Abstract
Palatable food intake reduces stress responses, suggesting that individuals may consume such “comfort” food as self-medication for stress relief. The mechanism by which palatable foods provide stress relief is not known, but likely lies at the intersection of forebrain reward and stress regulatory circuits. Forebrain opioidergic and gamma-aminobutyric acid (GABA)ergic signaling is critical for both reward and stress regulation suggesting that these systems are prime candidates for mediating stress relief by palatable foods. Thus, the current study aimed to determine 1) how palatable “comfort” food alters stress induced changes in the mRNA expression of inhibitory neurotransmitters in reward and stress neurocircuitry, and 2) identify candidate brain regions that may underlie comfort food-mediated stress reduction. We used a model of palatable “snacking” in combination with a model of chronic variable stress followed by in situ hybridization to determine forebrain levels of pro-opioid and glutamic acid decarboxylase (GAD) mRNA. The data identify regions within the extended amygdala, striatum, and hypothalamus as potential regions for mediating hypothalamic-pituitary-adrenal axis (HPA)-buffering following palatable snacking. Specifically, palatable snacking alone decreased enkephalin mRNA expression in the anterior bed nucleus of the stria terminalis and the nucleus accumbens, as well as decreasing GAD65 mRNA in the posterior bed nucleus of the stria terminalis. Chronic stress alone increased enkephalin mRNA in the hypothalamus, nucleus accumbens, amygdala, and hippocampus; increased dynorphin mRNA in the nucleus accumbens; increased GAD65 mRNA in the anterior hypothalamus and bed nucleus of the stria terminalis; and decreased GAD65 mRNA in the dorsal hypothalamus. Importantly, palatable food intake prevented stress-induced gene expression changes in subregions of the hypothalamus, bed nucleus of the stria terminalis, and nucleus accumbens. Overall, these data suggest that complex interactions exist between brain reward and stress pathways and that palatable snacking can mitigate many of the neurochemical alterations induced by chronic stress.
Keywords: dynorphin, enkephalin, GAD65/67, sucrose, hypothalamus, nucleus accumbens
INTRODUCTION
Stress, defined as a real or perceived threat to homeostasis, evokes a number of physiological responses. Responses to stress include hormone release from the hypothalamic-pituitary-adrenal axis (HPA), activation of the autonomic nervous system, and changes in behavior (Dallman, 1993, Strack et al., 1995, Herman and Cullinan, 1997, Strack et al., 1997, Denver, 2009, Ulrich-Lai and Herman, 2009). These stress response systems are managed, in large part, by the forebrain (Herman and Cullinan, 1997, Herman et al., 2003, Herman et al., 2005). In particular, forebrain regions important in stress modulation often employ inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA) and opioids (Bowers et al., 1998, Lucas et al., 2007, Poulin et al., 2009). While these stress responses can be protective during bouts of acute stress, repeated stimulation of these systems by chronic stress can be maladaptive and is implicated in a number of serious health problems, including depression, anxiety disorders, and obesity (Tsigos and Chrousos, 2002, Simon et al., 2006, Scott et al., 2008). For example, chronic stress elicits facilitation of HPA responses, increased autonomic tone, and increased anxiety-like and depressive-like behavior (Herman et al., 1995, Paskitti et al., 2000, Ostrander et al., 2006). In addition, chronic stress alters activity in stress regulatory brain regions (Herman and Cullinan, 1997, Herman et al., 2003, Herman et al., 2005). For example, chronic variable stress (CVS) increases the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD65/67) mRNA (Bowers et al., 1998) and pre-proenkephalin (ENK) and pre-prodynorphin (DYN) mRNA (Dumont et al., 2000, Chen et al., 2004) in key stress regulatory regions. This suggests that these neurotransmitter systems may be important for adaptation to chronic stress.
