Abstract
Hidradenitis suppurativa (HS) is a chronic debilitating disorder that can affect any areas bearing apocrine glands. Perineal HS is associated with high morbidity compared with other anatomic regions. Early-stage disease may mimic various other forms of cutaneous disorders, but as HS progresses pathognomonic skin changes occur. Clinical stage can guide the therapeutic approach, but the lowest recurrence rate is obtained by removing all involved skin and subcutaneous fat. Pruritus ani is a complex disease with a multitude of etiologies. Its management can be frustrating and disappointing for the patient and doctor alike. The key is to start with simple treatment options focusing on perianal hygiene and avoidance of the most common offending foods and beverages. If these measures fail, topical medications should be attempted before graduating to perianal injections of methylene blue as a last resort.
Keywords: Hidradenitis suppurativa, acne inversa, pruritus ani
HIDRADENITIS SUPPURATIVA
Hidradenitis suppurativa (HS) is a chronic recurrent inflammatory skin disorder affecting the hair and areas bearing apocrine sweat glands. It most commonly effects the axillae, perineum, and inframammary regions of postpubertal males and females.1 Perineal disease is more common in males and axillary disease is more common in females.2 There are two main theories that describe the pathogenesis of this disease, which often results in chronically draining wounds and sinus tracks.3,4 This may ultimately manifest as subcutaneous “pitlike” scarring, distorting contractures, and persistent induration of the skin. HS may lead to socioeconomic isolation and significantly decrease quality of life.5,6 Treatment is based on the stage of disease at presentation and ranges from medical therapy (antibiotics, antiandrogens, and immunosuppression) to more invasive procedures where total excision of all effected apocrine sweat gland areas may be required.7,8,9,10
Epidemiology
HS rarely occurs before puberty or after menopause and is most common between the second and fourth decade of life.11 The incidence of HS is thought to be underreported, but recent studies point to a prevalence of around 1%. Jemec et al estimated a 1-year prevalence of 1% with point prevalence estimated at 4.1%. Similarly, Revuz et al estimated the prevalence at 1% in a case–control French population study.11,12 Conflicting results have been reported in regards to sex and racial distribution of HS. Overall, HS is thought to be more common in females, but location may be a more decisive factor in gender distribution of this disease as published by Wiltz et al. In their series, 93% of patients with perianal HS were male.13 HS has been shown to affect all races, but 2 studies have suggested a higher incidence in African Americans.14,15 Homma et al looked at the number of apocrine glands in African Americans and compared with Caucasians found a 3-fold difference.14 African Americans have more apocrine glands. This may be somewhat conflicting as the etiology may not be of apocrine origin, but rather follicular occlusion.4
Pathogenesis
In 1839, Velpeau published a case report in which he described an inflammatory and abscess-forming process affecting the axillary, mammary, and perianal regions, which later was suggested to have originated in the sweat glands.16 Verneuil reported the first series of patients with this disease and gave it the name “hidradenitis suppurativa.”17 Other terminology can be found in the literature such as acne inversa or Verneuil disease. Sweat glands are classified into apocrine and eccrine. Eccrine glands serve a role in thermal regulation and secrete directly onto the skin. Apocrine glands are larger and extend through the dermis into the subcutaneous tissue. They have a coil-like base that secretes milky fluid into hair follicles via a secretory duct. Apocrine secretion can be stimulated by various emotions (sexual arousal, pain, anger) and is odorless. As the apocrine fluid comes into contact with the bacterial flora of the skin it becomes malodorous.15
The etiology of HS remains a matter of debate, but likely originates from occlusion of the apocrine and/or the hair follicular duct. This may be caused by hyperkeratosis or anatomically abnormal follicles resulting in obstruction of these pilosebaceous organs. The secondary effects of this process are stasis and dilatation of the follicle/apocrine gland followed by bacterial infiltration via the hair follicle. When the follicles/apocrine glands rupture into the subcutaneous space, abscesses are formed that ultimately may lead to complex subcutaneous sinuses and draining fistulas. Longstanding subcutaneous inflammation may result in subcutaneous “pitlike” scarring, distorting contractures, and persistent induration of the skin.3,4 Although approximately half of patients with HS have negative cultures, a wide variety of bacterial pathogens have been cultured from abscesses, fistulas, and sinus tracts.18 Among them are both gram-positive and gram-negative bacteria, but the most common in perineal HS is Streptococcus milleri.19,20 Environmental and genetic factors have been implicated to predispose patients to HS. Androgens, trauma, and exposure to chemicals in antiperspirants have been reported in retrospective studies, but many of these theories have been disputed.21,22,23 In addition, there is increased prevalence of HS in obese patients and smokers.11 Obesity is thought to predispose to follicular duct occlusion via shear forces that may trigger hyperkeratosis and desquamation of skin.24 Nicotine has been implicated in several studies. Wiltz et al found that 70% of their patients with perianal HS were smokers. This has been supported by a study by Hana et al, which examined skin tissue cultures and the effects of nicotine. Nicotine may inhibit normal glandular duct secretion and promote epithelial hyperplasia.13,25,26 There are also possible immunologic causes to HS. A defect in neutrophilic function, an autoimmune mechanism, and an increase in toll-like receptors (TLR) have been hypothesized as etiologic factors of chronic inflammation in this debilitating disease.27,28,29
Clinical Presentation
The initial presentation of HS has been described as painful subcutaneous nodules that may progress to coalesced abscesses that fistulize with a malodorous discharge through the dermis via one or multiple sites.15 If patients present with mild disease, limited regions are affected and spontaneous remission may occur.30 Recurrences are common and highest in perianal HS, which contribute to its more debilitating disease process compared with other sites of apocrine sweat glands.31 With each recurrence the region ultimately becomes distorted with scarring, dermal contraction, induration, and chronically malodorous draining sinus tracts. Perianal HS may extend to the dentate line as this corresponds to the distribution of apocrine glands and hair follicles in the anal canal.32 Perianal HS can coexist with other inflammatory disorders. Fistulization above the dentate line may give suspicion for coexcising cryptoglandular disease or Crohn disease. Church et al found a Crohn incidence of ~40% in their series of HS and hypothesized that Crohn may precipitate perianal HS. It surely complicated their management as 70% of these patients with coexisting disease required proctectomy and 91% fecal diversion.33
Two staging symptoms have been developed for HS. The Hurley system is a simplistic objective description of disease extent and separates patients into three groups based on presence of scar formation and sinus tracts (Table 1) and may be useful when deciding a therapeutic approach for HS.34
Table 1.
