Abstract
Anorectal abscess and fistula are among the most common diseases encountered in adults. Abscess and fistula should be considered the acute and chronic phase of the same anorectal infection. Abscesses are thought to begin as an infection in the anal glands spreading into adjacent spaces and resulting in fistulas in ~40% of cases. The treatment of an anorectal abscess is early, adequate, dependent drainage. The treatment of a fistula, although surgical in all cases, is more complex due to the possibility of fecal incontinence as a result of sphincterotomy. Primary fistulotomy and cutting setons have the same incidence of fecal incontinence depending on the complexity of the fistula. So even though the aim of a surgical procedure is to cure a fistula, conservative management short of major sphincterotomy is warranted to preserve fecal incontinence. However, trading radical surgery for conservative (nonsphincter cutting) procedures such as a draining seton, fibrin sealant, anal fistula plug, endorectal advancement flap, dermal island flap, anoplasty, and LIFT (ligation of intersphincteric fistula tract) procedure all result in more recurrence/persistence requiring repeated operations in many cases. A surgeon dealing with fistulas on a regular basis must tailor various operations to the needs of the patient depending on the complexity of the fistula encountered.
Keywords: Fistula, abscess, anorectal infection, sphincterotomy, fecal incontinence
Anorectal abscess–fistula is one of our most common afflictions. Because of the close association of abscess and fistula in etiology, anatomy, pathophysiology, therapy and morbidity, it is appropriate to consider both entities as one, i.e., abscess–fistula or a fistulous abscess. It is also appropriate to consider an abscess as the acute and a fistula as the chronic state of anorectal suppuration.
EPIDEMIOLOGY
Incidence
Most publications on the subject reflect authors' experience from a single institution; this does not address the incidence of the disease due to lack of a proper denominator. Also, it is difficult if not impossible to accurately access the incidence of anorectal abscesses because they often drain spontaneously or are incised and drained in a physician's office, emergency room, or a surgicenter. On the other hand, hospital discharges or a formal operation in an operating room are usually recorded and available for statistical evaluation. Thus among the 1000 patients presented to the Surgical Section of the Diagnostic Clinic at the University of Virginia, 150 had anorectal pathology, 4 (0.4%) had an abscess, and 8 (0.8%) had fistulas.1 This is very similar to the 532 fistulas treated in a population of 77,372 patients (0.69%) admitted to Brooklyn Hospital between 1930–1939.2 Buie reported an incidence of 5% anal fistulas in those with anorectal abscess seen at the Mayo Clinic.3 Using operating room data in Helsinki, Finland (1969–1978), the incidence of fistula was calculated to be 8.6 per 100,000 population per year (12.3% males and 5.6% females).4 Nelson is his meta-analysis equated this with 20,000 to 25,000 fistulas treated annually in the United States. Interestingly, the Ambulatory Care Survey of the National Center for Health Statistics recorded 24,000 patients with a primary diagnosis of fistulas treated in a U.S. hospital in 1979. This number has decreased significantly to 3,800 fistula operations in 1995 possibly due to more outpatient procedures.5
The incidence of anorectal abscess can be calculated and extrapolated from those of fistulas. In a large series of anorectal abscess treated in the operating room with simultaneous search for an anorectal fistula, the incidence of fistula was 34%.6 In two other single-institution series, the incidence of fistula following an abscess was 26% and 37%.7,8 If one is to extrapolate from fistula numbers, the incidence of anorectal abscess falls between 68,000 and 96,000 per annum in the United States.
Age and Sex
Data on age and sex are principally available from surgical series. Most patients present between the ages of 20 to 60 with the mean age of 40 in both sexes. Fistula in children is uncommon. Nine of 636 patients treated by Hill were less than 9 years old and all were boys.9 Similarly, 25 out of 1000 patients treated by Mazier were younger than 10 years and all but one were boys.10 Piazza and Radhakrishnan reported 33 boys and 7 girls with abscess fistula. Twenty-two of them were younger than 2 years old, 20 were under 9 months, and all were boys.11
Race
There are few epidemiologic studies regarding the racial distribution of anal fistulas. In a series of 474 patients from Cook County Hospital in Chicago, 92% of the patients were black; this closely corresponded with the racial distribution of patients at that time and in that Hospital. However, the patients were younger (61% ages 15–29) and the peak incidence was between 20 to 29 years of age.12
Seasonal Occurrence
No clear seasonal occurrence has been found in anorectal abscesses, although Vasilevsky and Gordon reported that they were more prevalent in June and at a minimum in August and September.8
Personal Hygiene and Sedentary Occupation
Although both have been implicated, personal hygiene and sedentary occupation have not been shown to be statistically significant.
