Abstract
Epilepsy is a common disease with significant morbidity and mortality. Approximately one-third of patients with epilepsy are refractory to available seizure medications, emphasizing the need to develop better drugs with novel mechanisms of action. Ezogabine, also known as retigabine, is a new potential adjunctive treatment for adults with intractable partial seizures. Ezogabine has a unique mechanism of action consisting of activating KCNQ2/3 (Kv7) potassium channels. Ezogabine has undergone a number of Phase II and III trials demonstrating efficacy at 600,900 and 1200 mg/day in a dose-dependent fashion. The most common adverse events with ezogabine are central nervous system effects, particularly dizziness and somnolence. Urologic symptoms, particularly urinary retention, represent a rare but unique side effect of ezogabine. Ezogabine is predominantly metabolized via glucuronidation. Its half-life is 8 hours, suggesting a need for three-times-a-day administration. Ezogabine exhibits minimal interactions with other seizure medications, except possibly lamotrigine. Ezogabine has potential for clinical applications in other medical conditions beyond epilepsy, such as neuropathic pain, neuromyotonia, and bipolar disease, but these are based primarily on experimental models.
Keywords: antiepileptic drug, epilepsy, ezogabine
Introduction
Epilepsy is a common disease with a cumulative lifetime risk of at least 3%.1 The majority of patients with epilepsy have partial seizures, accounting for about two-thirds of cases in epidemiologic studies in developed countries.2 The burden of disease is also quite high, involving significant morbidity and mortality. Individuals with epilepsy in developed countries have an up to three-fold increase in mortality compared with the general population.3 Adults with seizures have also been found to have lower levels of education, higher rates of unemployment as well as higher rates of physical ailments.4 While the aim of epilepsy treatment is complete seizure control with minimal adverse events from medication, approximately one-third of patients will have persistent seizures despite antiepileptic drug (AED) treatment and can be classified as medically intractable.5,6
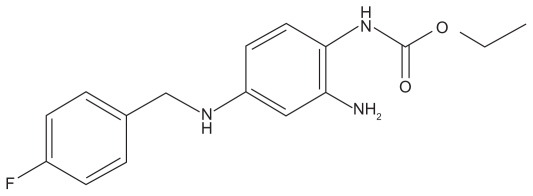
Ezogabine is an ethyl N-(2-amino-4-[{4-fluorophenyl}methylamino]phenyl carbamate) (Figure 1). Previously known as D-23129, it is also known by the international nonproprietary name of retigabine in Europe and most of the world, but the adopted name in the United States is ezogabine. This drug was recently approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for adjunctive treatment of partial-onset seizures in adults. It appears to work by a unique mechanism of action compared with other currently available AEDs.
Figure 1.
Chemical structure of ezogabine.
Mechanism of action
Ezogabine exerts its anticonvulsant effects through a novel mechanism of action that is unique among existing AEDs. In particular, ezogabine activates potassium currents in neurons,7 which should lead to hyperpolarization of the membrane potential and decreased neuronal excitability under physiological conditions. On the molecular level, ezogabine enhances the activation of a specific heteromeric potassium channel, consisting of KCNQ2 or KCNQ3 channel subunits, a member of the Kv7 family of potassium channels.8,9 Ezogabine binds to the activation gate or within the pore of the channel itself to stabilize the channel in the open position.10–12 KCNQ2/3 channels mediate a previously described potassium current, the M-current, in neurons. The M-current possesses unique electrophysiological properties, including slow activation and deactivation near the resting membrane potential, which cause a decrease in repetitive firing of neurons under excitatory conditions. While the M-current had previously been shown to be blocked by a number of pharmacological agents, such as the acetylcholine agonist, muscarine (and thus the origin of the name “M” current), ezogabine is the first reported drug that enhances opening of this channel,13,14 with the associated potential for anticonvulsant activity.
