Abstract
Storz, Jay F., and Hideaki Moriyama. Mechanisms of hemoglobin adaptation. High Alt. Med. Biol. 9:148–157, 2008.—Evidence from a number of vertebrate taxa suggests that modifications of hemoglobin (Hb) function may often play a key role in mediating an adaptive response to high altitude hypoxia. The respiratory functions of Hb are a product of the protein's intrinsic O2-binding affinity and its interactions with allosteric effectors such as protons, chloride ions, CO2, and organic phosphates. Here we review several case studies involving high altitude vertebrates where it has been possible to identify specific mechanisms of Hb adaptation to hypoxia. In addition to comparative studies of Hbs from diverse animal species, functional studies of human Hb mutants also suggest that there is ample scope for evolutionary adjustments in Hb–O2 affinity through alterations of the equilibrium constants of O2 binding to deoxy- and oxyHb or through changes in the allosteric equilibrium constants for the transition between the deoxy- and oxyHb quaternary structures. It may be the case that certain evolutionary paths are followed more often than others simply because they are subject to less stringent pleiotropic constraints.
Key words: adaptation, globins, hemoglobin, hypoxia, molecular evolution, physiological evolution
Introduction
High altitude environments pose a number of unique physiological challenges to animal life. In addition to the characteristically cold temperatures, high altitude environments are also characterized by lower partial pressures of oxygen (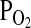 ) relative to low altitude environments at similar latitudes. Since the reduced
) relative to low altitude environments at similar latitudes. Since the reduced 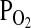 of inspired air will typically result in a concomitant reduction in the O2 saturation of arterial blood, a suite of compensatory physiological adjustments may be required to ensure an adequate supply of O2 to the cells of aerobically metabolizing tissues. These physiological adjustments may be manifest at multiple hierarchical levels of biological organization, from the level of cardiopulmonary organ systems to the molecular level of oxidative metabolism (Bouverot, 1985; Hochachka and Somero, 2002; Scott and Milsom, 2006; Weber, 2007). In species that are native to high altitude environments, the suite of derived physiological changes that contributes to hypoxia tolerance may often represent genetically based adaptations that have evolved under the influence of natural selection. In such cases, identifying the genetic basis of hypoxia tolerance can provide important insights into mechanisms of physiological evolution.
of inspired air will typically result in a concomitant reduction in the O2 saturation of arterial blood, a suite of compensatory physiological adjustments may be required to ensure an adequate supply of O2 to the cells of aerobically metabolizing tissues. These physiological adjustments may be manifest at multiple hierarchical levels of biological organization, from the level of cardiopulmonary organ systems to the molecular level of oxidative metabolism (Bouverot, 1985; Hochachka and Somero, 2002; Scott and Milsom, 2006; Weber, 2007). In species that are native to high altitude environments, the suite of derived physiological changes that contributes to hypoxia tolerance may often represent genetically based adaptations that have evolved under the influence of natural selection. In such cases, identifying the genetic basis of hypoxia tolerance can provide important insights into mechanisms of physiological evolution.
Since hypoxia impinges on well-characterized physiological pathways involved in the transport, storage, and cellular utilization of molecular oxygen (Hochachka and Somero, 2002; Powell, 2003), the identification of plausible candidate genes for hypoxia tolerance is greatly facilitated. Although the genetic basis of hypoxia tolerance has yet to be fully elucidated in any vertebrate species, evidence from a number of birds, mammals, and amphibians suggests that modifications of hemoglobin (Hb) function may often play a key role in mediating an adaptive response to high altitude hypoxia (Perutz, 1983; Monge and León-Velarde, 1991; Poyart et al., 1992; Weber, 1995, 2007; Weber and Fago, 2004; Storz, 2007).
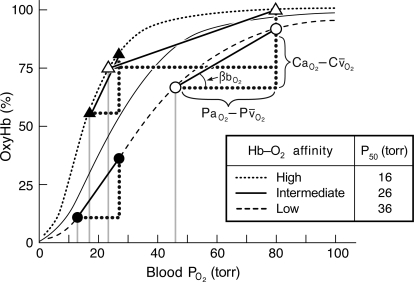
Under conditions of extreme hypoxia when pulmonary O2 loading is at a premium, an increased Hb–O2 affinity helps maximize the level of tissue oxygenation for a given difference in O2 tension between the sites of O2 loading (the pulmonary capillaries) and the sites of O2 unloading (the tissue capillaries; Fig. 1). However, the specific 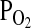 at which an increase in Hb–O2 affinity is advantageous depends on a number of taxon-specific physiological parameters (Turek et al., 1973, 1978; Bencowitz et al., 1982; Samaja et al., 1986, 2003). The O2 transport functions of Hb depend on homotropic effects (the subunit cooperativity of O2 binding) and heterotropic effects (the sensitivity of O2 binding to red cell concentrations of protons, chloride ions, CO2, and organic phosphates such as 2,3-diphosphoglycerate (2,3-DPG) in mammals, inositol pentaphosphate (IP5) in birds, adenosine triphosphate (ATP) in reptiles, and both DPG and ATP in amphibians).
at which an increase in Hb–O2 affinity is advantageous depends on a number of taxon-specific physiological parameters (Turek et al., 1973, 1978; Bencowitz et al., 1982; Samaja et al., 1986, 2003). The O2 transport functions of Hb depend on homotropic effects (the subunit cooperativity of O2 binding) and heterotropic effects (the sensitivity of O2 binding to red cell concentrations of protons, chloride ions, CO2, and organic phosphates such as 2,3-diphosphoglycerate (2,3-DPG) in mammals, inositol pentaphosphate (IP5) in birds, adenosine triphosphate (ATP) in reptiles, and both DPG and ATP in amphibians).
FIG. 1.
O2 equilibrium curves showing the theoretical influence of a change in Hb–O2 affinity on blood O2 transport under conditions of moderate hypoxia (open symbols) and severe hypoxia (filled symbols). Each curve is a plot of the O2 saturation of Hb (vertical axis) versus blood 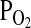 (horizontal axis), with paired values for arterial and venous blood connected by solid lines. For each pair of arterial and venous points, the
(horizontal axis), with paired values for arterial and venous blood connected by solid lines. For each pair of arterial and venous points, the 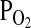 for venous blood (
for venous blood (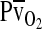 ) is marked by a vertical gray line that extends to the horizontal axis. The sigmoid O2 equilibrium curves are shown for high, intermediate, and low Hb–O2 affinities;
) is marked by a vertical gray line that extends to the horizontal axis. The sigmoid O2 equilibrium curves are shown for high, intermediate, and low Hb–O2 affinities; 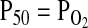 of blood at which the O2 saturation of Hb is at 50%. Each change in Hb–O2 affinity produces a shift in
of blood at which the O2 saturation of Hb is at 50%. Each change in Hb–O2 affinity produces a shift in 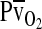 , but the
, but the 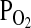 of arterial blood (
of arterial blood ( ) is assumed to remain constant.
) is assumed to remain constant.
In this graph, 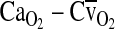 is the difference in O2 concentration between arterial and mixed venous blood, and
is the difference in O2 concentration between arterial and mixed venous blood, and 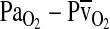 is the corresponding arterial–venous difference in
is the corresponding arterial–venous difference in 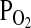 .
