Abstract
Microbial resistance has reached alarming levels, threatening to outpace the ability to counter with more potent antimicrobial agents. In particular, methicillin-resistant Staphylococcus aureus (MRSA) has become a leading cause of skin and soft-tissue infections and PVL-positive strains have been associated with necrotizing pneumonia. Increasing reports of growing resistance to glycopeptides have been noted, further limiting the efficacy of standard antibiotics, such as vancomycin. Ceftaroline is a novel fifth-generation cephalosporin, which exhibits broad-spectrum activity against Gram-positive bacteria, including MRSA and extensively-resistant strains, such as vancomycin-intermediate S. aureus (VISA), heteroresistant VISA (hVISA), and vancomycin-resistant S. aureus (VRSA). In addition to being an exciting new agent in the anti-MRSA armamentarium, ceftaroline provides efficacy against many respiratory pathogens including Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Ceftaroline (600 mg intravenously every 12 hours) has been shown effective in phase III studies in the treatment of complicated skin and soft tissue infections and community-acquired pneumonia. To date, this unique antibiotic exhibits a low propensity for inducing resistance and has a good safety profile, although further post-marketing data and clinical experience are needed. In summary, ceftaroline provides an additional option for the management of complex multidrug resistant infections, including MRSA.
Keywords: Ceftaroline, antibiotic, cephalosporin, methicillin-resistant Staphylococcus aureus, MRSA, multidrug resistant organisms
Introduction
Microbial pathogens have an extraordinary capacity to develop resistance to antimicrobial agents. Within the last two decades, resistance has escalated, occasionally at seemingly exponential rates, threatening to outpace the ability to counter with more potent antimicrobial agents. Methicillin-resistant Staphylococcus aureus (MRSA), first isolated in the 1960s, became a prominent nosocomial pathogen over the past three decades. The advent of community-associated MRSA (CA-MRSA), which arose de novo outside the healthcare environment, has dramatically heightened the importance of MRSA. Today, MRSA is the leading cause of community-acquired skin and soft tissue infections (SSTI) and a cause of necrotizing pneumonia.1,2 The dramatic escalation in MRSA, which is now globally ubiquitous, coupled to intrinsic resistance to many of the existing antimicrobial agents, renders this an enormous public health issue. MRSA has also recently exhibited an inexorable creep in minimum inhibitory concentrations (MIC) to the standard intravenous antibiotic (vancomycin) utilized in its management. In addition, S. aureus strains with vancomycin-intermediate resistance (VISA), heteroresistance (hVISA), and vancomycin resistance (VRSA) have been described.3 These resistant strains are associated with increased morbidity and mortality above that of methicillin-sensitive Staphylococcus aureus (MSSA), and often require surgical intervention coupled to a sparse selection of suitable antimicrobial therapy.4
Fortunately, alternatives to vancomycin have been developed in the past decade for the treatment of multidrug resistant (MDR) Gram-positive bacterial infections including an oxazolidinone (linezolid), a lipopeptide (daptomycin), a streptogramin (quinupristin-dalfopristin), and a glycylcycline (tigecycline).5,6 Telavancin is a recent addition to the Gram-positive arsenal, and is a lipoglycopeptide which inhibits both bacterial cell wall synthesis and cell-membrane function.7
Despite these novel agents, resistance continues to evolve, and strains resistant to linezolid, quinupristin/dalfopristin and daptomycin have been described.5,6,8 Moreover, there are disadvantages associated with these contemporary antibiotic classes. For example, linezolid has minimal Gram-negative activity (due to efflux pumps), although it does have some activity against anaerobes and Mycobacteria spp.9 Furthermore, linezolid is bacteriostatic and its long-term use (e.g., >2 weeks) has been associated with the development of peripheral neuropathy, lactic acidosis, and thrombocytopenia (as well as the potential for trilineage bone marrow suppression).10 Daptomycin lacks pulmonary activity, and may cause a pulmonary hypersensitivity reaction and myopathy.11,12 Additionally, daptomycin resistance has been noted in the setting of prior vancomycin therapy, especially with suboptimal dosing and sequestered infections including osteomyelitis, endocarditis, and device related infections.13–16 Daptomycin resistance had been linked to its inactivity in the setting of thickened cell walls in VISA and hVISA isolates, with reduced access to binding sites on the cell membrane, and to point mutations leading to amino acid substitutions in the MprF and YycG proteins16. Quinupristin-dalfopristin is limited by its administration via central venous access, its only modest activity against MRSA pneumonia, and a host of adverse side-effects including myalgias.6 Tigecycline is active against a range of both Gram-positive and -negative organisms (notably excluding P. aeruginosa), and approved for the treatment of SSTI and complicated intra-abdominal infections17. However, it exhibits low serum concentrations, accumulates in bone (contraindicated in children and pregnancy), and is often associated with significant nausea.18 Furthermore in a recent multicenter trial, tigecycline (+/− ceftazidime +/− aminoglycoside) versus imipenem (+/−vancomycin +/− aminoglycoside) had significantly lower cure rates for ventilator-associated pneumonia (VAP)19, and the FDA has issued a warning that tigecycline may be associated with an increased mortality risk compared to other drugs for treatment of a variety of serious infections including VAP. Telavancin may also cause nausea and vomiting, and it has been associated with infusion-related reactions (i.e., red-person syndrome).7 Finally, many of these unique agents (i.e., linezolid, daptomycin, and telavancin) are only active against Gram-positive bacteria.
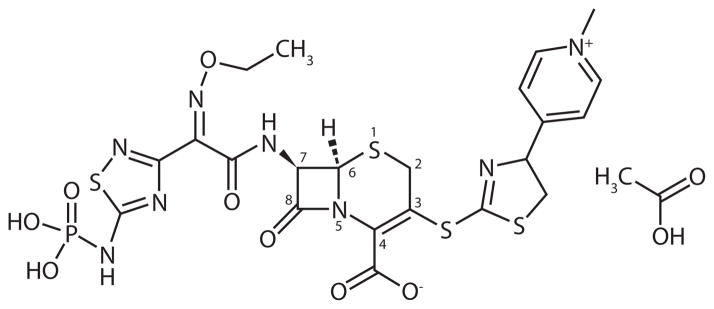
Ceftaroline fosamil (brand name Teflaro, previously referred to as PPI-0903M, T-91825, TAK-599) is a novel fifth-generation parental oxyimino cephalosporin with bactericidal activity against MRSA (Figure 1).20,21 In contrast to most of the aforementioned MRSA antimicrobials, ceftaroline fosamil (hereafter, ceftaroline) exhibits broad-spectrum activity against many of the important community-acquired Gram-positive and Gram-negative pathogens,20–22 similar to the sole other fifth-generation cephalosporin (ceftobiprole) in development.
Figure 1.
Chemical Structure of Ceftaroline fosamil acetate
Importantly, it has activity against MDR Gram-positive bacteria, including MRSA, VISA, hVISA, and VRSA.23,24 It also has efficacy against respiratory bacterial pathogens such as Streptococcus pneumoniae (including multidrug-resistant strains), Haemophilus influenzae, and Moraxella catarrhalis. Mirroring other broad-spectrum cephalosporins, ceftaroline does not possess activity against extensively-resistant Gram-negative bacteria and exhibits limited activity against most non-fermentative Gram-negative bacilli (e.g., Pseudomonas aeruginosa, Acinetobacter spp.) and many anaerobic species.20–23
A new drug application for ceftaroline (Forest Laboratories Inc., New York, NY) was submitted in December 2009, with the specific indications for the treatment of complicated SSTI and community-acquired pneumonia (CAP). This novel drug gained FDA approval in September 2010 and is expected to be available for use in early 2011.
Mechanism of Action
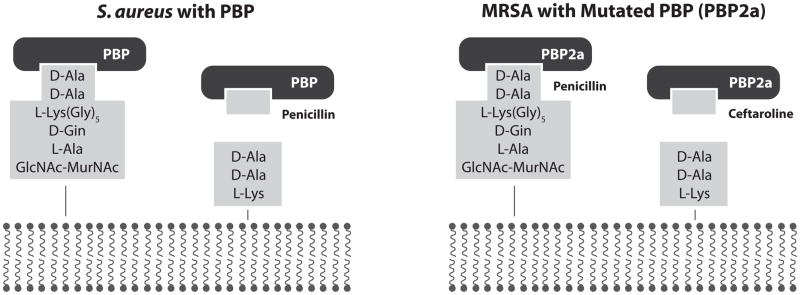
Like other β-lactams, ceftaroline’s mechanism of action is mediated by binding to the penicillin-binding protein (PBP), the enzyme mediating the cross-linking transpeptidation of the peptidoglycan which are the terminal steps in completing formation of the bacterial cell wall. MRSA strains have a mutated PBP2a (coded by the mecA gene residing on the staphylococcal chromosomal cassette), which prohibits β-lactam antibiotics from accessing its active site that mediates the transpeptidation reaction. The interaction of PBP2a at an allosteric site within peptidoglycan triggers conformational changes potentiating access to the active state. When not actively involved in transpeptidation, the active site is closed, effectively “shielded” from potential β-lactam antibiotics.25 Ceftaroline possesses an ethoxyimino side-chain mimicking a portion of a cell wall structure, which acts as a “Trojan horse”, allosterically opening and facilitating access to the active site of the PBP2a.25,26
More specifically, β-lactam antibiotics form a non-covalent complex with the transpeptidase enzymatic domain of the PBP. This is characterized by an equilibrium dissociation constant, KD, which is converted to the covalent acyl-enzyme form with a rateconstant, k2. The acyl-enzyme complex prevents transpeptidation, and as free enzyme regeneration via hydrolytic deacylation characterized by the rate constant (k3) is slow (eclipsing duration of cell viability) the bacteria undergoes lysis. Now the dissociation constant for the non-covalent interaction of the transpeptidase enzymatic region of PBP2a with the β-lactam is very high due to structural inaccessibility of the β-lactam due to the presence of a peptide loop shielding the active site of PBP2a. Binding of muropeptide of peptidoglycan to an allosteric site of the PBP2a potentiates a conformation change displacing the peptide loop enabling access of substrate for wall synthesis. Ceftaroline possesses a side chain mimetic of the muropeptide which can interact with the allosteric site of PBP2a duplicating the conformational change necessary to displace the peptide loop shielding access, allowing formation of the initial non-covalent interaction of the transpeptidase enzymatic region of PBP2a with the β-lactam.27 (Figure 2).