In addition to modulating potassium channels, ezogabine may have other mechanisms of action. There is minimal evidence of a clinically significant effect of ezogabine on voltage-gated sodium and calcium channels.15 However, ezogabine may interact with GABAA (γ-aminobutyric acid type A) receptors,16 although this effect may require relatively high concentrations and thus may only represent a minor contribution to the anticonvulsant actions of ezogabine.15
Prior to clinical studies of ezogabine in epilepsy patients, ezogabine was shown to be effective in a variety of animal models of seizures. For example, ezogabine displays efficacy in the traditional antiepileptic screening assays of acutely induced seizures: the electrically induced maximal electroshock seizure and chemical convulsant penetylenetetrazole models in rats or mice.17 Ezogabine also has anticonvulsant properties in genetic animal models of epilepsy.17,18 In addition to direct anticonvulsant effects in suppressing seizures, ezogabine may also have antiepileptogenic properties in delaying the development of seizures in the kindling model of epilepsy.19
Interestingly, genetic mutations in the KCNQ2/3 potassium channels have been identified as the cause of cases of benign familial neonatal convulsions.20–22 Defective potassium channel function represents a rational mechanistic explanation for brain hyperexcitability and seizures in this inherited epilepsy syndrome. Since ezogabine enhances the function of KCNQ2/3 channels, ezogabine represents a rare example in which a seizure medication may directly target the underlying molecular defect causing a specific type of epilepsy.
Efficacy
There have been three placebo-controlled, multicenter studies to assess safety and efficacy in adults (Table 1). All of these studies were performed on patients aged 18–75. In all of these studies, ezogabine was used as adjunctive therapy in patients with refractory partial seizures, who were already on at least one or two other AEDs, such as valproate, carbamazepine, phenytoin, topiramate, lamotrigine, gabapentin, oxcarbazepine, benzodiazepines, or barbiturates. The first of these studies looked at multiple dosing schedules, 600, 900, and 1200 mg/day, administered three times daily.23 Based on this study, the FDA recommended two confirmatory studies. RESTORE 2 addressed dosages of 600 and 900 mg/day administered three times daily,24 and RESTORE 1 addressed the dosage of 1200 mg/day divided three times daily.25
Table 1.
Summary of efficacy data from randomized, double-blind, placebo-controlled trials of ezogabine as adjunctive treatment of refractory partial seizures in adults
| Study/outcome measure | Placebo | Ezogabine
|
||
|---|---|---|---|---|
| 600 mg/d | 900 mg/d | 1200 mg/d | ||
| Porter et al23 | n = 96 | n = 99 | n = 95 | n = 106 |
| Median change in seizure frequency, % | −13.1 | −23.4a | −29.3a | −35.2a |
| Patients with >50% seizure reduction, % | 15.6 | 23.2 | 31.6a | 33.0a |
| RESTORE 2 (Brodie et al24) | n = 170 | n = 179 | n = 175 | |
| Median change in seizure frequency, % | −15.9 | −27.9a | −39.9a | |
| Patients with >50% seizure reduction, % | 18.9 | 38.6a | 47.0a | |
| RESTORE 1 (French et al25) | n = 152 | n = 153 | ||
| Median change in seizure frequency, % | −17.5 | −44.3a | ||
| Patients with >50% seizure reduction, % | 17.8 | 44.4a | ||
Note: Statistically significant difference compared to the placebo group.
In the first study there was an 8-week forced-titration phase, followed by an 8-week maintenance phase. A total of 537 subjects entered baseline, and 399 were randomized to a study treatment. 396 were included in an intention-to-treat analysis. The primary endpoint analyzed was change from baseline in monthly seizure frequency and was compared across all treatment arms. A secondary endpoint was responder rate, which was defined as a ≥50% reduction in seizure frequency. Seizure-free rates were not reported. When looking at median percentage change in monthly total seizure frequency, a significant dose-dependent effect was seen in the primary endpoint across all treatment groups: 600 mg/day (−23.4%), 900 mg/day (−29.3%), and 1200 mg/day (−35.2%) vs placebo (−13.1%).23 Significant responder rates in the secondary endpoint were seen compared with placebo in various forms of statistical analysis, with ranges from 23%–41%.23 This effect did also appear to be dose dependent with significant effects in the 900 and 1200 mg/day groups.23
In RESTORE 2, there were four phases: a prospective 8-week baseline, 4-week titration, 12-week maintenance, and a 4-week transition phase if patients elected to participate in the open-label extension phase. The primary efficacy measure for the FDA was change in total partial seizure frequency per 28 days between baseline and double-blind period in the treated patients vs placebo. An intention-to-treat analysis was used. A total of 696 patients were screened and 539 randomized. A total of 538 patients were included in the intention-to-treat analysis. The median percentage reduction was higher for both the 600 mg/day (27.9%, P = 0.007) and 900 mg/day (39.9%, P < 0.001) groups than placebo (15.9%).24 A significant responder rate (>50% reduction in seizure frequency) was seen in both the 600 mg/day (38.6%) and the 900 mg/day group (47.0%) as compared with placebo (18.9%, P < 0.001).24 Three to five percent of patients in the treatment groups became seizure-free, although this was not statistically significant compared with the placebo group.
