Abstract
Introduction:
GP88 (PC-Cell Derived Growth Factor, progranulin) is a glycoprotein overexpressed in breast tumors and involved in their proliferation and survival. Since GP88 is secreted, an exploratory study was established to compare serum GP88 level between breast cancer patients (BC) and healthy volunteers (HV).
Methods:
An IRB approved prospective study enrolled 189 stage 1–4 BC patients and 18 HV. GP88 serum concentration was determined by immunoassay.
Results:
Serum GP88 level was 28.7 + 5.8 ng/ml in HV and increased to 40.7 + 16.0 ng/ml (P = 0.007) for stage 1–3 and 45.3 + 23.3 ng/ml (P = 0.0007) for stage 4 BC patients. There was no correlation between the GP88 level and BC characteristics such as age, race, tumor grade, ER, PR and HER-2 expression.
Conclusion:
These data suggest that serial testing of serum GP88 levels may have value as a circulating biomarker for detection, monitoring and follow up of BC.
Keywords: progranulin, GP88, breast cancer, biomarker
Introduction
Breast cancer (BC) is the most common type of cancer diagnosed in women in the US.1 The majority of patients are diagnosed with early stage disease but many will develop systemic recurrence later on therefore the value of serial monitoring for recurrence of breast cancer with circulating tumor markers is of importance. Efforts to identify such serum biomarkers in BC have largely been unsuccessful and are best represented by the development of immunoassays for CA15-3, CA27-29 and carcinoembryonic antigen (CEA) to be utilized for monitoring of patients with early and advanced breast cancer.2,3 The CEA blood test measures the level of the antigen CEA by a sandwich enzyme linked immunoassay. CA15-3 and CA27-29 tests measure the serum level of a mucin-like membrane glycoprotein (MUC-1) that is shed from tumor cells into the bloodstream. The CA 15-3 epitope is recognized by two monoclonal antibodies in a double-determinant or sandwich immunoassay. The CA27-29 is a one epitope antibody test generated against MUC-1 protein. It is well established that 75% to 90% of patients with metastatic breast cancer will have elevated MUC-1 levels. Many studies have demonstrated that a rising CA15-3 or CA27-29 level can detect recurrence after primary treatment. Thus, tests measuring MUC-1 have been used in the management of patients with breast cancer.4,5 Hou et al2 showed that in patients with metastatic breast cancer the sensitivity and specificity was 85.7% for CA27-29, 82.8% for CA15-3 and 62.8% for CEA, respectively. In addition patients had significantly higher levels of CA27-29 than CEA, but they were similar to CA15-3 suggesting that CA27-29 is more sensitive and specific than CEA, but is similar to CA15-3 for metastatic breast cancer detection and monitoring.
In another study, patients with hormone (HR) sensitive and HER2 negative tumors were more likely to have elevated CA15-3 level at the time of diagnosis of metastatic disease than patients with other tumor types, 69% for HR+ Her2-primary tumors, 56% of HR+ Her2+++, 46% of HR- Her2+++ and 41% for triple-negative cases (P = 0.003).6 Safi et al7 assessed CA15-3 serum levels preoperatively in N = 1342 patients with benign breast conditions and various malignancies. CA15-3 levels were found to be over 50 U/ml in 0%, 2%, 13%, and 73% of the patients with stages 1, 2, 3, and 4 breast cancer respectively. Others showed that CA15-3 levels were highest in patients with liver metastases and increasing numbers of metastatic sites and that the increased CA15-3 concentration usually preceded the clinical diagnosis of the relapse with the median lead time of 9 months (range: 1–40) in 72.4% of patients with distant metastases due to breast carcinoma.5 The low detection rate in early stage BC has precluded the routine use of CA15-3 for screening for breast cancer recurrence, even though CA15-3 is utilized to monitor the effectiveness of treatments for metastatic BC in addition to imaging studies and clinical symptoms.