“Comfort food” is a colloquial term used to denote highly palatable, calorically dense foods sought out to provide relief from stress. Individuals appear to engage in consumption of palatable food to limit HPA, autonomic, and behavioral responses to stress (Dallman, 1993, Strack et al., 1995, Prasad and Prasad, 1996, Strack et al., 1997, Markus et al., 2000, la Fleur et al., 2005, Schiltz et al., 2007, Ulrich-Lai et al., 2007). Like chronic stress, repeated palatable food intake can elicit changes in HPA function and brain regulation in forebrain regions important in processing emotionally salient information (Prasad and Prasad, 1996, Strack et al., 1997, Markus et al., 2000, Kelley et al., 2003, Schiltz et al., 2007, Ulrich-Lai et al., 2007). Opioidergic and GABAergic circuitry regulate brain regions activated by palatable food intake (Will et al., 2003, Ikemoto and Wise, 2004, Kelley et al., 2005), and palatable food can modulate GABA and opioid brain signaling (Kelley et al., 2003, Schiltz et al., 2007).
Forebrain opioidergic and GABAergic signaling are ideal candidates for mediating the stress dampening properties of palatable foods. Thus, the goal of the current study was to determine how chronic palatable “snacking” affects CVS-induced changes in opioid and GAD gene expression in stress and reward brain regions. We hypothesized that the rewarding and/or metabolic effects resulting from regular access to palatable comfort food would reverse the effects of chronic stress on GAD, DYN, and ENK mRNA expression in brain regions important in stress and/or reward regulation. To test this hypothesis, the current study used a model of palatable “snacking” in combination with a model of CVS to assess stress-reward interaction in regulation of opioid and GAD mRNA expression in forebrain circuitry.
METHODS
Animals and Experimental Design
To directly compare neuroanatomical findings with the hormonal stress response and to conserve animal usage, the brain samples analyzed in this study were from adult, male Long-Evans rats used in experiments reported by Ulrich-Lai, et al. (Ulrich-Lai et al., 2007). All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee and were consistent with NIH guidelines. Rats were given ad libitum access to water and chow (LM-485 Mouse/Rat sterilizable diet; Harlan-Teklad, Madison, WI) for the duration of the study. Rats were given additional intermittent (up to 30-min) access twice-daily to 4 ml sucrose (30% Sigma Aldrich Co., St. Louis, MO), sodium saccharin (0.1%, Sigma Aldrich Co.), or water at 09:30 and 15:30 for 28 days. Saccharin, a non-caloric sweetener, was included to determine whether palatable food effects were due to sweet taste alone (saccharin) vs. sweet taste with calories. After two weeks of intermittent drink treatment, half of each group of rats was concurrently exposed to 14 days of chronic stress (for a total of 6 treatment groups). The chronic stress paradigm used was chronic variable stress (CVS), a well-characterized chronic stress protocol (Herman et al., 1995, Paskitti et al., 2000, Ostrander et al., 2006). The stressors have all been shown to promote activation of the stress axis without substantial injury, morbidity or mortality. CVS consisted of twice daily exposure (at unpredictable times, spaced a minimum of 4 hours apart) to one of several different stressors in an unpredictable order. Stressors included: 20-min hypoxia (8% oxygen, 92% nitrogen), 20-min warm (26-30°C) swim, 10-min cold (17-18°C) swim, 1 hour in cold (4°C) room (housed 2 per cage with no bedding), 5-min novel environment, and 1 hour in a cage atop a orbital platform shaker (90 rpm). In addition to the twice-daily stressors, CVS rats were individually housed overnight in large (guinea pig) cages on experiment days 17, 19, 22, and 25 and in small (mouse) cages on experiment days 16, 21, 24, and 27. Approximately 15 hours after the last stress exposure, rats did not receive their respective drink solution and all rats, both control and CVS, were given a novel restraint stress challenge. Rats were placed into well-ventilated restraint tubes (~21 cm in length, with ~5 cm opening) for 20 min. Animals were sacrificed by rapid decapitation without anesthesia at 60 min after the initiation of restraint. Brains were removed and snap frozen in isopentane on dry ice, then stored at -80°C. Brains were sectioned coronally at a thickness of 14 μm on a cryostat (Microm), thaw mounted to slides (Gold Seal Ultrastick Slides, Portsmouth, NH), and stored at -20°C until being pre-treated for in situ hybridization.