In RESTORE 1, there were two phases: an 8-week, prospective baseline phase and an 18-week double-blind treatment period (6-week initial dose titration phase). There were two primary endpoints: percentage change in 28-day total partial seizure frequency between baseline and double-blind period, and responder rate (≥50% reduction in 28-day total partial seizure frequency between baseline and maintenance phase). A total of 442 patients were screened and 306 were randomized. A total of 305 patients were used in the intention-to-treat analysis. Median percentage reduction in monthly seizure frequency was 44.3% in the treated group (1200 mg/day) vs 17.5% in the placebo group (P < 0.001).25 Responder rate was 44.4% in the treated group vs 17.8% in the placebo group (P < 0.001).25 Five percent of patients in the treated group were seizure-free during the maintenance phase compared with 1% in the placebo group, a difference that was not quite statistically significant. However, the percentage of seizure-free days in the treated group was significantly greater than in the placebo group.
Overall, the published placebo-controlled multicenter studies indicate that ezogabine is effective as an adjunctive therapy for adults with refractory partial seizures. However, very few patients became seizure-free on ezogabine, and the degree of reduction in seizure frequency is comparable to most other AEDs studied as add-on therapy for intractable epilepsy. Furthermore, future studies are needed to assess the potential efficacy of ezogabine for the pediatric population and for other seizure types or epilepsy syndromes, such as generalized seizures.
Safety
Data on the safety and side effects of ezogabine were also derived from the three placebo-controlled, multicenter studies testing ezogabine as adjunctive therapy in adult patients with refractory partial seizures (Table 2). In the first study, common adverse events (>10%) could be mostly categorized as central nervous system (CNS) effects and occurred more frequently in the ezogabine arms. These included somnolence, confusion, dizziness, tremor, amnesia, thinking abnormal, vertigo, and speech disorder.23 In the second study, common adverse effects (>10%) all could be categorized again as CNS effects and were described as dizziness, somnolence, headache, and fatigue.24 In the third study, CNS effects were again the most common, with 40.5% complaining of dizziness and 31.4% complaining of somnolence in the ezogabine group.25 Additional common treatment emergent adverse events were fatigue, confusion, dysarthria, ataxia, blurred vision, and tremor. Nausea was also reported in 10.5% of ezogabine patients compared with 6.6% in the placebo group.25 This was higher than seen in the RESTORE 2 study, at 6.1% in the 600 mg/day group and 6.7% in the 900 mg/day group.24 Urinary tract infections were seen in 11.8% of subjects on ezogabine compared with 8.6% in the placebo group.25
Table 2.
Summary of common adverse events (>10% of patients) reported in ezogabine-treated patients from randomized, double-blind, placebo-controlled trials as adjunctive treatment of refractory partial seizures in adults
| Dizziness |
| Somnolence |
| Fatigue |
| Confusion |
| Headache |
| Dysarthria |
| Tremor |
| Vertigo |
| Ataxia |
| Amnesia |
| Thinking abnormal |
| Speech disorder |
| Blurry vision |
| Asthenia |
| Nausea |
| Urinary tract infection |
As ezogabine has been demonstrated to have effects on bladder function in a rat model, extra attention was paid to urinary system side effects in RESTORE 1 and 2.26,27 In RESTORE 2, three patients on ezogabine experienced chromaturia (occasional reddish-brown discoloration of urine) and three patients exited the study because of adverse events of the urinary tract (one nephritis and two urinary retention).24 In RESTORE 1, it was observed that 15 of the ezogabine-treated patients had increased post-void residual volumes of >100 mL compared with six of the placebo-treated patients.25 Other adverse events observed included urinary tract infection, urinary hesitation, dysuria, and chromaturia, all of which were infrequent but occurred more commonly in the ezogabine-treated group than placebo.25
In summary, common adverse events of ezogabine are CNS effects, with the most common of these being dizziness and somnolence. All adverse events appeared to have some dose dependency. No significant cardiac, hematologic, or liver adverse events have been reported.23–25 Some attention should be paid to potential adverse effects on the urinary tract. There are no reported safety data in the pediatric population.