Bowel Habits
Vasilevsky and Gordon reported that of the 103 abscess patients, diarrhea was the presenting symptom in 7%.8 In most published series of anorectal fistula in adults, diarrhea and constipation are infrequent symptoms.10
ETIOLOGY OF ANAL SEPSIS
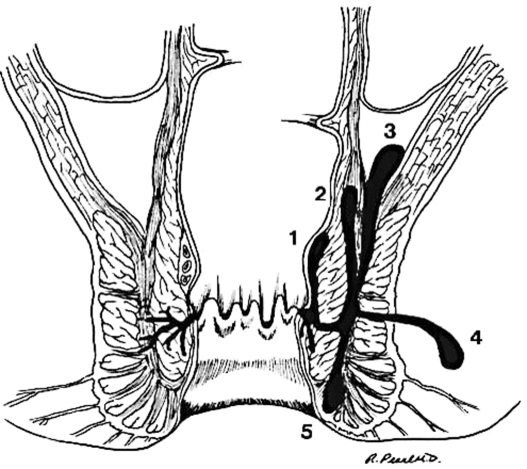
Anorectal abscess is believed to originate from an infection in the anal glands. In 1880, Hermann and Desfosses demonstrated branching of the anal glands within the internal sphincter, submucosa, and opening into the anal crypts.13 They were the first to suggest that infection in the anal glands results in extension of sepsis through the intersphincteric space to the perianal tissues.13 Tucker and Hellwig demonstrated definitively that anal sepsis originated in the anal ducts, which allows the infection to extend from the anal lumen into the wall of the anal canal.14 Eisenhammer in 1956 ascribed almost all anal fistulas to anal intermuscular gland infection.15 The infection may extend between the internal and external sphincter, reach the anal verge to become a perianal abscess. Or it may rupture through the external sphincter and become an ischiorectal abscess. If the abscess extends cephalad in the rectal wall a high intermuscular abscess will result and extension of abscess above the levators will produce a supralevator abscess. A deep postanal abscess may extend to either or both ischiorectal fossae resulting in a horseshoe abscess (Figs. 1 and 2).
Figure 1.
(Left) Anal glands opening in anal crypts. (Right) Extension of abscess to adjacent spaces. 1, submucosal; 2, high intermuscular; 3, supralevator; 4, ischiorectal; 5, perianal. (Illustration provided by Russell K. Pearl, M.D., Department of Surgery, University of Illinois at Chicago.)
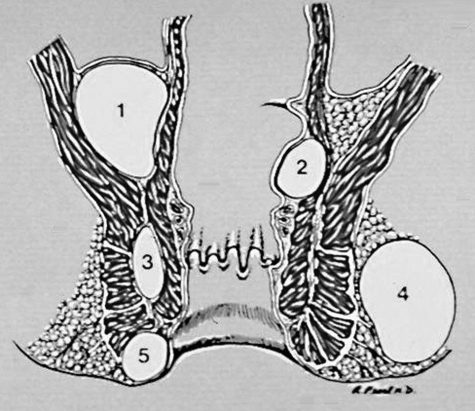
Figure 2.
High abscesses. 1, supralevator; 2, submucosal/intermuscular. Low abscesses. 3, intersphincteric; 4, ischiorectal; 5, perianal. (Illustration provided by Russell K. Pearl, M.D., Department of Surgery, University of Illinois at Chicago.)
There are rare causes of supralevator abscess, which result from a pelvic sepsis due to appendicitis, diverticulitis, or gynecologic sepsis. These may extend into the rectum or spread downward through the levators into the ischiorectal fossa. Crohn disease of the anorectal region may extend transmurally into the perirectal or perianal space. Similarly, suppuration may occur with perforation of the anorectum from impacted chicken or fish bones, from externally penetrating trauma (stab or gunshot wounds), low rectal cancer or cancer originating in the anal glands. Specific infections related to oxyuris vermicularis, tuberculosis, or fungal infections are relatively uncommon and of historic interest.
Anorectal fistulas arise from a preexisting abscess in the majority of cases. A previously drained abscess may heal permanently, heal and recur in the same location, or remain unhealed draining intermittently or continuously. In both latter cases, a diagnosis of anal fistula is almost certain. In a study of 100 recurrent anorectal abscesses, an underlying fistula was demonstrated in 68% of the patients.16 Fistulas may occur with much less frequency from trauma, iatrogenic perforation, posthemorrhoidectomy, infected episiotomy or repair of a fourth-degree sphincter tear during delivery, infected anal fissure, or Crohn disease.