Figure 2.
Hence, ceftaroline’s anti-MRSA efficacy stems from high affinity for the MRSA-associated PBP2a (perhaps ≥256-fold over other β-lactams). For example, the MIC50 for the PBP2a for ceftaroline is 0.90 μg/ml compared with 408 μg/ml for oxacillin, 677 μg/ml for ceftriaxone, and 57 μg/ml for imipenem. The inhibition of PBP by ceftaroline results in cell wall irregularities and eventual bacterial cell death.28 Ceftaroline also demonstrates superior affinity for all the prominent PBPs utilized within both sensitive (PBP 1-3) and resistant strains of S. aureus. Furthermore, it has activity to the mutable PBPs of S. pneumoniae including multiple drug-resistant S. pneumoniae (MDRSP) (PBP1a, PBP2a, PBP2b, PBP2x, PBP3) and the PBP3 of Gram-negative bacteria.28, 29 Finally, ceftaroline remains effective in the setting of the cell wall changes which mediate resistance within VISA, hVISA, VRSA, and daptomycin-resistant isolates.22,24,30
Pharmacokinetics
Ceftaroline is the bioactive metabolite of ceftaroline fosamil, an N-phosphonoamino water-soluble cephalosporin prodrug, which is rapidly converted in vivo upon the hydrolysis of the phosphonate group by plasma phosphatises.23 Ceftaroline’s chemical stability and water solubility is attributed in part from improved crystallization and hygroscopicity imparted by innovated chemical modifications, necessitating administration as a prodrug via intravenous or intramuscular routes.
Following single 500 mg and 750 mg intravenous doses, ceftaroline reaches peak serum concentration (Cmax) of 16.5 and 23 μg/ml, respectively, and steady state AUC values of 44.7 and 56.9 μg/hour/ml, respectively. Escalating single doses of ceftaroline fosamil (50 to 1000 mg) administered intravenously as one-hour infusions to healthy male individuals (n = 48) yielded ceftaroline concentrations ranging from 1.5 to 30.2 μg/ml; mean half-lives of ceftaroline fosamil, ceftaroline, and the major metabolite (ceftaroline-M-1) were 0.4, 2.4, and 4.5 hours, respectively.31
Multiple escalating doses of ceftaroline fosamil were administered intravenously in healthy male subjects as 300 and 600 mg, respectively, every 12 hours for 14 days, and 800 mg every 24 hours for 7 days. Ceftaroline again formed rapidly after dosing, exhibiting a half-life of 2.6 (range 2.3–2.9) hours. The values of Cmax, AUC and clearance for the three respective groups were: Cmax: 8.4, 21, and 31 μg/ml; AUC: 24, 56, and 73 μg/hour/ml; clearance: 183, 159, and 161 ml/min, respectively. For multiple intravenous doses of 600 mg given over one hour every 12 hours for 14 days, the maximum plasma concentration was 19.0 μg/ml and 21.0 μg/ml for first and last doses, respectively, without evidencing drug accumulation with multiple dosing.31,32
The intramuscular route of delivery is attractive, given its potential convenience of administration. In animal models, intramuscular administration exhibited similar pharmacokinetics to that of intravascular administration with almost 100% bioavailability.26,33 The AUCs for the intramuscular route was comparable to that achieved with intravenous dosing in both rabbits (mean AUC 7.3% greater) and monkeys (12.7% greater), indicating excellent bioavailability via this route.33 In addition, the half-lives of the two routes were comparable. The time to achieve Cmax was slightly longer, with intramuscular administration and initial peak levels slightly lower, perhaps due to the slower release of the pro-drug from the intramuscular site. Data showing near equivalence regarding intramuscular and intravenous routes have also been noted in human studies.34
Ceftaroline’s volume of distribution is an estimated 0.37 L/kg, corresponding to the extracellular fluid volume of about 16–17 liters with plasma protein binding of <20%.23,35,36 Establishing pulmonary tissue penetration was imperative in seeking approval for an indication to treat CAP. The mean pulmonary penetration in a rabbit model was 42% (+/− 11.2%) relative to plasma levels over two hours; intravenous dosing was administered at 20 mg/kg for 30 minutes, with plasma and lung tissue concentrations of 41.0 mg/L and 18.7 mg/kg, respectively. Further, the pulmonary concentrations exceeded the MICs of most respiratory pathogens.37 Assessment of pulmonary penetration in human studies is pending. Furthermore, pharmacokinetic studies await evaluation of cerebrospinal fluid penetration. If ceftaroline provides adequate CSF penetration, coupled to its impressive anti-MDRSP activity,38 ceftaroline would offer a promising option against bacterial meningitis.
Elimination (drug clearance) occurs primarily through renal excretion, exhibiting classical two-compartmental linear pharmacokinetics with upwards of 75% of drug recovered in urine (52 +/− 33%). After conversion from the prodrug ceftaroline fosamil to ceftaroline, a small fraction of the latter is converted to an inactive metabolite, ceftaroline-M-1. Approximately 50% of ceftaroline (clearance ranging from 90.0 to 129.2 ml/min) and 7% of ceftaroline-M-1 are excreted in the urine.31,32,35
In a small study (n=18, 6 per group), individuals with normal renal function (creatinine clearance (CrCl) >80 ml/min), mild renal impairment (CrCl of 51–80 ml/min), or moderate renal impairment (CrCl of 31–50 ml/min) received ceftaroline fosamil (600 mg) as an one-hour intravenous infusion, with subsequent plasma and urine collections for up to 48 hours. Ceftaroline exhibited an increasing plasma half-life with increased renal impairment from 2.8, 3.6, to 4.5 hours, respectively. The Cmax of ceftaroline was unaffected by renal function, ranging from 27 to 31 μg/ml, while AUC values increased with worsening renal function: 68 to 120 μg/hour/ml, with a commensurate reduction in the clearance from 126 to 74 ml/min. The renal clearance of ceftaroline and ceftaroline-M-1 was decreased significantly by 65% and 84%, respectively.35
Based on Monte Carlo simulations, dosage adjustment is recommended for patients with moderate renal impairment (creatinine clearance 31–50 ml/min) at 400 mg intravenously (infused over one hour) every 12 hours. No dosage adjustment is necessary for mild renal impairment (CrCl >50 ml/min).35,39 There are no recommendations for dosing in severe renal dysfunction (CrCl <30 ml/min) or hemodialysis available at this time, but some pharmacokinetic data suggest that a dose reduction of at least 50%, or doubling of the dosing interval will be warranted among these patients.40,41
Ceftaroline lacks a p450-dependent mechanism of metabolism and is unlikely to interfere with drugs metabolized through cytochromes in the liver.42 Minimal ceftaroline was recovered in the bile or intestines after administration, further confirming that most of the drug is excreted renally and suggesting minimal hepatic influence on pharmacokinetics.43 In addition, hepatic impairment will likely have minimal influence on ceftaroline dosing.
Pharmacodynamics
The %T > MIC is the most important pharmokinetic/pharmacodynamic parameter, predicting ceftaroline’s clinical efficacy consistent with the β-lactam antibiotic class. Target attainment studies performed with cephalosporins reported that bacteriostatic and bactericidal effects are achieved for staphylococci when free drug concentrations exceed the MIC for 30% or 50% of the dosing interval, respectively. As true for the cephalosporin class, superior pharmacokinetic/pharmacodynamic efficacy correlates with the duration (and not the peak concentrations) eclipsing the MIC.23,26,44 The %T > MIC necessary to produce 1 log killing were 43 ± 9% (S. pneumoniae), 33 ± 9% (S. aureus), and 41 ± 11% (Gram-negative bacilli). The data for 2 log killing were 50 ± 10% (S. pneumoniae), 45 ± 13% (S. aureus), and 54 ± 3% (Gram-negative Enterobacteriaceae bacilli), respectively, in a murine model.
A population pharmacokinetic analysis of data from phase I and II trials for ceftaroline found that the probability of target attainment for %T > MIC of 50% for a 1-μg/ml target was 96% and 50% for a 2-μg/ml target, assuming subjects with normal renal function and administered 600 mg ceftaroline over a one-hour infusion every 12 hours. The ceftaroline MIC distribution for susceptible bacteria is narrow, with only 4.5% of the strains displaying a MIC of ≤0.25 μg/ml and 1.5% with a MIC of ≥2 μg/ml. The MIC50 and MIC90 rarely deviate significantly, as minimum bactericidal concentration (MBC) values are consistently equal to, or within a single dilution higher than, their respective MICs for 86% of tested organisms, while 90% of strains had an ideal MBC/MIC ratio of ≤4.38 Kill-curve kinetic studies corroborated MBC determinations for ceftaroline, as bactericidal (≥3 log10 CFU/ml reductions) action could be demonstrated in the majority of strains at up to eight times the reference MIC tested.45
Ceftaroline breakpoints have been proposed, but not confirmed, since the final MIC values and disc diffusion breakpoints await analyses of the results from Phase III clinical trials.22,26 Susceptible Gram-positive quality control strains all had zone diameters exceeding >20 mm for the 10–100 μg disc concentrations and a corresponding MIC ≤0.5 μg/ml. The maximum zone diameter differences of approximately 10 mm were achieved between susceptible and possibly resistant strains utilizing the 10 or 30 μg disc. Therefore, the 10 or 30 μg disc content may be a reasonable choice for potential correlation of MIC breakpoints of ≤1–4 μg/ml.22,45,46
As PBP affinity correlates with the MIC, predictably, ceftaroline enjoys superior efficacy (i.e., reduced MICs) to that of contemporary β-lactams.28 This is best illustrated with high binding affinity of ceftaroline to PBP2a associated with superior MICs against MRSA (0.05–2 μg/ml).24 An apparent relatively lengthy post antibiotic effect (PAE) has been noted in treatment of Gram-positive organisms, especially S. aureus, which could prevent bacterial re-growth when ceftaroline levels in serum fall below the MIC.47 Predictably and consistent with the cephalosporin class, ceftaroline fails to achieve a significant PAE against most other types of bacteria.26 Bacterial re-growth has been uncorrelated to resistance, drug instability, or tolerance, thus far. Preliminary data suggest that the in vivo activity of ceftaroline parallels the in vitro MICs.48
Microbiology
Ceftaroline, in contradistinction to other drugs within the cephalosporin class, has good efficacy against MRSA, VISA, hVISA, and VRSA; linezolid- and daptomycin-resistant S. aureus; and MDRSP, while retaining efficacy against numerous Gram-negative pathogens including respiratory and non-extended-spectrum β-lactamases (ESBL) producing Enterobacteriaceae (Table 1).20,21,23,38
Table 1.