Clinical Staging of Hidradenitis Suppurativa
| Stage | Description |
|---|---|
| I | Abscess formation, single or multiple, without scarring or sinus tracts |
| II | Recurrent abscesses with tract formation and scarring, single or multiple, widely separated lesions |
| III | Multiple interconnected tracts and abscesses throughout entire area |
A second staging system has been developed by Sartorius et al that incorporates involved anatomic regions, number and type of lesions, distance between lesions, and the presence of normal skin in between lesions. In addition, they suggest using a visual analogue scale for pain assessment or the dermatology life quality index (DLQS). Due to its complexity, this system likely is better for use in conducting clinical trials than in planning treatment strategies.35
Differential Diagnosis
HS is a clinical diagnosis and various stages of this disease entity may have a similar presentation to other cutaneous lesions. The characteristic age of onset and distribution of lesions of HS is likely the best differentiator and subcutaneous “pitlike” scarring, distorting contractures, and induration of the skin is pathognomonic for end-stage HS. Alikan et al in a review article summarized the differential diagnosis of HS in two categories based on early and late lesions (Table 2).36
Table 2.
Differential Diagnosis of Hidradenitis Suppurativa
| Early Lesions | Late Lesions |
|---|---|
| Acne | Actinomycosis |
| Carbuncles | Anal fistula |
| Cellulitis | Cat scratch disease |
| Cutanous blastomycosis | Crohn disease |
| Dermoid cyst | Granuloma inguinale |
| Erysipelas | Ishciorectal abscess |
| Furuncles | Lymphogranuloma venereum |
| Inflamed epidermoid cysts | Nocardia infection |
| Lymphadenopathy | Noduloulcerative syphilis |
| Perirectal abscess | Pilonidal disease |
| Pilonidal cyst | Tuberculous abscess |
| Tularemia |
Despite its rarity, malignant transformation to squamous cell carcinoma in longstanding HS (> 20 years) has been published in over 25 case reports.37 The incidence is thought to be 3.2% in HS, more common in perianal, perineal, and inguinal regions and has shown to have an aggressive course compared with de novo squamous cell cancers with rapid growth and early metastasis.38,39 In addition local complications from HS in the perineal region may manifest itself as lymphoedema, fistulas, and strictures in the scrotum, urethra, anus, and sphincter mechanism resulting in incontinence.40,41
Treatment
Various subspecialties have published case reports and outcome series of their management strategies for HS. Dermatologists, plastic surgeons, and colorectal surgeons are among a few. Based on presentation these treatment strategies can be categorized broadly into medical and surgical. The Hurley clinical staging system may be useful for such measures and patients may require a series of different treatments in an attempt to control symptoms of HS. The best way to ensure the lowest recurrence rate is aggressive removal of all apocrine-bearing areas affected. As has been stated, initial occurrence may present only with a painful inflammatory nodule. Warm compresses, weight loss, and topical cleaning agents may be used in an attempt to reduce friction and skin bacterial load.
Antibiotics, retinoids, hormones, and immunosuppressive agents have been used to treat HS. All have shown success in reducing symptoms temporarily but none long-term. For perianal and perineal HS, antibiotics must be chosen to cover both aerobic and anaerobic bacteria. Topical clindamycin and oral clindamycin with rifampin, in addition to tetracycline, erythromycin, and doxycycline have shown efficacy at reducing symptoms of HS.42,43 Long-term treatment is required ranging from 2–12 weeks to achieve remission. Evidence though is lacking that antibiotics change the natural course of this disease. Isotretinoin has been used with variable success, but should not be used in women of child-bearing age due to its teratogenecity.44,45 Mortimer et al looked at combined hormonal therapy in a double-blinded crossover study in 24 women. One-third achieved remission lasting up to 18 months. Finasteride, at a dose of 5 mg/day, was found effective in a small series of 7 patients, who were not responding to antibiotic treatment. All improved somewhat and remission lasted 8–18 months.46 Immunosuppressive agents like prednisone and cyclosporine have resulted in temporary remission of HS, but recurrence is high once treatment is discontinued.47,48 Infliximab and etanercept (TNF-α inhibitors) are biologics that have shown promise in improving symptoms of HS, but one must consider the long- and short-term morbid effects of their use (malignancy, infection).49,50
Radiation, cryosurgery, and laser therapy has been effective in a small series of patients with early stages of HS, but their long-term effects have yet to be proven as have their effect on disease progression.51,52,53
Upon diagnosis of HS the extent and stage of disease should guide surgical approach. For Hurley stages 2–3, surgery is regarded as the most effective treatment for HS. Localized fluctuating abscesses should be drained by incision and drainage for symptomatic relieve with the understanding that recurrence rates are high.54 For perianal HS, unroofing of sinus tracts and marsupialization has been described in a small series of 4 patients by Brown et al. Despite their success it may serve better as a temporizing measure to more definitive surgical treatment as disease progresses.55 Other temporizing measures have been described such as local excision with and without primary closure. The benefits are that this method avoids the morbidity of more extensive wounds but again recurrences are frequent (up to 50%) and often at different anatomic regions.56 Radical excision or en bloc excision has the lowest recurrence rate in HS.57 The success of this strategy is based on removing all pilosebaceous glands within an effected anatomic region. The method described for en bloc resection requires excision of skin and subcutaneous fat down to fascia. Diversion is seldom required.31,58 This approach may result in large complex wounds in the perineal and inguinal area; a staged approach may be considered.58 Wounds may be allowed to heal by secondary intent or may require skin-grafting or flaps for closure.31,59,60 Rompel et al looked at long-term results of en bloc excision of HS in 106 patients and stated that the complication of recurrence is related to adequacy of resection not the selected method of wound closure.61
Hidradenitis suppurativa (HS) is a chronic debilitating disorder that can affect any apocrine-gland bearing skin area of the body. Perineal HS is associated with high morbidity compared with other anatomic regions. Early-stage disease may mimic various other forms of cutaneous disorders, but as HS progresses pathognomonic skin changes occur. Clinical stage can guide therapeutic approach, but the lowest recurrence rate is obtained by removing all involved skin and subcutaneous fat.