In a study of 1023 patients Ramanujam et al found 219 intersphincteric, 75 supralevator, 437 perianal, 233 ischiorectal, and 59 high intermuscular variants. The incidence of fistula in these abscess subsets was 47.4%, 42.6%, 24.5%, 25.3%, and 15.2%, respectively. With unroofing and primary fistulotomy, when deemed safe, the incidence of recurrent infection was only 3.7%.6
CLASSIFICATION OF FISTULAS
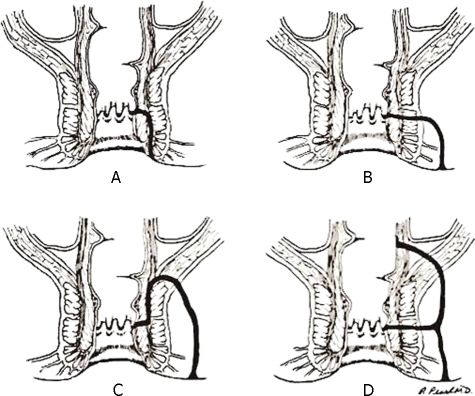
Throughout the years many classifications have been proposed (low vs high, simple vs complex, with or without extension). Nowadays, the classification of Park, Gordon, and Hardcastle is the one commonly used because not only does it describe accurately the anatomic track of the fistula, but it is also important in predicting the complexity of the operation, the need for varying degree of sphincterotomy, and the potential for continence disturbance.17 Intersphincteric fistulas begin at the dentate line and end at the anal verge, tracking between internal and external sphincters. Transsphincteric fistulas track through the external sphincter into the ischiorectal fossa. Suprasphincteric fistulas originate at the anal crypt and circle the entire sphincter mechanism before ending at the ischiorectal fossa. Extrasphincteric fistulas are usually very high in location, traverse the entire sphincter mechanism as well as the levators, and may originate anywhere in the anorectum—not solely from an anal crypt. Each of these types of fistulas may be associated with an adjacent communicating high blind tract (Fig. 3).
Figure 3.
Classification of fistulas after Parks, Gordon, Hardcastle.17 (A) Intersphincteric. (B) Transsphincteric. (C) Suprasphincteric. (D) Extrasphincteric. (Illustration provided by Russell K. Pearl, M.D., Department of Surgery, University of Illinois at Chicago.)
DIAGNOSIS
The principle symptom of anorectal abscess is pain. As such, it has to be differentiated from other causes of anal pain including anal fissure, thrombosed hemorrhoids, levator spasm, sexually transmitted disease, proctitis, and cancer. Low (intersphincteric, perianal, and ischiorectal) abscesses are usually associated with swelling, cellulites, and exquisite tenderness, but few systemic symptoms. High (submucosal, supralevator) abscesses may have few local symptoms, but significant systemic (fever, toxicity) symptoms. If an abscess is suspected, but cannot be diagnosed with certainty due to patient's resistance, examination under anesthesia (EUA) must be arranged as soon as possible. Rarely, imaging is needed to diagnoses an acute abscess.
A clinically draining fistula is easily diagnosed and anoscopy should be performed looking for the internal opening. Goodsall's rule is still applicable unless the anatomy has been distorted with prior operations and fibrosis.18 Probing the fistula tract in the office is painful and unnecessary. If intraoperatively a primary opening cannot be easily identified, injection of a dilute hydrogen peroxide solution with or without a few drops of methylene blue is often helpful. If one or more EUAs have not resulted in identification of the internal opening, anal fistulography, endoanal ultrasound with injection of peroxide, computed tomography (CT), or magnetic resonance imaging (MRI) may be utilized.
In patients with long-standing fistulas there is a risk of developing cancer. In one study, six cancers were found in fistulas which had been present for an average of 13.8 years.19 No intraluminal tumor was found in any of the six patients. This condition must be differentiated from perforating cancer causing secondary fistulas.
OBJECTIVES IN MANAGEMENT
Abscess
The goal of surgical therapy of an abscess is to drain the abscess expeditiously, drain any associated sepsis in adjacent anatomic spaces, identify a fistula tract and either proceed with primary fistulotomy to prevent recurrence (if sphincterotomy is deemed safe) or mark the fistula track with a loose seton for future consideration.