Other recent serum-based tumor markers in BC, also measured by enzyme immunoassays, include the plasminogen activator (PA) system which is comprised of the 2 serine proteases, urokinase PA (uPA) and tissue PA (tPA), the 2 serpin inhibitors, PAI-1 and PAI-2 and the uPA receptor (uPAR; CD87). High levels of uPA, PAI-1, uPA-PAI-1 complex and uPAR in BC tissue are associated with poor prognosis, while high levels of tPA or PAI-2 correlate with good prognosis.10 Measurements of the extracellular domain of Her-2 (HER2- ECD) have also been found to be prognostic for predicting response to anti-Her2 therapy and monitoring recurrence.11 Our laboratory is focused on characterizing functional biological markers of breast cancer with a particular attention to proteins produced by the cancer cells and released in the circulation. Such proteins’ function is to stimulate tumor growth and survival or inhibit apoptosis which often results in acquisition of resistance to therapeutic interventions. Such targets would be ideal as tissue-based or serum-based biomarkers as well as a base for development of targeted therapy. One such marker is the autocrine growth/survival factor PC cell derived growth factor (PCDGF also called GP88). GP88 (also known as progranulin, acrogranin or granulin-epithelin precursor) is the largest member of a unique family of cysteine-rich polypeptides growth modulators that include the 6 kDa epithelins or granulins.12,13 The 88 kda glycoprotein contains a 63 kDa core protein with a 17 amino-acid signal peptide for targets GP88 for secretion. Our laboratory was the first to demonstrate the biological activity of 88 kDa GP88 as a growth promoter for tumorigenic cells including human breast cancer cells.14,15 Others later demonstrated growth-promoting activity of the precursor for other mesenchymal and epithelial cells as well as for pre-implantation embryos.16–18 The 88 kDa protein has been shown to play a role in tumorigenesis, including stimulation of proliferation, survival, migration, angiogenesis invasion and matrix metallo-protease activity.19 In addition, in normal tissues, it plays a role in wound healing and in inflammation.20,21 The pathways involved in GP88 signaling include both the mitogen-activated protein kinase (MAP Kinase Erk 1/2), phosphatidylinositol 3-kinase (PI-3 Kinase), and focal adhesion kinase (FAK), leading to the activation of the cell cycle regulatory proteins Cyclin D1 and Cyclin B.15,17,22–25
Screening of human breast adenocarcinoma cell lines indicated that GP88 was expressed in correlation with tumorigenic properties. Conversely, inhibition of GP88 expression by antisense cDNA transfection in ER– human breast carcinoma resulted in a greater than 90% reduction of tumor incidence and tumor size when injected in nude mice,15 implicating GP88 as a major factor in the maintenance of tumor phenotype. In ER-positive (ER+) cells, GP88 expression was associated with the acquisition of resistance to the to the anti-estrogen Tamoxifen both in vitro and in vivo.26,27 In addition, GP88 overexpression has been associated with resistance to Trastuzumab and to doxorubicin.28,29 Pathological studies in paraffin embedded human BC biopsies indicated that GP88 was overexpressed in 80% of invasive ductal carcinoma, where it correlated with clinical parameters of poor prognosis such as tumor grade, p53 expression and Ki67 index.26 In contrast, normal mammary tissues and benign lesions were negative for GP-88 expression.26
Based on these properties and since GP88 is overexpressed in BC tissues and is targeted for secretion, we elected to conduct a clinical correlation study to determine whether GP88 can be measured/detected in blood samples from healthy volunteers (HV) and BC patients and if the measured level is statistically elevated in BC patients compared to HV. We also planned to look at the accepted BC prognostic factors such as age, stage, ER, PR, HER2 and tumor grade in relation to the GP88 serum level.
Materials and Methods
A prospective, IRB approved blood sampling protocol was established at the University of Maryland Marlene and Stewart Greenebaum Cancer Center (UMGCC) and enrolled HV and stage 1–4 BC patients undergoing therapy or coming for routine follow-ups to the UMGCC’s Breast Cancer Clinic. HV and BC patients were sampled once to test the hypothesis that the serum GP88 can be measured via our method. Study eligibility criteria included histologically confirmed diagnosis of BC, age greater than 18, stage 1–4, status post lumpectomy or mastectomy. Stage 4 patients were sampled during systemic therapy (chemotherapy, hormonal therapy or HER2 targeted therapy). The majority of early stage patients were sampled after completion of adjuvant/neoadjuvant chemotherapy but while still receiving adjuvant hormonal therapy if indicated based on tumor characteristics. Patients with stage 4 BC were eligible to participate in this study irrespective of the number of prior therapies received for treatment of MBC.