PRURITUS ANI
Pruritus ani (PA) is a dermatologic disorder characterized by intense itching and burning sensation in the perianal area. Patients are typically diagnosed by their primary care physician who attempts initial management. If symptoms persist patients are often referred to any of the following specialists: dermatologist, gastroenterologist, or colorectal surgeon. The incidence of PA ranges from 1–4% with a 4:1 male predominance and frequently debuts in the fourth to sixth decades of life.62,63,64a
PA can be subdivided into idiopathic and secondary. Idiopathic is more common, accounting for up to 90% of cases. This diagnosis can only be made after etiologic factors have been ruled out; however, 70% of patients will have a coexisting diagnosis which is usually not the cause of their PA. The problem is further complicated by the fact that treatment of some of the coexisting conditions can actually worsen mild idiopathic symptoms. Secondary PA has a complex variety of causes and is usually divided into perianal infections, dermatologic diagnosis, neoplasms, local irritation, foods, colorectal, systemic and psychological disorders.62 In all cases, the condition is exacerbated by the scratch–itch cycle, where intense scratching increases skin breakdown, irritation/inflammation, excoriations, and sometimes infections, which in turn increases the itching frequency.
Causes
Over 100 causes have been named in the English literature (Table 3).65
Table 3.
| Anorectal disorders | Fissures |
| Hemorrhoids | |
| Proctitis | |
| Abscess | |
| Fistula | |
| Rectal cancer | |
| Colon cancer | |
| Adenomatous | |
| Infections | Candida albicans |
| Dermatophytes (Malassezia furfur) | |
| Staphylococcus aureus | |
| Beta-hemolytic streptococcus | |
| Corynebacterium munitissimum (erythrasma) | |
| Human papilloma virus | |
| Herpes simplex | |
| Sacroptes scabei (scabies) | |
| Enterobius vermicularis (pinworms) | |
| Fecal soiling | Encopresis |
| Incontinence | |
| Chronic diarrhea | |
| Poor hygiene | |
| Transient relaxation of internal sphincter | |
| Prolapsed, hemorrhoids, etc. | |
| Local irritation | Soaps and detergents |
| Topical creams and medications | |
| Dermatologic disorders | Psoriasis |
| Contact dermatitis | |
| Atopic dermatitis | |
| Bowen's disease, Paget's disease | |
| Hidradenitis | |
| Systemic disease | Diabetes mellitus |
| Leukemia | |
| Thyroid disorders | |
| Liver disease | |
| Renal failure | |
| Psychological | Depression |
| Stress | |
| Anxiety | |
| Medications | Colchicine |
| Antibiotics | |
| Quinidine | |
| Mineral oil | |
| Witch hazel | |
| Local anesthetic |
INFECTIONS
Fungal infections are the most common perianal infections. These include Candida albicans and dermatophytes.66 Eradication is imperative to achieve cure. Topical and systemic antifungal agents have been used. Bacterial infections such as Streptococcus, Staphylococcus aureus, and Corynebacterium minutissimum (erythrasma) have all been implicated.67 Erythrasma often presents with itching and infects other sites like the toes and groin. It is best diagnosed by a Wood's lamp, which reveals a coral-red fluorescence.62 False-negative results can occur with the Wood's lamp if the patient has recently showered. Corynebacterium minutissimum is susceptible to erythromycin and tetracycline.62 Sexually transmitted diseases like herpes simplex, gonorrhea, and Condyloma accuminata can also present with itching.
Parasites are also common causes for PA especially in the tropics and in children. Pinworms (Enterobius vermicularis) are often implicated in the pediatric population. Itching occurs mainly at night and is diagnosed by a cellophane tape test. The adult worms and eggs can be identified on the tape. Mebendazole is the treatment of choice. Perianal topical application of albendazole as well as a single oral dose has been demonstrated to provide immediate relief.68
IRRITATING FACTORS
A multitude of irritants have been associated with PA. Although the obvious culprit is fecal contamination, feces haven't been shown to have the same effect on other sites of the body as it does on perianal skin. An example is ostomy sites, where the skin around ostomies does not develop pruritus in the same manner as perianal skin despite frequent and lengthy contact with feces. Contrary to this observation Caplan et al demonstrated that skin on other sites of the body react similarly to perianal skin. They performed skin patch testing using autologous feces on other sites of the body. They were able to elicit “anal symptoms” in a third of patients with PA and 53% of asymptomatic patients.69 Feces alone are not responsible for PA. In fact other local irritants like moisture and anal sphincter pressure may also be culprits. Anal manometric studies have shown an increase in anal sphincter relaxation and subsequent leakage following rectal distention in patients with PA.70 Diarrhea will increase the contamination with increased moisture and maceration, which will potentiate the scratch–itch cycle.62 Anal surgical procedures may affect normal anal morphology resulting in fecal soiling. This is usually due to inability to incompletely empty the rectal vault and retained fecal matter can seep and irritate the perianal area resulting in PA. Other irritants include moisture, soaps, detergents, synthetic underwear, vigorous scrubbing and medication like quinine, topical nitroglycerin,71 steroid creams, peppermint oil, colchicin, gemcitabine,72 colpermin, and mineral oil. Some of these agents are allergens and produce an allergic reaction with histamine release causing pruritus.73
DERMATOLOGIC DISORDERS
Numerous dermatologic disorders manifest differently in the perianal region. The incidence of psoriasis in patients with PA is between 5–55%. The psoriasis plaques may look different due to the persistent scratching thereby making the diagnosis difficult. These lesions are sometimes referred to as inverse psoriasis because they are without scales and tend to be paler.62 Lichen sclerosis et atrophicus is more common in women and usually involves the vulva extending into the perianal area posteriorly. It is very itchy and initially often scratched raw and heals with sclerosis and atrophy. On physical examination, one can find a figure-8 pattern around the vulva and anus. The clitoris and labia may be involved. Treatment of choice is topical steroids.62 Neurodermatitis is due to the scratch–itch cycle. This cycle is very difficult to break with the repetitive trauma resulting in scaly skin known as lichenification. When lichenification has occurred the lesions become increasingly resistant to therapy. Treatment is with topical steroids, but resistant cases have been treated with tacrolimus. Atopic dermatitis is a chronic skin disorder. It is caused by dry skin with intense scratching, which results in a red papular rash and is considered an allergic reaction. Patients usually have a family history of the disease, which is associated with hay fever, asthma, and eczema. Dry and cold weather, stress, and skin infections worsen symptoms. Diagnosis is highly reliant on a careful and detailed history and physical exam.74 Seborrhoic dermatitis is a very rare cause of PA, but it deserves a mention here because it is relatively easy to treat once diagnosed. It is an inflammatory skin disorder affecting the areas of skin rich in sebum, namely the scalp, face, trunk, nasolabial folds, and perineum. These lesions are scaly, itchy, flaky, and red. The responsible organism is a fungus called Malassezia furfur. It is managed by antidandruff shampoo or any other topical antifungal agent.74,75,76,77
NEOPLASMS
Cloacogenic carcinoma, Bowen's disease, Paget's disease, and squamous cell carcinoma of the anal margin have all presented with refractory PA. It is mandatory to biopsy any lesions that do not respond to standard treatment for PA.78,79
DIET
Several different foods have been implicated in the etiology of idiopathic PA without much scientific support. However current literature suggests elimination of suspected foods from the diet. Some of these foods like coffee reduces anal sphincter pressure and produces exaggerated anal reflexes; undigested foods can sensitize the perianal skin.63 Relaxed anal sphincter pressure combined with exaggerated anal reflexes lead to liquid stools, quicker transit time, and increased frequency of bowel movements. Ultimately, soiling progresses as does perianal trauma from repetitive cleaning. If a food or beverage is found to exacerbate symptoms, it should be avoided (Table 4).70
Table 4.