As shown previously, the rats that did not receive chronic stress drank sucrose and saccharin in amounts approaching the maximum permitted, whereas rats receiving chronic stress showed markedly reduced saccharin intake (see Figure 1) (Ulrich-Lai et al., 2007).
Figure 1.
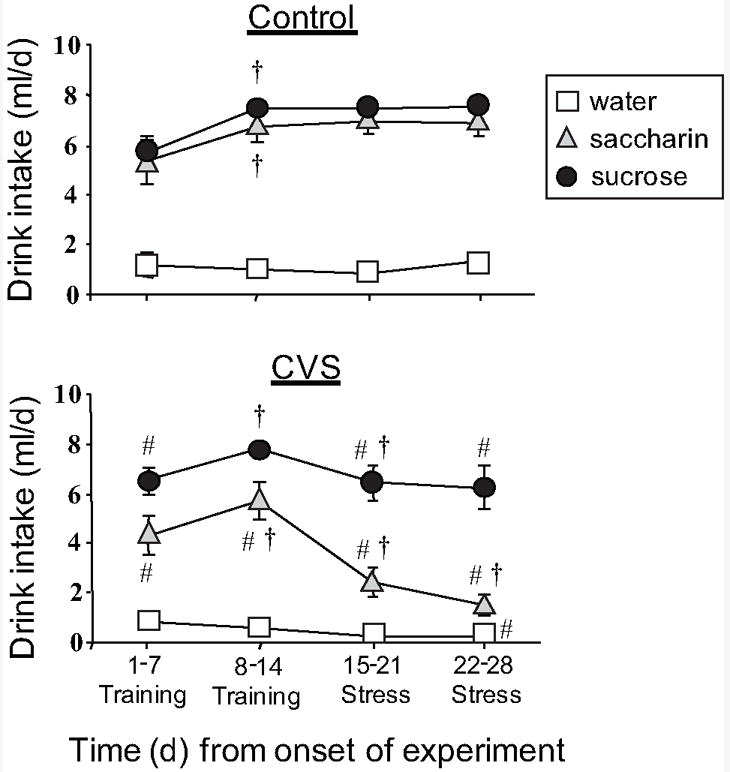
Timecourse of mean daily drink intake of sucrose, saccharin or water (8ml/day max) in control (a) and CVS (b) rats receiving twice daily access to these drink solutions in addition to ad libitum food and water. Palatable drink intake was reduced during chronic stress. All sucrose and saccharin points were different from water (p<0.05). An # indicates p<0.05 vs. water and a cross indicates p<0.05 vs. the previous timepoint by post-hoc analysis. Abbreviations: chronic variable stress (CVS). Graph reproduced from Ulrich-Lai, et al. 2007, Copyright 2007 The Endocrine Society.
Additionally, the chronic stress treatment groups demonstrated thymic involution, a decrease in body weight gain, and the appropriate hormonal responses indicative of a chronic stress condition (Ulrich-Lai et al, 2007).
In situ Hybridization
A one-in-ten series of brain sections was fixed in phosphate-buffered paraformaldehyde (4%) for 10 min, then was rinsed twice for 5-min in DEPC-treated 5mM potassium phosphate-buffered saline (KPBS), followed by two 5-min rinses in KPBS plus 0.2% glycine, and an additional two 5 min rinses in KPBS. The sections were then treated with 0.25% acetic anhydride (in 0.1M triethanolamine, pH8) for 10 min and rinsed twice in 2X saline sodium citrate (SSC) for 5 min before being dehydrated in a graded alcohol series.
Anti-sense cRNA probes for DYN (Curran and Watson, 1995), ENK (Curran and Watson, 1995), GAD65 (Bowers et al., 1998), and GAD67 (Bowers et al., 1998) were in vitro transcribed using 35S-UTP. The transcription reactions consisted of the following: 1X transcription buffer, 62.5 μCi 35S-UTP, 330 μM ATP, 330 μM GTP, 330 μM CTP, 10 μM cold UTP, 66.6 mM dithiothreitol (DTT), 40 U ribonuclease inhibitor, 20 U of the appropriate RNA polymerase (T3 or T7), and 2.5 μg of the appropriate linearized DNA. The reaction was incubated for 1 hour at 37°C, then was DNAse treated, and had tRNA added. Finally, the labeled probe was separated from the free nucleotide by ammonium acetate precipitation.