. 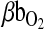 , the blood O2 capacitance coefficient, is defined as the ratio
, the blood O2 capacitance coefficient, is defined as the ratio 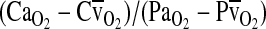 (= the slope of the line connecting the arterial and venous points on the O2 equilibrium curve). The interrelationships of these parameters are summarized by the following Fick's equation:
(= the slope of the line connecting the arterial and venous points on the O2 equilibrium curve). The interrelationships of these parameters are summarized by the following Fick's equation: 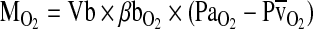 , where
, where 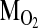 is the total O2 consumption and Vb is the total cardiac bloodflow. The product
is the total O2 consumption and Vb is the total cardiac bloodflow. The product 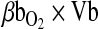 is the circulatory conductance of O2 in the bloodstream. Note that under conditions of moderate hypoxia the right-shifted curve produces the greatest
is the circulatory conductance of O2 in the bloodstream. Note that under conditions of moderate hypoxia the right-shifted curve produces the greatest 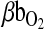 and results in a less severe drop in
and results in a less severe drop in 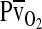 , the overall index of tissue oxygenation. By contrast, under severe hypoxia, the left-shifted curve produces the greatest values of
, the overall index of tissue oxygenation. By contrast, under severe hypoxia, the left-shifted curve produces the greatest values of  and
and 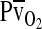 . When the kinetics of O2 transfer across the alveolar gas–blood barrier become a limiting step (diffusion limitation), a left-shifted O2 equilibrium curve may also be advantageous under conditions of less severe hypoxia (Bencowitz et al., 1982; Bouverot, 1985).
. When the kinetics of O2 transfer across the alveolar gas–blood barrier become a limiting step (diffusion limitation), a left-shifted O2 equilibrium curve may also be advantageous under conditions of less severe hypoxia (Bencowitz et al., 1982; Bouverot, 1985).
Amino acid residues that play especially important roles in controlling Hb–O2 affinity are located at heme–protein contacts, the intersubunit contact surfaces (which mediate the transition in quaternary structure between the oxy and deoxy states of the Hb tetramer), and binding sites for heterotropic ligands that are mainly located at the N- and C-termini of the subunit polypeptides and in the central cavity of the Hb protein. Functional studies of human Hb mutants and comparative studies of Hbs from diverse animal species suggest that there is ample scope for evolutionary adjustments in Hb–O2 affinity through alterations of the equilibrium constants of O2 binding to deoxy- and oxyHb or through changes in the allosteric equilibrium constants for the transition between the deoxy- and oxyHb structures.
Here we review several case studies of Hb adaptation to hypoxia in high altitude vertebrates. Specifically, we highlight several case studies involving endothermic and ectothermic vertebrates where it has been possible to identify specific mechanisms of Hb adaptation. We focus mainly on modifications of Hb function that alter O2-binding affinity, although it is important to keep in mind that several other aspects of Hb function may also play an important role in physiological adaptation to hypoxia. These additional protein functions might include the transport of vasoactive nitric oxide and the regulation of red cell glycolysis (Weber and Fago, 2004; Weber et al., 2004). Also, changes in Hb stability that increase resistance to pH-induced oxidative degradation and denaturation may also be important under conditions of high altitude hypoxia. In reviewing these case studies of Hb adaptation, we will concern ourselves with the following question: Do functionally equivalent modifications of Hb–O2 affinity in disparate taxa typically involve the same underlying mechanisms? If so, then parallel and convergent evolution of Hb function may be pervasive among representatives of different vertebrate classes that have independently colonized high altitude environments. Conversely, if a given change in Hb function can be accomplished in myriad different ways through different mechanisms, then even similar selective pressures may yield idiosyncratic outcomes in different evolutionary lineages. We first provide an overview of Hb structure and function, and we then describe different mechanisms by which Hb–O2 affinity can be fine-tuned to match O2 supply and O2 demand under different environmental conditions.
Structure of Vertebrate Hemoglobin
Vertebrate Hbs are heterotetramers, consisting of two α-chain subunits and two β-chain subunits (141 and 146 amino acids, respectively). The α-chain polypeptide folds into seven α-helices and the β-chain folds into eight. These α-helices are labeled A-H (the D helix is missing from the α-chain) and are linked together by short interhelical segments, labeled AB, BC, and so on. The N- and C- terminal extensions of each chain are labeled NA and HC, respectively (Dickerson and Geis, 1983). In each polypeptide chain, individual residues are labeled according to their helical position and their sequential number from the N-terminus. For example, α58(E7)His refers to the histidine that occupies the 58th residue position of the α-chain and the seventh position of the E helix.
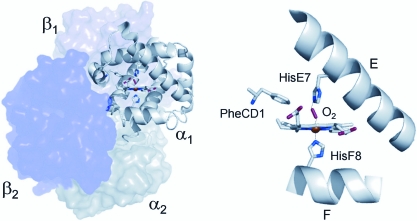
The folded globin polypeptide forms a cleft in which the E, F, and G helices and the CD corner enclose a hydrophobic pocket containing the heme group, a porphyrin ring with a ferrous iron atom capable of reversibly binding a single dioxygen molecule (Fig. 2). Within this hydrophobic pocket, the heme is held in place by a coordination bond between the iron atom and the Nέ atom of His F8 (α87, β92), the proximal histidine. The residue Phe CD1(α43, β42) assists by wedging the heme into a stable position. His F8 and Phe CD1 are among the few residues that are completely invariant among all vertebrate Hb chains (Dickerson and Geis, 1983). The reversible binding of O2 is further facilitated by the His E7(α58, β63) residue (the distal histidine), which lies opposite His F8 on the other side of the heme plane and helps stabilize the Fe–O2 bond, along with other residues lining the heme pocket that confer an appropriate polarity to the cavity. The other nearly invariant position in vertebrate Hb is Leu F4(α83, β88), which prevents hydrolysis of the Fe–His F8 bond by restricting solvent access.
FIG. 2.
Three-dimensional structure of the Hb tetramer (left) and a detailed view of the heme–ligand complex (right). The distal histidine (E7) and proximal histidine (F8) residues are shown above and below the heme plane, respectively.
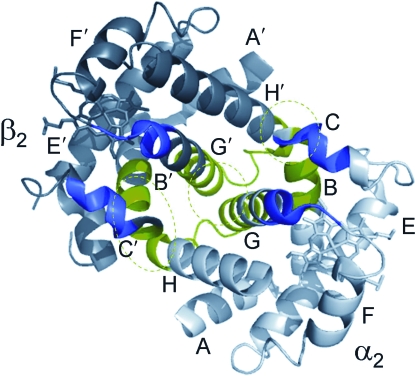
With regard to quaternary structure, the Hb tetramer is made up of two semirigid αβ dimers that rotate around each other by 15° during the transition between the deoxy [low affinity (T)] structure and the oxy [high affinity (R)] structure (Perutz, 1972; Baldwin and Chothia, 1979; Shaanan, 1980; Fermi and Perutz, 1981; Fermi et al., 1984). The mutual rotation of the α1β1 and α2β2 dimers involves no appreciable change in the intradimer contact surfaces, but substantial changes occur in intersubunit interactions at the α1β2 and α2β1 contact surfaces. The intradimer (α1β1 and α2β2) packing contacts involve 34 residues concentrated in the H and G helices and the BC corner, whereas the less extensive interdimer (α1β2 and α2β1) sliding contacts involve 19 residues concentrated in helices C and G and the FG corner (Fig. 3). Most of the free-energy difference between the T- and R-states is concentrated in the sliding contacts (Pettigrew et al., 1982). In some cases, this free-energy difference can be abolished by a single amino acid substitution (Dickerson and Geis, 1983). It is therefore not surprising that these intersubunit contacts are among the most highly conserved sites in vertebrate Hb.