Beverages and Foods Associated with Pruritus Ani
| Beverages | Foods |
|---|---|
| Coffee | Chocolate |
| Tea | Tomatoes including ketchup |
| Cola and other caffeinated beverages | Dairy products |
| Beer and wine | Peanuts and nuts |
| Spices | |
| Citrus fruits | |
| Grapes | |
| Popcorn | |
| Spicy foods | |
| Prunes | |
| Figs |
SYSTEMIC DISEASE
A variety of systemic diseases can lead to PA. The most common ones are diabetes mellitus, liver disease, leukemia, renal failure, hypothyroidism, iron deficiency anemia, pellagra, and vitamin A and D deficiencies.62,63,65,75
PSYCHOLOGICAL FACTORS
PA has been associated with stress, anxiety, and depression. Despite these associations scientific evidence is lacking, in fact Smith et al using the Minnesota Multiphasic Personality Inventory failed to demonstrate emotional disorders in these patients. PA tends to worsen with stress and depression. If any of these conditions are present they should be dealt with concurrently.70 Short-term anxiolytic medications may benefit some patients especially at bedtime.
History and Physical
History should be focused and directed toward relevant etiologies. Coexisting skin and perianal conditions should be questioned. Atopy, urticaria, hay fever, allergies, and family history are key components as are the use of over-the-counter medication. Common colon and rectal symptoms like diarrhea, constipation, etc., and prior history of anorectal surgical procedures is vital information. Physical examination should be complete. Evaluate the whole body for skin lesions to exclude other dermatologic disorders. Dermatologic diseases are usually not limited to just one site of the body. A detailed examination of the perianal region including anoscopy is mandatory.80 There might be evidence of lichenification. Lichen sclerosis almost always involves the labia and perineum. Moisture and maceration present in the gluteal folds should be noted. Excoriations are not unusual. Valsalva maneuver can exclude mucosal prolapse. Palpate groins for lymph nodes and biopsy asymmetric lesions.
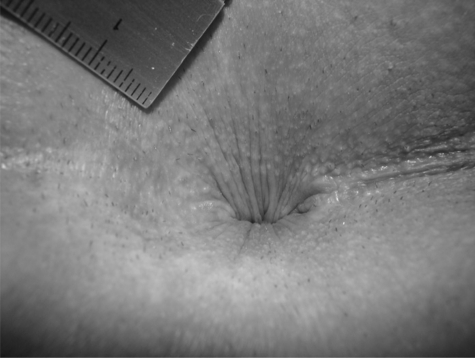
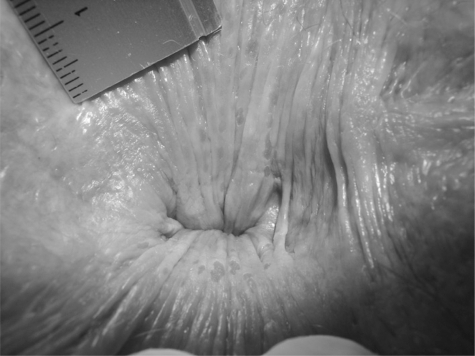
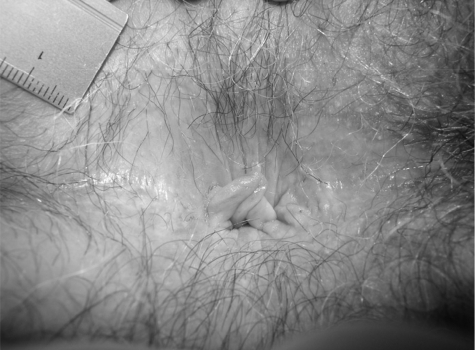
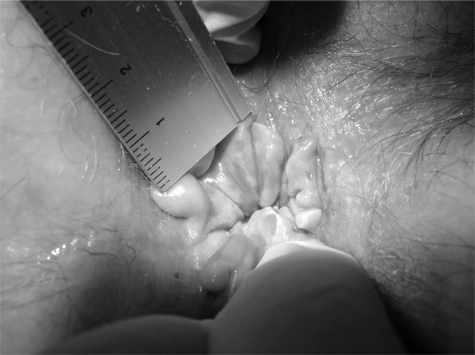
PA can be graded into four stages, ranging from mild symptoms in stage one to severe and chronic symptoms in stage four (Table 5) (Figs. 1–7).74
Table 5.
Clinical Staging of Pruritus Ani
| Grading | Terms and Definition |
|---|---|
| Mild | Stage 1. No lesion is seen at inspection of anal verge but patient finds palpation and anoscopy painful. Other anal lesions have been excluded |
| Moderate | Stage 2. Red dry skin only, at times weeping skin with superficial round splits and longitudinal superficial fissures. |
| Severe | Stage 3. Reddened weeping skin, with superficial ulcers and excoriations disrupted by pale, whitish areas with no more hairs |
| Chronic | Stage 4. Pale, whitened, thickened, dry leathery, scaly, skin with no hairs and no superficial ulcers or excoriations |
Figure 1.
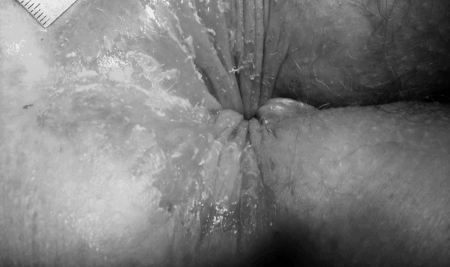
Stage 1 pruritus ani. Unremarkable, looks normal but painful on examination. Anal verge with hairs shaved (tiny black spots around the anus).