Ceftraroline’s Mean Inhibitory Concentrations (MICs) for Selected Organisms
| Organism | MIC90 (μg/ml) |
|---|---|
|
Gram-Positive Bacteria | |
| Staphylococcus aureus | |
| MSSA | 0.25 μg/ml (≤0.03–1.0) |
| MRSA | 0.5–2.0 |
| hVISA | 0.25–4.0 |
| Quinupristin-dalfopristin-resistant | 1 |
| Staphylococcus epidermidis | |
| Oxacillin-sensitive | 0.12 (0.06–0.12) |
| Oxacillin-resistant | 0.5 (0.25–2.0) |
| Vancomycin-intermediate (VISE) | ≤0.016–2.0 |
| Quinupristin-dalfopristin-resistant | 1.0 |
| Linezolid-resistant | 0.5 |
| Streptococcus pneumoniae | |
| Penicillin-susceptible | 0.015 |
| Penicillin-intermediate | 0.06 |
| Penicillin-resistant (MDRSP) | (0.12–0.25) |
| Ceftazidime-resistant | 1.0 |
| Ceftriaxone- and cefotaxime-resistant | 0.25 |
| Erythromycin-resistant | 0.25 |
| Levofloxacin-resistant | 0.12 |
| Streptococcus (β hemolytic) | <0.008–0.016 |
| Enterococcus faecalis | 2.0–4.0 |
| Enterococcus faecium | 16–64 |
| Gram-Negative Bacteria | |
| H. influenzae | <0.016–0.03 |
| Enterobacteriaceae | |
| No β-lactamases | 0.06–4.0 |
| ESBL positive | >32 |
| AmpC positive | >128 |
| Citrobacter freundii | 2.0 |
| E. coli | |
| All isolates | 0.12 |
| − TEM/SHV | 0.015–0.03 |
| +TEM/SHV | 0.5–2.0 |
| Klebsiella pneumoniae | 0.5 |
| Morganella morganii | 0.12 |
| Proteus mirabilis | 0.12 |
| Serratia marcescens | 2.0 |
| Non-Enterobacteriaceae | |
| Pseudomonas spp. | >32 |
| Acinetobacter spp. | 4–>128 |
| Stenotrophomonas maltophila | >32 |
| Anaerobes | |
| Peptostreptococcus spp. | 0.12 |
| Propionibacterium spp | 0.12 |
| Bacteroides spp. | >32 |
| Prevotella spp. | >32 |
| Pasteurella multocida | 0.06 |
| Clostridium difficile | 4 |
Gram-Positive Organisms
Ceftaroline has 16-fold greater activity than ceftriaxone against MSSA isolates. For example, ceftaroline’s MIC90 is consistently reported to be 0.25 μg/ml (≤0.03–1 μg/ml) for MSSA, compared with 4 μg/ml for ceftriaxone, 1 μg/ml for vancomycin, and ≤0.12 μg/ml for imipenem. Ceftaroline demonstrated up to four-fold greater activity than vancomycin against MRSA isolates, independent of the isolate’s source (blood, skin, or respiratory tract), demonstrating MIC and MBC values ranging between 0.125 to 2 μg/ml and 0.5 to 2 μg/ml for ceftaroline and vancomycin, respectively.20, 21, 49 As expected, ceftaroline was ≥8-fold more potent than cefepime and ≥16-fold more active than ceftriaxone against MRSA strains.42 Ceftaroline MIC90 values against MRSA were 0.5–2 μg/ml, similar to that of linezolid and vancomycin (MIC90 of 1–2 μg/ml).49 Moreover, the MBC against MRSA strains were 1, 2, and >64 μg/ml, respectively, for ceftaroline, vancomycin, and linezolid.
Ceftaroline’s superiority over vancomycin was evident in hVISA, VISA, and VRSA as well as MRSA strains concomitantly resistant to linezolid and daptomycin.50–52 The MICs and MBCs for hVISA strains (n=100 isolates) were 2 (0.25–4 μg/ml) and 2 μg/ml, respectively, for ceftaroline. The corresponding MICs and MBCs were 4 and 8 μg/ml, respectively, for vancomycin and 1 and 16 μg/ml, respectively, for linezolid.44 Ceftaroline yielded MICs of 1–4 μg/ml against both linezolid-sensitive and -resistant S. aureus isolates. Additionally, ceftaroline exhibited bactericidal effects, as opposed to the slowly bactericidal activity exhibited by vancomycin and the bacteriostatic activity of linezolid, and has synergy in combination with tobramycin.50 Finally, ceftaroline’s MIC values against quinupristin-dalfopristin-resistant strains were similar in activity to that described for MRSA (MIC50 and MIC90, 1 μg/ml).21
Ceftaroline is also active against coagulase-negative Staphylococcus epidermidis (CoNS). Ceftaroline exhibited MIC90 of 0.12 (0.06–0.12) and 0.5 (0.25–2.0) μg/ml for oxacillin-susceptible and oxacillin-resistant isolates of CoNS, respectively.20, 21,49 Ceftaroline demonstrated MICs of ≤0.016 to 2 μg/ml against CoNS strains having reduced susceptibility to vancomycin (MIC of 4 μg/ml).21 Ceftaroline was also active against 15 quinupristin-dalfopristin- and linezolid-nonsusceptible isolates (MIC90, 1.0 μg/ml and 0.5 μg/ml), respectively.21
Consistent with other cephalosporins, the MIC90 value is lower against penicillin-susceptible strains of S. pneumoniae (MIC90 = 0.015 μg/ml) than against penicillin-intermediate (0.06 μg/ml) or penicillin-resistant strains (0.12–0.25 μg/ml).38,53,54 Moreover, MICs (both MIC50 and MIC90) varied between <0.008 and 0.5 μg/ml against 891 clinical human pneumococcal isolates collected from 22 centers in the United States in 2008.53,54 Ceftaroline remained highly active, regardless of penicillin-susceptibility status (MIC90 ≤0.5 μg/ml), levofloxacin- susceptibility, and MDR strains, remaining 2–16 fold more active than other β-lactam comparators, including cefotaxime, ceftriaxone (MIC = 1 to 2 μg/ml), amoxicillin (8 μg/ml), meropenem, cefepime, and the new cephalosporin ceftobiprole (1 μg/ml).20,21,40, 42,54,55 The MBC/MIC ratios for ceftaroline were also lower than all comparators to penicillin-susceptible and penicillin non-susceptible isolates.55 Ceftaroline (MIC90 0.03 μg/ml) was superior in isolates containing known mutations within the PBPs (i.e., 1A, 2B, and 2X) exhibiting MIC90 values against MDRSP of 0.25 μg/ml.42,45 Ceftaroline maintained MICs of 1.0 μg/ml against penicillin- and ceftazidime-resistant S. pneumoniae and MIC90 of 0.5 μg/ml (0.125–2.0 μg/ml) against highly cephalosporin-resistant clinical isolates of S. pneumoniae (cefotaxime and ceftriaxone MIC90 ≥4–16 μg/ml).49 Against amoxicillin- and cefotaxime-resistant strains, the ceftaroline MIC90 (0.25 μg/ml) was four and 16 times lower, respectively, than that of ceftriaxone (1 and 4 μg/ml, respectively). Ceftaroline’s MIC90 against erythromycin- and levofloxacin-resistant strains were 0.25 μg/ml and 0.12 μg/ml, respectively.53–56
Ceftaroline exhibits excellent potency against β-hemolytic streptococci, including Streptococcus pyogenes and Streptococcus agalactiae, with the vast majority of strains inhibited at a MIC90 ≤0.008–0.016 μg/ml, irrespective of macrolide- and levofloxacin-susceptibility status.42 Ceftaroline retained MIC50 and MIC90 of 0.03 and 0.5 μg/ml for penicillin-susceptible and penicillin-resistant viridans group streptococci strains, respectively, irrespective of levofloxacin-susceptibility status.20,56 Quinupristin-dalfopristin-nonsusceptible Streptococcus bovis and S. mitis strains were also sensitive to ceftaroline, exhibiting MICs varying widely from ≤0.016–8 μg/ml.21
Ceftaroline exhibits an MIC90 of 4 μg/ml (0.25–8 μg/ml) for Enterococcus faecalis, irrespective of vancomycin-, linezolid-, quinupristin-dalfopristin-, or ampicillin-susceptibility status. Ceftaroline MICs varied from 2–4 μg/ml against vancomycin- sensitive and -resistant E. faecalis strains (including Vanr).20,21,48 However, ceftaroline yielded minimal activity against vancomycin-susceptible or - resistant Enterococcus faecium isolates with MIC90 of 16–64 μg/ml.21,42,48,49
Gram-Negative Organisms
MICs against Enterobacteriaceae isolates without β-lactamases range from 0.06–4 μg/ml (typically with a MIC90 of 1 μg/ml, Table 1), exhibiting similar to modestly inferior activity compared to cefepime, ceftazidime, cefotaxime, and ceftriaxone.20,21,42 Example MICs for non-ESBL producing Enterobacteriaceae isolates include Citrobacter freundii (MIC50 0.15 μg/ml; MIC90, 2 μg/ml), E. coli (MIC50 0.06 μg/ml; MIC90, 0.12 μg/ml), Klebsiella pneumoniae (MIC50 0.06 μg/ml; MIC90, 0.5 μg/ml), Morganella morganii (MIC50 0.06 μg/ml; MIC90, 0.12 μg/ml), Proteus mirabilis (MIC50 0.06–0.5 μg/ml; MIC90, 0.12 μg/ml), and Serratia marcescens (MIC50 0.12–1 μg/ml; MIC90, 2.0 μg/ml). Ceftaroline also exhibits potent activity in vitro against the respiratory pathogens, H. influenzae and M. catarrhalis regardless of β-lactamase production (including ampicillin-resistant strains). For example, the MIC90 is ≤ 0.016–0.03 μg/ml for H. influenzae.20,21
Mirroring its predecessor oxyimino cephalosporins, ceftaroline lacks activity against ceftazidime non-susceptible Enterobacteriaceae. In addition, ceftaroline demonstrated generally poor activity (i.e., MIC90 of ≥32 μg/ml), similar to ceftriaxone and inferior to cefepime, ceftazidime, and imipenem against a diverse group of nonfermentative Gram-negative bacilli.