Fistulas
The goal of surgical therapy of a fistula is to define the anatomy accurately, drain associated sepsis (undrained abscess), eradicate the fistula tract if possible, prevent recurrence, and preserve sphincter integrity and continence. Fistulotomy is preferable to fistulectomy. Excision of the entire fistula tract is not only unnecessary, but also will result in a wider and deeper gap in the sphincter mechanism and worsening fecal incontinence.
TREATMENT OF ANORECTAL ABSCESS
An anorectal abscess requires a surgical procedure for early, adequate, and dependent drainage. A superficial abscess may be amenable to drainage in the office or emergency room, however, a more complex ischiorectal abscess needs to be examined under anesthesia and drained appropriately. General or regional anesthesia allows the surgeon to adequately examine the patient, identify the entire extent of the abscess, and provide wide, dependent drainage. Even if a large abscess has drained spontaneously, EUA and wide drainage is still indicated, because so often the thick proteinaceous abscess content will plug the small opening and the abscess will continue to smolder.
There is no place for antibiotic therapy alone as “conservative” management of an anorectal abscess. The abscess wall contains occluded and necrotic blood vessels and the antibiotic will never penetrate into the abscess cavity. The old technique of incision of abscess, curettage, and instillation of antibiotics and primary closure advocated in the 1950s by Goligher19a has long been abandoned. Antibiotics in addition to adequate drainage are indicated in diabetic patients, those with morbid obesity or immunosuppression (e.g., human immunodeficiency virus, acquired immunodeficiency syndrome, chemotherapy, posttransplant surgery, etc.). If a patient remains febrile, continues to have signs and symptoms of cellulitis or persistent elevated white blood cell count, the patient should be returned to the operating room to search for undrained pus rather than continuing with the same or a newer regimen of antibiotics.
A large abscess should be drained with multiple counter incisions rather than a long incision, which will create a step-off deformity and delay wound healing. Packing is not recommended due to severe pain inflicted on the patient during its removal, unless there is continuous oozing of blood from the depth of the wound, which is difficult to control directly, but stops with packing and compression. Similarly, horseshoe abscesses should be drained with counter incisions on both sides rather than a long curved incision connecting the two sides. It is preferable to make as many incisions as necessary to drain a large abscess, leaving the intervening skin intact and encircling it with a Penrose drain sutured onto itself to avoid premature extrusion. The drains will delay the healing of superficial tissues allowing the abscess to fill in and close from the depth. The drains can be removed in an outpatient setting 2 to 3 weeks later as the surgeon sees fit.
Should a surgeon search for a fistula at the time of the initial incision and drainage (I & D) of an abscess? This is controversial. McElwain and McLean reported 1000 primary fistulotomies during drainage of an abscess with no adverse results.20 However, this report preceded the advent of anal physiologic studies and endoanal ultrasonography to determine the extent of the sphincter injury and its consequences. Others have reported a good success rate with primary fistulotomy.6 If the surgeon is not familiar with anorectal anatomy or pathology, anal fistula should not be searched for. However, an experienced surgeon may probe the corresponding anal crypt gently, looking for a fistula. If a fistula is identified and is quite superficial, primary fistulotomy may be attempted.6 If the surgeon is not certain of the thickness of the sphincter muscle involved in the fistula, a loose seton of braided, nonabsorbable suture should be inserted into the fistula tract, tied loosely to act as a drain as well as a marker for future fistula surgery.21
In horseshoe abscesses, the deep postanal space should be unroofed. The abscess on either side is drained and a Penrose drain is inserted and secured on either side for prolonged drainage. If a midline posterior fistula is identified, a seton can be placed and tied loosely to be addressed in the future.
Anorectal abscess in patients with Crohn disease should be drained as close to the anal canal as possible while avoiding an incision in the external sphincter. This will result in a shorter rather than longer fistula track subsequently and makes the future management of the patient easier.
An alternate method of drainage of abscess popular at the Cleveland Clinic was to place a mushroom catheter in the abscess, keeping it for as long as necessary for prolonged drainage. The catheter can be used for a sinogram or a contrast CT examination. If the abscess is considered complex or recurrent, imaging might be helpful to provide a road map for subsequent operations.
Prolonged drainage from an I & D site of an abscess beyond 2 to 3 months should raise suspicion of a fistula. Similarly, if an abscess heals and recurs at the same location a fistula should be strongly suspected. Prolonged therapy with the same or different antibiotics and cauterization of a fistula track is usually fruitless and delays the inevitable need for reexploration. The appearance of an abscess on the opposite side should alert the surgeon of the presence of a horseshoe abscess–fistula originating in the midline, most commonly posterior midline. Confirmation of diagnosis with CT, endoanal ultrasound (with or without peroxide injection), or MRI is usually unnecessary. Fistulography has been replaced by modern imaging techniques especially MRI, which can be a very accurate diagnostic tool in experienced hands.