Figure 2.
Stage 2 pruritus ani. Weeping anal skin with superficial round splits and longitudinal superficial lesions at everted distal anal canal. Normally, the distal anal canal is closed so that these tiny lesions are not seen. Lesions diameter: 1–3 mm.
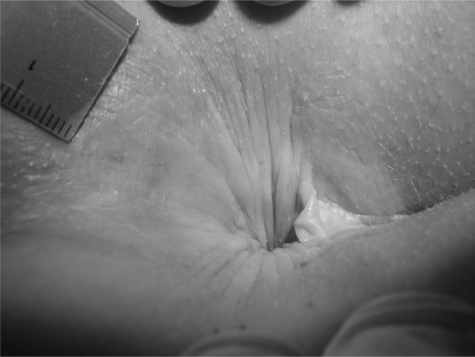
Figure 3.
Stage 2 pruritus ani. Erythematous dry skin with bleeding spots of a patient with a hairy anus.
Figure 4.
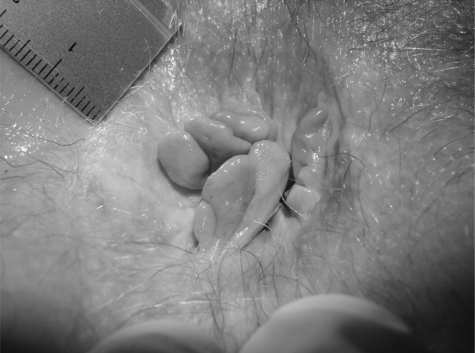
Stage 3 pruritus ani. Superficial lesions found within whitish areas of anal skin. This cover is skin tags surrounding the anus.
Figure 5.
Stage 3 pruritus ani. Erythematous, weeping skin, with superficial ulcers and excoriations disrupted by pale, whitish hairless areas. Note the loss of hair.
Figure 6.
Stage 4 pruritus ani. Whitish, pale, dry anal skin at anal verge with thick, leathery surrounding skin tags.
Figure 7.
Stage 4 pruritus ani. Pale, white, thick, dry, leathery, scaly skin without hair and no superficial ulcers or excoriations.
Treatment
Successful management depends on accurate diagnosis and ruling out coexisting disorders. Address precipitating and exacerbating factors and break the scratch–itch cycle.73 Secondary PA should be treated by managing the primary disorder. Inform the patient about the chronic and complex nature of this disease. Maintain realistic expectations to avoid major frustration and disappointment. Some patients present with stress and anxiety. They are worried the itching is a symptom of “cancer.” Where there is no suspicion of neoplasm do well to inform the patient promptly as this can be reassuring and will hopefully alleviate some of their anxiety.70 Patients with dermatologic disorders should be referred to a dermatologist.79
The first step in management is to ensure perianal hygiene. Recommend only water to cleanse the perianal area after bowel movements, avoiding soaps and toilet tissue, two very common irritants. The goal is to alleviate the irritation. Dry perianal area with a dry towel and apply corn starch powder or talc to ensure the intergluteal fold remains dry. Avoid cornstarch powder if there is suspicion of a fungal infection as fungus is known to thrive very well in cornstarch. Sweat increases moisture and irritation in the perianal area. Sweating should be kept to a minimum by avoiding vigorous exercises and wearing thin cotton fabrics which air easily. Eliminate possible precipitating foods, the most common being coffee, chocolate and fruits, until symptoms resolve. Encourage patients to keep a diary and then reintroduce the foods one at a time in an attempt to determine the offending foods. Recommend a high-fiber diet. Antidiarrheal agents like lomotil and imodium can be used for frequent loose stools. Excessive scratching occurs mostly at night when the patient is asleep and unaware of their actions. An antihistamine taken at bedtime can be helpful. Treat depressed patients concomitantly. Topical agents are often effective. Topical steroids have been a mainstay of treatment over the years. However, long-term use of topical steroids can lead to thinning of the perianal skin.81 Oztas et al demonstrated that perianal cleansers were as good as topical steroids, 1% hydrocortisone is recommended.82 Topical capsaicin has been shown to be an effective agent against PA compared with placebo83 and is thought to break the vexing scratch–itch cycle. If no etiology is found and all the above treatment measures have failed, another option in management involves the injection of methylene blue into the subcutaneous tissue of the perianal region. Mentes et al injected 30 consecutive patients with 7–8 mLl of 2% methylene blue mixed with an equal volume of 0.5% lidocaine. Using a 1% methylene blue solution with a 22-gauge needle, they targeted the dermo–epidermal junction and infiltrated the entire perianal area with as few punctures as possible. Eighty percent (24 patients) were symptom free after one month with 5 patients having partial response. These patients were reinjected with a similar solution, which resulted in complete relief in 4 of 5. The total early response rate was 28 of 30 or 93%. At 12-month follow-up there were five recurrences reducing the success rate to 76%.84 Botterill et al had similar results with 88% of patients becoming symptom free.85,86 Hypnosis has been tried with moderate results.87
In conclusion, pruritus ani is a complex disease with a multitude of etiologies. Its management can be frustrating and disappointing for the patient and doctor alike. The key is to start with simple treatment options focusing on perianal hygiene and avoidance of the most common offending foods and beverages. If these measures fail, topical medications should be attempted before graduating to perianal injections of methylene blue as a last resort.