Saliently, ceftaroline does not exhibit reliable activity against Pseudomonas spp., Acinetobacter spp., or Stenotrophomonas spp. The MIC50 against Pseudomonas aeruginosa ranges from 2–16 μg/ml, while the MIC90 exceeds 32 μg/ml; hence, ceftaroline is not considered active against this organism. The MICs for Acinetobacter spp. isolates ranges from 4 – >128 μg/ml, and for Stenotrophomonas maltophilia, the MIC90 is typically ≥32 μg/ml.20,21
Against classical β-lactamases, such as TEM-1, TEM-2, or SHV-1, MICs have a significant variability ranging from 2–16 μg/ml. Additionally, ceftaroline exhibits (rather uniquely for an oxyimino cephalosporin) mild labiality to classic TEM and SHV β-lactamases, exhibiting four-fold elevations in its MICs, with high inoculums or with isolates upregulating their expression (demonstrated in many isolates of E. coli, P. mirabilis, and Klebsiella spp.). For example, ceftaroline MICs varied from 0.015–0.03 μg/ml to 0.5–2.0 μg/ml in E. coli isolates with and without classical TEM/SHV β-lactamases.49
Consistent with the cephalosporin class, ceftaroline exhibits little activity and is inactivated by ESBL-producing Enterobacteriaceae isolates (MIC90 ≥32 μg/ml), particularly compromised against CTX-M ESBL (the predominant ESBL in much of Europe, Asia, and South America). Ceftaroline also exhibits high MICs (>128 μg/ml) against bacteria containing AmpC enzymes (derepressed or constitutively expressed) and carbapenemases (OXA-48, KPC, K1, and metallo-β-lactamases).20,21,49
Studies are underway to examine the potential protection with a β-lactamase inhibitors (clavulanic acid and tazobactam), which could markedly reduce the MICs of ceftaroline potentially restoring activity against ESBL-producing isolates, including classical- and extended-spectrumclass A (TEM, SHV) and D (OXA) β-lactamases, as well as the K1 carbapenemases. Forest Laboratories is developing a combination product consisting of ceftaroline and NXL104, a novel β-lactamase inhibitor, to enhance activity against ESBLs and AmpCs; the effectiveness of this combination is under evaluation. Preliminary chequerboard analysis suggests potentiation of ceftaroline activity against Enterobacteriaceae producing AmpCs, KPCs (K1 enzyme), and non-metalloenzymatic β-lactamases (including OXA-48 carbapenemases), including isolates with impermeability.57 Furthermore, NXL104 has been shown to potentiate ceftazidime activity against non-fermenting Pseudomonas aeruginosa (including isolates producing AmpC with MICs decreased to <8 μg/ml) and ESBLs (except those exhibiting up-regulated efflux).58
Anaerobic Organisms
Ceftaroline possesses activity against Gram-positive anaerobes, including Peptostreptococcus spp., Propionibacterium spp., and non-difficile Clostridium spp. similar to that of amoxicillin-clavulanate, and 4–8 fold superior to ceftriaxone (Table 1). It also has good activity against Pasteurella multocida with an MIC90 of 0.06 μg/ml.20 It has minimal activity against Bacteroides spp. and Prevotella spp. (MIC90 ≥32 μg/ml). It possesses similar activity to that of ceftriaxone against Gram-negative non-β-lactamase producing anaerobes, and possesses insignificant activity against Clostridium difficile (MIC50, 2 μg/ml; MIC90, 4 μg/ml).21,59
Animal Studies
Animal studies on the efficacy of ceftaroline are summarized in Table 2. In a murine pyomyositis model, ceftaroline and linezolid were both superior to vancomycin (p ≤0.01).60 Ceftaroline demonstrated superior efficacy to vancomycin and linezolid in a rabbit model of joint infection due to MRSA and VISA isolates by reducing the CFU/gram tissue of MRSA in synovium by −1.98 log10. Finally, ceftaroline and linezolid (but not vancomycin) significantly reduced bacterial counts by means of −2.95 and −2.69 log10 CFU/gram in bone marrow tissue, and −2.83 and −2.25 log10 CFU/gram in bone, respectively. Overall, ceftaroline was the only intervention demonstrating homogeneous in vivo activity against MRSA and VISA isolates in all three tissues (i.e., synovium, bone, and bone marrow).60,61
Table 2.
Summary of Animal Studies Evaluating the Efficacy of Ceftaroline
| Study Reference | Study Type/Methods | Infection | Sample Size | Antibiotics | Results |
|---|---|---|---|---|---|
| Iizawa 2004 [60] | Murine Antibiotic administered at 2, 20, and 26 hours after infection Thigh muscle infected removed day 7 and homogenized in trypticase soy broth and bacterial counts estimated |
Pyomyositis | 10 mice per group | Ceftaroline Linezolid Vancomycin *All dosed at 20mg/kg |
All three drugs reduced bacterial counts compared to placebo Ceftaroline and linezolid reduced counts to ≤0.1% and were superior to vancomycin |
| Jacqueline 2010 [61] | Rabbit Knee injected with bacteria (MRSA) Antibiotic administered 3-days later following I&D for duration 4 days Comparison of bacterial load prior to antibiotics and at day 7 in the synovial fluid |
Septic Arthritis | Controls (8), ceftaroline (10), linezolid (8), and vancomycin (10) | Ceftaroline *dosed to mimic 10 mg/kg or 600 mg bid Vancomycin continuous infusion to achieve 20x the MIC Linezolid *mimic 10mg/kg or 600 mg bid |
Bacterial load in joint fluid: Mean +/− SD log10 CFU/g (D7–D3) Ceftaroline*: −1.98 +/− 1.00 Vancomcyin −0.19+/− 1.19 Linezolid −0.77 +/−1.39 Control 0.09+/−0.59 *P<0.05 vs. all comparisons |
| Izawa 2004 [60] | Murine Antibiotic administered one day after neutropenic pneumonia Bacterial counts detection at day 3 |
Pneumonia | 10 mice per group | Ceftaroline Vancomycin Linezolid *All dosed at 20mg/kg tid subcutaneously |
Ceftaroline decreased counts 99.9% from control (p<0.01) and was superior to both comparators (p<0.01) |
| Jacqueline 2007 [52] | Rabbit Antibiotic administered 24 hours after infection of aortic valve with MRSA and continued 4 days Valves then excised and homogenized for culture |
Endocarditis | Control (6), ceftaroline (10), linezolid (7), and vancomycin (6) | Ceftaroline 58 mg/kg over 12 hours to mimic human dose of 600 mg bid Vancomycin continuous infusion to achieve 20x the MIC Linezolid 10mg/kg to mimic human dose of 600 mg bid |
Bacterial CFU/g Mean log10 +/− SD (number with sterile vegetation) a,bCeftaroline: 2.5 +/− 0.3 (9/10) a,bVancomycin 2.7+/− 0.8 (4/6) aLinezolid 7.1 +/− 0.6 (0/7) Control 8.9+/−0.5 (0/6) aP<0.001 vs. controls bP<0.001 vs. linezolid |
| Jacqueline 2007 [52] | Rabbit Antibiotic administered 24 hours after infection of aortic valve with VISA and continued 4 days Valves then excised and homogenized for culture |
Endocarditis | Control (6), ceftaroline (10), linezolid (8), and vancomycin (5) | Ceftaroline 58 mg/kg over 12 hours to mimic human dose of 600 mg bid Vancomycin continuous infusion to achieve 20x the MIC Linezolid at 10 mg/kg to mimic human dose of 600 mg bid |
a,bCeftaroline: 3.0 +/− 0.9 (6/10) bVancomycin 6.7+/− 0.4 (0/5) bLinezolid 6.9 +/− 0.4 (0/8) Control 9.4+/−0.3 (0/6) aP<0.001 vs. control bP<0.001 vs. linezolid and vancomycin |
| Jacqueline 2009 [48] | Rabbit Vancomycin-sensitive E. faecalis Antibiotic administered 24 hours after infection of aortic valve and continued 4 days Valves then excised and homogenized for culture |
Endocarditis | Control (8), ceftaroline (7), linezolid (7), and vancomycin (8) | Ceftaroline to mimic human dose of 10 mg/kg/12 hr Vancomycin continuous infusion to achieve 20x the MIC Linezolid to mimic dose of 10 mg/kg/12hr |
Bacterial CFU/g Mean log10 +/− SD a,bCeftaroline: 5.68 +/− 0.49 Vancomcyin 6.70 +/− 0.25 Linezolid 6.88 +/− 0.7 Control 8.56+/−0.74 aP<0.001 vs. control bP<0.05 vs. linezolid and vancomycin |
| Jacqueline 2009 [48] | Rabbit Vancomycin-resistant E. faecalis Antibiotic administered 24 hours after infection of aortic valve and continued 4 days Valves then excised and homogenized for culture |
Endocarditis | Control (9), ceftaroline (9), linezolid (9), and Vancomycin (8) | Ceftaroline to mimic human dose of 10 mg/kg/12 hour Vancomycin continuous infusion to achieve 20x the MIC Linezolid to mimic dose of 10 mg/kg/12hour |
a,Ceftaroline: 3.98 +/− 0.85 Vancomycin 8.01 +/− 0.76 Linezolid 6.88 +/− 0.77 Control 8.60 +/− 0.54 aP<0.001 vs. all comparators |
In a murine MRSA pneumonia model, ceftaroline had similar efficacy in decreasing MRSA bacteria counts than that of vancomycin and linezolid when the drugs were begun within two hours of infection. However, ceftaroline started one day after infection demonstrated more than 99.9% reduction in bacterial counts by day 3 in a murine MRSA neutropenic pneumonia model, whereby linezolid and vancomycin had no effect.60