Healthy volunteers had to be more than 18 years of age and without current or prior history of malignancy and were required to sign informed IRB approved consent. For patients actively receiving adjuvant chemotherapy, blood samples were drawn before the chemotherapy was given on the day of treatment. The majority of early stage BC patients in our study were not sampled while receiving active adjuvant chemotherapy but while they were coming for routine follow-up visits to the oncology clinic after completion of active chemotherapy. Non fasting blood was collected into serum preparation tubes (BD Diagnostics) at the UMGCC and immediately transferred on ice, unprocessed; to A&G Pharmaceutical in Columbia, Maryland for serum preparation and analysis of the GP88 serum levels. Password protected clinical database of study participants was established at the UMGCC under the UM IRB and HIPAA guidelines. Participants (HV and BC patients) were assigned study numbers and these were used for further analysis of the GP88 levels of study participants at AG Pharmaceutical. The GP88 levels were reported back to the principal investigator at the UMGCC who then collected this information prospectively together with other patient and tumor specific information. The statistical analysis of the data was performed by an independent University of Maryland faculty member statistician.
Serum GP88 EIA
Whole blood samples received from UMGCC were processed, spun at 3000RPM for 10 minutes to collect serum. Once aliquoted, serum samples were kept at –80 °C before assaying for GP88. Measurement of GP88 levels in serum was carried out in triplicates using a GP88 sandwich enzyme immunoassay (EIA) kit developed by A & G Pharmaceutical, comprising a combination of capture and detection anti-human GP88 antibodies with increasing amounts of human GP88 used for calibration curve. Serum calibrators consisting of human sera spiked with known amount of GP88 were also used as internal controls in the assays; specifically, 96-well plates were coated with 1μg/well of capture anti-GP88 mouse monoclonal antibody. After washing, serum samples or standard human GP88 were incubated for 2 hours at 37 °C. Following washing, 100 μl of detecting rabbit anti-human GP88 antibody was added and incubated at 37 °C for 1 hr. Following washing, HRP-conjugated goat anti-rabbit secondary IgG, was added. After 1 hr of incubation, plates were washed and 100 μl of HRP substrate was added. OD at A620 was read with a microtiter plate reader. Amount of GP88 in serum samples from BC and HV was calculated from the standard human GP88 curve.
Statistical Analysis
Descriptive statistics were used to summarize patients’ characteristics. These were reported by proportions (for categorical variables) and median (range) for continuous variables. The mean level of GP88 among HV and different stages of BC patients were compared using Analysis of Variance (ANOVA) and T test. The associations of GP88 with patient and tumor characteristics were assessed by t test or ANOVA. A Receiver Operating Characteristic (ROC) Curve was generated by plotting the sensitivity (the true positive rate) versus 1-specificity (the false positive rate) using GP88 cutoffs to classify breast cancer patients (BC) versus healthy volunteers (HV).
Results
Patient characteristics
The demographics and clinico-pathological parameters of the 189 BC patients enrolled in this study are as follows: median age is 51, (age ranging from 29 to 86); 56% of participants were aged ≥ 50. Of interest, our clinical trial cohort had an almost equal representation of African American and Caucasian patients (48.7% and 48.1% respectively), well within the ethnic distribution of the population of Baltimore and the State of Maryland. The majority of patients had invasive ductal carcinoma and 50% were lymph node (LN) negative and had tumors <2 cm in diameter while 28% had tumors 2–5 cm in diameter at diagnosis; 54% of participants were stage 1 and 2 and 46% stage 3 and 4; 57% of patients were ER-positive and 46% PR-positive; 63% were HER2/neu negative, 40% of patients had grade 3 tumors (Table 1).
Table 1.
