Abstract
Biological markers are already used in the diagnosis and treatment of cardiovascular disease and cancer. Biomarkers have great potential use in the clinic as a noninvasive means to make more accurate diagnoses, monitor disease progression, and create personalized treatment regimes. Asthma is a heterogeneous disease with several different phenotypes, generally triggered by multiple gene-environment interactions. Pulmonary function tests are most often used objectively to confirm the diagnosis. However, airflow obstruction can be variable and thus missed using spirometry. Furthermore, lung function measurements may not reflect the precise underlying pathological processes responsible for different phenotypes. Inhaled corticosteroids and β2-agonists have been the mainstay of asthma therapy for over 30 years, but the heterogeneity of the disease means not all asthmatics respond to the same treatment. High costs and undesired side effects of drugs also drive the need for better targeted treatment of asthma. Biomarkers have the potential to indicate an individual’s disease phenotype and thereby guide clinicians in their decisions regarding treatment. This review focuses on biomarkers of airway inflammation which may help us to identify, monitor, and guide treatment of asthmatics. We discuss biomarkers obtained from multiple physiological sources, including sputum, exhaled gases, exhaled breath condensate, serum, and urine. We discuss the inherent limitations and benefits of using biomarkers in a heterogeneous disease such as asthma. We also discuss how we may modify our study designs to improve the identification and potential use of potential biomarkers in asthma.
Keywords: asthma, inflammation, airway biomarkers, urinary biomarkers, serum biomarkers
Background
Modern technology allows the measurement of a myriad of biological parameters. Many of these biological markers (biomarkers) have found clinical and regulatory acceptance as substitutes for clinical endpoints. Such surrogate endpoints are particularly useful when the clinical endpoint in question is extreme or relatively rare, eg, survival, myocardial infarction, or cancer recurrence. Reduction of elevated arterial blood pressure has been used for decades to indicate a lower incidence of congestive heart failure and stroke. Serum cholesterol is also commonly used as an indirect measure of coronary artery disease without the need for invasive exploratory surgery. Thus, a biomarker is a candidate surrogate endpoint, able to indicate presence of disease without waiting for extreme clinical endpoints to occur, and avoiding the need for potentially invasive surgery.
The National Institutes of Health defines a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”.1
The ability to diagnose or treat a disease by measuring a biological molecule from a noninvasive source, such as blood, urine, or exhaled breath, has a great advantage over traditional pathological techniques because direct access to diseased tissue is not required. If we were designing a “perfect” biomarker, it should be a mediator in a biochemical pathway necessary for development of the disease rather than an epiphenomenon and, as such, should act as a potential target for therapy. Levels of the marker should be low and stable in normal individuals and measurably higher in patients with disease, a difference which should be consistent between individuals with disease. Lastly, the technique required to measure the biomarker should be rapid, straightforward, and relatively inexpensive.
In the cardiovascular field, Maisel describes the ideal biomarker as a “tool that should aid the physician in one or more of the following: diagnosis and subsequent risk stratification, risk stratification for secondary prevention, guiding selection of therapy, and, finally, in some cases, serving as a target for therapy”.2 In addition to diagnosis and treatment, targeting a biomarker can act as a predictor. If a rise in a biomarker precedes a worsening of a condition, this advance warning may allow enough time for treatment to be adjusted to avoid an exacerbation. In chronic obstructive pulmonary disease (COPD) for example, plasma levels of the cardiac dysfunction biomarkers, troponin-T and N-terminal probrain natriuretic peptide, are often raised in patients suffering from acute exacerbations and are associated with increased mortality.3 Although this was a retrospective study of patients admitted to hospital after the onset of a COPD exacerbation, it is possible that a rise in plasma levels of these two biomarkers may precede the worsening of symptoms, and therefore act as a predictor of COPD exacerbation.
Clinical assessment of asthma
Pulmonary function tests are routinely used clinically to diagnose asthma. Forced expiratory volume in one second (FEV1) is a measure of airflow and is influenced largely by resistance in the airways. During an “asthma attack”, the airways narrow as a result of mucus hypersecretion and smooth muscle contraction, which results in a sharp increase in airway resistance and reduction in FEV1. In the clinic, this can be reproduced by exposing patients with asthma to nonspecific smooth muscle contractile agonists such as methacholine. A fall in FEV1 at a relatively low dose of methacholine indicates airway hyperresponsiveness. In asthmatic patients, this drop in FEV1 can be reversed by inhaled bronchodilators, such as β2-agonists (eg, salbutamol), which relax airway smooth muscles. Airway hyperresponsiveness is often used as a diagnostic criterion for asthma.
Asthma is rarely fatal but often leads to significant patient morbidity. For over 30 years, the mainstay of therapy for asthma has been inhaled β2-agonists, which relax the airway smooth muscles, causing bronchodilation, and inhaled corticosteroids which ameliorate airway inflammation and reduce the risk of asthma exacerbations. Steroids are anti-inflammatory drugs but also have many unwanted side effects, particularly at high doses, including weight gain due to changes in body metabolism, and a reduction in bone mineral density, potentially leading to brittle bones. Therefore, the decision to treat a patient with steroids should not be taken lightly.