Regarding the treatment of endocarditis, ceftaroline demonstrated bactericidal activity in a rabbit model by showing a 6 log10 decrease in MRSA and VISA isolates after four days of treatment.52 Ceftaroline was superior to linezolid and comparable to vancomycin in an aortic endocarditis rabbit model with MRSA (108 CFU), decreasing counts to 2.5 +/− 0.3 log10 CFU/gram vegetation compared to 7.1+/− 0.6 log10 CFU/gram in linezolid, 2.7 +/− 0.8 log10 CFU/gram in vancomycin, and 8.9 +/− 0.5 log10 CFU/gram vegetation in controls.52 Ceftaroline was the only bactericidal agent against VISA isolates (wherein both vancomycin and linezolid proved to be bacteriostatic). Regarding sterilization rates (no bacterial growth after 48 hours of incubation), ceftaroline achieved sterilization in 90% (9/10) of MRSA and 60% (6/10) of VISA compared to vancomycin, which achieved 67% (4/6) and 0% (0/5), respectively, and linezolid achieving 0% (0/7 and 0/8) against both isolates.52
In the same rabbit endocarditis model, ceftaroline was superior in decreasing bacterial vegetations (5.68 log10 CFU/gram) induced by vancomycin-susceptible E. faecalis strains compared to linezolid (6.88 log10 CFU/gram, p <0.05), vancomycin (6.70 log10 CFU/gram, p<0.05), and the control group (vs. 8.56 log10 CFU/gram, p<0.001). Results were more impressive evaluating results against a vancomycin-resistant E. faecalis strain: ceftaroline vs. linezolid (3.98 vs. 6.88 log10 CFU/gram, p<0.001), ceftaroline vs. vancomycin (vs. 8.01 log10 CFU/gram, p<0.001), and the control group (vs. 8.60 log10 CFU/gram, p<0.001). In a rat endocarditis model, ceftaroline at 20 mg/kg IV twice daily was compared to control, vancomycin 120 mg/kg subcutaneously twice/daily, and daptomycin 10 mg/kg subcutaneously, daily administered for three days. Ceftaroline decreased bacterial densities significantly compared with controls in the vegetation (4.88 vs. 9.87 log10 CFU/gram, p<0.0005), kidney (4.09 vs. 7.28 log10 CFU/gram, p<0.0005), and spleen (3.63 vs. 6.53 log10 CFU/gram, p<0.0005). Vancomycin and daptomycin decreased bacterial densities in the vegetation, liver, and spleen to 6.76 and 7.64 log10 CFU/gram, 4.15 and 5.53 log10 CFU/gram, and 4.28 and 5.49 log10 CFU/gram, respectively.48
Clinical Efficacy
To date, phase III trials have been completed evaluating the efficacy of ceftaroline for the treatment of SSTI and CAP (Table 3). Regarding the treatment of SSTI, ceftaroline (600 mg intravenously every 12 hours) was noninferior to vancomycin (1 gram intravenously every 12 hours) plus aztreonam (1 gram intravenously every 8 hours) administered for 5–14 days. Two phase III trials, named CANVAS I and II (Ceftaroline versus Vancomycin in Skin and Skin-Structure Infection), investigated complicated SSTI (most commonly extensive cellulitis, major abscess, and infected wounds) among 1,378 subjects comparing ceftaroline to vancomycin +/− aztreonam.62 CANVAS I and II were randomized, double-blind, multinational phase III trials. Fifty-five study sites in 10 countries participated in CANVAS I from February to November 200763 and 56 study sites in 12 countries participated in CANVAS II from March to December 2007.64 Eligibility requirements included age ≥18 years and SSTI requiring ≥5 days IV antibiotics. Four percent had concurrent bacteremia, and the most common cause of the SSTI was S. aureus. The clinical cure rates were 92% and 93% (non-significant difference), and microbiological eradication rates were 92% and 94% for ceftaroline vs. the comparator. Response rates for MRSA infections were also similar. Ceftaroline was inferior to the comparator in Gram-negative SSTI, particularly for P. aeruginosa. Results from an earlier phase II trial (n=100, randomized 2:1) showed similar results - ceftaroline achieved clinical cure rates of 97% versus 89% for the comparator. In addition, the microbiological cure rates were comparable: 95% for ceftaroline (including all MRSA isolates identified) versus 86% for the comparator.65
Table 3.
Human Clinical Studies on the Efficacy of Ceftaroline
| Study Reference | Study Type | Infection | Sample | Antibiotics | Clinical Response | Microbiologic Response | Miscellaneous |
|---|---|---|---|---|---|---|---|
| CANVAS I [63] | Phase III Randomized Double-blind Multicenter Multinational noninferior |
SSTI | 702 | Ceftaroline (600 mg IV bid) Versus Vancomycin 1g IV bid +/− Aztreonam 1g IV bid (5–14 days) |
91.1%, (288/316) Versus 93.3%, (280/300) 95% CI: −2.2 (−6.6, 2.1) |
92.2% (225/244) Versus 94.7% (215/227) 95% CI −2.5 (−7.2, 2.1) |
MRSA 95.1% (78/82) for Ceftaroline 95.2% (59/62) for Vancomycin plus Aztreonam |
| CANVAS II [64] | Phase III Randomized Double-blind Multicenter noninferior |
SSTI | 694 | Ceftaroline (600 mg IV bid) Versus Vancomycin 1g IV bid +/− Aztreonam 1g IV bid (5–14 days) |
92.2%, (271/294) Versus 92.1%, 269/292; 95% CI: (−4.4, 4.5) |
93.9% (170/181) Versus 94.4% (168/178) −2.1% 95% CI (−6.9, 2.5) |
ME: MRSA 96.6% (56/58) for Ceftaroline 94.2% (49/52) for Vancomycin plus Aztreonam |
| Pooled CANVAS [62] | Phase III Randomized Double-blind Multicenter noninferior |
SSTI | 1378 | Ceftaroline (600 mg IV bid) Versus Vancomycin 1g IV bid +/− Aztreonam 1g IV bid (5–14 days) |
91.6% 92.7% | 92.3% 93.7% | Ceftaroline inferior to comparator for Gram-negative organisms such as Pseudomonas spp. |
| FOCUS 1 [66,67] | Phase III Randomized Double-blind Multicenter |
CAP PORT III/IV Hospitalized No ICU |
606 | Ceftaroline (600 mg IV q12) Versus Ceftriaxone (1 g IV daily) 5–7 days |
86.6% (194/224) Versus 78.2% (183/234) Difference: 8.4%, 95% CI (1.4%–15.4%) |
89.9% (62/69) Versus 76.1% (54/71) Difference: 13.8%, 95% CI (1.3–26.4) |
|
| FOCUS 2 [66,67] | Phase III Randomized Double-blind Multicenter |
CAP PORT III/IV Hospitalized No ICU |
562 | Ceftaroline (600 mg IV q12) Versus Ceftriaxone (1 g IV daily) 5–7 days |
82.1% 193/235 Versus 77.2% 166/215 Difference: 4.9%, 95% CI (−2.5%–12.5%) |
81.2% 69/85 Versus 75% 57/76 Difference: 6.2%, 95% CI (−6.7 – 19.2%) |
|
| Pooled FOCUS [66,67] | Phase III Randomized Double-blind Multicenter |
CAP PORT III/IV Hospitalized No ICU |
1228 | Ceftaroline (600 mg IV q12) Versus Ceftriaxone (1 g IV daily) 5–7 days |
84.3% 77.7% Difference: 6.7%, 95% CI (1.6%–11.8%) |
87.0% 81.0% Difference: 6.1%, 95% CI (−2.3% to 14.6%) |
Clinical response rates were 86% vs. 69% for all S. pneumoniae and 100% vs. 22% for MDRSP favouring ceftaroline |
Clinical trials have demonstrated efficacy utilizing ceftaroline for treating CAP (FOCUS 1 and 2: Ceftaroline Community Acquired Pneumonia Trial vs. Ceftriaxone in Hospitalized Patients).66,67 In these two phase III randomized double-blind multicenter trials, 1,228 hospitalized (but not admitted to the ICU) adults with moderate to severe (PORT risk class III or IV) CAP were randomized to ceftaroline (600 mg intravenously every 12 hours) or ceftriaxone (1 gram intravenously daily) for 5–7 days (Table 3). The overall clinical cure rates were similar (84% in the ceftaroline group and 78% in the ceftriaxone group), as well as the overall microbiological response rate (87% for ceftaroline and 81% for ceftriaxone). The response rates were 86% and 69% against S. pneumoniae isolates and 100% (4/4) and 22% (2/9) against MDRSP for ceftaroline and ceftriaxone, respectively. Both drugs exhibited similar clinical cure rates against MSSA and Gram-negative respiratory pathogens, such as H. influenzae and K. pneumoniae.66,67 Therefore, individual and pooled analyses of the FOCUS trials demonstrate ceftaroline to be efficacious, well tolerated, and comparable in efficacy and adverse effects to ceftriaxone in the treatment of CAP.
Although the number of cases were small, ceftaroline appears to be superior to ceftriaxone in the treatment of MDRSP as predicted by its superior affinity to the PBP2x (implicated in β-lactam resistance).67 As expected, ceftaroline is not targeted for hospital-acquired or aspiration pneumonia, as it lacks activity against many Gram-negative pathogens including those expressing AmpC- or ESBL, Pseudomonas, and Acinetobacter spp., as well as many anaerobes. Finally, given the paucity of MRSA cases in the FOCUS studies, data on the efficacy of ceftaroline for MRSA pneumonia are needed.