Breast cancer patient characteristics (N = 189).
| Characteristic | No. of patients | Median (range) or percent | |
|---|---|---|---|
| Age | 51 (29–86) | ||
| Age group (%) | <50 | 83 | 43.9 |
| ≥50 | 106 | 56.1 | |
| Race (%) | Caucasian | 91 | 48.1 |
| African American | 92 | 48.7 | |
| Other races | 6 | 3.2 | |
| Stage (%) | 1 | 48 | 25.4 |
| 2 | 52 | 27.5 | |
| 3 | 26 | 13.8 | |
| 4 | 63 | 33.3 | |
| ER (%) | Positive | 109 | 57.7 |
| Negative | 69 | 36.5 | |
| Unknown | 11 | 5.8 | |
| PR (%) | Positive | 88 | 46.6 |
| Negative | 89 | 47.1 | |
| Unknown | 12 | 6.3 | |
| HER2 (%) | Positive | 34 | 18.0 |
| Negative | 119 | 63.0 | |
| Unknown | 36 | 19.0 | |
| Grade (%) | 1 | 30 | 15.9 |
| 2 | 43 | 22.7 | |
| 3 | 76 | 40.2 | |
| Unknown | 40 | 21.2 | |
| Tumor type (%) | Ductal | 152 | 80.4 |
| Lobular | 18 | 9.5 | |
| Other* | 19 | 10.1 | |
| Lymph node status (Stage 1–3 patients) | Negative | 63 | 50.0 |
| Positive | 45 | 35.7 | |
| Unknown | 18 | 14.37 | |
| Tumor size (cm) Stage 1–3 patients | <2 cm | 63 | 50.0 |
| 2–5 cm | 36 | 28.6 | |
| >5 cm | 12 | 9.5 | |
| Unknown | 15 | 11.9 |
Note:
Other includes: medullary, mucinous, tubular, colloid, and unknown.
Abbreviations: ER, estrogen receptor; PR, Progesterone Receptor; Her-2, human epidermal growth factor receptor-2.
GP88 Level in Serum of Breast Cancer Patients
We determined the serum GP88 level of BC patients and HV by sandwich EIA as described in the material and methods section. The enrollment of 18 healthy volunteers at the University of Maryland under the same IRB protocol used to enroll BC patients allowed us to establish a baseline serum GP88 level. Breast cancer patients were stratified in two categories: early stage (patients with stage 1, 2 and 3 disease) and stage 4 or metastatic patients. GP88 level was measured in triplicates for each subject enrolled in the study. These values were then used to calculate mean and median values for subjects in the three groups: HV, early stage BC patients (stage 1, 2, and 3) and BC patients stage 4, respectively (Table 2).
Table 2.
Comparison of mean serum GP-88 level in breast cancer patients by stage with healthy volunteers (HV).
| Group | Median GP88 (range) ng/ml | Mean GP-88 (±SD) ng/ml | P-value* |
|---|---|---|---|
| HV | 28.4 (16.6–38.2) | 28.7 (±5.8) | NA |
| BC Stage 1–3 | 38.7 (6.4–100.0) | 40.7 (±16.0) | 0.007 |
| BC Stage 4 | 39.9 (9.8–158.4) | 45.3 (±23.3) | 0.0007 |
Note:
P values are computed based on t tests.
As shown in Table 2, GP88 was measurable in the serum of HV and BC patients. The mean level of GP88 for healthy volunteers was 28.7 ng/ml ± 5.8 ng/ml. For stage 1–3 BC patients, mean GP88 serum level was 40.7 ± 16.0 ng/ml whereas it was 45.3 ± 23.3 ng/ml in stage 4 BC patients.
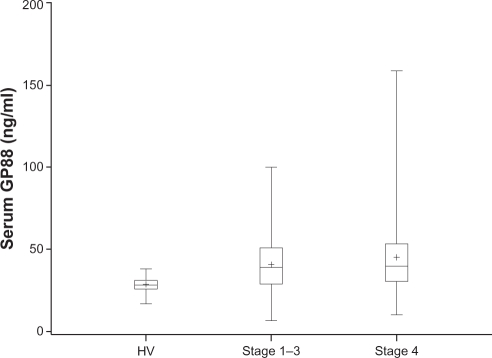
Statistical analysis of the results in Table 2 indicated that serum GP88 values were significantly elevated in both early stage (P = 0.007) and stage 4 patients (P = 0.0007) when compared to the GP88 serum level of healthy subjects. In addition, as shown in Figure 1, the range of GP88 values was much larger in BC patients than in healthy individuals reaching 100 ng/ml for stage 1–3 patients and 158 ng/ml in stage 4 patients when compared HV where the highest values was 39 ng/ml.