FIG. 3.
The α2β2 dimer (one-half of a functional Hb tetramer) shown in a side view. The intradimer α2β2 packing contacts are shown in green and the residues participating in interdimer (α1β2 and α2β2)sliding contacts are shown in purple.
Hemoglobin Function
Homotopic effects: cooperative O2 binding
The binding of O2 at each of the four heme irons in the Hb tetramer exhibits a positive cooperativity, meaning that O2 binding at one site increases the O2 binding affinity at each remaining site. Likewise, O2 unloading at one site decreases the O2 binding affinity at the remaining sites. This cooperativity among the four globin subunits of each Hb molecule enhances the efficiency of O2 loading and unloading for a given difference in pulmonary–tissue O2 tensions and is manifest in the sigmoid shape of the O2 equilibrium curve (Fig. 1). The cooperativity of O2 binding results from the fact that the binding of O2 to the heme iron of a given subunit produces a localized change in tertiary structure that is transmitted to adjacent subunits, thereby triggering the shift in quaternary structure (Perutz, 1970, 1979; Arnone, 1974; Baldwin and Chothia, 1979; Gelin et al., 1983; Perutz et al., 1987; Liddington et al., 1988). This oxygenation-linked shift in quaternary structure between the T- and R-states is central to the allosteric function of Hb as an O2 transport molecule.
Heterotropic effects: binding of allosteric effectors
The respiratory functions of Hb are a product of its intrinsic O2-binding affinity and its interactions with allosteric effectors such as protons, chloride ions, CO2, and organic phosphates. These effectors exert their influence on Hb–O2 affinity by binding more strongly to deoxy Hb, mainly at sites located at the N- and C-termini, thereby stabilizing the low-affinity T structure through the formation of additional salt bridges within and between subunits (Perutz, 1970, 1989; Bettati et al., 1983).
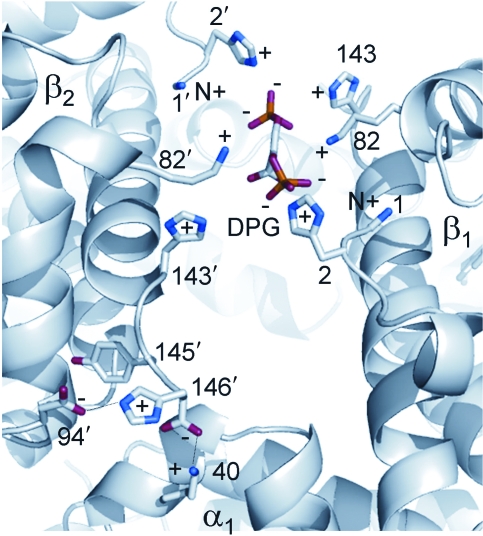
The allosteric effect of proton binding accounts for the typical reduction in Hb–O2 affinity at low pH (the Bohr effect) and facilitates O2 unloading under conditions of metabolic acidosis in working muscles. At physiological pH, the Bohr effect of human Hb is primarily attributable to proton binding at the following residues: α1(NA1)Val, α122(H5)His, β2(NA2)His, β82(EF6)Lys, β143(H21)His, and β146(HC3)His (Perutz et al., 1969; Kilmartin et al., 1978; Ho and Russu, 1987; Lukin and Ho, 2004). Chloride ions bind to one α-chain site between α1(NA1)Val and α131(H14)Ser and one β-chain site between β1(NA1)Val and β82(EF6)Lys (Riggs, 1988). Similarly, CO2 combines with the N-terminal 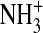 residues of each subunit chain of deoxyHb (Arnone, 1974; Perutz, 1983). It has also been hypothesized that Cl− may modulate O2 affinity through delocalized electrostatic effects that do not involve binding at specific residues (Perutz et al., 1994). According to this view, Cl− partially neutralizes the excess of positive charges between the β-chains of deoxy Hb, thereby stabilizing the T-state conformation. If this view is correct, then it is possible that Hb–O2 affinity could be increased not just by substitutions at specific Cl− binding sites, but also by substitutions at a number of residue positions that increase the net electropositivity of the central cavity. 2,3-DPG carries four negative charges, which allows it to bind between the β-chains of deoxyHb by charge–charge interactions with the β1(NA1)Val residue of one chain and with β2(NA2)His, β82(EF6)Lys, and β143(H21)His of both chains (Fig. 4). In principle, substitutions at any one of these binding sites can alter the sensitivity of Hb to the various allosteric effectors, thereby altering the equilibrium between the T- and R-state quaternary structures. Since the binding of allosteric effectors typically stabilizes T-state deoxyHb, substitutions that inhibit effector binding will typically increase Hb–O2 affinity by shifting the equilibrium in favor of R-state oxyHb.
residues of each subunit chain of deoxyHb (Arnone, 1974; Perutz, 1983). It has also been hypothesized that Cl− may modulate O2 affinity through delocalized electrostatic effects that do not involve binding at specific residues (Perutz et al., 1994). According to this view, Cl− partially neutralizes the excess of positive charges between the β-chains of deoxy Hb, thereby stabilizing the T-state conformation. If this view is correct, then it is possible that Hb–O2 affinity could be increased not just by substitutions at specific Cl− binding sites, but also by substitutions at a number of residue positions that increase the net electropositivity of the central cavity. 2,3-DPG carries four negative charges, which allows it to bind between the β-chains of deoxyHb by charge–charge interactions with the β1(NA1)Val residue of one chain and with β2(NA2)His, β82(EF6)Lys, and β143(H21)His of both chains (Fig. 4). In principle, substitutions at any one of these binding sites can alter the sensitivity of Hb to the various allosteric effectors, thereby altering the equilibrium between the T- and R-state quaternary structures. Since the binding of allosteric effectors typically stabilizes T-state deoxyHb, substitutions that inhibit effector binding will typically increase Hb–O2 affinity by shifting the equilibrium in favor of R-state oxyHb.
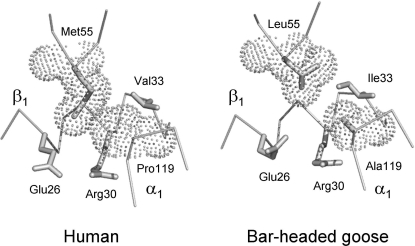
FIG. 4.
Dot representation of van der Waals radii at an intradimer α1β1 contact in human Hb (left) and bar-headed goose Hb (right). Note that in the bar-headed goose Hb the replacement of Ala for Pro at the α119(H2) residue position results in a loss of atomic contact between the α1 and β1 subunits. The disruption of this interchain van der Waals contact destabilizes the T-state deoxyHb quaternary structure and therefore results in an increased Hb–O2 affinity, because the allosteric equilibrium is shifted in favor of the R-state oxyHb structure.