Safety
Based on clinical trial data to date, ceftaroline appears to be safe and well-tolerated. Since ceftaroline is a cephalosporin, it has caused serious hypersensitivity reactions in patients who are allergic to cephalosporins and among some patients with penicillin allergies. Hence, a careful history of prior antibiotic allergies should be obtained prior to the use of ceftaroline.
Side effects and drug discontinuation rates were similar to the comparator arm in the CANVAS studies. Among those receiving ceftaroline, the most common side effects were 6% with nausea, 5% headache, 5% diarrhea, 4% pruritis, and 3% rash. Forty-five percent had at least one adverse event (most were mild), but only 3% had to discontinue the drug, most commonly ascribed to a possible allergic reaction.62 All adverse events were similar to that of vancomycin/aztreonam, except the latter group had a higher incidence of pruritis. No cases of neutropenia, thrombocytopenia, hemolytic anemia, or significant liver dysfunction were identified during these trials.62 Elevations in laboratory parameters occurred infrequently for blood creatine kinase (8%), alanine aminotransferase (6%), and aspartate aminotransferase (6%) levels, but were typically asymptomatic.63 Development of a positive direct Coombs’ test has been noted, but no known cases of haemolytic anemia have been documented, thus far. Furthermore, EKG data have not noted QT interval prolongation.63 In summary, ceftaroline has had an excellent safety profile to date; further post-marketing assessments are needed to ensure the safety of this new drug.
Ceftaroline is excreted renally, thus studies have shown minimal impact on the fecal microflora after seven days administration in healthy young adults. For example, in one study, minimal disruption was noted in the stool ecologic flora, with modest decreases observed in E. coli, Bifidobacteria, and Lactobacillus isolates, and no changes were found within Candida, Bacterioides, or Enterococcus spp.43 However, like all antibiotics, C. difficile infection may occur with ceftaroline - in the CANVAS I and II trials, two patients (of 693) developed a C. difficile infection (compared to one in the comparator group).62
Regarding drug-drug interactions, no formal studies have been conducted with ceftaroline, to date. Given its metabolism through the kidneys, ceftaroline likely exhibits minimal inhibition of the P450 system, suggesting limited propensity for drug interactions among medications metabolized via this system. It has no known antagonism with other antibiotics and has possible synergy with diverse antibiotic classes, to include aminoglycosides (tobramycin), piperacillin/tazobactam, aztreonam, and meropenem.42,50 Up to now, there are no specific data on the use of ceftaroline in paediatrics or pregnant/breastfeeding women, hence, the safety of this novel antibiotic in these settings is currently unknown.
Resistance Barrier
The barrier to resistance appears sizable for Gram-positive bacteria with resistance rarely reported to date. It has a comparable profile to other oxyimino cephalosporins for Gram-negative bacteria based on investigations of the spontaneous mutation frequency and change in MIC in single-step mutant selection and serial passage studies.23 For example, ceftaroline did not select for resistant variants of S. aureus in vivo.52 In vitro passage studies have demonstrated low rates of acquired resistance of Staphylococcus spp. to ceftaroline.51 Ceftaroline at concentrations of four times the MIC failed to select mutants atdetectable frequencies from tested MRSA, VISA, and MDRSP isolates.49 Ceftaroline also appeared immune to multi-step mutational induction attempts.49 In synopsis, ceftaroline has demonstrated minimal changes in MIC in serial passage studies in Gram-positive isolates, but demonstrates similar potential to resistance development as cefotaxime to Gram-negative organisms.23 Although these data are promising, information regarding the evolution of resistance to this novel antibiotic in clinical practice are needed. Furthermore, as previously noted, ceftaroline exhibits poor activity against ESBL and AmpC producing strains.
Ceftobiprole
There is an additional novel fifth-generation cephalosporin with activity against MRSA, ceftobiprole, currently under investigation. Ceftobiprole medocaril, the pro-drug of ceftobiprole (formerly BAL9141), is a parental investigational cephalosporin (pyrrolidinone-3-ylidene-methyl cephalosporin) for the treatment of SSTI with a recommended dose of 500 mg every 8 hours for 7–14 days. 68,69 Ceftobiprole exhibits activity against a wide-range of Gram-positive organisms including (MRSA) and Gram-negative organisms mirroring cefepime and ceftazidime.70–71 Per time-kill studies, ceftobiprole exhibits primarily bactericidal activity with an MBC/MIC < 4 for the majority of tested isolates. As with ceftaroline, ceftobiprole’s activity against MRSA hinges upon its affinity and interaction with PBP2a. It acylates PBP2a rapidly forming a more stable acyl-enzyme complex than other cephalosporins leading to 100% inhibition68. It also exhibits strong affinity for PBP2x providing activity against MDRSP, PBP2, PBP3 (E. coli), PBP1a-b, PBP2, PBP3, PBP4 (P. aeruginosa). Interestingly, ceftobiprole has no activity against Enterococcus faecium due to a lack of activity against PBP5.69 Ceftobiprole exhibits an MIC90 <2 μg/mL against MRSA and E. faecalis; and 0.25 μg/mL for sensitive S. pneumoniae and <0.5 μg/mL against penicillin-resistant S. pneumoniae.68,70,71 Similar to ceftaroline, ceftobiprole exhibits vulnerability to many β-lactamases resulting in a wide range of MICs for the Enterobacteriaceae.71,72 Ceftobiprole is resistant to the TEM-1 and SHV-1 β-lactamases, but similar to ceftaroline, is susceptible to a host of higher order β-lactamases including AmpC β-lactamase; CTX-M-15 ESBL; and the KPC-2 carbapenemase.
Similar to ceftaroline, ceftobiprole has demonstrated noninferiority to vancomycin with or without ceftazidime in two large-scale studies with both interventions achieving clinical cure rates of >90%73,74. In the first phase III clinical trial, overall clinical cure rates for SSTI were 93% and 94% in the ceftobiprole and vancomycin groups, respectively (95% CI, −4.4% to 3.9%).73 A second phase III clinical trial noted overall cure rates of 91% versus 90% compared to vancomycin plus ceftazidime without significant differences in adverse events.74 Ceftobiprole is approved for the treatment of SSTI in Switzerland and Canada (Zevtera). However, the drug has not been approved by the FDA and is pending further evaluation.75
Conclusions
Ceftaroline is a novel, broad-spectrum cephalosporin, which exhibits bactericidal activity against Gram-positive bacteria, including MRSA and MDRSP. Ceftaroline offers an exciting addition to the anti-MRSA armamentarium, including activity against VISA, hVISA, VRSA, and daptomycin- and linezolid-resistant strains. Unique among many anti-MRSA agents, ceftaroline additionally provides activity against Gram-negative respiratory pathogens including H. influenzae and M. catarrhalis. Since ceftaroline is not effective against organisms with AmpC- or ESBLs, research investigating combination with β-lactamase inhibitors to provide potential activity against these Gram-negative organisms are underway. To date, ceftaroline has demonstrated an excellent safety profile comparable to contemporary cephalosporins and exhibits an inherently low propensity to inducing resistance, especially among Gram-positive organisms; however, long-term data and clinical experience with this novel agent are needed. Ceftaroline is currently FDA approved for the treatment of both STTIs and CAP.
Footnotes
Conflict of Interest: None. The authors have no financial interest in this work. Both authors contributed to the content of the manuscript and concurred with the decision to submit it for publication.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This work is original and has not been published elsewhere.