References
- 1.Singer M, Cintron J R. Hidradentis suppuratuva. Clin Colon Rectal Surg. 2002;14:233–242. [Google Scholar]
- 2.Anderson M J, Jr, Dockerty M B. Perianal hidradenitis suppurativa; a clinical and pathologic study. Dis Colon Rectum. 1958;1(1):23–31. doi: 10.1007/BF02616510. [DOI] [PubMed] [Google Scholar]
- 3.Shelley W B, Levy E J, Weidman F D. Apocrine sweat retention in man. III. Apocrine retention cysts. AMA Arch Derm. 1955;72(2):171–172. doi: 10.1001/archderm.1955.03730320073011. [DOI] [PubMed] [Google Scholar]
- 4.Yu C C, Cook M G. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122(6):763–769. doi: 10.1111/j.1365-2133.1990.tb06264.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson B B, Cadogan C A, Gangadharam D. Hidradenitis suppurativa of the perineum, scrotum, and gluteal area: presentation, complications, and treatment. J Natl Med Assoc. 1982;74(10):999–1003. [PMC free article] [PubMed] [Google Scholar]
- 6.von der Werth J M, Jemec G B. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144(4):809–813. doi: 10.1046/j.1365-2133.2001.04137.x. [DOI] [PubMed] [Google Scholar]
- 7.Clemmensen O J. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22(5):325–328. doi: 10.1111/j.1365-4362.1983.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 8.Mortimer P S, Dawber R P, Gales M A, Moore R A. Mediation of hidradenitis suppurativa by androgens. Br Med J (Clin Res Ed) 1986;292(6515):245–248. doi: 10.1136/bmj.292.6515.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose R F, Goodfield M J, Clark S M. Treatment of recalcitrant hidradenitis suppurativa with oral cyclosporin. Clin Exp Dermatol. 2006;31(1):154–155. doi: 10.1111/j.1365-2230.2005.01983.x. [DOI] [PubMed] [Google Scholar]
- 10.Rompel R. [Orientation guides for excision lines and flap design] J Dtsch Dermatol Ges. 2006;4(10):888–890. doi: 10.1111/j.1610-0387.2006.06143.x. [DOI] [PubMed] [Google Scholar]
- 11.Revuz J E, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Jemec G B, Ganslandt C, Ortonne J P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2008;59(3):455–463. doi: 10.1016/j.jaad.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Wiltz O, Schoetz D J, Jr, Murray J J, Roberts P L, Coller J A, Veidenheimer M C. Perianal hidradenitis suppurativa. The Lahey Clinic experience. Dis Colon Rectum. 1990;33(9):731–734. doi: 10.1007/BF02052316. [DOI] [PubMed] [Google Scholar]
- 14.Homma H. On apocrine seat glands in white and Negro men and women. Bull Johns Hopkins Hosp. 1926;38:365–371. [Google Scholar]
- 15.Paletta C, Jurkiewicz M J. Hidradenitis suppurativa. Clin Plast Surg. 1987;14(2):383–390. [PubMed] [Google Scholar]
- 16.Velpeau A. Un repertoire general des sciences medicales sous la rapport theorique et practique. Dictionnaire de Medecine. 1839;2(2):91–109. [Google Scholar]
- 17.Verneuil A. De l'hidrosadenite phlegmoneuse et des asbces sudoripares. Arch Gen Med. 1864;2:537–557. [Google Scholar]
- 18.Jemec G B, Heidenheim M, Nielsen N H. Hidradenitis suppurativa—characteristics and consequences. Clin Exp Dermatol. 1996;21(6):419–423. doi: 10.1111/j.1365-2230.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 19.Bendahan J, Paran H, Kolman S, Neufeld D M, Freund U. The possible role of Chlamydia trachomatis in perineal suppurative hidradenitis. Eur J Surg. 1992;158(4):213–215. [PubMed] [Google Scholar]
- 20.Highet A S, Warren R E, Weekes A J. Bacteriology and antibiotic treatment of perineal suppurative hidradenitis. Arch Dermatol. 1988;124(7):1047–1051. [PubMed] [Google Scholar]
- 21.Harrison B J, Read G F, Hughes L E. Endocrine basis for the clinical presentation of hidradenitis suppurativa. Br J Surg. 1988;75(10):972–975. doi: 10.1002/bjs.1800751011. [DOI] [PubMed] [Google Scholar]
- 22.Jemec G B. The symptomatology of hidradenitis suppurativa in women. Br J Dermatol. 1988;119(3):345–350. doi: 10.1111/j.1365-2133.1988.tb03227.x. [DOI] [PubMed] [Google Scholar]
- 23.Morgan W P, Leicester G. The role of depilation and deodorants in hidradenitis suppurativa. Arch Dermatol. 1982;118(2):101–102. [PubMed] [Google Scholar]
- 24.Attanoos R L, Appleton M A, Douglas-Jones A G. The pathogenesis of hidradenitis suppurativa: a closer look at apocrine and apoeccrine glands. Br J Dermatol. 1995;133(2):254–258. doi: 10.1111/j.1365-2133.1995.tb02624.x. [DOI] [PubMed] [Google Scholar]
- 25.Hana A, Booken D, Henrich C, et al. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007;80(24-25):2214–2220. doi: 10.1016/j.lfs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Parks R W, Parks T G. Pathogenesis, clinical features and management of hidradenitis suppurativa. Ann R Coll Surg Engl. 1997;79(2):83–89. [PMC free article] [PubMed] [Google Scholar]
- 27.Lapins J, Asman B, Gustafsson A, Bergström K, Emtestam L. Neutrophil-related host response in hidradenitis suppurativa: a pilot study in patients with inactive disease. Acta Derm Venereol. 2001;81(2):96–99. doi: 10.1080/00015550152384209. [DOI] [PubMed] [Google Scholar]
- 28.Hunger R E, Surovy A M, Hassan A S, Braathen L R, Yawalkar N. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br J Dermatol. 2008;158(4):691–697. doi: 10.1111/j.1365-2133.2007.08425.x. [DOI] [PubMed] [Google Scholar]
- 29.Giamarellos-Bourboulis E J, Pelekanou E, Antonopoulou A, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158(3):567–572. doi: 10.1111/j.1365-2133.2007.08372.x. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee A K. Surgical treatment of hidradenitis suppurativa. Br J Surg. 1992;79(9):863–866. doi: 10.1002/bjs.1800790905. [DOI] [PubMed] [Google Scholar]
- 31.Thornton J P, Abcarian H. Surgical treatment of perianal and perineal hidradenitis suppurativa. Dis Colon Rectum. 1978;21(8):573–577. doi: 10.1007/BF02586399. [DOI] [PubMed] [Google Scholar]
- 32.Culp C E. Chronic hidradenitis suppurativa of the anal canal. A surgical skin disease. Dis Colon Rectum. 1983;26(10):669–676. doi: 10.1007/BF02553341. [DOI] [PubMed] [Google Scholar]