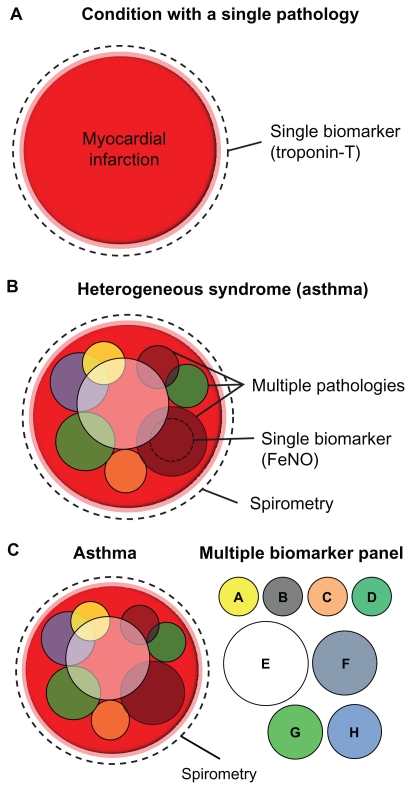
Asthma is increasingly considered a syndrome, with diverse overlapping pathologies and phenotypes contributing to significant heterogeneity in clinical manifestation, disease progression, and treatment response.4 Severe uncontrolled asthmatics make up approximately 5%–10% of the population of asthmatics, yet they consume around 50% of the treatment resources.5 Spirometric tests are incapable of exposing the underlying pathologies or phenotypes which combine within an individual to produce asthma. In contrast, specific biomarkers may enable more accurate subphenotyping of disease by indicating the pathology in an individual, and assist clinicians to tailor better the type and/or dose of therapy. In this way, biomarkers have the potential to guide more effective personalized treatment regimes in asthma (Figure 1).
Figure 1.
Heterogeneous diseases require multiple biomarkers for accurate diagnosis. (A) A condition with a single dominant underlying pathology such as myocardial infarction can be accurately diagnosed using a single biomarker (eg, troponin T). (B) A heterogeneous disease such as asthma is a result of multiple overlapping pathologies, which may vary between individuals. Spirometry can be used to diagnose asthma in its broadest clinical terms, but cannot distinguish between different subphenotypes of disease. A single biomarker (such as FeNO) can only identify a single subphenotype of a heterogeneous disease. (C) A panel of biomarkers is required to diagnose the subtype of asthma accurately in an individual.
Abbreviation: FeNO, fractional exhaled nitric oxide.
Airway remodeling in asthma
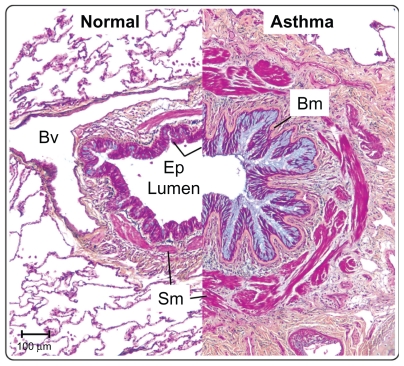
Although asthma is considered an inflammatory disease, there are many structural changes in the airways. Figure 2 shows cross-sections through the large airways of two patients, one normal and one a severe asthmatic. The Movat’s pentachrome stain clearly highlights the various architectural remodeling events occurring in the asthmatic airway. Obstruction of the airways by excessive mucus production is a common finding in severe asthmatics, and blue staining in the epithelium and lumen demonstrates mucous cell hyperplasia, with excessive mucus deposition into the airway. Under the asthmatic epithelium, a thicker basement membrane is present which contains several different extracellular matrix factors (including tenascin-C) compared with normals.6 Deeper into the airway, red-stained muscle mass is increased in the asthmatic, owing to a combination of smooth muscle hypertrophy and hyperplasia.7 The airways of severe asthmatics also demonstrate a fibrotic response, with increased connective tissue deposition and fibroblast and myofibroblast proliferation. Opinion is divided as to whether inflammation precedes airway remodeling, or whether the two occur in parallel.8–10 Evidence tends to favor the latter because, firstly, remodeling occurs very early on in the disease, in some cases in the absence of inflammation,11 secondly, there is only a weak link between airway inflammation and symptoms,12 and, thirdly, epidemiological data demonstrate that steroids do not work in all asthmatics.13 In reality, it is likely that effective therapies will need to target both airway inflammation and remodeling.
Figure 2.
The airways in asthma undergo significant structural remodeling. Medium-sized airways from a normal individual and a severe asthmatic patient were sectioned and stained using Movat’s pentachrome stain. The epithelium in asthma shows mucous hyperplasia and hypersecretion (blue), and significant basement membrane (Bm) thickening. Smooth muscle (Sm) volume is also increased in asthma. Scale bar 100 μm.
Abbreviations: Bv, blood vessel; Ep, epithelium; Bm, basement membrane; Sm, smooth muscle.