TREATMENT OF ANORECTAL FISTULA
If a drainage site of an abscess does not heal in 2 to 3 months or breaks down after healing, a fistula should be strongly suspected and the patient should be reexplored under anesthesia. Imaging studies can be utilized for complex or recurrent cases, but is often unnecessary and quite costly.
Preoperatively a fistula can be diagnosed by gentle palpation of the tract connecting the external opening to the anal canal. A lubricated gloved finger should be able to detect the fistula track (like the extensor tendons of the hand). In the operating room with the patient under anesthesia or monitored anesthesia care, the secondary opening is probed with a blunt tipped fistula probe and the corresponding area of the dentate line inspected through an operating anoscope. Compression of the fistula tract might yield a droplet of pus from the primary opening. Lateral traction on the external opening will help straighten the fistula tract and facilitate probing. If this proves ineffective, a dilute solution of plain hydrogen peroxide or with a few drops of methylene blue should be injected gently into the external opening with an angiocath and following the Goodsall's rule, the dentate line is inspected for leakage of the injected solution. More than a century since its original description, the Goodsall's rule is still a very reliable guide for identification of the internal opening of the fistula, unless the anatomy has been distorted with previous operations and scarring.18
Once the fistula is identified, the surgeon must decide what to do with it. In general, the goal of fistula surgery is primary healing. If the surgeon is too aggressive, the fistula may be cured at a cost of incontinence or worse, yet a false passage may be created that further complicates the clinical picture. Nevertheless, being conservative and doing less harm to the sphincter contributes to the persistence or recurrence of a fistula.
When treating a fistula the classification proposed by Parks, Gordon, and Hardcastle (Fig. 3) is invaluable not only to assess the complexity of the fistula, but also to predict the ease or the difficulty of the operation, risk of recurrence, or incontinence. These issues must be discussed with the patient preoperatively. If the risks versus benefits of surgery have not been discussed with the patient, it is appropriate to abort the procedure and postpone definitive surgery until the patient understands the risk and benefits of a proposed operation and is able to decide and give informed consent. If the surgeon is not confident or certain about the nature and complexity of a fistula, a second opinion from an experienced colorectal surgeon should be sought.
Intersphincteric fistulas are amenable to a simple distal internal sphincterotomy much like lateral internal sphincterotomy done for anal fissure, this procedure results in minor disturbance of continence (5% or less).
Extrasphincteric fistulas, though rare, are too complex to be treated with fistulotomy and may need diversion and complicated procedures to close the primary opening of the fistula.
Transsphincteric and suprasphincteric fistulas are difficult when it comes to the choice of procedures to be used in treatment. In general, two types of operations can utilized for these fistulas: those employing sphincterotomy and those without sphincterotomy.
FISTULOTOMY WITH SPHINCTEROTOMY
Only low (distal) transsphincteric fistulas involving the distal one third to one half of the external sphincter are amenable to sphincterotomy. This will definitely cause some disturbance of continence, which can be estimated based on the amount of external sphincter divided to be in the range of 17 to 33%.22
A partial external sphincterotomy can be utilized as a definitive procedure for low transsphincteric fistulas.
A staged fistulotomy implies that a portion of the sphincter mechanism is divided at the first operation, a marking (loose) seton is placed around the remaining portion of the external sphincter involved in the fistula. After 6 to 8 weeks, the patient is reexamined in the operating room and if the initial fistulotomy incision is healed by fibrosis, the remainder of the sphincter is divided, removing the seton. In a study of 480 patients, Ramanajam et al reported good results with staged fistulotomy with minimal impairment of continence.21 However, this report preceded the anal physiologic studies and availability of endoanal ultrasound.
Using a cutting seton, the skin overlying the fistula is removed, fibrotic tissue, if any, is excised and a rubber band is inserted around the external sphincter and tied loosely to produce additional drainage.22 After the suppuration has subsided, the rubber band is tightened with serial sutures or a hemorrhoidal ligator every 2 weeks until the rubber band Seton cuts through the muscle completely.23 In a small series, a cutting seton was associated with low recurrence rates (0.8%) and minor incontinence (2–6%) and major incontinence of 5–10%.24
Using a chemical seton, a seton coated with latex is impregnated with Kshara, an alkaline compound, and is introduced in the fistula tract and tightened progressively, allowing the chemical to cut through the fistula and allowing the tissue to reunite behind the cutting seton. In a comparison of 237 patients with fistulotomy compared with 265 treated with Kshara sutra, the healing time was faster in the fistulotomy group (4 vs 8 weeks), the recurrence rate was lower (4% vs 11%) and the incontinence rate was similar.25
TREATMENT OF FISTULA WITHOUT SPHINCTEROTOMY
To preserve continence, different methods have been designed in the last two decades to close the fistula tract without sphincterotomy. The problem with all of these techniques is that they have a variable success rate averaging 40 to 70% and the surgeon and the patient trade cure for preservation of continence and the need for further and often multiple operations.