Figure 1.
Comparison of the GP88 level in healthy volunteers (HV) and breast cancer patients separated by stages (Stage 1–3) and stage 4. GP88 serum levels in healthy volunteers and breast cancer patients stage 1–3 and stage 4 were represented by box and whisker plots. The mean is represented by a + sign. The median is represented by the line inside the box. The bottom and top of the box represent the 25th and 75th percentiles. The vertical line extends to the minimum and maximum data values.
No statistically significant difference in the mean of serum GP88 level was observed between the early stage and late stage disease groups. We then examined the possible association between patients GP88 serum level and their clinico-pathological parameters such as age, race, lymph node status, steroid receptor expression, (ER and PR), Her-2 expression, and tumor grade. No statistical correlation was observed between GP88 level and these parameters (Table 3).
Table 3.
Association of serum GP-88 levels with patient and tumor characteristics.
| Characteristic | Median (range) ng/ml | Mean GP88 ng/ml | P-value* |
|---|---|---|---|
| Age | |||
| <50 | 37.8 (9.8–105.8) | 40.2 | |
| ≥50 | 39.5 (6.4–158.4) | 43.8 | 0.19 |
| Race | |||
| Caucasian | 38.3 (9.8–100.0) | 40.7 | 0.27 |
| African American | 39.4 (6.4–158.4) | 43.8 | |
| ER | |||
| + | 38.0 (15.1–158.4) | 42.0 | 0.96 |
| – | 38.7 (6.4–105.8) | 42.2 | |
| PR | |||
| + | 37.9 (15.1–85.3) | 41.4 | 1.0 |
| – | 38.7 (6.4–105.8) | 41.4 | |
| HER2 | |||
| + | 37.4 (9.8–85.3) | 41.0 | 0.99 |
| – | 39.0 (6.4–105.8) | 40.9 | |
| Grade | |||
| 1 | 41.2 (15.1–158.4) | 45.6 | 0.45 |
| 2 | 39.0 (16.1–100.0) | 41.8 | |
| 3 | 37.9 (6.4–105.8) | 40.3 |
Notes:
P values are computed based on t tests for all comparisons except for comparison among grades, which is based on analysis of variance.
Discussion
Glycoprotein GP88 (progranulin, PCDGF) is a growth and survival factor that has been shown to play an important role in breast cancer tumorigenesis.20,21 We previously showed that GP88 can be measured in breast cancer tumor tissue by immunohistochemistry staining with an anti-human GP88 antibody developed in our laboratory. These data showed that GP88 was expressed at higher level in breast cancer tissues (invasive ductal carcinoma) when compared to normal mammary tissue.30 In addition, GP88 tumor overexpression is associated with worse outcomes (disease-free survival and overall survivals) in early stage BC patients (Serrero et al manuscript in preparation). We established an IRB approved prospective blood sampling study to examine by sandwich EIA the level of serum GP88 in BC patients and in healthy volunteers with study participants being sampled once. We showed that by our technique, GP88 is measurable in the non-fasting blood samples of HV and patients with stage 1–4 BC. A baseline serum GP88 level was established for healthy volunteers. When compared to this baseline, BC patients with both early and late stage diseases have a statistically significant increase in the serum GP88 level. An analysis of ROC curve for control and BC patients GP88 values indicated that by using a GP88 cut off value of 30.4 ng/ml, specificity and sensitivity of the assay were 72% which is in line with the sensitivity of CA15-3 and CA27-29.7
We found a statistically significant difference in the serum levels of GP88 between individuals without history of breast cancer and BC patients regardless of the stage. We did not however find a statistically significant difference in the serum GP88 level between patients with different stages of BC and this could certainly be explained by the fact that patients were sampled at different points of their treatment for BC as described in the method section. The purpose of the investigation initially was to establish that GP88 can be detected and measured by our assay and we were able to establish this in our study. Interestingly we found that GP88 serum level was elevated in early stage BC patients and this makes this test attractive for further investigation in more structured prospective protocol in this patient population. The objective of the present study was not to differentiate between the stages or other prognostic factors for early stage BC since patients were not enrolled at the time of initial diagnosis of early or advanced BC. Sixty seven percent of participants had early stage BC and were sampled after completion of lumpectomy or mastectomy surgery. We did not find statistically significant correlation between the GP-88 serum level and the tumor expression of ER, PR, HER2 and tumor grade. In addition age and race did not seem to have an effect on the level of the GP-88.