Inflammation in asthma
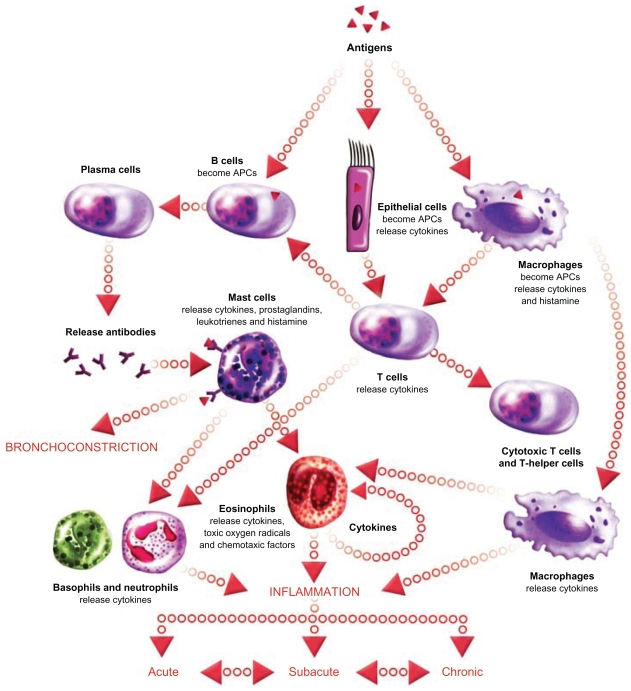
As previously mentioned, the rationale for treating severe asthmatics with steroids is based on the assumption that the disease is driven by uncontrolled airway inflammation. The classical paradigm of the acute inflammatory response in allergic asthma is shown in Figure 3. Briefly, inhaled allergenic antigens are captured by antigen-presenting dendritic cells, macrophages, or epithelial cells. Antigens presented by these cells are recognized by T cells which proliferate and differentiate. Primed Th2 cells bind to B cells and release cytokines, that trigger the maturation of antigen-specific B cell populations into plasma cells. Plasma B cells release antigen-specific IgE that binds to IgE receptors on mast cells in the airways, causing release of histamine-containing granules. Extracellular histamine released from mast cells binds to membrane receptors on airway smooth muscle cells, triggering a rise in intracellular calcium, muscle contraction, and airway narrowing. Although this paradigm has gained acceptance as an important mechanism in asthma, there is significant variation in the specific inflammatory cell types involved. In the setting of chronic asthma, eosinophils are thought to play a major role in maintaining airway inflammation in the long term. Neutrophils are occasionally the predominant inflammatory cell present in the airways of chronic asthmatics, suggesting multiple underlying pathologies of disease. Neutrophilic versus eosinophilic asthma are clinically indistinguishable by pulmonary function testing, but such pathological variation may have important implications regarding treatment because patients with neutrophilic inflammation tend to be relatively unresponsive to steroid treatment. 13 A rapid and definitive method for determining the nature of airways inflammation in a diagnosed asthmatic is therefore needed to guide selection of therapy.
Figure 3.
The inflammatory cascade in asthma. Acute-phase inflammation is triggered when inhaled allergens are captured by antigen-presenting cells (epithelial cells, dendritic cells, macrophages) and presented to T cells. Activated Th2 cells trigger B cells to become antibody-producing plasma cells. Plasma cells release antigen-specific IgE which binds to IgE receptors on mast cells. Activated mast cells degranulate releasing histamine which binds to receptors on airway smooth muscle cells, triggering contraction and airway narrowing. Repeated bouts of acute inflammation can lead to chronic inflammation with persistent airway eosinophilia and/or neutrophilia. Image courtesy of EPG Online at www.epghealthmedia.com.
Abbreviation: APC, antigen-presenting cell.
Direct measures of airway inflammation
Tables 1 and 2 summarize the advantages and disadvantages of current and potential future biomarkers of airway inflammation for the diagnosis and monitoring of asthma in the clinic. These are discussed in more detail below.
Table 1.
Advantages and disadvantages of currently used asthma biomarkers
| Biomarker | Advantage | Disadvantage |
|---|---|---|
| Pulmonary function tests (PFTs) (FEV1, AHR) | Non-invasive, well validated, sensitive – ie, will detect all asthmatics, reproducible, PFTs will change rapidly with treatment. | Unable to identify sub-phenotypes of asthmatics, PFTs do not reflect pathology, PFTs cannot predict treatment response. |
| Tissue biopsy | Definitive measure of airway inflammation. | Highly invasive, disconnect between cell counts and symptoms, time consuming, requires high level of expertise. |
| Induced sputum (differential inflammatory cell counts) | Less invasive than tissue biopsy, reliable indicator of airway inflammation. | Very uncomfortable process, limited to children >8 yrs, requires expertise, reproducibility problems. |
| Exhaled nitric oxide (FeNO) | Non-invasive, simple measurement methods, indicates treatment (steroid) response. | Proven only for a sub-set of asthmatics, expensive equipment, a single biomarker reflects a single pathology. |
Table 2.