A loose seton placed at the time of original drainage of abscess or in a “high” fistula in ano, provides drainage, prevents recurrence of abscess, and acts as a marker for future fistula surgery, e.g., two-stage fistulotomy procedures.26
The fibrin sealant technique originally entailed using an autologous material that has now been replaced with a commercially available sealant. The fistula opening is cannulated with a double-channel catheter from a secondary opening toward the primary opening. The injection of fibrinogen through one arm and thrombin through the other results in the production of a pearly clot at the internal opening. The catheter is then gradually withdrawn through the fistula tract and fibrin sealant is injected continuously until the tract is completely sealed and a similar clot is seen at the external opening. The wound is covered with Vaseline gauze and the patient is followed on a biweekly basis until either the fistula closure occurs or failure is documented beyond 12 weeks. Early enthusiasm with this technique with a report of 80% has dwindled to 30 to 40% success rates.27 The advantage of fibrin sealant is that it may be repeated and the salvage procedure increases the success rate by an additional 10 to 15%.28 This technique is more suitable for longer fistula tracks. Rectovaginal fistula due to the short straight track is especially unsuitable for fibrin sealant.27
An anal fistula plug (AFP) is an acellular porcine submucosal collagen designed to allow growth of fibroblasts into its scaffolding, resulting in closure of fistulas. Originally, success rates of up to 80 to 85% were reported; however, more recent reports have lowered the success to 30 to 50%.29 As in fibrin sealant, failed cases can be subjected to a salvage procedure using AFP. There is evidence from a consensus conference that placing a seton for 2 to 3 months allowing the fistula tract to mature improves success rates.30 A newer fistula plug made of PTFE is under investigation and long-term results are not yet available.
An endorectal advancement flap (ERAF), originally designed for low rectovaginal or anoperineal fistulas secondary to obstetric injury have also been utilized for anorectal fistulas. In this procedure, a flap of rectal wall including mucosa, submucosal, and superficial smooth muscle is raised cephalad. The primary opening of the fistula is closed and the flap is brought down and sutured to the anoderm. The fistula tract is drained with a size #10–12 mushroom catheter for 10 to 14 days. If the internal opening of the fistula is too distal, ERAF will result in ectropion and a “wet anus,” which will be interpreted as incontinence by the patient. ERAF is suitable for fistulas in the lateral or anterior quadrant, but it is difficult to raise a posterior midline flap due to the acute angulation of the rectum rendering the proximal extent of the flap relatively inaccessible. If the rectal mucosa is normal, ERAF can be used in Crohn fistulas as well.31
A dermal island flap anoplasty (DIFA) is a procedure for resurfacing the anal canal in low anal strictures. Nelson and colleagues modified the procedure to cover the primary opening of the fistula after it was closed with absorbable sutures. The raised dermal flap is then advanced in the anal canal and sutured to the lower rectal wall with full-thickness bites. The procedure can be done in all quadrants and even in Crohn fistulas as long as the low rectal mucosa is normal. Recurrence/persistence is always seen early (6–18 months) and may be addressed with a repeat (salvage) flap. A success rate as high as 80% was reported by Nelson et al.32
Ligation of the intersphincteric fistula tract (LIFT) is the most recent attempt in closure of transsphincteric fistula tracts. An incision is made parallel to the anal verge and deepened, separating the internal and external sphincters until the fistula tract is encountered. An indwelling probe facilitates identification of the tract. The tract is then encircled, ligated at both the proximal and distal ends with absorbable sutures, and the fistula tract is divided. A segment of the tract can be excised (similar to a vasectomy procedure). The external end of the tract is left alone if short and can be drained with a small mushroom catheter if it is long. Although this is a relatively new procedure, success rates of 57% and 89% have been reported.33,34 Long-term follow-up is needed to validate these results, but this operation makes sense as a definitive method of closure of transsphincteric fistulas without sphincterotomy.