Pharmacology
Ezogabine is metabolized primarily in the liver by acetylation to the mono-acetylated metabolite AWD21–360 and more significantly by glucuronidation to an N-glucuronide28–31 with subsequent predominant renal clearance. The cytochrome P450 system does not appear to play a significant role in the metabolism of ezogabine.29 Glucuronidation appears to be predominantly by the UGT1A1, UGT1A9, and less so, UGT1A4 enzymes.30,31
A variety of studies have assessed the pharmacokinetics of ezogabine with the majority of the data being obtained in a study by Ferron et al that assessed the pharmacokinetics of ezogabine at multiple doses up to 700 mg/day in 45 healthy male volunteers.32 It is rapidly absorbed after oral administration, with a peak plasma concentration at 1.5 hours.32 With food, maximal plasma concentration is delayed to approximately 2 hours. Mean terminal half-life was 8 hours.32 Oral clearance was 0.7 L/hour/kg in white subjects and volume of distribution was 8.7 L/kg.32 In black subjects, clearance and volume of distribution were 25% and 30% lower, respectively. 32 Pharmacokinetics was linearly dose proportional for both ezogabine and the metabolite AWD21–360.32 No clinically significant effect has been seen with gender.33 However, a decreased rate of clearance has been observed in elderly subjects, possibly related to decline in renal function.33
Drug interactions
In vitro studies have demonstrated little or no potential for ezogabine to inhibit the major cytochrome P450 isoenzymes. No clinically significant interactions have been found between ezogabine and propofol, valproate, lamotrigine, or imipramine. 31 An in vivo study of 29 healthy volunteers given both lamotrigine and ezogabine demonstrated some limited interactions.34 In the presence of lamotrigine, ezogabine rate of clearance was slightly decreased, possibly due to competition for renal clearance.34 Interestingly, lamotrigine clearance was slightly increased, raising the possibility that ezogabine acted as an inductor of glucuronidation.34 An in vivo study has demonstrated lack of interaction between phenobarbitone and ezogabine.35 In clinical studies, no significant pharmacokinetic effect has been seen on phenytoin, carbamazepine, valproic acid, or topiramate.36 Finally, one study showed women on a maintenance dose of ezogabine 750 mg/day for 19 days as well as a combination oral contraceptive did not have a decrease in contraceptive hormone exposure, suggesting lack of clinically significant interaction.36 Overall, ezogabine has minimal clinically significant interactions with other AEDs, as well as other drugs metabolized by the P450 system.
Other clinical applications
A variety of additional clinical applications beyond epilepsy have been proposed for ezogabine. One logical consideration is the possibility of treatment of peripheral nerve hyperexcitability with ezogabine. The role of potassium channels in some forms of peripheral nerve hyperexcitability has been understood for some time. For instance, Isaac’s syndrome, a disorder that includes neuromyotonia, has been found in some cases to be autoimmune with anti-voltage- gated potassium channel antibodies.37 Interestingly, two families have been described with both benign familial neonatal convulsions and myokymia, supportive of KCNQ2/3 mutations as causative.38,39 Furthermore, a single case has been reported of peripheral nerve hyperexcitability, secondary to a KCNQ2 mutation.40 Interestingly, in this same case report, an in vitro study was performed that demonstrated potential beneficial effect of ezogabine on peripheral nerve hyperexcitability. 40 As some familial cases of episodic ataxia have also been attributed to potassium channel mutations, theoretically, ezogabine may represent a rational treatment for ataxia in these cases, although published clinical data are lacking.
Based on rat models, ezogabine has also been proposed as a potential treatment for neuropathic pain,41,42 mania,43 and addiction to psychostimulants.44 Another rodent model demonstrated a possible antidystonic effect.45 An in vitro study has demonstrated a possible anxiolytic effect of ezogabine.46 As remarked above, ezogabine has been studied in a rat model to look at effects on bladder function, with resultant suggestion that it may be effective for detrusor overactivity.26,27
Conclusion
Epilepsy is a significant cause of morbidity and mortality, with approximately one-third of patients being refractory to available medications. Ezogabine is a new potential treatment that has a unique mechanism of action consisting of activating KCNQ2/3 channels. It demonstrates reasonable efficacy for adjunctive partial seizure control, although ezogabine appears to make few intractable patients seizure-free and has similar effects on seizure frequency as other AEDs tested as add-on therapy for refractory epilepsy. Based on clinical trials, ezogabine appears to be relatively safe and well tolerated, although long-term post-marketing surveillance data are needed. Furthermore, while there have been minimal serious or life-threatening adverse events reported, it has potential for significant urologic side effects. Early data demonstrate minimal interaction with medications other than lamotrigine. Future studies may demonstrate whether ezogabine will play a role in other neuropsychiatric or even urologic diseases. In addition, further studies are being considered at this time for ezogabine’s role in the treatment of pediatric partial epilepsy and Lennox–Gastaut syndrome.36
Footnotes
Disclosure
Dr Weisenberg reports no conflicts of interest in this work. Dr Wong has received preclinical research grant funding from Pfizer.
