapies must be examined closely to avoid potential drug antagonism.
Figure 5.
Delphinidin reduces anti-proliferative activity of HER2-targeted therapies: potential for drug antagonism. (A) BT474 cells were treated with 5, 10, or 20 μg/mL Herceptin (HCT), 6, 12, or 24 μg/mL delphinidin (delph), or combination HCT + delph. (B) BT474 cells were treated with 100, 200, or 400 nM lapatinib (lap), 6, 12, or 24 μg/mL delphinidin (delph), or combination lap + delph. (C) SKBR3 cells were treated with 5, 10, or 20 μg/mL Herceptin (HCT), 6, 12, or 24 μg/mL delphinidin (delph), or combination HCT + delph. (D) SKBR3 cells were treated with 100, 200, or 400 nM lapatinib (lap), 6, 12, or 24 μg/mL delphinidin (delph), or combination lap + delph. Control cells were treated with DMSO at the volume found in the highest concentration of delphinidin and/or lapatinib. After 6 d, MTS colorimetric assays were performed to measure cell proliferation.
Notes: Values are expressed as a percentage of control DMSO group per line. Error bars represent standard deviation between six replicates. Statistical significance of differences between treatment groups was determined by ANOVA; ##P<0.005. Statistical significance of each treatment versus control was determined per cell line by student’s t-test; *P ≤ 0.05.
Discussion
The biological effects of delphinidin in breast cancer cell lines included inhibition of proliferation, as measured by MTS assay, and increased apoptosis, as measured by DNA fragmentation and reduced survivin levels. Delphinidin did not promote cell death in non-transformed MCF10A breast epithelial cells. However, MCF10A cells that were chemically transformed by chronic exposure to the PhIP carcinogen were sensitive to the growth inhibitory action of delphinidin. The most pronounced biological effects of delphinidin included inhibition of anchorage-independent growth, induction of apoptosis, and prevention of PhIP-mediated transformation. These results provide compelling evidence that delphinidin specifically targets breast cancer cells without non-specific toxicity to non-cancerous breast epithelial cells.
Our data suggested that delphinidin reduced proliferation of triple negative, ER-positive, and HER2-overexpressing breast cancer molecular subtypes. Consistent with our results, delphinidin has been shown to inhibit proliferation of the ER-positive MCF7 breast cancer cell line.17 Growth inhibition of ER-positive breast cancer may occur independent of ER-targeted effects, however, as published data suggested that delphinidin does not reduce ER alpha or ER beta expression levels.17 In fact, delphinidin-mediated inhibition of MAPK signaling may be a critical molecular mechanism of growth inhibition. We observed reduced Erk1/2 phosphorylation after delphinidin exposure in triple negative HCC1806 and MDA468 cells and in HER2-overexpressing SKBR3 cells. Reduced Erk1/2 signaling has been reported in delphinidin-treated A431 cells downstream of EGFR and HER2 inhibition,14 consistent with our data in SKBR3 cells. We found that delphinidin did not reduce phosphorylation of other MAPK proteins (p38 and JNK) in HCC1806 cells. However, delphinidin has previously been shown to inhibit UV-induced Erk1/2, p38, and JNK MAPK phosphorylation in mouse epidermal cells.13,18 In addition, delphinidin did not significantly block Akt phosphorylation in MDA468 or HCC1806 triple negative cells. In contrast, UV-induced Akt phosphorylation was reduced by delphinidin in epidermal cells.18 Collectively, these results indicate that delphinidin targets the MAPK signaling pathway, but also suggest that delphinidin has potential cell type-specific or context-specific molecular effects.
Cellular transformation by carcinogens that induce MAPK signaling appears to be inhibited by delphinidin. Kang et al13 showed that 12-O-tetradecanoylphorbol-13-acetate (TPA)–induced neoplastic transformation of mouse epidermal cells was blocked by delphinidin. The authors found that delphinidin directly blocked TPA-induced phosphorylation of MEK-Erk1/2. Similarly, we found that the carcinogen PhIP activated Erk1/2 phosphorylation, consistent with past findings.19 Delphinidin blocked PhIP-mediated Erk1/2 activation, and inhibited anchorage-independent growth of PhIP-transformed MCF10A cells. PhIP is one of the most abundant heterocyclic amines consumed by humans in cooked meat and fish, and has been shown to have carcinogenic activity in the mammary gland, colon, and prostate of rats.15,19–22 Natural antioxidant compounds such as those derived from green tea have been shown to block PhIP-mediated carcinogenesis.24 Our data further support the role of polyphenolic compounds such as delphinidin in blocking cellular transformation by environmental or diet-derived carcinogens such as PhIP.
Delphinidin-mediated apoptosis was induced at the highest levels in HER2-overexpressing cell lines. Use of delphinidin in HER2-positive disease would possibly be limited to single agent treatment, however, since combining delphinidin with Herceptin or lapatinib appeared to reduce efficacy of these currently approved HER2-targeted therapies. Our molecular data indicated that delphinidin inhibits HER2 signaling. Similarly, delphinidin has been reported to be a potent inhibitor of EGFR and HER2 phosphorylation in the human vulva carcinoma cell line A431.14,23,24 Inhibition of HER2 signaling may be an important growth inhibitory mechanism of delphinidin in HER2-overexpressing breast cancer since these cancers have oncogenic addiction to HER2, and since the combination of delphinidin with HER2-targeted drugs did not show synergy. If delphinidin affected multiple signaling pathways independently of HER2, we might expect synergy between HER2 inhibitors and delphinidin since multiple molecular pathways would be blocked. Instead, the combination did not produce additive or synergistic effects, but yielded slightly antagonistic effects. Thus, delphinidin-mediated HER2 inhibition is likely to be an important molecular effect of delphinidin in this breast cancer subtype.