The York–Mason Procedure is an operation that allows an excellent exposure of the midrectum. The procedure, originally designed for rectoprostatic urethral fistula, may be utilized for extrasphincteric fistulas with or without covering colostomy. The advantage of this approach, despite its complexity, is that it puts the surgeon in virgin territory, especially after multiple prior failed operations.
CONCLUSION
An anal fistula is an affliction that tests the patience of the surgeon and the patient alike. Approaching the fistulas with the intent to cure, the surgeon must always keep in mind the fine balance between an aggressive approach resulting in cure and incontinence and a conservative approach, preserving continence and resulting in recurrence or persistence and the need for additional operative procedures. Respecting the classic adage of “primum non nocere,” the surgeon must be familiar with all available alternatives in treating a fistula and try to tailor the operation to the patient and not vice versa. In these litigious times, recurrence or persistence of a fistula is surely preferable to incontinence.
References
- 1.Shrum R C. Anorectal pathology in 1000 consecutive patients with suspected surgical disorders. Dis Colon Rectum. 1959;2:469–472. doi: 10.1007/BF02616939. [DOI] [PubMed] [Google Scholar]
- 2.Buda A M. General candidates of fistula in ano: the role of foreign bodies as causative factors fistulas. Am J Surg. 1941;54:384–387. [Google Scholar]
- 3.Buie S L., Sr Practice Proctology. 2nd ed. Springfield, IL: Charles C Thomas; 1960. [Google Scholar]
- 4.Sainio P. Fistula in ano in a defined population: incidence and epidemiology of patients. Ann Chir Gynaecol. 1984;73:219–224. [PubMed] [Google Scholar]
- 5.Nelson R L. Anorectal abscess fistula: what do we know? Surg Clin North Am. 2002;82(6):1139–1151. v–vi. doi: 10.1016/s0039-6109(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 6.Ramanujam P S, Prasad M L, Abcarian H, Tan A B. Perianal abscesses and fistulas. A study of 1023 patients. Dis Colon Rectum. 1984;27(9):593–597. doi: 10.1007/BF02553848. [DOI] [PubMed] [Google Scholar]
- 7.Scoma J A, Salvati E P, Rubin R J. Incidence of fistulas subsequent to anal abscesses. Dis Colon Rectum. 1974;17(3):357–359. doi: 10.1007/BF02586982. [DOI] [PubMed] [Google Scholar]
- 8.Vasilevsky C A, Gordon P H. The incidence of recurrent abscesses or fistula-in-ano following anorectal suppuration. Dis Colon Rectum. 1984;27(2):126–130. doi: 10.1007/BF02553995. [DOI] [PubMed] [Google Scholar]
- 9.Hill J R. Fistulas and fistulous abscesses in the anorectal region: personal experience in management. Dis Colon Rectum. 1967;10(6):421–434. doi: 10.1007/BF02616813. [DOI] [PubMed] [Google Scholar]
- 10.Mazier W P. The treatment and care of anal fistulas: a study of 1,000 patients. Dis Colon Rectum. 1971;14(2):134–144. doi: 10.1007/BF02560060. [DOI] [PubMed] [Google Scholar]
- 11.Piazza D J, Radhakrishnan J. Perianal abscess and fistula-in-ano in children. Dis Colon Rectum. 1990;33(12):1014–1016. doi: 10.1007/BF02139215. [DOI] [PubMed] [Google Scholar]
- 12.Read D R, Abcarian H. A prospective survey of 474 patients with anorectal abscess. Dis Colon Rectum. 1979;22(8):566–568. doi: 10.1007/BF02587008. [DOI] [PubMed] [Google Scholar]
- 13.Hermann G, Desfosses L. Sur la muquese de la region cloacale de rectum. (III) Compts Rend Acad Sci. 1880;90:1301–1302. [Google Scholar]
- 14.Tucker C C, Hellwing C A. Histopathology of anal glands. Surg Gynecol Obstet. 1933;58:145–149. [Google Scholar]
- 15.Eisenhammer S. The internal anal sphincter and the anorectal abscess. Surg Gynecol Obstet. 1956;103(4):501–506. [PubMed] [Google Scholar]
- 16.Chrabot C M, Prasad M L, Abcarian H. Recurrent anorectal abscesses. Dis Colon Rectum. 1983;26(2):105–108. doi: 10.1007/BF02562586. [DOI] [PubMed] [Google Scholar]