Hemoglobin Adaptation Involving Changes in Homotropic Effects
One of the most celebrated case studies of high altitude adaptation involves a pair of distantly related waterfowl species, the bar-headed goose (Anser indicus) and the Andean goose (Chloephaga melanoptera), that have independently evolved exceptionally high Hb–O2 affinities. The bar-headed goose spends the breeding season on high alpine lakes at 4000 to 6000 m on the Tibetan Plateau and spends the winter months in wetland habitats in different parts of the Indian subcontinent. This requires an annual roundtrip migratory flight over the crest of the Himalaya at altitudes of nearly 10,000 m where ambient 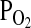 is less than one-third of that at sea level. As might be expected for an animal capable of sustaining powered flight at such altitudes, the bar-headed goose is characterized by an exceptionally high Hb–O2 affinity relative to its lowland sibling species, the greylag goose (Anser anser; P50, the
is less than one-third of that at sea level. As might be expected for an animal capable of sustaining powered flight at such altitudes, the bar-headed goose is characterized by an exceptionally high Hb–O2 affinity relative to its lowland sibling species, the greylag goose (Anser anser; P50, the 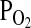 at 50% saturation of Hb = 29.7 vs. 39.5 torr at 37°C, pH 7.4). The observed difference in P50 is attributable to a small difference in the intrinsic Hb–O2 affinity that is amplified by differential sensitivity to IP5 (Petschow et al., 1977; Rollema and Bauer, 1979). Adult Hbs of the two species are distinguished by three amino acid substitutions in the α-chains, 18(A16) Gly → Ser, 63(E12)Ala → Val, and 119(H2)Pro → Ala, and one substitution in the β-chains, 125(H3)Glu → Asp (greylag → bar-headed in each case). The α119(H2)Pro → Ala substitution is unique among all avian Hbs studied to date, possibly because it disrupts an important intradimer van der Waals contact between the Cγ of the 119(H2)Pro residue on the α-chain and the 55(D6)Leu residue on the β-chain (Fig. 5). Since studies of human Hb mutants have demonstrated that the loss of atomic contacts at intersubunit contact surfaces typically destabilizes the T-state conformation, Perutz (1983) hypothesized that the loss of this van der Waals contact is responsible for the elevated O2 affinity of bar-headed goose Hb.
at 50% saturation of Hb = 29.7 vs. 39.5 torr at 37°C, pH 7.4). The observed difference in P50 is attributable to a small difference in the intrinsic Hb–O2 affinity that is amplified by differential sensitivity to IP5 (Petschow et al., 1977; Rollema and Bauer, 1979). Adult Hbs of the two species are distinguished by three amino acid substitutions in the α-chains, 18(A16) Gly → Ser, 63(E12)Ala → Val, and 119(H2)Pro → Ala, and one substitution in the β-chains, 125(H3)Glu → Asp (greylag → bar-headed in each case). The α119(H2)Pro → Ala substitution is unique among all avian Hbs studied to date, possibly because it disrupts an important intradimer van der Waals contact between the Cγ of the 119(H2)Pro residue on the α-chain and the 55(D6)Leu residue on the β-chain (Fig. 5). Since studies of human Hb mutants have demonstrated that the loss of atomic contacts at intersubunit contact surfaces typically destabilizes the T-state conformation, Perutz (1983) hypothesized that the loss of this van der Waals contact is responsible for the elevated O2 affinity of bar-headed goose Hb.
FIG. 5.
Binding of 2,3-DPG in the central cavity between the β1 and β2 chains of deoxyHb. Also shown (lower-left corner) is an intrachain salt bridge that is formed in deoxyHb between the imidazole ring of the N-terminal β146(HC3)His and the negatively charged β94(FG1)Asp. This bond increases the affinity of FG1 Asp for protons and therefore contributes to the Bohr effect. The protons are released upon transition to R-state oxyHb.
Remarkably, a disruption of this same van der Waals contact also appears to be responsible for the high Hb–O2 affinity of the Andean goose (P50 = 33.9 torr at 40°C, pH 7.1), which is a year-round resident of lakes and marshes in the high Andes at altitudes > 3000 m. Similar to the case with the bar-headed goose, the Andean goose exhibits a much higher Hb–O2 affinity than its lowland sibling species, the mallard duck, Anas platyrhynchos. The adult Hbs of the Andean goose and the mallard duck are distinguished by five amino acid substitutions in the α-chains and five substitutions in the β-chains (Hiebl et al., 1987a). It is the β-chain 55(D6)Leu → Ser substitution in Andean goose Hb that eliminates the same intersubunit contact as the α-chain 119(H2)Pro → Ala substitution in bar-headed goose Hb. To test Perutz's (1983) hypothesis about the mechanism underlying the increased Hb–O2 affinity, Jessen et al. (1991) and Weber et al. (1993) used site-directed mutagensis to synthesize and functionally characterize two recombinant human Hb mutants: one that contained the bar-headed goose α-chain mutation 119(H2)Ala and one that contained the Andean goose β-chain mutation 55(D6)Ser. These studies revealed that each engineered Hb mutant was characterized by much higher O2-binding affinities than in wild-type human Hb and confirmed that the observed differences were primarily attributable to the loss of a single α1β1 interchain contact (Jessen et al., 1991; Weber et al., 1993). Thus, in each of these two species, the evolution of increased Hb–O2 affinity can be attributed to a similar mechanism: destabilization of T-state deoxyHb through the disruption of intersubunit contacts. However, in each species the same functional outcome was achieved through distinct mutational pathways in different subunit polypeptides of the Hb tetramer. Golding and Dean (1998:360) provided an apt summary of this phenomenon: “different species, different genes, different replacements—same mechanism, same effect.”
The fact that the independent evolution of increased Hb–O2 affinity in these two bird species involved the same mechanism for favoring the T-to-R transition in quaternary structure suggests that there may be relatively few ways in which a given change in Hb–O2 affinity may be accomplished. If this is the case, we might expect to see this same mechanism replicated in other taxa that have independently evolved an increased Hb–O2 affinity as an adaptive response to environmental hypoxia. As explained later, the disruption of intersubunit contacts clearly does not represent the only possible mechanism of Hb adaptation to hypoxia, as several studies have documented evolutionary changes in Hb–O2 affinity that have been accomplished by altering Hb sensitivity to allosteric effectors.
Hemoglobin Adaptation Involving Changes in Heterotropic Effects
The relatively high Hb–O2 affinity of Andean camelids (llama, vicuña, alpaca, and guanaco) is attributable to a β2(NA2)His → Asn substitution that eliminates two of the seven DPG-binding sites per tetramer. Within this group of highland camelids, the especially high Hb–O2 affinity of the vicuña (Vicugna vicugna) appears to be attributable to a α130(H13)Ala → Thr substitution, which introduces a polar hydroxyl group that inhibits Cl− binding at the neighboring α131Asn residue (Kleinschmidt et al., 1986; Piccinini et al., 1990; Weber, 2007).
Modifications of Cl− binding sites also appear to be responsible for the increased Hb–O2 affinity of frogs in the genus Telmatobius that live in snowmelt streams at altitudes of 3000 to 4600 m in the Andes. A study of Hb function in the species Telmatobius peruvianus from 3800 m (Weber et al., 2002) revealed that the high Hb–O2 affinity of the major Hb isoform (isoHb) of this species is attributable to two modifications of α-chain Cl− binding sites: acetylation of the NH2-terminal residue and an amino acid substitution at residue 131(H14) where nonpolar Ala replaces the ancestral polar residue (= Thr in the lowland clawed-frog, Xenopus laevis).