Based on our data, delphinidin may serve as an important lead compound for developing novel therapies for breast cancer. The concentrations of delphinidin that we used in this study were in the μg/mL range. These are likely to be physiologically tolerable doses as humans are estimated to consume between 80–215 mg of anthocyanidins daily,25,26 with delphinidin being a major source of anthocyanidins. Delphinidin has demonstrated anti-inflammatory and anti-angiogenic activities in prostate cancer models,7 and appears to be a broad-spectrum inhibitor of multiple kinase signaling pathways including HER2 and downstream MAPK in multiple cell types.13,23,24 Our own data indicates that delphinidin is an inhibitor of HER2 and Erk1/2 signaling, with growth inhibition and apoptosis observed in multiple molecular subtypes of breast cancer. Thus, delphinidin could represent a novel lead agent for development of future breast cancer chemotherapeutic drugs.
Abbreviations
- ER
estrogen receptor;
- EGF
epidermal growth factor;
- VEGF
vascular endothelial growth factor;
- NF-kB
nuclear factor-kappa B;
- AP-1
activator protein-1;
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine;
- DMSO
dimethylsulfoxide
- h
hours
Footnotes
Funding
Grant Support is acknowledged from National Cancer Institute K01CA118174 and Georgia Cancer Coalition Distinguished Cancer Scholar Award to R Nahta.
Disclosure
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 3.Millar EK, Graham PH, O’Toole SA, McNeil CM, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–8. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen PL, Taghian AG, Katz MS, Niemierko A, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–8. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 5.Haffty BG, Yang Q, Reiss M, Kearney T, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 6.Prior RL. Fruits and vegetables in the prevention of cellular oxidative DNA damage. Am J Clin Nutr. 2003;78:570S–8. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- 7.Hafeez BB, Siddiqui IA, Asim M, Malik A, et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–72. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzè MC, Savio M, Pizzala R, Cazzalini O, et al. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis. 2004;25:1427–33. doi: 10.1093/carcin/bgh138. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–72. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Bin Hafeez B, Asim M, Siddiqui IA, Adhami VM, et al. Delphinidin, a dietary anthocyanidin in pigmented fruits and vegetables: a new weapon to blunt prostate cancer growth. Cell Cycle. 2008;7:3320–6. doi: 10.4161/cc.7.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun JM, Afaq F, Khan N, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol Carcinog. 2009;48:260–70. doi: 10.1002/mc.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou DX, Kai K, Li JJ, Lin S, et al. Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis. 2004;25:29–36. doi: 10.1093/carcin/bgg184. [DOI] [PubMed] [Google Scholar]
- 13.Kang NJ, Lee KW, Kwon JY, Hwang MK, et al. Delphinidin attenuates neoplastic transformation in JB6 Cl41 mouse epidermal cells by blocking Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling. Cancer Prev Res (Phila Pa) 2008;1:522–31. doi: 10.1158/1940-6207.CAPR-08-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meiers S, Kemény M, Weyand U, Gastpar R, et al. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J Agric Food Chem. 2001;49:958–62. doi: 10.1021/jf0009100. [DOI] [PubMed] [Google Scholar]
- 15.Nagao M, Ushijima T, Wakabayashi K, Ochiai M, et al. Dietary carcinogens and mammary carcinogenesis. Induction of rat mammary carcinomas by administration of heterocyclic amines in cooked foods. Cancer. 1994;74:1063–9. doi: 10.1002/1097-0142(19940801)74:3+<1063::aid-cncr2820741514>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Creton SK, Zhu H, Gooderham NJ. The cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine activates the extracellular signal regulated kinase mitogen-activated protein kinase pathway. Cancer Res. 2007;67:11455–62. doi: 10.1158/0008-5472.CAN-07-2821. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes I, Faria A, Azevedo J, Soares S, et al. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J Agric Food Chem. 2010;58:3785–92. doi: 10.1021/jf903714z. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JY, Lee KW, Kim JE, Jung SK, et al. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis. 2009;30:1932–40. doi: 10.1093/carcin/bgp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito N, Hasegawa R, Sano M, Tamano S, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1991;12:1503–6. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa R, Sano M, Tamano S, Imaida K, et al. Dose-dependence of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine (PhIP) carcinogenicity in rats. Carcinogenesis. 1993;14:2553–7. doi: 10.1093/carcin/14.12.2553. [DOI] [PubMed] [Google Scholar]
- 21.Shirai T, Sano M, Tamano S, Takahashi S, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–8. [PubMed] [Google Scholar]
- 22.Hirose M, Akagi K, Hasegawa R, Yaono M, et al. Chemoprevention of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine (PhIP)-induced mammary gland carcinogenesis by antioxidants in F344 female rats. Carcinogenesis. 1995;16:217–21. doi: 10.1093/carcin/16.2.217. [DOI] [PubMed] [Google Scholar]
- 23.Fridrich D, Teller N, Esselen M, Pahlke G, Marko D. Comparison of delphinidin, quercetin and (-)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res. 2008;52:815–22. doi: 10.1002/mnfr.200800026. [DOI] [PubMed] [Google Scholar]
- 24.Teller N, Thiele W, Boettler U, Sleeman J, Marko D. Delphinidin inhibits a broad spectrum of receptor tyrosine kinases of the ErbB and VEGFR family. Mol Nutr Food Res. 2009;53:1075–83. doi: 10.1002/mnfr.200800524. [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J Agric Food Chem. 1999;47:1083–91. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 26.Seeram NP, Zhang Y, Nair MG. Inhibition of proliferation of human cancer cells and cyclooxygenase enzymes by anthocyanidins and catechins. Nutr Cancer. 2003;46:101–6. doi: 10.1207/S15327914NC4601_13. [DOI] [PubMed] [Google Scholar]