Alternative biomarkers for asthma diagnosis and management
| Biomarker source | Advantages | Disadvantages |
|---|---|---|
| Exhaled breath condensate (EBC) – pH & proteins (Inflammatory markers; IL-6, IL-8, TNF-α, H2O2, leukotrienes, 8-isoprostane. Non-inflammatory markers; actin, cytokeratins, albumin) | Non-invasive, multiple biomarkers in sample therefore sub-phenotyping possible. | Collection techniques drastically affect proteins in sample, reproducibility a problem, salivary contamination, unproven clinical effectiveness. |
| Serum proteins (Leptin/adiponectin, eosinophillic cationic protein, chemokines, chitinases) | Less invasive, multiple biomarkers = sub-phenotyping, standardised collection and processing techniques. | Less sensitive and slower response to airway changes, unproven clinical effectiveness. |
| Urinary metabolites (>70) endpoints | Non-invasive, multiple biomarkers = sub-phenotyping, standarised collection and processing, good sensitivity and specificity. | Unproven clinical effectiveness (although preliminary data is positive), limited access to NMR equipment. |
Tissue biopsies
To date the most accurate method to assess lung inflammation (and remodeling) is by histological examination of lung tissue. Subtypes of inflammatory cells can be identified in tissue sections using specific stains. Unfortunately the process of taking bronchial biopsies via bronchoscopy is invasive and requires experienced, skilled pathologists for tissue examination. In addition, clinical studies demonstrate a disconnect between the numbers of inflammatory cells counted in airway biopsies, and lung function in asthmatics.12
Induced sputum
One alternative method of directly assessing airway inflammation is by sputum induction. A patient inhales nebulized hypertonic saline to trigger sputum production in the airways. The sputum is then coughed out along with any inflammatory cells present in the airway lumen. The main assumption is that the inflammatory infiltrate in the airway lumen reflects that in the tissue. Cytospins of the resulting sputum samples are then stained using similar techniques to tissue biopsies to examine the specific type of cellular infiltrate in the sputum. The principal output is the differential inflammatory cell count, expressed as a percentage, based on the manual counting of 400 cells (eosinophils, neutrophils, lymphocytes, macrophages, epithelial cells). The upper limit of eosinophilic proportion in normals is approximately 1.9% and this fraction rises up to seven-fold in asthmatics after allergen challenge, before exacerbations, and following inhaled corticosteroid withdrawal.14 This technique has been used to identify subpopulations of asthmatics who demonstrate neutrophilic rather than eosinophilic asthma, and are therefore less likely to respond to steroids.13 In patients with moderate to severe asthma, the absence of sputum eosinophilia cannot distinguish between patients who suffer from noneosinophilic asthma versus those whose eosinophilia is controlled by steroids. A recent study has shown that the eosinophilic proteins, eosinophil cationic protein and eosinophil peroxidase, can be identified in cultured macrophages that have consumed apoptotic eosinophils.15 In patients, eosinophil cationic protein/eosinophil peroxidase-positive macrophages were increased in the sputum of moderate to severe asthmatics treated with inhaled steroids regardless of sputum eosinophilia, in individuals who suffer from eosinophilia upon steroid withdrawal, and in patients with eosinophilic bronchitis. Thus, sputum macrophage eosinophil protein content is an indirect biomarker of airway eosinophilia that can identify asthmatics whose disease stems from airway eosinophilia, even in patients undergoing steroid treatment.15
The protein content of induced sputum may also have some value in diagnosing asthma. High mobility group box-1, a ligand of the receptor for advanced glycation end products (RAGE), is a mediator in many inflammatory disorders. High mobility group box-1 is increased in the sputum of asthmatic patients, along with endogenous secretory RAGE, a soluble receptor that inhibits RAGE signaling.16 Sputum induction is considerably less invasive than a tissue biopsy, but this technique is nonetheless uncomfortable for the patient. Given that many children are unwilling to undergo sputum collection at follow-up visits, this technique tends to be limited to patients aged eight years and older (although sputum induction has been performed successfully on younger children).14 The techniques of sputum induction and processing are well validated, but they are time-consuming, require skilled people, and results are often difficult to reproduce and vary across centers.
Exhaled biomarkers
Less invasive means of obtaining airway biomarkers are desirable, particularly as diagnostic or disease monitoring tools in pediatric patients. The composition of exhaled breath is correlated with various disease states. This has been exploited by cancer researchers who have pioneered the use of gas sensor arrays, or “electronic noses”, to aid the diagnosis of lung cancer.17 Because asthma is also a disease of the airways, it follows that the composition of exhaled breath will also be altered in the disease. The advent of so-called “breathomics” promises to revolutionize the way clinicians diagnose and treat asthmatics.
Fractional exhaled nitric oxide
To date, fractional exhaled nitric oxide (FeNO) is the most widely used exhaled biomarker of airway inflammation in asthma. Levels of nitric oxide in exhaled breath can be measured relatively quickly in the clinic, although the gas analyzers required are expensive.14 FeNO is often increased in steroid-naïve asthmatics and severe asthmatics, and is correlated with airway eosinophilia.18,19 FeNO is derived from the action of inducible nitric oxide synthase expressed by the airway epithelium,20 although the precise mechanism of how eosinophilia triggers inducible nitric oxide synthase activity in epithelial cells is undefined. Advocates of FeNO use in asthma suggest that it can be used as a noninvasive biomarker to indicate likelihood of response to steroid therapy.21–25 A defined schema has been proposed for FeNO-directed asthma diagnosis and treatment. Briefly, in steroid-naïve asthmatics, FeNO should be used as an indicator of steroid responsiveness. A low FeNO level of less than 25 parts per billion indicates that the patient is unlikely to suffer from eosinophilic asthma and is less likely to respond to steroids. A high FeNO (>50 parts per billion) strongly suggests airway eosinophilia and steroid responsiveness. In difficult asthmatics already undergoing inhaled corticosteroid treatment, FeNO can indicate whether poor disease control is due to uncontrolled airway inflammation or to noninflammatory causes, thus assisting clinicians to direct inhaled corticosteroid dosage. Despite this logic, several randomized controlled trials have concluded that FeNO has little value over and above clinical symptoms when optimizing the dose of steroid used for asthma treatment.26 It is important to note that each of the six trials reviewed used different FeNO values as cutoff points to direct treatment decisions, ranging from 20 to 45 parts per billion. Because the predictive value of FeNO for steroid responsiveness varies greatly depending on the cutoff value used, it is difficult to compare the results of trials which used different designs. Nevertheless, taken together, these studies suggest that FeNO alone cannot provide reliable information on the exact dosage of inhaled corticosteroid required in a given patient. Rather, FeNO is best used to determine whether the present dose of inhaled corticosteroid is adequate to control airway inflammation.21 If inadequate, clinicians may choose to increase the dose of inhaled corticosteroids or add additional anti-inflammatory therapies, eg, leukotriene receptor modifiers. There is a large variation in FeNO levels between individuals, which may reflect the natural heterogeneity in baseline epithelial nitric oxide synthase activity and/or the contribution of other noneosinophilic factors to epithelial nitric oxide synthase activity. Interindividual variation in FeNO combined with the inherent heterogeneity of asthma increases the background noise, which renders FeNO a relatively insensitive tool for guiding therapy in all asthmatics. However, FeNO values in an individual are highly reproducible, so it may be more successful to use personalized cutoff values for each subject, rather than using a single cutoff value for all patients.27 It is possible that there are certain subgroups of asthmatics in whom FeNO measurement would have an excellent signal to noise ratio. In the future, with improved phenotyping, identification of such subgroups may be possible.