The suppression of DPG binding that accounts for the high Hb–O2 affinity of Andean camelids also accounts for the high O2-binding affinity of human fetal Hb (HbF). The β-chain subunits of HbF are encoded by the γ-globin gene, which is distinguished from the adult β-globin gene by the substitution β143(H21)His → γ143Ser, a key DPG-binding site. The increased affinity of HbF relative to adult Hb is advantageous, because it facilitates placental O2 transfer from the maternal circulation to the fetal circulation. Likewise, as pointed out by Weber et al. (2002), the suppression of Cl− binding that accounts for the high Hb–O2 affinity of Andean frogs also accounts for the high O2-binding affinity of human embryonic Hb (HbE). The α-chain subunits of HbE are encoded by the ζ-globin gene, which is distinguished from the adult α-globin gene by the substitution α131(H21)Ser → ζ131Val, a key Cl− binding site. The increased affinity of HbE relative to adult Hb may be important for O2 uptake from the amniotic fluid and for facilitating the transition to a placental circulatory system (Brittain, 2002).
Other Mechanisms of Hemoglobin Adaptation
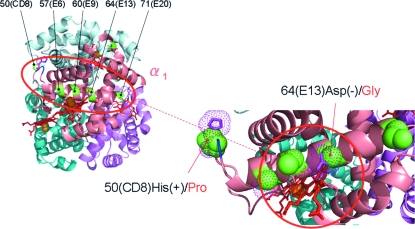
Studies of human Hb mutants indicate that Hb–O2 affinity can also be adjusted through subtle changes in the polarity of the heme pocket (Dickerson and Geis, 1983). This mechanism may underlie allelic variation in Hb–O2 affinity between high and low altitude populations of deer mice (Peromyscus maniculatus) in western North America. Deer mice possess triplicated copies of the adult α-globin gene, and each of the three copies segregate functionally distinct alleles that exhibit pronounced allele frequency differences between high and low altitude populations (Snyder et al., 1988; Storz, 2007; Storz et al., 2007, 2008). Allelic variation at these α-globin genes contributes to fitness-related variation in aerobic performance in natural populations of deer mice (Chappell and Snyder, 1984; Chappell et al., 1988; Hayes and O'Connor, 1999), and the abrupt altitudinal shifts in allele frequencies appear to be attributable to spatially varying selection that drives the divergent fine-tuning of Hb–O2 affinity between different elevational zones (Snyder, 1981; Snyder et al., 1988; Storz et al., 2007). Two of the three α-globin genes, HBA-T1 and HBA-T2, segregate the same two alternative protein alleles (the shared polymorphism between the two closely linked gene duplicates is attributable to gene conversion, a form of recombination between nonallellic sequences). At both HBA-T1 and HBA-T2, the two alternative alleles are distinguished from one another by substitutions at five solvent-exposed amino acid residues that span the E-helix domain (Fig. 6). These five residues line the opening of the heme pocket and contribute to the polarity of the cavity. The high affinity allele, which predominates in high altitude populations, is characterized by the five-site combination: 50(CD8)Pro, 57(E6)Gly, 60(E9)Ala, 64(E13)Gly, and 71(E20)Gly. The low affinity allele, which predominates in low altitude populations, is characterized by an alternative combination of amino acids at these same five sites: His, Ala, Gly, Asp, and Ser, respectively. Of the five substitutions that distinguish the high- and low-affinity allele classes, the 64(E13)Asp → Gly substitution is predicted to have the most important effects on O2-binding affinity. At this site, substitution of the uncharged Gly residue for the negatively charged Asp residue involves substituting a single H atom side chain for a much larger carboxylic acid side chain. The affinity-enhancing effects of this substitution are corroborated by functional studies of the same 64(E13)Gly mutant in human Hb (Hb Guangzhou–Hangzhou), a high-affinity Hb mutant found at low frequency among East Asians. In deer mice, the other charge-changing substitution that distinguishes the alternative allele classes, 50(CD8)His → Pro, may also have an important effect on the geometry of the heme pocket, because Pro introduces a sharper turn angle in the CD interhelical segment that could alter the orientation of the E helix.
FIG. 6.
Homology-based structural model of deer mouse oxyHb showing the location of five amino acid replacement polymorphisms that span the E-helix domain of the α-chain subunit. Two charge-changing substitutions at α50(CD8) and α64(E13) are highlighted in the inset figure.
Effects of Hemoglobin Isoform Differentiation on Blood O2 Transport
Since the individual subunit polypeptides of the Hb tetramer are encoded by genes that may be present in multiple copies, many species are capable of synthesizing multiple iso- Hbs. All gnathostome vertebrates synthesize functionally distinct isoHbs during different stages of development, and some species are known to synthesize functionally distinct isoHbs during the same stage of development. In some cases, functional differentiation among coexpressed globin genes may provide the basis for a cascade mechanism of blood O2 transport where circulating red cells contain a mixture of isoHbs with different O2-binding affinities. Adjustments in the composition stoichiometry of the different isoHbs may have important effects on blood O2 transport under hypoxic conditions (van Vliet and Huisman, 1964; Weber, 1990, 2000, 2007). The high-affinity isoHbs may be specialized for pulmonary O2 loading at low 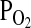 , whereas the low-affinity isoHbs may be specialized for O2 unloading in the peripheral circulation (Weber et al., 1988a, 1988b; Weber, 2007).
, whereas the low-affinity isoHbs may be specialized for O2 unloading in the peripheral circulation (Weber et al., 1988a, 1988b; Weber, 2007).
The expression of multiple isoHbs with graded O2 affinities is expected to broaden the permissible range of arterial O2 tensions for pulmonary-tissue O2 transport and may thus provide a regulatory reserve of O2 transport capacity. This cascade mechanism of blood O2 transport appears to have played an important role in the evolution of hypoxia tolerance in birds that are capable of flying at extremely high altitudes (Hiebl et al., 1987a, 1987b, 1987c, 1988; Weber et al., 1988a). One of the most striking examples of the role of isoHb differentiation in high altitude respiration involves a high-soaring African vulture called Rüppell's griffon, Gyps rueppelli. As a result of tandem duplication and functional divergence of the αA- and αD-globin genes, the red blood cells of these birds contain a mixture of four distinct α-chain iso- Hbs (HbA, HbA′, HbD, and HbD′) with graded O2-binding affinities (Hiebl et al., 1988; Weber et al., 1988a). Under hypoxic conditions, the high-affinity αD-chain isoHbs appear to help safeguard arterial O2 saturation, whereas the relatively low affinity αA-chain isoHbs ensure efficient O2 delivery to the cells of respiring tissues (Weber et al., 1988a). The differentiation in isoHb O2 affinities appears to be primarily attributable to α-chain substitutions that produce isoform-specific shifts in the allosteric equilibrium between the T- and R-states. The reduced O2 affinity of HbA relative to the other three isoHbs is largely attributable to an αAchain substitution at an intradimer α1β1 contact, α134(B15)–β1125(H3), whereas the increased O2 affinity of HbD and HbD′ relative to HbA and HbA’ is largely attributable to separate αD- and αD′-chain substitutions at the same interdimer α1β2 contact, α138(C3)–β297(FG4)/99(FG6) (Weber et al., 1988a; Weber, 2007).