References
- 1.Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: a population-based study in Rochester, Minnesota. Neurology. 2011;76:23–27. doi: 10.1212/WNL.0b013e318204a36a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarrelli MM, Beghi E, Rocca WA, Hauser WA. Incidence of epileptic syndromes in Rochester, Minnesota. Epilepsia. 1999;40:1708–1714. doi: 10.1111/j.1528-1157.1999.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 3.Forsgren L, Hauser WA, Olafsson E, Sander JWAS, Sillanpaa M, Tomson T. Mortality of epilepsy in developed countries: a review. Epilepsia. 2005;46(Suppl 11):18–27. doi: 10.1111/j.1528-1167.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- 4.Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U S adults with seizures: results from the 2002 national health interview survey. Epilepsia. 2005;46:1133–1139. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 6.Mohanraj R, Brodie MJ. Outcomes in newly diagnosed localization-related epilepsies. Seizure. 2005;14:318–323. doi: 10.1016/j.seizure.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Rundfelt C. The new anticonvulsant retigabine (D-23129) acts as an opener of K+ channels in neuronal cells. Eur J Pharmacol. 1997;336:243–249. doi: 10.1016/s0014-2999(97)01249-1. [DOI] [PubMed] [Google Scholar]
- 8.Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anticonvulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- 9.Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Schenzer A, Friedrich T, Pusch M, et al. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci. 2005;25:5051–5060. doi: 10.1523/JNEUROSCI.0128-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–1017. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- 12.Lange W, Geissendorfer J, Schenzer A, et al. Refinement of the binding site and mode of action of the anticonvulsant Retigabine on KCNQ K+ channels. Mol Pharmacol. 2009;75:272–280. doi: 10.1124/mol.108.052282. [DOI] [PubMed] [Google Scholar]
- 13.Rundfeldt C, Netzer R. The novel anticonvulsant retigabine activates M-currents in Chinese hamster ovary-cells transfected with human KCNQ2/3 subunits. Neurosci Lett. 2000;282:73–76. doi: 10.1016/s0304-3940(00)00866-1. [DOI] [PubMed] [Google Scholar]
- 14.Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rundfeldt C, Netzer R. Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung. 2000;50:1063–1070. doi: 10.1055/s-0031-1300346. [DOI] [PubMed] [Google Scholar]
- 16.van Rijn CM, Willems-van Bree E. Synergy between retigabine and GABA in modulating the convulsant site of the GABAA receptor complex. Eur J Pharmacol. 2003;464:95–100. doi: 10.1016/s0014-2999(03)01426-2. [DOI] [PubMed] [Google Scholar]
- 17.Rostock A, Tober C, Rundfeldt C, et al. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23:211–223. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 18.De Sarro G, Di Paola ED, Conte G, Pasculli MP, De Sarro A. Influence of retigabine on the anticonvulsant activity of some antiepileptic drugs against audiogenic seizures in DBA/2 mice. Naynyn Schmiedebergs Arch Pharmacol. 2001;363:330–336. doi: 10.1007/s002100000361. [DOI] [PubMed] [Google Scholar]
- 19.Mazarati A, Wu J, Shin D, Kwon YS, Sankar R. Antiepileptogenic and antiictogenic effects of retigabine under conditions of rapid kindling: an ontogenic study. Epilepsia. 2008;49:1777–1786. doi: 10.1111/j.1528-1167.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biervert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 21.Charlier C, Singh NA, Ryan SG, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18:53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- 22.Singh NA, Charlier C, Stauffer D, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 23.Porter RJ, Partiot A, Sachedo R, Nohria C, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- 24.Brodie MJ, Lerche H, Gil-Nagel A, et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75:1817–1824. doi: 10.1212/WNL.0b013e3181fd6170. [DOI] [PubMed] [Google Scholar]
- 25.French JA, Abou-Khalil BW, Leroy RF, et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76:1555–1563. doi: 10.1212/WNL.0b013e3182194bd3. [DOI] [PubMed] [Google Scholar]
- 26.Streng T, Christoph T, Andersson KE. Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J Urol. 2004;172:2054–2058. doi: 10.1097/01.ju.0000138155.33749.f4. [DOI] [PubMed] [Google Scholar]