Atopy, ie, the genetic predisposition to develop allergic diseases such as eczema, allergic rhinitis, and asthma, is a possible confounding factor when measuring FeNO in asthma. Atopy is often accepted as the major trigger of asthma, particularly in children, but its association with the disease may have been exaggerated. Epidemiological studies have demonstrated that the proportion of asthmatic cases attributable to atopy in the general population is usually less than 50% and many atopics do not develop asthma.28 Various studies demonstrate that FeNO is increased in atopic individuals with and without asthma, suggesting FeNO acts as a biomarker of atopy and the “allergic asthma” phenotype.29–32 Contradictory studies have found that atopy, as defined by positive skin prick testing, or the presence of various allergic diseases, is not related to FeNO and thus elevated FeNO is a relatively specific marker of asthmatic disease.33,34 With such polarized opinion as to whether atopy is indeed a confounding factor, it is probably wise at present to consider the coexistence of atopy when assessing a patient’s asthmatic status based on FeNO.
Exhaled breath condensate
The collection of exhaled breath condensate and subsequent measurement of inflammatory biomarkers is a relatively recent development. Exhaled breath condenses when it comes into contact with a cooled collector, allowing the collection of respiratory particles, droplets, and water vapor. The pH of exhaled breath condensate has been shown to relate to airway inflammation. Low exhaled breath condensate pH indicates poorly controlled eosinophilic asthma in a similar manner to high FeNO.25 It is unlikely that a single biomarker will be able to reflect the various pathologies present in a heterogeneous disease such as asthma. Therefore, additional markers of airway inflammation will be needed to provide information complementary to that gained from FeNO measurements. Many proteins are present in exhaled breath condensate, and this method has been proposed as a means to allow the objective proteomic analysis of exhaled breath. As with pH, several of these proteins are markers of oxidative stress, including cysteinyl leukotrienes, leukotriene B4, 8-isoprostane, and hydrogen peroxide, although inflammatory proteins, such as interleukin (IL)-6, IL-8, and tumor necrosis factor alpha may also be useful markers.14,35 Other proteins which do not obviously fit into any inflammatory pathway, including actin, cytokeratins, albumin, and hemoglobin, have also been shown to be increased in the exhaled breath condensate of asthmatic patients.36
Currently there is no standard type of condenser used to collect exhaled breath condensate. The differing surface properties of each condenser type causes significant variation in the nature of the particles collected, although exercise and environmental conditions also contribute.37 For this reason, standardization of collector apparatus and protocols are required to allow exhaled breath condensate measurements to be comparable between sites.
Non-exhaled biomarkers
Serum proteins
As described earlier, biomarker studies in asthma have tended to concentrate on changes to the composition of exhaled breath. Although primarily a disease of the airways, there is mounting evidence to suggest there is also a systemic component to asthma.38 If this is the case, then circulating metabolites may be able to act as biomarkers of airways disease. Blood collection, serum isolation, and analysis are highly standardized techniques of a minimally invasive nature and are therefore an ideal source of reproducible data. Serum proteins are already gaining credence as biomarkers in other inflammatory lung diseases. In COPD, for example, not all smokers develop the disease, so being able to identify those who are at risk would be useful. Studies have shown circulating levels of pulmonary and activation-regulated chemokine/CCL-18 are increased in COPD patients,39 and those patients who show a rapid fall in FEV1 tend to have high circulating levels of fibrinogen.40 Even accepted markers of cardiac dysfunction, eg, cardiac troponin-T and N-terminal probrain natriuretic peptide, have been associated with increased mortality in patients with COPD, suggesting a cardiac component to the disease.3 Several serum biomarkers have been demonstrated to be associated with asthma, including eosinophil cationic protein. Eosinophil cationic protein levels increase in response to allergen challenge, and decrease after allergen avoidance or inhaled corticosteroid therapy, albeit in a less responsive manner than sputum eosinophils or FeNO.14 However, in the clinic, serum eosinophil cationic protein levels do not reflect treatment-induced functional changes in chronic asthmatics, and serum eosinophil cationic protein is unable to predict steroid responsiveness.14 One randomized trial demonstrated that patients whose asthma management was based on serum eosinophil cationic protein levels experienced no improvement in symptoms compared with those treated using traditional monitoring techniques.41 In addition, raised serum eosinophil cationic protein levels may not be a specific marker of asthma, as studies in pediatric patients have demonstrated serum eosinophil cationic protein is also raised in cystic fibrosis and viral bronchiolitis.42
Asthma is to a large extent a disease of gene-environment interaction. One important environmental factor is obesity, with several studies demonstrating an association with asthma. The leptin/adiponectin balance is an important regulator of body fat metabolism and this ratio is altered in obesity. Studies have shown that levels of circulating leptin and adiponectin are altered in obese and nonobese patients with asthma.43 Interestingly, a recent study in a Swedish cohort demonstrated that obese patients who wheeze have reduced FeNO compared with obese patients without asthma symptoms.44 FeNO is not related to body mass index in patients without airways disease,44,45 suggesting the underlying disease mechanisms, and consequently asthma phenotype, may be different in patients with a high body mass index.