Similar types of isoHb differentiation have also been described in mammals. Under conditions of high-altitude hypoxia, adult alpacas (Vicugna pacos) and yaks (Bos grunniens) are known to upregulate a fetal β-like globin gene, which results in the synthesis of a relatively high affinity fetal Hb (Reynafarje et al., 1975; Sarkar et al., 1999). This high-affinity fetal Hb is adapted to placental-tissue O2 transport in the hypoxic intrauterine environment and apparently can be co-opted for pulmonary-tissue O2 transport under hypoxic conditions during postnatal life. In addition to the coexpression of fetal and adult Hbs under hypoxic conditions, yaks also possess multiple adult isoHbs due to functional differentiation among tandemly duplicated α- and β-globin genes (Lalthantluanga et al., 1985; Weber et al., 1988b). Since yaks inhabit alpine environments at elevations of 3000 to 6000 m on the Tibetan Plateau, the cascaded O2 affinities of the fetal and adult isoHbs appear to play an important role in the hypoxia tolerance of these animals during both pre- and postnatal life. The differentiation in O2-binding affinity among α-chain isoHbs of deer mice may play a similar role in hypoxia tolerance (Storz et al., 2007, 2008).
Pleiotropic Constraints on Hemoglobin Evolution
In addition to comparative studies of Hb function in animal species that are native to high and low altitude environments, the wealth of functional information about human Hb mutants also provides insight into molecular mechanisms that underlie changes in intrinsic Hb–O2 affinity and sensitivity to allosteric effectors (Dickerson and Geis, 1983). Some of the same mutations that alter Hb–O2 affinity of human Hbs may be involved in adaptive modifications of Hb function in other species. In humans, high-affinity Hb mutants are typically associated with reduced levels of tissue oxygenation, which results in polycythemia due to the increased production of erythropoietin. However, affinity-enhancing mutations that have deleterious effects in humans living under normoxic conditions may be physiologically advantageous in animal species that inhabit hypoxic environments where the preservation of arterial O2 saturation is at a premium.
Functional information about human Hb mutations can also provide insights into the nature of pleiotropic constraints on Hb evolution (where pleiotropy here refers to the case where a single mutation has multiple effects on different aspects of protein structure or function). Information on pleiotropic constraints is important because it can provide clues as to why certain evolutionary pathways are followed more often than others. For example, even if mutation X and mutation Y produce identical effects on one particular aspect of Hb function, such as O2-binding affinity, the two mutations may have different pleiotropic effects on other aspects of Hb structure or function. Thus, the two mutations may differ with respect to their net effects on physiological performance (and fitness).
The majority of human Hb mutants that increase O2-binding affinity do so by destabilizing the T-state (Dickerson and Geis, 1983). This is typically accomplished by disrupting hydrogen bonds or salt bridges at the chain termini of deoxyHb. For example, three different α-chain mutations (Hbs Tarrant, Suresnes, and Legnano) favor the T-to-R transition by disrupting an important salt bridge between 126(H9)Asp and the C-terminal 141(HC3)Arg in deoxyHb. This is accomplished by eliminating a side chain at either one of the two residue positions. Other mutations impair DPG binding by adding a negative charge at the binding site (Hbs Shepherds Bush, Ohio) or by eliminating a positive charge (Hbs Rahere, Helsinki, Little Rock, Syracuse). In many cases the disruption is so severe that the Hbs undergo spontaneous oxidation and precipitate to form insoluble inclusions (known as Heinz bodies) within the red cell, ultimately resulting in hemolytic anemia.
The available data on human Hb mutants indicate that there are many potential mutational changes that can produce an increased Hb–O2 affinity. However, many of these changes will have negative pleiotropic effects on structural stability. Thus, even if an advantageous change in Hb–O2 affinity can be attained by means of a single substitution at a critical residue position, such changes may often require additional compensatory substitutions to offset negative side effects of the original change.
Interpretative Challenges Associated with the Study of Molecular Adaptation
As illustrated by the case studies described above, there are a number of different mechanisms by which Hb–O2 affinity can be fine-tuned to optimize blood-O2 transport under hypoxia. In several high altitude species, the evolution of increased Hb–O2 affinity is attributable to amino acid substitutions that shift the allosteric equilibrium in favor of the R-state oxyHb conformation. If it is true that adaptive modifications of Hb function are typically attributable to a very small number of amino acid substitutions at key positions, as suggested by Perutz (1983), then it may often be possible to pinpoint the causal substitutions in comparisons of Hb variation between highland and lowland species or between high and low altitude populations of the same species. The other implication of Perutz's (1983) argument is that there may be a very limited number of solutions to the problem of optimizing blood O2 transport under hypoxic conditions, so the same evolutionary pathways may be exploited time and time again in different lineages. If Perutz is correct, we should therefore expect that many additional examples of convergent or parallel evolution of Hb function will be revealed by comparative studies of high altitude animals. However, it is important to keep in mind that adaptive modifications of protein function may often involve two steps forward and one step back, as physiologically advantageous changes in one aspect of protein function may often have pleiotropic effects that need to be compensated for by multiple auxiliary changes. If this proves generally to be the case, it suggests that it may not always be easy to identify causal substitutions in comparisons of Hb variation within or between species. This is because it will often be difficult or impossible to determine which substitutions were responsible for producing the initial adaptive improvement in protein function and which substitutions represent compensatory changes that only became advantageous in the context provided by previous modifications of the genetic background.
As stated by Golding and Dean (1998:355): “The study of molecular adaptation has long been fraught with difficulties, not the least of which is identifying out of hundreds of amino acid replacements those few directly responsible for major adaptations.” Even in cases where it is possible to identify a single substitution of major effect (as in the comparison between bar-headed goose and greylag goose Hbs), it would be interesting to know whether the additional amino acid changes accentuate the main effect of this substitution (in an additive or epistatic fashion) or whether they represent compensatory changes that offset negative side effects associated with the initial substitution.
Acknowledgments
We thank T. Zera and two anonymous reviewers for helpful comments and suggestions, and we thank Dr. J. B. West for the invitation to contribute this review. We also wish to acknowledge funding from the National Institutes of Health (1 R01 HL087216-01A2), National Science Foundation to JFS (DEB-0614342), a Layman Award to JFS, and an Interdisciplinary Research Grant to JFS and HM from the Nebraska Research Council.