- 27.Rode F, Svalo J, Sheykhzade M, Ronn LCB. Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur J Pharmacol. 2010;638:121–127. doi: 10.1016/j.ejphar.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 28.McNeilly RJ, Torchin CD, Anderson LW, Kapetanovic IM, Kupferberg HJ, Strong JM. In vitro glucuronidation of D-23129, a novel anticonvulsant, by human liver microsomes and liver slices. Xenobiotica. 1997;27:431–441. doi: 10.1080/004982597240424. [DOI] [PubMed] [Google Scholar]
- 29.Hempel R, Schupke H, McNeilly PJ, et al. Metabolism of retigabine (D-23129), a novel anticonvulsant. Drug Metab Dispos. 1999;27:613–622. [PubMed] [Google Scholar]
- 30.Hiller A, Nguyen N, Strassburg C, et al. Retigabine N-glucuronidation and its potential role in enterohepatic circulation. Drug Metab Dispos. 1999;27:605–612. [PubMed] [Google Scholar]
- 31.Borlak J, Gasparic A, Locher M, Schupke H, Hermann R. N-glucuronidation of the antiepileptic drug retigabine: results from studies with human volunteers, heterologously expressed human UGTs, human liver, kidney, and liver microsomal membranes of Crigler Najjar type II. Metabolism. 2006;5:711–721. doi: 10.1016/j.metabol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Ferron GM, Paul J, Fruncillo R, et al. Multiple-dose, linear, dose-proprotional, pharmacokinetics of retigabine in healthy volunteers. J Clin Pharmacol. 2002;42:175–182. doi: 10.1177/00912700222011210. [DOI] [PubMed] [Google Scholar]
- 33.Hermann R, Ferron GM, Erb K, et al. Effects of age and sex on the disposition of retigabine. Clin Pharmacol Ther. 2003;73:61–70. doi: 10.1067/mcp.2003.12. [DOI] [PubMed] [Google Scholar]
- 34.Hermann R, Knebel NG, Niebch G, Richards L, Borlak J, Locher M. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2003;58:795–802. doi: 10.1007/s00228-003-0558-6. [DOI] [PubMed] [Google Scholar]
- 35.Ferron GM, Patat A, Parks V, Rolan P, Troy SM. Lack of pharmacokinetic interaction between retigabine and phenobarbitone at steady-state in healthy subjects. Br J Clin Pharmacol. 2003;56:39–45. doi: 10.1046/j.1365-2125.2003.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Epilepsy Res. Vol. 83. Progress; 2009. report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX) pp. 1–43. [DOI] [PubMed] [Google Scholar]
- 37.Shillito P, Molenaar PC, Vincent A, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. 1995;38:714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- 38.Auger RG, Daube JR, Gomez MR, Lambert EH. Hereditary form of sustained muscle activity of peripheral nerve origin causing generalized myokymia and muscle stiffness. Ann Neurol. 1984;15:13–21. doi: 10.1002/ana.410150104. [DOI] [PubMed] [Google Scholar]
- 39.Dedek K, Kunath B, Kananura C, Reuner U, Jentsch TJ, Steinlein OK. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc Natl Acad Sci U S A. 2001;98:12272–12277. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuttke TV, Jurkat-Rott K, Paulus W, Garnkarek M, Lehrman-Horn F, Lerche H. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 2007;69:2045–2053. doi: 10.1212/01.wnl.0000275523.95103.36. [DOI] [PubMed] [Google Scholar]
- 41.Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviors in rat models of persistent and neuropathic pain. Eur J Pharmacol. 2003;460:109–116. doi: 10.1016/s0014-2999(02)02924-2. [DOI] [PubMed] [Google Scholar]
- 42.Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology. 2007;53:609–618. doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Dencker D, Dias R, Pedersen ML, Husum H. Effect of the new antiepileptic drug retigabine in a rodent model of mania. Epilepsy Behav. 2008;12:49–53. doi: 10.1016/j.yebeh.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Hansen HH, Andreasen JT, Weikop P, Mirza M, Scheel-Kruger J, Mikkelsen JD. The neuronal KCNQ channel opener retigabine inhibits locomotor activity and reduces forebrain excitatory responses to the psychostimulants cocaine, methylphenidate and phencyclidine. Eur J Pharmacol. 2007;570:77–88. doi: 10.1016/j.ejphar.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Richter A, Sander SE, Rundfelt C. Antidystonic effects of Kv7 (KCNQ) channel openers in the dtsz mutant, an animal model of primary paroxysmal dystonia. Br J Pharmacol. 2006;149:747–753. doi: 10.1038/sj.bjp.0706878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314:282–292. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]