The fact that there was no significant difference in GP88 values between patients aged < 50 and ≥50 years old suggests that GP88 measurement can be used in both pre- and postmenopausal women. Similarly, the lack of difference in GP88 values in African-American and Caucasian would support the wide use of GP88 measurement across diverse breast cancer populations. Concerning tumor characteristics, our previous IHC study showed that GP88 was expressed in both ER positive and ER negative tumors. Additionally, the tissue based study indicated that GP88 was an independent biomarker from Her-2.30 These results could possibly explain why we did not find any difference in serum GP88 level with ER, PR and Her-2 status.
Our findings suggest that subsequent studies to explore the GP88 serum level testing as a screening blood test for breast cancer in otherwise healthy population of women are warranted. Our results also support further studies in breast cancer patients to examine the correlation of the serum level of GP88 with disease-free and overall survival parameters and with respect to other tumor characteristics (ER, PR, HER2, tumor grade, size and LN status) in patients enrolled at the initial diagnosis. Further studies of the association of the serum level of GP-88 and the response to systemic therapy, time to progression and survival in patients with advanced breast cancer are also warranted based on our initial results. Our efforts are currently focused on the prospective serial blood sampling study in patients with advanced breast cancer. In addition we are prospectively sampling patients with early stage breast cancer who are currently undergoing therapy and post therapy for 2–3 years when the risk of recurrence is the highest. We believe that this approach is more likely to show the correlation of the GP-88 level with the various BC clinicopathologic prognostic factors.
In summary, this study established that GP88 can be measured successfully in the blood of HV and BC patients and the fact that GP88 expression can also be measured in tumor tissue and is considered a negative prognostic factor makes it an interesting biomarker to study further in different clinical settings.
Acknowledgments
The authors wish to thank Dr. Jun Hayashi for helpful discussion. The study was supported by grants 1R43CA124179-01, 2R44CA124179-02 from the National Cancer Institute and 07-2007-064 from the Avon Foundation to Ginette Serrero and from MIPS.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.American Cancer Society. Cancer Society. 2010. Cancer Facts and Figures 2010. Atlanta, American; pp. 1–62. [Google Scholar]
- 2.Hou MF, Tsai LY, Tsai SM, et al. Evaluation of serum CA27.29, CA15-3 and CEA in patients with breast cancer. Kaohsiung J Med Sci. 1999;15:520–8. [PubMed] [Google Scholar]
- 3.Einarsson R, Lindman H, Bergh J. Use of TPS and CA15-3 assays for monitoring chemotherapy in metastatic breast cancer patients. Anticancer Res. 2000;20:5089–93. [PubMed] [Google Scholar]
- 4.Hayes DF, Zurawski VR, Jr, Kufe DW. Comparison of circulating CA15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986;4:1542–50. doi: 10.1200/JCO.1986.4.10.1542. [DOI] [PubMed] [Google Scholar]
- 5.Wojtacki J, Kruszewski WJ, Sliwińska M, et al. Elevation of serum CA15-3 antigen: an early indicator of distant metastasis from breast cancer. Retrospective analysis of 733 cases. Przegl Lek. 2001;58:498–503. [PubMed] [Google Scholar]
- 6.Bensouda Y, André F, Boulet T, et al. Prevalence of elevated serum CA15-3 at time of metastatic relapse of breast cancer and correlation with hormone receptor status. Bull Cancer. 2009;96:923–8. doi: 10.1684/bdc.2009.0919. [DOI] [PubMed] [Google Scholar]
- 7.Safi F, Kohler I, Röttinger E, Suhr P, Beger HG. Comparison of CA15-3 and CEA in diagnosis and monitoring of breast cancer. Int J Biol Markers. 1989;4:207–14. doi: 10.1177/172460088900400405. [DOI] [PubMed] [Google Scholar]