- 33.Church J M, Fazio V W, Lavery I C, Oakley J R, Milsom J W. The differential diagnosis and comorbidity of hidradenitis suppurativa and perianal Crohn's disease. Int J Colorectal Dis. 1993;8(3):117–119. doi: 10.1007/BF00341181. [DOI] [PubMed] [Google Scholar]
- 34.Randall K, Roenigk H H. Dermatologic Surgery, Principles and Practice. New York: Marcel Dekker; 1989. [Google Scholar]
- 35.Sartorius K, Lapins J, Emtestam L, Jemec G B. Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa. Br J Dermatol. 2003;149(1):211–213. doi: 10.1046/j.1365-2133.2003.05390.x. [DOI] [PubMed] [Google Scholar]
- 36.Alikhan A, Lynch P J, Eisen D B. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60(4):539–561. quiz 562–563. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Diaz D, Calvo-Serrano M, Mártinez-Hijosa E, et al. Squamous cell carcinoma complicating perianal hidradenitis suppurativa. Int J Colorectal Dis. 1995;10(4):225–228. doi: 10.1007/BF00346224. [DOI] [PubMed] [Google Scholar]
- 38.Anstey A V, Wilkinson J D, Lord P. Squamous cell carcinoma complicating hidradenitis suppurativa. Br J Dermatol. 1990;123(4):527–531. doi: 10.1111/j.1365-2133.1990.tb01460.x. [DOI] [PubMed] [Google Scholar]
- 39.Radcliffe K W. Hidradenitis suppurativa. Genitourin Med. 1991;67(1):58. doi: 10.1136/sti.67.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams S T, Busby R C, DeMuth R J, Nelson H. Perineal hidradenitis suppurativa: presentation of two unusual complications and a review. Ann Plast Surg. 1991;26(5):456–462. [PubMed] [Google Scholar]
- 41.Konety B R, Cooper T, Flood H D, Futrell J W. Scrotal elephantiasis associated with hidradenitis suppurativa. Plast Reconstr Surg. 1996;97(6):1243–1245. doi: 10.1097/00006534-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Krbec A C. Current understanding and management of hidradenitis suppurativa. J Am Acad Nurse Pract. 2007;19(5):228–234. doi: 10.1111/j.1745-7599.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 43.Mendonça H, Rebelo C, Fernandes A, Lino A, Garcia e Silva L. Squamous cell carcinoma arising in hidradenitis suppurativa. J Dermatol Surg Oncol. 1991;17(10):830–832. doi: 10.1111/j.1524-4725.1991.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 44.Brown C F, Gallup D G, Brown V M. Hidradenitis suppurativa of the anogenital region: response to isotretinoin. Am J Obstet Gynecol. 1988;158(1):12–15. doi: 10.1016/0002-9378(88)90766-1. [DOI] [PubMed] [Google Scholar]
- 45.Boer J, Gemert M J van. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40(1):73–76. doi: 10.1016/s0190-9622(99)70530-x. [DOI] [PubMed] [Google Scholar]
- 46.Joseph M A, Jayaseelan E, Ganapathi B, Stephen J. Hidradenitis suppurativa treated with finasteride. J Dermatolog Treat. 2005;16(2):75–78. doi: 10.1080/09546630510031403. [DOI] [PubMed] [Google Scholar]
- 47.Buckley D A, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88(5):289P–290P. [PMC free article] [PubMed] [Google Scholar]
- 48.Bolanowski A, Mannon R B, Holland S M, et al. Successful renal transplantation in patients with chronic granulomatous disease. Am J Transplant. 2006;6(3):636–639. doi: 10.1111/j.1600-6143.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan T P, Welsh E, Kerdel F A, Burdick A E, Kirsner R S. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149(5):1046–1049. doi: 10.1111/j.1365-2133.2003.05663.x. [DOI] [PubMed] [Google Scholar]
- 50.Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis suppurativa. Br J Dermatol. 2006;154(4):726–729. doi: 10.1111/j.1365-2133.2005.07067.x. [DOI] [PubMed] [Google Scholar]
- 51.Zeligman I. Temporary x-ray epilation therapy of chronic axillary hidradenitis suppurativa. Arch Dermatol. 1965;92(6):690–694. [PubMed] [Google Scholar]
- 52.Lapins J, Sartorius K, Emtestam L. Scanner-assisted carbon dioxide laser surgery: a retrospective follow-up study of patients with hidradenitis suppurativa. J Am Acad Dermatol. 2002;47(2):280–285. doi: 10.1067/mjd.2002.124601. [DOI] [PubMed] [Google Scholar]
- 53.Bong J L, Shalders K, Saihan E. Treatment of persistent painful nodules of hidradenitis suppurativa with cryotherapy. Clin Exp Dermatol. 2003;28(3):241–244. doi: 10.1046/j.1365-2230.2003.01238.x. [DOI] [PubMed] [Google Scholar]
- 54.Ritz J P, Runkel N, Haier J, Buhr H J. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13(4):164–168. doi: 10.1007/s003840050159. [DOI] [PubMed] [Google Scholar]
- 55.Brown S C, Kazzazi N, Lord P H. Surgical treatment of perineal hidradenitis suppurativa with special reference to recognition of the perianal form. Br J Surg. 1986;73(12):978–980. doi: 10.1002/bjs.1800731210. [DOI] [PubMed] [Google Scholar]
- 56.Watson J D. Hidradenitis suppurativa—a clinical review. Br J Plast Surg. 1985;38(4):567–569. doi: 10.1016/0007-1226(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 57.Harrison B J, Kumar S, Read G F, Edwards C A, Scanlon M F, Hughes L E. Hidradenitis suppurativa: evidence for an endocrine abnormality. Br J Surg. 1985;72(12):1002–1004. doi: 10.1002/bjs.1800721223. [DOI] [PubMed] [Google Scholar]
- 58.Kagan R J, Yakuboff K P, Warner P, Warden G D. Surgical treatment of hidradenitis suppurativa: a 10-year experience. Surgery. 2005;138(4):734–740. discussion 740–741. doi: 10.1016/j.surg.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 59.Ramasastry S S, Conklin W T, Granick M S, Futrell J W. Surgical management of massive perianal hidradenitis suppurativa. Ann Plast Surg. 1985;15(3):218–223. doi: 10.1097/00000637-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Solanki N S, Roshan A, Malata C M. Pedicled gracilis myocutaneous flap for treatment of recalcitrant hidradenitis suppurativa of the groin and perineum. J Wound Care. 2009;18(3):111–112. doi: 10.12968/jowc.2009.18.3.39811. [DOI] [PubMed] [Google Scholar]