Disclosures
This manuscript has been read and approved by both authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Moran G, Krishnadasan A, Gorwitz R, et al. Methicillin-resistant S. aureus Infections Among Patients in the Emergency Department. N Engl J Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary Manifestations in Children with Invasive Community-acquired Staphylococcus aureus Infection. Clin Infect Dis. 2005;41(5):583–590. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 3.Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis. 2008;46(5):668–674. doi: 10.1086/527392. [DOI] [PubMed] [Google Scholar]
- 4.Stryjewski ME, Corey GR. New Treatments for Methicillin-resistant Staphylococcus aureus. Curr Opin Crit Care. 2009;15(5):403–412. doi: 10.1097/MCC.0b013e32832f0a74. [DOI] [PubMed] [Google Scholar]
- 5.Pan A, Lorenzotti S, Zoncada A. Registered and Investigational Drugs for the Treatment of Methicillin-resistant Staphylococcus aureus Infection. Recent Pat Antiinfect Drug Discov. 2008;3(1):10–33. doi: 10.2174/157489108783413173. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi R, Sabbatani S. Novel Pharmaceutical Molecules Against Emerging Resistant Gram-positive Cocci. Braz J Infect Dis. 2010;14(1):96–108. doi: 10.1590/s1413-86702010000100020. [DOI] [PubMed] [Google Scholar]
- 7.Stryjewski ME, Graham DR, Wilson SE, et al. Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. Telavancin Versus Vancomycin for the Treatment of Complicated Skin and Skin-structure Infections Caused by Gram-positive Organisms. Clin Infect Dis. 2008;46(11):1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 8.Stefani S, Goglio A. Methicillin-resistant Staphylococcus aureus: Related Infections and Antibiotic Resistance. Int J Infect Dis. 2010;14S4:S19–S22. doi: 10.1016/j.ijid.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Livermore D. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother. 2003;51(S2):S9–S16. doi: 10.1093/jac/dkg249. [DOI] [PubMed] [Google Scholar]
- 10.Manfredi R. Update on the Appropriate Use of Linezolid in Clinical Practice. Ther Clin Risk Manage. 2006;2(4):455–463. doi: 10.2147/tcrm.2006.2.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nannini E, Murray BE, Arias CA. Resistance or Decreased Susceptibility to Glycopeptides, Daptomycin, and Linezolid in Methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol. 2010;10(5):516–521. doi: 10.1016/j.coph.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Koplowicz YB, Schwartz BS, Guglielmo BJ. Development of Daptomycin-susceptible, Methicillin-resistant Staphylococcus aureus Pneumonia During High-dose Daptomycin Therapy. Clin Infect Dis. 2009;49(8):1286–1287. doi: 10.1086/605690. [DOI] [PubMed] [Google Scholar]
- 13.Marty F, Yeh W, Wennersten C, et al. Emergence of a Clinical Daptomycin-Resistant Staphylococcus aureus Isolate during Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia and Osteomyelitis. J Clin Microbiol. 2006;44(2):595–597. doi: 10.1128/JCM.44.2.595-597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden MK, Rezai K, Hayes RA, Lolans K, Quinn JP, Weinstein RA. Development of Daptomycin Resistance In Vivo in Methicillin-Resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5285–5287. doi: 10.1128/JCM.43.10.5285-5287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering R, Eliopoulos G. Induction of Daptomycin Heterogenous Susceptibility in Staphylococcus aureus by Exposure to Vancomycin. Antimicrob Agents Chemother. 2006;50(4):1581–1585. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose W, Leonard S, Sakoulas G, et al. Daptomycin Activity against Staphylococcus aureus following Vancomycin Exposure in an In Vitro Pharmacodynamic Model with Simulated Endocardial Vegetation. Antimicrob Agents Chemother. 2008;52(3):831–836. doi: 10.1128/AAC.00869-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson LR. A review of tigecycline-the first glycylcycline. Int J Antimicrob Agents. 2008;32(S4):S215–222. doi: 10.1016/S0924-8579(09)70005-6. [DOI] [PubMed] [Google Scholar]
- 18.Reygaert W. Antibiotic Optimization in the Difficult-to-treat Patient with Complicated Intra-abdominal or Complicated Skin and Skin Structure Infections: Focus on Tigecycline. Ther Clin Risk Manag. 2010;6:419–430. doi: 10.2147/tcrm.s9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freire A, Melnyk V, Kim M, et al. Comparison of Tigecycline with Imipenem/Cilastatin for the Treatment of Hospital-acquired Pneumonia. Diagn Microbiol Infect Dis. 2010;68(2):140–151. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Ge Y, Biek D, Talbot G, Sahm D. In Vitro Profiling of Ceftaroline Against a Collection of Recent Bacterial Clinical Isolates from Across the United States. Antimicrob Agents Chemother. 2008;52(9):3398–3407. doi: 10.1128/AAC.00149-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sader H, Fritsche T, Kaniga K, Ge Y, Jones R. Antimicrobial Activity and Spectrum of PPI-0903M (T-91825), a Novel Cephalosporin, Tested Against a Worldwide Collection of Clinical Strains. Antimicrob Agents Chemother. 2005;49(8):3501–3512. doi: 10.1128/AAC.49.8.3501-3512.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steed M, Rybak MJ. Ceftaroline. A New Cephalosporin with Activity Against esistant Gram-positive Pathogens. Pharmacotherapy. 2010;30(4):375–389. doi: 10.1592/phco.30.4.375. [DOI] [PubMed] [Google Scholar]
- 23.Zhanel G, Sniezek G, Schweizer F, et al. Ceftaroline: A Novel Broad-spectrum Cephalosporin with Activity Against Methicillin-resistant Staphylococcus aureus. Drugs. 2010;69(7):809–831. doi: 10.2165/00003495-200969070-00003. [DOI] [PubMed] [Google Scholar]
- 24.Vidaillac C, Leonard S, Rybak M. In Vitro Activity of Ceftaroline Against Methicillin-resistant Staphylococcus aureus and Heterogeneous Vancomycin-intermediate S. aureus in a Hollow Fiber Model. Antimicrob Agents Chemother. 2009;53(11):4712–4717. doi: 10.1128/AAC.00636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villegas-Estrada A, Lee M, Hesek D, Vakulenko S, Mobashery S. Co-opting the Cell Wall in Fighting Methicillin-resistant Staphylococcus aureus: Potent Inhibition of PBP 2a by Two Anti-MRSA β-lactam Antibiotics. J Am Chem Soc. 2008;130(29):9212–9213. doi: 10.1021/ja8029448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanafani Z, Corey R. Ceftaroline: A Cephalosporin with Expanded Gram- positive Activity. Future Microbiol. 2009;4(1):25–33. doi: 10.2217/17460913.4.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Llarrull L, Fisher J, Mobashery S. Molecular Basis and Phenotype of Methicillin Resistance in Staphylococcus aureus and Insights into New beta-lactams that Meet the Challenge. Antimicrob Agents Chemother. 2009;53(10):4051–4063. doi: 10.1128/AAC.00084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moisan H, Pruneau M, Malouin F. Binding of Ceftaroline to Penicillin-binding Proteins of Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother. 2010;65(4):713–716. doi: 10.1093/jac/dkp503. [DOI] [PubMed] [Google Scholar]
- 29.Kosowska-Shick K, McGhee PL, Appelbaum PC. Affinity of Ceftaroline and Other Beta-lactams for Penicillin-binding Proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2010;54(5):1670–1677. doi: 10.1128/AAC.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sader H, Fritsche T, Jones R. Antimicrobial Activities of Ceftaroline and ME1036 Tested Against Clinical Strains of Community-acquired Methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(3):1153–1155. doi: 10.1128/AAC.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge Y, Floren L, Redman R, Wikler M, Liao S. Single-dose Pharmacokinetics (PK) of Ceftaroline (PPI-0903) in Healthy Subjects [abstract A-1936]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 27–30, 2006; San Francisco CA. [Google Scholar]
- 32.Ge Y, Redman R, Floren L, Liao S, Wilker M. The Pharmacokinetics (PK) and Safety of Ceftaroline (PPI-0903) in Healthy Subjects Receiving Multiple-dose Intravenous (IV) Infusions [abstract A-1937]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 27–30, 2006; San Francisco CA. [Google Scholar]
- 33.Ge Y, Maynard D, Rickert DE. Comparative Pharmacokinetics of Ceftaroline in Rats, Rabbits, and Monkeys Following a Single Intravenous or Intramuscular Injection. Antimicrob Agents Chemother. 2010;54(2):912–914. doi: 10.1128/AAC.00645-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccobene T, Fang E, Thye D. A Single- and Multiple-dose Study to Determine the Safety, Tolerability, and Pharmacokinetics (PK) of Ceftaroline (CPT) Administered by Intramuscular (IM) Injection to Healthy Subjects [abstract A-1888]. 48th International Conference on Antimicrobial Agents and Chemotherapy/Infectious Disease Society of America 46th Annual Meeting; October 25–28, 2008; Washington DC. [Google Scholar]
- 35.Ge Y, Thye D, Liao S, et al. Pharmacokinetics (PK) of Ceftaroline (PPI0903) in Subjects with Mild or Moderate Renal Impairment (RI) [abstract A-1939]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 27–30, 2006; San Francisco CA. [Google Scholar]
- 36.Ge Y, Liao S, Talbot GH. Population Pharmacokinetics (PK) Analysis of Ceftaroline (CPT) in Volunteers and Patients with Complicated Skin and Skin Structure Infections (CSSSI) [abstract A-34]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 27–30, 2006; San Francisco CA. [Google Scholar]
- 37.Jacqueline C, Caillon J, Miegeville Penetration of Ceftaroline (PPI-0903), a New Cephalosporin, into Lung Tissues: Measurement of Plasma and Lung Tissue Concentrations after a Short IV Infusion in the Rabbit [abstract A-1938]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 27–30, 2006; San Francisco CA. [Google Scholar]
- 38.McGee L, Biek D, Ge Y, et al. In vitro Evaluation of the Antimicrobial Activity of Ceftaroline Against Cephalosporin-resistant Isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2009;53(2):552–556. doi: 10.1128/AAC.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge Y, Liao S, Thye DA, et al. Ceftaroline (CPT) Dose Adjustment Recommendations for Subjects with Mild or Moderate Renal Impairment (RI). [abstract A-35]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 27–30, 2006; San Francisco CA. [Google Scholar]
- 40.Bazan JA, Martin SI, Kaye KM. Newer Beta-lactam Antibiotics: Doripenem, Ceftobiprole, Cetaroline and Cefepime. Infect Dis Clin N Am. 2009;23:983–996. doi: 10.1016/j.idc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Riccobene T, Jakate A, Rank D, Thye DA. An Open-label, Pharmacokinetic, Safety and Tolerability Study of Single-dose Intravenous Ceftaroline in Subjects with End-stage Renal Disease on Intermittent Haemodialysis. Poster Presentation at the European Congress of Clinical Microbiology and Infectious Diseases; Helsinki, Finland. May 16–19, 2009. [Google Scholar]