Other novel biomarkers are also altered in the circulation of patients with asthma. CCL-17 is a chemokine released from dendritic cells and epithelial cells after allergen contact, and is involved in the recruitment of Th2 cells into the lungs. Sputum levels of CCL-17 are increased in the lungs of asthmatic adults,46 whilst serum levels of CCL-17 are increased in children with asthma and lowered in steroid-treated children with asthma.47 The chitinase-like protein, YKL-40, has also been shown to be increased in the serum of adult asthmatics and is correlated with sputum levels and various clinical markers of asthma severity.48,49 In asthma, structural cells of the airway, including the epithelium, release various proinflammatory chemokines, such as IL-6 and IL-8, which trigger subsequent infiltration by immune cells. The epithelium also releases proteinases which are capable and necessary for the cleavage and activation of chemokinetic activity for molecules such as fas-ligand and IL–25.50,51 It is possible that circulating levels of these chemokines and proteinases may provide an early warning for an imminent asthma exacerbation, or even indicate the particular subtype of inflammation occurring in the patient.
Urinary metabolites
The aforementioned data suggest that the metabolism of patients with asthma is altered compared with normals. Urine is possibly the least invasive biofluid for biomarker measurements, and is therefore highly suitable for the study and assessment of asthma in young children. Clinical studies have shown urinary biomarkers are potentially useful in asthma. Levels of the downstream histamine metabolite, N-methylhistamine, is increased in the urine of patients with asthma, is increased after allergen challenge or exacerbation, and is reduced in asthmatic children taking antiallergy medication.52,53
One recent study used nuclear magnetic resonance spectroscopy (NMR) to measure levels of 70 metabolites in the urine of children with and without asthma. NMR is an attractive method for urinary biomarker examination because it is able to provide qualitative and quantitative data on multiple compounds in a complex biofluid, without requiring significant pretreatment of the sample. Urine was collected from control children without asthma, children with stable asthma in the outpatient department, and from children with unstable disease hospitalized for an asthma exacerbation. NMR examination of urinary metabolites showed a 94% success rate in identifying outpatient department asthmatic children versus children without asthma, and a similar success rate was seen when diagnosing an asthma exacerbation versus outpatient department asthma.54 This study suggests that measurement of urinary metabolites is a potentially valuable technique to help clinicians diagnose and monitor asthma in children.
Future for biomarkers in asthma
In recent years, it has become increasingly accepted that pulmonary function testing has its limitations. Spirometry can identify a broad spectrum of asthmatics, but it is incapable of discerning the various subtypes of disease and therefore which individuals will respond to normal treatment regimes. Tissue biopsies and inflammatory cell counting in induced sputum are accepted measures of determining airway inflammation, but both techniques are invasive, expensive, and difficult to standardize, making them unsuitable for routine clinical use. Biomarkers hold the promise of being able to diagnose and monitor various subtypes of asthma rapidly and specifically in a noninvasive manner.
Before considering biomarkers for use in the clinic, we must appreciate that asthma is a heterogeneous disease. Expecting a single biomarker to improve the treatment of all asthmatics regardless of their underlying disease phenotype is unrealistic. The most well developed biomarker in current use is FeNO. However, as previously discussed, its utility is lost when it is applied to all asthmatics because of the underlying heterogeneity of the phenotypes. In practice, a panel of biomarkers is needed to indicate the various different underlying disease pathologies, thus enabling the definitive and objective categorization of asthmatics into distinct subphenotypes (see Figure 1). Standardization of protocols is vital when designing a panel of asthma biomarkers for clinical use. FeNO is a promising biomarker, but at present there is disagreement about what is a normal or abnormal FeNO level. Defining appropriate cutoff points is crucial to guide the appropriate clinical response.
Exhaled breath condensate is a potentially rich source of airway biomarkers, but again standard collection protocols are required before use in the clinic is even considered. In contrast, protocols for biofluid (serum and urine) collection and processing are well developed, and indeed several studies have shown that monitoring systemic or secreted metabolites may be the best way to define multiple biomarkers for the diagnosis and design of treatment regimes for asthma.
To date, the majority of asthma biomarkers have reflected airways inflammation, but inflammation is not the only pathological component of the disease. Airways remodeling plays a major role in asthma pathology, and so markers designed to indicate structural changes, such as epithelial damage, mucous hyperplasia, myofibroblast proliferation, and smooth muscle growth, may also prove useful to define a disease phenotype accurately. Regardless of the complexity or completeness of a future panel of asthma biomarkers, it is highly unlikely that they will completely replace pulmonary function testing in the clinic. Indeed, biomarker testing will be designed to complement rather than replace existing methods of clinical diagnosis and disease monitoring.