References
- Arnone A. Mechanism of action of hemoglobin. Ann. Rev. Med. 1974;25:123–130. doi: 10.1146/annurev.me.25.020174.001011. [DOI] [PubMed] [Google Scholar]
- Baldwin J. Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 1979;129:175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Bettati S. Mozzarelli A. Perutz M.F. Allosteric mechanism of hemoglobin: rupture of salt bridges raises oxygen affinity of the T-structure. J. Mol. Biol. 1983;281:581–585. doi: 10.1006/jmbi.1998.1983. [DOI] [PubMed] [Google Scholar]
- Bencowitz H.Z. Wagner P.D. West J.B. Effect of change in P50 on exercise tolerance at high-altitude: a theoretical study. J. Appl. Physiol. 1982;53:1487–1495. doi: 10.1152/jappl.1982.53.6.1487. [DOI] [PubMed] [Google Scholar]
- Bouverot P. Springer-Verlag; Berlin: 1985. Adaptation to Altitude-Hypoxia in Vertebrates. [Google Scholar]
- Brittain T. Molecular aspects of embryonic hemoglobin function. Mol. Aspects Med. 2002;23:293–342. doi: 10.1016/s0098-2997(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Chappell M.A. Snyder L.R.G. Biochemical and physiological correlates of deer mouse α chain hemoglobin polymorphisms. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5484–5488. doi: 10.1073/pnas.81.17.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M.A. Hayes J.P. Snyder L.R.G. Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): physiology of beta-globin variants and alpha-globin recombinants. Evolution. 1988;42:681–688. doi: 10.1111/j.1558-5646.1988.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Dickerson R.E. Geis I. Hemoglobin: Structure, Function, Evolution, and Pathology. Benjamin/Cummings Publishing; Menlo Park, CA: 1983. [Google Scholar]
- Fermi G. Perutz M.F. Clarendon Press; Oxford, UK: 1981. Atlas of Molecular Structures in Biology: Haemoglobin and Myoglobin. [Google Scholar]
- Fermi G. Perutz M.F. Shaanan B. Fourme R. The crystal structure of deoxyhaemoglobin at 1.7 A resolution. J. Mol. Biol. 1984;175:159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Gelin B.R. Lee A.W.M. Karplus M. Hemoglobin tertiary structural change on ligand binding: its role in the cooperative mechanism. J. Mol. Biol. 1983;171:489–559. doi: 10.1016/0022-2836(83)90042-6. [DOI] [PubMed] [Google Scholar]
- Golding G.B. Dean A.M. The structural basis of molecular adaptation. Mol. Biol. Evol. 1998;15:355–369. doi: 10.1093/oxfordjournals.molbev.a025932. [DOI] [PubMed] [Google Scholar]
- Hayes J.P. O'Connor C.S. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution. 1999;53:1280–1287. doi: 10.1111/j.1558-5646.1999.tb04540.x. [DOI] [PubMed] [Google Scholar]
- Hiebl I. Braunitzer G. Schneeganss D. The primary sequence of the major and minor hemoglobin components of adult Andean goose (Chloephaga melanoptera, Anatidae): The mutation Leu-Ser in position 55 of the β chains. Biol. Chem. Hoppe–Seyler. 1987a;368:1385–1390. doi: 10.1515/bchm3.1987.368.2.1559. [DOI] [PubMed] [Google Scholar]
- Hiebl I. Kosters J. Braunitzer G. The primary structures of the major and minor hemoglobin component of adult goshawk (Accipiter gentilis, Accipitrinae) Biol. Chem. Hoppe–Seyler. 1987b;368:333–342. doi: 10.1515/bchm3.1987.368.1.333. [DOI] [PubMed] [Google Scholar]
- Hiebl I. Schneeganss D. Grimm F. Kosters J. Braunitzer G. The primary structures of the major and minor hemoglobin components of adult European black vulture (Aegypius monachus, Aegypiinae) Biol. Chem. Hoppe-Seyler. 1987c;368:11–18. doi: 10.1515/bchm3.1987.368.1.11. [DOI] [PubMed] [Google Scholar]
- Hiebl I. Weber R.E. Schneeganss D. Kosters J. Braunitzer G. Structural adaptations in the major and minor hemoglobin components of adult Ruppell's griffon (Gyps ruepellii, Aegypiinae): a new molecular pattern for hypoxia tolerance. Biol. Chem. Hoppe–Seyler. 1988;369:217–232. doi: 10.1515/bchm3.1988.369.1.217. [DOI] [PubMed] [Google Scholar]
- Ho C. Russu I.M. How much do we know about the Bohr effect of hemoglobin? Biochemistry. 1987;26:6229–6305. doi: 10.1021/bi00394a001. [DOI] [PubMed] [Google Scholar]
- Hochachka P.W. Somero G.N. Oxford University Press; New York: 2002. Biochemical Adaptation. [Google Scholar]
- Jessen T.H. Weber R.E. Fermi G. Tame J. Braunitzer G. Adaptation of bird hemoglobins to high-altitudes—demonstration of molecular mechanism by protein engineering. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6519–6522. doi: 10.1073/pnas.88.15.6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J.V. Imai K. Jones R.T. Farouqui A.R. Fogg J. Baldwin J.M. Role of Bohr group salt bridges in cooperativity in hemoglobin. Biochim Biophys. Acta. 1978;534:15–25. doi: 10.1016/0005-2795(78)90471-3. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt T. Marz J. Jurgens K.D. Braunitzer G. Interaction of allosteric effectors with α-globin chains and high altitude respiration in mammals. The primary structure of two tylopod hemoglobins with high oxygen affinity: vicuna (Lama vicugna) and alpaca (Lama pacos) Biol. Chem. Hoppe–Seyler. 1986;367:153–160. doi: 10.1515/bchm3.1986.367.1.153. [DOI] [PubMed] [Google Scholar]
- Lalthantluanga R. Wiesner H. Braunitzer G. Studies on yak hemoglobin (Bos grunniens, Bovidae): structural basis for high intrinsic oxygen affinity. Biol. Chem. Hoppe–Seyler. 1985;366:63–68. doi: 10.1515/bchm3.1985.366.1.63. [DOI] [PubMed] [Google Scholar]
- Liddington R.C. Derewenda Z. Dodson G. Harris D. Structure of liganded T-state of hemoglobin identifies the origin of co-operative oxygen binding. Nature. 1988;331:725–728. doi: 10.1038/331725a0. [DOI] [PubMed] [Google Scholar]
- Lukin J.A. Ho C. The structure–function relationship of hemoglobin in solution at atomic resolution. Chem. Rev. 2004;104:1219–1230. doi: 10.1021/cr940325w. [DOI] [PubMed] [Google Scholar]
- Monge C. León-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol. Res. 1991;71:1135–1172. doi: 10.1152/physrev.1991.71.4.1135. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Stereochemistry of co-operative effects in haemoglobin. Nature. 1970;228:726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Haem–haem interactions. Nature. 1972;237:495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Regulation of oxygen affinity of haemoglobin: influence of structure of the globin on the haem. Ann. Rev. Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Species adaptation in a protein molecule. Mol. Biol. Evol. 1983;1:1–28. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Mechanisms of Co-operative and Allosteric Regulation in Proteins. Cambridge University Press; Cambridge, U.K: 1989. [Google Scholar]
- Perutz M.F. Muirhead H. Mazzarella L. Crowther R.A. Greer J. Kilmartin J.V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969;222:1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Fermi G. Luisi B. Shaanan B. Liddington R.C. Stereochemistry of co-operative effects in hemoglobin. Accs. Chem. Res. 1987;20:309–321. [Google Scholar]
- Perutz M.F. Shih D.T.B. Williamson D. The chloride effect in human haemoglobin, a new kind of allosteric mechanism. J. Mol. Biol. 1994;239:555–560. doi: 10.1006/jmbi.1994.1394. [DOI] [PubMed] [Google Scholar]