- 8.Uehara M, Kinoshita T, Hojo T, et al. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3) in breast cancer. Int J Clin Oncol. 2008;13:447–51. doi: 10.1007/s10147-008-0773-3. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MJ, Evoy D, McDermott EW. CA15-3: Uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–74. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Meijer-van Gelder ME, Look MP, Peters HA, et al. Urokinase-type plasminogen activator system in breast cancer: association with tamoxifen therapy in recurrent disease. Cancer Res. 2004;64:4563–8. doi: 10.1158/0008-5472.CAN-03-3848. [DOI] [PubMed] [Google Scholar]
- 11.Esteva FJ, Cheli CD, Fritsche H, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7:R436–43. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89:1715–9. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plowman GD, Green JM, Neubauer MG, et al. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem. 1992;267:13073–8. [PubMed] [Google Scholar]
- 14.Zhang H, Serrero G. Inhibition of tumorigenicity of the teratoma PC cell line by transfection with antisense cDNA for PC cell-derived growth factor (PCDGF) Proc Natl Acad Sci U S A. 1998;95:14202–7. doi: 10.1073/pnas.95.24.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu R, Serrero G. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc Natl Acad Sci U S A. 2000;97:3993–8. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu SQ, Tang D, Chamberlain S, et al. The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J Biol Chem. 1998;273:20078–83. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59:3222–9. [PubMed] [Google Scholar]
- 18.Díaz-Cueto L, Stein P, Jacobs A, Schultz RM, Gerton GL. Modulation of mouse preimplantation embryo development by acrogranin (epithelin/granulin precursor. Dev Biol. 2000;217:406–18. doi: 10.1006/dbio.1999.9564. [DOI] [PubMed] [Google Scholar]
- 19.Tangkeangsirisin W, Serrero G. PC-cell derived growth factor (PCDGF/GP88) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–9. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–12. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 21.Serrero G. Autocrine growth factor revisited: PC-cell-derived growth factor (progranulin), a critical player in breast cancer tumorigenesis. Biochem Biophys Res Commun. 2003;308:409–13. doi: 10.1016/s0006-291x(03)01452-9. [DOI] [PubMed] [Google Scholar]
- 22.Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–40. [PubMed] [Google Scholar]
- 23.Jones MB, Michener CM, Blanchette JO, et al. The granulin-epithelin precursor: a putative new growth factor for ovarian cancer. Gynecol Oncol. 2003;88:S136–9. doi: 10.1006/gyno.2002.6704. [DOI] [PubMed] [Google Scholar]
- 24.Pizarro GO, Zhou XC, Koch A, et al. Prosurvival function of the granulin-epithelin precursor is important in tumor progression and chemoresponse. Int J Cancer. 2007;120:2339–43. doi: 10.1002/ijc.22559. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Xi L, Liao G, et al. Inhibition of PC cell-derived growth factor (PCDGF)/granulin-epithelin precursor (GEP) decreased cell proliferation and invasion through downregulation of cyclin D and CDK4 and inactivation of MMP-2. BMC Cancer. 2007;7:22. doi: 10.1186/1471-2407-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu R, Serrero G. Mediation of estrogen mitogenic effect in human breast cancer MCF-7 cells by PC-cell-derived growth factor (PCDGF/granulin precursor) Proc Natl Acad Sci U S A. 2001;98:142–7. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tangkeangsirisin W, Hayashi J, Serrero G. PC cell-derived growth factor mediates tamoxifen resistance and promotes tumor growth of human breast cancer cells. Cancer Res. 2004;64:1737–43. doi: 10.1158/0008-5472.can-03-2364. [DOI] [PubMed] [Google Scholar]
- 28.Kim WE, Serrero G. PC-Cell Derived Growth Factor (PCDGF/GP88, progranulin) stimulates proliferation and confers Herceptin resistance to Her-2 overexpressing breast cancer cells. Clin Can Res. 2006;12:4192–9. doi: 10.1158/1078-0432.CCR-05-2663. [DOI] [PubMed] [Google Scholar]
- 29.Kudoh K, Ramanna M, Ravatn R, et al. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000;60:4161–6. [PubMed] [Google Scholar]
- 30.Serrero G, Ioffe O. Expression of the novel autocrine growth factor PC-cell derived growth factor in human breast cancer tissue. Human Pathology. 2003;34:1148–54. doi: 10.1016/s0046-8177(03)00425-8. [DOI] [PubMed] [Google Scholar]