Acknowledgments
The authors wish to thank Dr M Elliott and A Samra for their assistance and advice regarding tissue histology. We also thank the iCAPTURE biobank and the International Institute for the Advancement of Medicine for human tissue.
Footnotes
Disclosure
Supported by operating grants from the Canadian Institutes of Health Research, the Canadian/British Columbia Lung Associations, National Sanatorium Association, and Allergen, NCE and personal support awards from the Michael Smith Foundation for Health Research (D.R.D.) and the Canadian Institutes of Health Research (D.R.D.), D.D.S is holder of a Canadian Research Chair.
References
- 1.Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.Maisel A. Biomarkers in heart failure. Does prognostic utility translate to clinical futility? J Am Coll Cardiol. 2007;50(11):1061–1063. doi: 10.1016/j.jacc.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011 April 7; doi: 10.1136/thx.2010.155333. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 5.Adcock IM, Ito K. Steroid resistance in asthma: a major problem requiring novel solutions or a non-issue? Curr Opin Pharmacol. 2004;4(3):257–262. doi: 10.1016/j.coph.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med. 1997;156(3 Pt 1):951–958. doi: 10.1164/ajrccm.156.3.9610084. [DOI] [PubMed] [Google Scholar]
- 7.Hirst SJ. Airway smooth muscle as a target in asthma. Clin Exp Allergy. 2000;30 (Suppl 1):54–59. [PubMed] [Google Scholar]
- 8.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105(2 Pt 1):193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 9.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007;7(1):63–68. doi: 10.1097/ACI.0b013e328013d61b. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. The role of inflammation and anti-inflammatory medication in asthma. Respir Med. 2002;96 (Suppl A):S9–S15. [PubMed] [Google Scholar]
- 11.Malmstrom K, Pelkonen AS, Malmberg LP, et al. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66(2):157–162. doi: 10.1136/thx.2010.139246. [DOI] [PubMed] [Google Scholar]
- 12.Sont JK, Han J, van Krieken JM, et al. Relationship between the inflammatory infiltrate in bronchial biopsy specimens and clinical severity of asthma in patients treated with inhaled steroids. Thorax. 1996;51(5):496–502. doi: 10.1136/thx.51.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni NS, Hollins F, Sutcliffe A, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126(1):61.e3–69.e3. doi: 10.1016/j.jaci.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105(4):519–525. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico A, Pennazza G, Santonico M, et al. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer. 2010;68(2):170–176. doi: 10.1016/j.lungcan.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1376–1381. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 19.Schleich FN, Seidel L, Sele J, et al. Exhaled nitric oxide thresholds associated with a sputum eosinophil count ≥3% in a cohort of unselected patients with asthma. Thorax. 2010;65(12):1039–1044. doi: 10.1136/thx.2009.124925. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Malavia N, Suresh V, George SC. Nitric oxide gas phase release in human small airway epithelial cells. Respir Res. 2009;10:3. doi: 10.1186/1465-9921-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor DR. Nitric oxide as a clinical guide for asthma management. J Allergy Clin Immunol. 2006;117(2):259–262. doi: 10.1016/j.jaci.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130(5):1319–1325. doi: 10.1378/chest.130.5.1319. [DOI] [PubMed] [Google Scholar]
- 23.Smith AD, Cowan JO, Brassett KP, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005;172(4):453–459. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 24.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60(3):215–218. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostikas K, Papaioannou AI, Tanou K, et al. Exhaled NO and exhaled breath condensate pH in the evaluation of asthma control. Respir Med. 2011;105(4):526–532. doi: 10.1016/j.rmed.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Petsky HL, Cates CJ, Li A, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2009;(4):CD006340. doi: 10.1002/14651858.CD006340.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Holz O, Magnussen H. Cutoff values for FENO-guided asthma management. Am J Respir Crit Care Med. 2009;180(3):281–282. doi: 10.1164/ajrccm.180.3.281a. [DOI] [PubMed] [Google Scholar]
- 28.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54(3):268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hervas D, Milan JM, Garde J. Differences in exhaled nitric oxide in atopic children. Allergol Immunopathol (Madr) 2008;36(6):331–335. doi: 10.1016/s0301-0546(08)75865-8. [DOI] [PubMed] [Google Scholar]
- 30.Chan EY, Ng DK, Chan CH. Measuring FENO in asthma: coexisting allergic rhinitis and severity of atopy as confounding factors. Am J Respir Crit Care Med. 2009;180(3):281. doi: 10.1164/ajrccm.180.3.281. [DOI] [PubMed] [Google Scholar]