- 42.Parish D, Scheinfeld N. Ceftaroline Fosamil, A Cephalosporin Derivative for the Potential Treatment of MRSA Infection. Curr Opin Invest Drugs. 2008;9(2):201–209. [PubMed] [Google Scholar]
- 43.Panagiotidis G, Backstrom T, Asker-Hagelberg C, Jandourek A, Weintraub A, Nord CE. Effect of Ceftaroline on Normal Human Intestinal Microflora. Antimicrob Agents Chemother. 2010;54(5):1811–1814. doi: 10.1128/AAC.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andes D, Craig WA. Pharmacodynamics of a New Cephalosporin, PPI-0903 (TAK-599), Active Against Methicillin-resistant Staphylococcus aureus in Murine Thigh and Lung Infection Models: Identification of an In Vivo Pharmacokinetic-pharmacodynamic Target. Antimicrob Agents Chemother. 2006;50(4):1376–1383. doi: 10.1128/AAC.50.4.1376-1383.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown S, Traczewski M. In vitro Antimicrobial Activity of a New Cephalosporin, Ceftaroline, and Determination of Quality Control Ranges for MIC Testing. Antimicrob Agents Chemother. 2009;53(3):1271–1274. doi: 10.1128/AAC.01021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones R, Fritsche T, Ge Y, Kaniga K, Sader H. Evaluation of PPI-0903M ( T91825), A Novel Cephalosporin: Bactericidal Activity, Effects of Modifying In Vitro Testing Parameters and Optimization of Disc Diffusion Tests. J Antimicrob Chemother. 2005;56(6):1047–1052. doi: 10.1093/jac/dki362. [DOI] [PubMed] [Google Scholar]
- 47.Pankuch G, Appelbaum P. Postantibiotic Effect of Ceftaroline Against Gram- positive Organisms. Antimicrob Agents Chemother. 2009;53(10):4537–4539. doi: 10.1128/AAC.00785-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacqueline C, Caillon J, Le Mabecque V, et al. In vivo Activity of a Novel Anti-methicillin-resistant Staphylococcus aureus Cephalosporin, Ceftaroline, Against Vancomycin-susceptible and -resistant Enterococcus faecalis Strains in a Rabbit Endocarditis model: A Comparative Study with Linezolid and Vancomycin. Antimicrob Agents Chemother. 2009;53(12):5300–5302. doi: 10.1128/AAC.00984-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mushtaq S, Warner M, Ge Y, Kaniga K, Livermore D. In Vitro Activity of Ceftaroline (PPI-0903M, T-91825) Against Bacteria with Defined Resistance Mechanisms and Phenotypes. J Antimicrob Chemother. 2007;60(2):300–311. doi: 10.1093/jac/dkm150. [DOI] [PubMed] [Google Scholar]
- 50.Vidaillac C, Leonard S, Rybak M. In Vitro Evaluation of Ceftaroline Alone and in Combination with Tobramycin Against Hospital-acquired Methicillin-Resistant Staphylococcus aureus (HA-MRSA) Isolates. Int J Antimicrob Agents. 2010;35(6):527–530. doi: 10.1016/j.ijantimicag.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Saravolatz L, Pawlak J, Johnson L. In Vitro Activity of Ceftaroline Against Community Associated Methicillin Resistant, Vancomycin Intermediate, Vancomycin Resistant, and Daptomycin Non Susceptible Staphylococcus aureus Isolates. Antimicrob Agents Chemother. 2010;54(7):3027–3030. doi: 10.1128/AAC.01516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacqueline C, Caillon J, Le Mabecque V, et al. In Vivo Efficacy of Ceftaroline (PPI-0903), a New Broad-spectrum Cephalosporin, Compared with Linezolid and Vancomycin Against Methicillin-resistant and Vancomycin-intermediate Staphylococcus aureus in a Rabbit Endocarditis Model. Antimicrob Agents Chemother. 2007;51(9):3397–3400. doi: 10.1128/AAC.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobs M, Good C, Windau A, et al. Activity of Ceftaroline Against Recent Emerging Serotypes of Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 2010;54(6):2716–2719. doi: 10.1128/AAC.01797-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel S, Pillai D, Pong-Porter S, McGeer A, Green K, Low DE. In Vitro Activity of Ceftaroline, Ceftobiprole and Cethromycin Against Clinical Isolates of Streptococcus pneumoniae Collected from Across Canada Between 2003 and 2008. J Antimicrob Chemother. 2009;64(3):659–660. doi: 10.1093/jac/dkp231. [DOI] [PubMed] [Google Scholar]
- 55.Fenoll A. In Vitro Activity of Ceftaroline Against Streptococcus pneumoniae Isolates Exhibiting Resistance to Penicillin, Amoxicillin, and Cefotaxime. Antimicrob Agents Chemother. 2008;52(11):4209–4210. doi: 10.1128/AAC.00712-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrissey I, Ge Y, Janes R. Activity of the New Cephalosporin Ceftaroline Against Bacteraemia Isolates from Patients with Community-acquired Pneumonia. Int J Antimicrob Agents. 2009;33(6):515–519. doi: 10.1016/j.ijantimicag.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Mushtaq S, Warner M, Williams G, Crithcley I, Livermore D. Activity of Checquerboard Combinations of Ceftaroline and NXL104 Versus Beta-lactamase Producing Enterobacteriaceae. J Antimicrob Chemother. 2010;65(7):1428–1432. doi: 10.1093/jac/dkq161. [DOI] [PubMed] [Google Scholar]
- 58.Mushtaq S, Warner M, Livermore D. In Vitro Activity of Ceftazidime+NXL104 Against Pseudomonas aeruginosa and Other Non-fermenters. J Antimicrob Chemother. 2010;65(11):2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 59.Citron D, Tyrrell K, Merriam C, Goldstein E. In Vitro Activity of Ceftaroline Against 623 Diverse Strains of Anaerobic Bacteria. Antimicrob Agents Chemother. 2010;54(4):1627–1632. doi: 10.1128/AAC.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iizawa Y, Nagai J, Ishikawa T, et al. In vitro Antimicrobial Activity of T-91825, a Novel Anti-MRSA Cephalosporin, and In Vivo Anti-MRSA Activity of its Prodrug, TAK-599. J Infect Chemother. 2004;10(3):146–156. doi: 10.1007/s10156-004-0309-3. [DOI] [PubMed] [Google Scholar]
- 61.Jacqueline C, Amador G, Caillon J, et al. Efficacy of the New Cephalosporin Ceftaroline in the Treatment of Experimental Methicillin-resistant Staphylococcus aureus Acute Osteomyelitis. J Antimicrob Chemother. 2010;65(8):1749–1752. doi: 10.1093/jac/dkq193. [DOI] [PubMed] [Google Scholar]
- 62.Corey GR, Wilcox M, Talbot GH, Friedland HD, Baculik T, Witherell GW, Critchley I, Das AF, Thye D. Integrated Analysis of CANVAS 1 and 2: Phase 3, Multicenter, Randomized, Double-blind Studies to Evaluate the Safety and Efficacy of Ceftaroline Versus Vancomycin Plus Aztreonam in Complicated Skin and Skin-structure Infection. Clin Infect Dis. 2010;51(6):641–650. doi: 10.1086/655827. [DOI] [PubMed] [Google Scholar]
- 63.Corey GR, Wilcox MH, Talbot GH, Thye D, Friedland D, Baculik T CANVAS 1 investigators. CANVAS 1: The First Phase III, Randomized, Double-blind Study Evaluating Ceftaroline fosamil for the Treatment of Patients with Complicated Skin and Skin Structure Infections. J Antimicrob Chemother. 2010;65(S4):S41–51. doi: 10.1093/jac/dkq254. [DOI] [PubMed] [Google Scholar]
- 64.Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, Baculik T CANVAS 2 investigators. CANVAS II: The Second Phase III, Randomized, Double-blind Study Evaluating Ceftaroline fosamil for the Treatment of Patients with Complicated Skin and Skin Structure Infections. J Antimicrob Chemother. 2010;65(S4):S53–S65. doi: 10.1093/jac/dkq255. [DOI] [PubMed] [Google Scholar]
- 65.Talbot G, Thye D, Das A, Ge Y. Phase 2 Study of Ceftaroline Versus Standard Therapy in Treatment of Complicated Skin and Skin Structure Infections. Antimicrob Agents Chemother. 2007;51(10):3612–3616. doi: 10.1128/AAC.00590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckburg P, Friedland HD, Lee J, Llorens L, Critchley IA, Thye DA. FOCUS 1 and 2: Randomized, Double-blinded, Multicenter Phase 3 Trials of the Efficacy and Safety of Ceftaroline (CPT) VS Ceftriaxone (CRO) in Community-acquired Pneumonia (CAP) [Abstract LI-345a]. Poster presentation at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. September 12–15, 2009. [Google Scholar]
- 67.File TM, Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. Integrated analysis of FOCUS 1 and FOCUS 2: Randomized Double-Blinded, Multicenter Phase 3 Trials of the Efficacy and Safety of Ceftaroline fosamil versus Ceftriaxone in Patients with Community-acquired Pneumonia. Clin Infect Dis. 2010;51(2):1395–1405. doi: 10.1086/657313. [DOI] [PubMed] [Google Scholar]
- 68.Dauner D, Nelson R, Taketa D. Ceftobiprole: A novel, Broad-spectrum Cephalosporin with Activity Against Methicillin-resistant Staphylococcus aureus. Am J Health Syst Pharm. 2010;67(12):983–993. doi: 10.2146/ajhp090285. [DOI] [PubMed] [Google Scholar]
- 69.Barbour A, Derendorf H. Resistance and the Management of Complicated Skin and Skin Structure Infections: the Role of Ceftobiprole. Ther Clin Risk Manag. 2010;6:485–495. doi: 10.2147/TCRM.S5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Betriu C, Culebras E, Gomez M, Lopez-Fabal F, Rodriguez-Avial I, Picazo JJ. Comparative in vitro Activity of Ceftobiprole against Gram-positive Cocci. Int J Antimicrob Agents. 2010;36(2):111–113. doi: 10.1016/j.ijantimicag.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Fritsche TR, Sader HS, Jones RN. Antimicrobial activity of Ceftobiprole, a Novel Anti-methicillin-resistant Staphylococcus aureus Cephalosporin, Tested against Contemporary Pathogens: Results from the SENTRY Antimicrobial Surveillance Program (2005–2006) Diagn Microbiol Infect Dis. 2008;61(1):86–95. doi: 10.1016/j.diagmicrobio.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Silva N, Radhouani H, Goncalves A, Araujo C, Rodrigues J, Igrejas G, Poeta P. In vitro Activity of Ceftobiprole against Gram-positive and Gram-negative Bacteria Isolated from Humans and Animals. J Antimicrob Chemother. 2010;65(4):801–803. doi: 10.1093/jac/dkq011. [DOI] [PubMed] [Google Scholar]
- 73.Noel GJ, Strauss RS, Amsler K, Heep M, Pypstra R, Solomkin JS. Results of a Double-blind, Randomized Trial of Ceftobiprole Treatment of Complicated Skin and Skin Structure Infections caused by Gram-positive Bacteria. Antimicrob Agents Chemother. 2008;52(1):37–44. doi: 10.1128/AAC.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. A Randomized, Double-blind Trial Comparing Ceftobiprole medocaril with Vancomycin plus Ceftazidime for the Treatment of Patients with Complicated Skin and Skin-structure Infections. Clin Infect Dis. 2008;46(5):647–655. doi: 10.1086/526527. [DOI] [PubMed] [Google Scholar]
- 75.Chahine E. Ceftobiprole: Farewell or Just a Delay? Am J Health Syst Pharm. 2010;67(12):981. doi: 10.2146/ajhp100123. [DOI] [PubMed] [Google Scholar]