- Petschow D. Wurdinger I. Baumann R. Duhm J. Braunitzer G. Bauer C. Causes of high blood O2 affinity of animals living at high altitude. J. Appl. Physiol. 1977;42:139–143. doi: 10.1152/jappl.1977.42.2.139. [DOI] [PubMed] [Google Scholar]
- Pettigrew D.W. Romeo P.H. Tsapis A. Thillet J. Smith M.L. Turner B.W. Ackers G.K. Probing the energetics of proteins through structural perturbation: sites of regulatory energy in human hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 1982;79:1849–1853. doi: 10.1073/pnas.79.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini M. Kleinschmidt T. Jurgens K.L. Braunitzer G. Primary structure and oxygen binding properties of the hemoglobin from guanaco (Lama guanacoe, Tylopoda) Biol. Chem. Hoppe–Seyler. 1990;371:641–648. doi: 10.1515/bchm3.1990.371.2.641. [DOI] [PubMed] [Google Scholar]
- Powell F.L. Functional genomics and the comparative physiology of hypoxia. Ann. Rev. Physiol. 2003;65:203–230. doi: 10.1146/annurev.physiol.65.092101.142711. [DOI] [PubMed] [Google Scholar]
- Poyart C. Wajcman H. Kister J. Molecular adaptation of hemoglobin function in mammals. Resp. Physiol. 1992;90:3–17. doi: 10.1016/0034-5687(92)90130-o. [DOI] [PubMed] [Google Scholar]
- Reynafarje C. Faura J. Villavicencio D. Curaca A. Reynafarje B. Oyola L. Contreras L. Vallenas E. Faura A. Oxygen transport of hemoglobin in high-altitude animals (Camelidae) J. Appl. Physiol. 1975;38:806–810. doi: 10.1152/jappl.1975.38.5.806. [DOI] [PubMed] [Google Scholar]
- Riggs A.F. The Bohr effect. Ann. Rev. Physiol. 1988;50:181–204. doi: 10.1146/annurev.ph.50.030188.001145. [DOI] [PubMed] [Google Scholar]
- Rollema H.S. Bauer C. The interaction of inositol pentaphosphate with the hemoglobins of highland and lowland geese. J. Biol. Chem. 1979;254:12038–12043. [PubMed] [Google Scholar]
- Samaja M. di Prampero P.E. Cerretelli P. The role of 2,3-DPG in the oxygen transport at altitude. Resp. Physiol. 1986;64:191–202. doi: 10.1016/0034-5687(86)90041-1. [DOI] [PubMed] [Google Scholar]
- Samaja M. Crespi T. Guazzi M. Vandegriff K.D. Oxygen transport in blood at high altitude: role of the hemoglobin–oxygen affinity and impact of the phenomena related to hemoglobin allosterism and red cell function. Eur. J. Biochem. 2003;90:351–359. doi: 10.1007/s00421-003-0954-8. [DOI] [PubMed] [Google Scholar]
- Sarkar M. Pal R.N. Das D.N. Mondal D.B. Mohanty T.K. Pattanaik S. Postnatal persistence of foetal haemoglobin in yaks. Aust. Vet. J. 1999;77:190. doi: 10.1111/j.1751-0813.1999.tb11234.x. [DOI] [PubMed] [Google Scholar]
- Scott G.R. Milsom W.K. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Resp. Physiol. Neurobiol. 2006;154:284–301. doi: 10.1016/j.resp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Shaanan B. The crystal structure of oxyhaemoglobin at 2.1 Å resolution. J. Mol. Biol. 1980;142:531. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Snyder L.R.G. Deer mouse hemoglobins: is there genetic adaptation to high altitude? Bioscience. 1981;31:299–304. [Google Scholar]
- Snyder L.R.G. Chappell M.A. Hayes J.P. α-chain hemoglobin polymorphisms are correlated with altitude in the deer mouse, Peromyscus maniculatus. Evolution. 1988;42:689–697. doi: 10.1111/j.1558-5646.1988.tb02487.x. [DOI] [PubMed] [Google Scholar]
- Storz J.F. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J. Mammal. 2007;88:24–31. [Google Scholar]
- Storz J.F. Sabatino S.J. Hoffmann F.G. Gering E.J. Moriyama H. Ferrand N. Monteiro B. Nachman M.W. The molecular basis of high-altitude adaptation in deer mice. PloS Genetics. 2007;3(e45):448–459. doi: 10.1371/journal.pgen.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J.F. Hoffmann F.G. Opazo J.C. Moriyama H. Adaptive functional divergence among triplicated α-globin genes in rodents. Genetics. 2008;177:1623–1638. doi: 10.1534/genetics.107.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek Z. Kreuzer F. Hoofd L. Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. A theoretical study comparing man and rat. Pflügers Arch. 1973;342:185–187. doi: 10.1007/BF00591367. [DOI] [PubMed] [Google Scholar]
- Turek Z. Kreuzer F. Ringnalda B.E.M. Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflügers Arch. 1978;376:7–13. doi: 10.1007/BF00585241. [DOI] [PubMed] [Google Scholar]
- van Vliet G. Huisman T.H. Changes in the haemoglobin types of sheep as a response to anaemia. Biochem. J. 1964;93:401–409. doi: 10.1042/bj0930401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R.E. Functional significance and structural basis of multiple hemoglobins with special reference to ectothermic vertebrates. In: Truchot J.-P., editor; Lahlou B., editor. Animal Nutrition and Transport Processes. 2. Transport, Respiration, Excretion: Comparative, Environmental Aspects, Vol. 6. Karger, Basel: 1990. pp. 58–75. [Google Scholar]
- Weber R.E. Hemoglobin adaptations to hypoxia and altitude—the phylogenetic perspective. In: Sutton J.R., editor; Houston C.S., editor; Coates G., editor. Hypoxia and the Brain: Proceedings of the 9th International Hypoxia Symposium. Queen City Printers; Burlington, VT: 1995. pp. 31–44. [Google Scholar]
- Weber R.E. Adaptations for oxygen transport: lessons from fish hemoglobins. In: Di Prisco G., editor; Giardina B., editor; Weber R.E., editor. Hemoglobin Function in Vertebrates: Molecular Adaptation in Extreme and Temperate Environments. Springer-Verlag; New York: 2000. pp. 23–37. [Google Scholar]
- Weber R.E. High-altitude adaptations in vertebrate hemoglobins. Res. Physiol. Neurobiol. 2007;158:132–142. doi: 10.1016/j.resp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Weber R.E. Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Res. Physiol. Neurobiol. 2004;144:141–159. doi: 10.1016/j.resp.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Weber R.E. Hiebl I. Braunitzer G. High altitude and hemoglobin function in the vultures Gyps rueppellii and Aegypius monachus. Biol. Chem. Hoppe–Seyler. 1988a;369:233–240. [PubMed] [Google Scholar]
- Weber R.E. Lalthantluanga R. Braunitzer G. Functional characterization of fetal and adult yak hemoglobins: an oxygen binding cascade and its molecular basis. Arch. Biochem. Biophys. 1988b;263:199–203. doi: 10.1016/0003-9861(88)90628-5. [DOI] [PubMed] [Google Scholar]
- Weber R.E. Jessen T.H. Malte H. Tame J. Mutant hemoglobins (α119-Ala and β55-Ser)—functions related to high-altitude respiration in geese. J. Appl. Physiol. 1993;75:2646–2655. doi: 10.1152/jappl.1993.75.6.2646. [DOI] [PubMed] [Google Scholar]
- Weber R.E. Ostojic H. Fago A. Dewilde S. Van Hauwaert M.L. Moens L. Monge C. Novel mechanism for high-altitude adaptation in hemoglobin of the Andean frog Telmatobius peruvianus. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2002;283:R1052–R1060. doi: 10.1152/ajpregu.00292.2002. [DOI] [PubMed] [Google Scholar]
- Weber R.E. Voelter W. Fago A. Echner H. Campanella E. Low P.S. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2004;287:R454–R464. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]