- 31.Banovcin P, Jesenak M, Michnova Z, et al. Factors attributable to the level of exhaled nitric oxide in asthmatic children. Eur J Med Res. 2009;14 (Suppl 4):9–13. doi: 10.1186/2047-783X-14-S4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott M, Raza A, Karmaus W, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65(3):258–262. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouhos A, Kainu A, Karjalainen J, et al. Atopic sensitization to common allergens without symptoms or signs of airway disorders does not increase exhaled nitric oxide. Clin Respir J. 2008;2(3):141–148. doi: 10.1111/j.1752-699X.2007.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordeiro D, Rudolphus A, Snoey E, Braunstahl GJ. Utility of nitric oxide for the diagnosis of asthma in an allergy clinic population. Allergy Asthma Proc. 2011;32(2):119–126. doi: 10.2500/aap.2011.32.3419. [DOI] [PubMed] [Google Scholar]
- 35.Gessner C, Rechner B, Hammerschmidt S, et al. Angiogenic markers in breath condensate identify non-small cell lung cancer. Lung Cancer. 2010;68(2):177–184. doi: 10.1016/j.lungcan.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Bloemen K, Van Den Heuvel R, Govarts E, et al. A new approach to study exhaled proteins as potential biomarkers for asthma. Clin Exp Allergy. 2011;41(3):346–356. doi: 10.1111/j.1365-2222.2010.03638.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmeyer F, Raulf-Heimsoth M, Bruning T. Exhaled breath condensate and airway inflammation. Curr Opin Allergy Clin Immunol. 2009;9(1):16–22. doi: 10.1097/ACI.0b013e32831d8144. [DOI] [PubMed] [Google Scholar]
- 38.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 39.Sin DD, Miller BE, Duvoix A, et al. Serum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(9):1187–1192. doi: 10.1164/rccm.201008-1220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84(2):210–215. [PubMed] [Google Scholar]
- 41.Lowhagen O, Wever AM, Lusuardi M, et al. The inflammatory marker serum eosinophil cationic protein (ECP) compared with PEF as a tool to decide inhaled corticosteroid dose in asthmatic patients. Respir Med. 2002;96(2):95–101. doi: 10.1053/rmed.2001.1218. [DOI] [PubMed] [Google Scholar]
- 42.Dosanjh A, Gamst A, Phillipson J, Broughton A. Elevated serum eosinophil cationic protein levels in cystic fibrosis, pediatric asthma, and bronchiolitis. Pediatr Asthma Allergy Immunol. 1996;10(4):169–173. [Google Scholar]
- 43.Lessard A, St-Laurent J, Turcotte H, Boulet LP. Leptin and adiponectin in obese and non-obese subjects with asthma. Biomarkers. 2011;16(3):271–273. doi: 10.3109/1354750X.2010.550013. [DOI] [PubMed] [Google Scholar]
- 44.Berg CM, Thelle DS, Rosengren A, Lissner L, Toren K, Olin AC. Decreased fraction of exhaled nitric oxide in obese subjects with asthma symptoms: data from the population study INTERGENE/ADONIX. Chest. 2011;139(5):1109–1116. doi: 10.1378/chest.10-1299. [DOI] [PubMed] [Google Scholar]
- 45.Kim SH, Kim TH, Lee JS, et al. Adiposity, adipokines, and exhaled nitric oxide in healthy adults without asthma. J Asthma. 2011;48(2):177–182. doi: 10.3109/02770903.2010.529223. [DOI] [PubMed] [Google Scholar]
- 46.Sekiya T, Yamada H, Yamaguchi M, et al. Increased levels of a TH2-type CC chemokine thymus and activation-regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy. 2002;57(2):173–177. doi: 10.1034/j.1398-9995.2002.5720256.x. [DOI] [PubMed] [Google Scholar]
- 47.Leung TF, Wong CK, Chan IH, Ip WK, Lam CW, Wong GW. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J Allergy Clin Immunol. 2002;110(3):404–409. doi: 10.1067/mai.2002.126378. [DOI] [PubMed] [Google Scholar]
- 48.Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357(20):2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 49.Hartl D, Lee CG, Da Silva CA, Chupp GL, Elias JA. Novel biomarkers in asthma: chemokines and chitinase-like proteins. Curr Opin Allergy Clin Immunol. 2009;9(1):60–66. doi: 10.1097/ACI.0b013e32831f8ee0. [DOI] [PubMed] [Google Scholar]
- 50.Wadsworth SJ, Atsuta R, McIntyre JO, Hackett TL, Singhera GK, Dorscheid DR. IL-13 and TH2 cytokine exposure triggers matrix metalloproteinase 7-mediated Fas ligand cleavage from bronchial epithelial cells. J Allergy Clin Immunol. 2010;126(2):366–374. doi: 10.1016/j.jaci.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Goswami S, Angkasekwinai P, Shan M, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10(5):496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephan V, Zimmermann A, Kuhr J, Urbanek R. Determination of N-methylhistamine in urine as an indicator of histamine release in immediate allergic reactions. J Allergy Clin Immunol. 1990;86 (6 Pt 1):862–868. doi: 10.1016/s0091-6749(05)80147-2. [DOI] [PubMed] [Google Scholar]
- 53.Takei S, Shimago A, Iwashita M, Kumamoto T, Kamuro K, Miyata K. Urinary N-methylhistamine in asthmatic children receiving azelastine hydrochloride. Ann Allergy Asthma Immunol. 1997;78(5):492–496. doi: 10.1016/S1081-1206(10)63237-1. [DOI] [PubMed] [Google Scholar]
- 54.Saude EJ, Skappak CD, Regush S, et al. Metabolomic profiling of asthma: diagnostic utility of urine nuclear magnetic resonance spectroscopy. J Allergy Clin Immunol. 2011;127(3):757–764. doi: 10.1016/j.jaci.2010.12.1077. [DOI] [PubMed] [Google Scholar]