- 17.Parks A G, Gordon P H, Hardcastle J D. A classification of fistula-in-ano. Br J Surg. 1976;63(1):1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 18.Goodsall D M. Anorectal fistula. In: Goodsall D M, Miles W E, (eds.), editors. Diseases of Anus and Rectum. London: Longman and Green; 1900. p. 92. [Google Scholar]
- 19.Nelson R L, Prasad M L, Abcarian H. Anal carcinoma presenting as a perirectal abscess or fistula. Arch Surg. 1985;120(5):632–635. doi: 10.1001/archsurg.1985.01390290106019. [DOI] [PubMed] [Google Scholar]
- 19a.Goligher J C, editor. Abscess. Surgery of the Anus, Rectum and Colon. 3rd ed. Springfield, IL: Charles C Thomas; 1975. pp. 200–201. [Google Scholar]
- 20.McElwain J R, Alexander R M, Maclean M D, et al. Experience with primary fistulectomy of anorectal abscess: a report of 1000 cases. Dis Colon Rectum. 1975;18:646–649. doi: 10.1007/BF02604266. [DOI] [PubMed] [Google Scholar]
- 21.Ramanujam P S, Prasad M L, Abcarian H. The role of seton in fistulotomy of the anus. Surg Gynecol Obstet. 1983;157(5):419–422. [PubMed] [Google Scholar]
- 22.Parks A G, Stitz R W. The treatment of high fistula-in-ano. Dis Colon Rectum. 1976;19(6):487–499. doi: 10.1007/BF02590941. [DOI] [PubMed] [Google Scholar]
- 23.Hanley P H. Rubber band seton in the management of abscess-anal fistula. Ann Surg. 1978;187(4):435–437. doi: 10.1097/00000658-197804000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg S M, Garcia-Aguilar J. The cutting seton in anal fistula. In: Phillip R K, Lunniss P J, (eds.), editors. Anal Fistula. London: Chapman and Hall Medical; 1995. p. 101. [Google Scholar]
- 25.Shukla N K, Narang R, Nair NGK, et al. Indian Council of Medical Research Multicentric randomized controlled clinical trial of Kshaarasootra (Ayurvedic medicated thread) in the management of fistula-in-ano. Indian J Med Res. 1991;94:177–185. [PubMed] [Google Scholar]
- 26.Pearl R K, Andrews J R, Orsay C P, et al. Role of the seton in the management of anorectal fistulas. Dis Colon Rectum. 1993;36(6):573–577. discussion 577–579. doi: 10.1007/BF02049864. [DOI] [PubMed] [Google Scholar]
- 27.Cintron J R, Park J J, Orsay C P, et al. Repair of anorectal fistulae with fibrin sealant – long-term follow-up. Dis Colon Rectum. 2000;43:944–950. doi: 10.1007/BF02237355. [DOI] [PubMed] [Google Scholar]
- 28.Sentovich S M. Fibrin glue for all anal fistulas. J Gastrointest Surg. 2001;5(2):158–161. doi: 10.1016/s1091-255x(01)80028-7. [DOI] [PubMed] [Google Scholar]
- 29.Ellis C N, Rostas J W, Greiner F G. Long-term outcomes with the use of bioprosthetic plugs for the management of complex anal fistulas. Dis Colon Rectum. 2010;53:798–802. doi: 10.1007/DCR.0b013e3181d43b7d. [DOI] [PubMed] [Google Scholar]
- 30.Corman M, Abcarian H, Bailey H R, et al. The Surgisis AFP anal fistula plug: report of a consensus conference. Colorectal Dis. 2008;10:17–20. doi: 10.1111/j.1463-1318.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 31.Ozuner G, Hull T L, Cartmill J, Fazio V W. Long-term analysis of the use of transanal rectal advancement flaps for complicated anorectal/vaginal fistulas. Dis Colon Rectum. 1996;39(1):10–14. doi: 10.1007/BF02048261. [DOI] [PubMed] [Google Scholar]
- 32.Nelson R L, Cintron J, Abcarian H. Dermal island-flap anoplasty for transsphincteric fistula-in-ano: assessment of treatment failures. Dis Colon Rectum. 2000;43(5):681–684. doi: 10.1007/BF02235588. [DOI] [PubMed] [Google Scholar]
- 33.Bleier J I, Moloo H, Goldberg S M. Ligation of the intersphincteric fistula tract: an effective new technique for complex fistulas. Dis Colon Rectum. 2010;53(1):43–46. doi: 10.1007/DCR.0b013e3181bb869f. [DOI] [PubMed] [Google Scholar]
- 34.Shanwani A, Nor A M, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53(1):39–42. doi: 10.1007/DCR.0b013e3181c160c4. [DOI] [PubMed] [Google Scholar]