- 61.Rompel R, Petres J. Long-term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg. 2000;26(7):638–643. doi: 10.1046/j.1524-4725.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 62.Markell K W, Billingham R P. Pruritus ani: etiology and management. Surg Clin North Am. 2010;90(1):125–135. doi: 10.1016/j.suc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Siddiqi S, Vijay V, Ward M, Mahendran R, Warren S. Pruritus ani. Ann R Coll Surg Engl. 2008;90(6):457–463. doi: 10.1308/003588408X317940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stermer E, Sukhotnic I, Shaoul R. Pruritus ani: an approach to an itching condition. J Pediatr Gastroenterol Nutr. 2009;48(5):513–516. doi: 10.1097/MPG.0b013e31818080c0. [DOI] [PubMed] [Google Scholar]
- 64a.Vincent C. Anorectal pain and irritation: anal fissure, levator syndrome, proctalgia fugax and pruritus ani. Prim Care. 1999;26(1):53–68. doi: 10.1016/s0095-4543(05)70101-9. [DOI] [PubMed] [Google Scholar]
- 65.Lacy B E, Weiser K. Common anorectal disorders: diagnosis and treatment. Curr Gastroenterol Rep. 2009;11(5):413–419. doi: 10.1007/s11894-009-0062-y. [DOI] [PubMed] [Google Scholar]
- 66.Kränke B, Trummer M, Brabek E, Komericki P, Turek T D, Aberer W. Etiologic and causative factors in perianal dermatitis: results of a prospective study in 126 patients. Wien Klin Wochenschr. 2006;118(3-4):90–94. doi: 10.1007/s00508-006-0529-x. [DOI] [PubMed] [Google Scholar]
- 67.Jongen J, Eberstein A, Peleikis H G, Kahlke V, Herbst R A. Perianal streptococcal dermatitis: an important differential diagnosis in pediatric patients. Dis Colon Rectum. 2008;51(5):584–587. doi: 10.1007/s10350-008-9237-0. [DOI] [PubMed] [Google Scholar]
- 68.Singh S P, Panda C, Rout N, Mishra A P. Anal albendazole application for pruritus ani in threadworm infestation. J Trop Pediatr. 2005;51(6):386. doi: 10.1093/tropej/fmi046. [DOI] [PubMed] [Google Scholar]
- 69.Caplan R M. The irritant role of feces in the genesis of perianal itch. Gastroenterology. 1966;50(1):19–23. [PubMed] [Google Scholar]
- 70.Smith L E, Henrichs D, McCullah R D. Prospective studies on the etiology and treatment of pruritus ani. Dis Colon Rectum. 1982;25(4):358–363. doi: 10.1007/BF02553616. [DOI] [PubMed] [Google Scholar]
- 71.McKenna K E. Allergic contact dermatitis from glyceryl trinitrate ointment. Contact Dermat. 2000;42(4):246. [PubMed] [Google Scholar]
- 72.Hejna M, Valencak J, Raderer M. Anal pruritus after cancer chemotherapy with gemcitabine. N Engl J Med. 1999;340(8):655–656. doi: 10.1056/NEJM199902253400814. [DOI] [PubMed] [Google Scholar]
- 73.Pfenninger J L, Zainea G G. Common anorectal conditions. Part I. Symptoms and complaints. Am Fam Physician. 2001;63(12):2391–2398. [PubMed] [Google Scholar]
- 74.Kuehn H G, Gebbensleben O, Hilger Y, Rohde H. Relationship between anal symptoms and anal findings. Int J Med Sci. 2009;6(2):77–84. doi: 10.7150/ijms.6.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuccati G, Lotti T, Mastrolorenzo A, Rapaccini A, Tiradritti L. Pruritus ani. Dermatol Ther. 2005;18(4):355–362. doi: 10.1111/j.1529-8019.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- 76.Weichert G E. An approach to the treatment of anogenital pruritus. Dermatol Ther. 2004;17(1):129–133. doi: 10.1111/j.1396-0296.2004.04013.x. [DOI] [PubMed] [Google Scholar]
- 77.Dasan S, Neill S M, Donaldson D R, Scott H J. Treatment of persistent pruritus ani in a combined colorectal and dermatological clinic. Br J Surg. 1999;86(10):1337–1340. doi: 10.1046/j.1365-2168.1999.01231.x. [DOI] [PubMed] [Google Scholar]
- 78.Handa Y, Watanabe O, Adachi A, Yamanaka N. Squamous cell carcinoma of the anal margin with pruritus ani of long duration. Dermatol Surg. 2003;29(1):108–110. doi: 10.1046/j.1524-4725.2003.29003.x. [DOI] [PubMed] [Google Scholar]
- 79.Mazier W P. Hemorrhoids, fissures, and pruritus ani. Surg Clin North Am. 1994;74(6):1277–1292. doi: 10.1016/s0039-6109(16)46480-7. [DOI] [PubMed] [Google Scholar]
- 80.Gopal D V. Diseases of the rectum and anus: a clinical approach to common disorders. Clin Cornerstone. 2002;4(4):34–48. doi: 10.1016/s1098-3597(02)90004-9. [DOI] [PubMed] [Google Scholar]
- 81.Al-Ghnaniem R, Short K, Pullen A, Fuller L C, Rennie J A, Leather A J. 1% hydrocortisone ointment is an effective treatment of pruritus ani: a pilot randomized controlled crossover trial. Int J Colorectal Dis. 2007;22(12):1463–1467. doi: 10.1007/s00384-007-0325-8. [DOI] [PubMed] [Google Scholar]
- 82.Oztaş M O, Oztaş P, Onder M. Idiopathic perianal pruritus: washing compared with topical corticosteroids. Postgrad Med J. 2004;80(943):295–297. doi: 10.1136/pgmj.2003.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lysy J, Sistiery-Ittah M, Israelit Y, et al. Topical capsaicin—a novel and effective treatment for idiopathic intractable pruritus ani: a randomised, placebo controlled, crossover study. Gut. 2003;52(9):1323–1326. doi: 10.1136/gut.52.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mentes B B, Akin M, Leventoglu S, Gultekin F A, Oguz M. Intradermal methylene blue injection for the treatment of intractable idiopathic pruritus ani: results of 30 cases. Tech Coloproctol. 2004;8(1):11–14. doi: 10.1007/s10151-004-0043-y. [DOI] [PubMed] [Google Scholar]
- 85.Sutherland A D, Faragher I G, Frizelle F A. Intradermal injection of methylene blue for the treatment of refractory pruritus ani. Colorectal Dis. 2009;11(3):282–287. doi: 10.1111/j.1463-1318.2008.01587.x. [DOI] [PubMed] [Google Scholar]
- 86.Botterill I D, Sagar P M. Intra-dermal methylene blue, hydrocortisone and lignocaine for chronic, intractable pruritus ani. Colorectal Dis. 2002;4(2):144–146. doi: 10.1046/j.1463-1318.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 87.Rucklidge J J, Saunders D. Hypnosis in a case of long-standing idiopathic itch. Psychosom Med. 1999;61(3):355–358. doi: 10.1097/00006842-199905000-00015. [DOI] [PubMed] [Google Scholar]