35S-UTP labeled probes were re-suspended in DEPC water and diluted in hybridization buffer (50% formamide, 20 mM Tris-HCl pH 7.5, 1 mM EDTA, 335 mM NaCl, 1x Denhardt’s solution, 200 μ g/ml herring sperm DNA, 100 μg/ml yeast tRNA, 20 mM DTT, and 10% dextran sulfate) to generate a solution of 100,000 cpm per 50 μl of buffer. Each slide was treated with 50 μl of diluted probe, coverslipped, and incubated overnight at 55°C in chambers humidified with 50% formamide. The next day, coverslips were carefully removed in 2X SSC and slides were rinsed in 2X SSC for 5 min. Slides were transferred into 100 μg/ml ribonuclease A solution and incubated at 37°C for 30 min. Slides were rinsed twice in 2X SSC for 5 min, three times in 0.2X SSC for 10 min, and once in 0.2X SSC at 65°C for 1 hour. Slides were dehydrated through a graded alcohol series and exposed to Kodak Biomax MR-2 film. Sense probes and RNase-treated tissue were run for all probes as negative controls. On each radiograph, an ARC 146-14C standard slide (American Radiolabeled Chemicals, Inc., St. Louis, MO) was included as an internal control to verify that the film exposure was constant between films and was not saturated.
Due to the complexity of the experimental design, all rats in this study received an acute restraint stress one hour prior to tissue collection. Basal levels of ENK, DYN, GAD65 and GAD67 mRNAs are relatively high in all brain regions examined, and show either no, or a slight increase at 60 minutes after an acute stimulus (Dumont et al., 2000) (Bowers et al., 1998, Wang et al., 2003, Lucas et al., 2007). The authors acknowledge that that the rats in the current study were not naive, however these studies suggest that the mRNA levels assessed in the present study are predominantly reflective of expression prior to the acute stress challenge, thereby representing primarily the effects of the chronic palatable drink and stress treatments.
Image Analysis
Semi-quantitative gray-scale densitometric analysis of the x-ray autoradiographs was performed using Scion Image 1.62 software (Scion, Frederick, MD). Anatomical regions of interest were targeted on the basis of known involvement in stress and reward brain circuits, and were delineated on the basis of the Swanson and the Paxinos and Watson rat brain atlases (Paxinos G, 1986, Swanson, 1998). Because recent data suggest differentially involvement of subareas of many brain regions in stress or reward (e.g., BST, nucleus accumbens), areas were parcellated into known subdivisions on the basis of anatomical landmarks defined in the atlases. A region was required to have more than twice the background to be included for analysis. Labeled mRNA was measured in the amygdala (central (CeA), anterior medial (anterior MeA), posterior medial (posterior MeA), and basolateral nucleus (BLA) regions); the NAc (medial shell (NAc medial shell), lateral shell (NAc lateral shell), and core (NAc core) subregions); dorsal striatum; dentate gyrus of hippocampus (DG); hypothalamus (dorsomedial nucleus (DMH), ventrolateral part of the ventromedial nucleus (VMNvl), medial preoptic area (mPOA), anterior hypothalamic nucleus (AHN), lateral hypothalamic area (LHA), and PVN subregions); the zona inserta (ZI); and the bed nucleus of the stria terminalis (anterodorsal subregion (BSTad), anteroventral subregion (BSTav), posteriordosal subregion (BSTpd) and posterioventral subregion (BSTpv)). Background signal was determined from a non-hybridized portion of the tissue in each section, and then subtracted from the total gray level to give corrected gray level. The mean corrected gray level was determined bilaterally from 2-4 sections per region. All image analysis was done blind to treatment and the n for each group ranged from 5-9 animals depending on variations in tissue integrity and availability.
Statistical Analysis
Data are presented as mean corrected gray level ± standard error of the mean (SEM). ENK, DYN, GAD65 and GAD67 mRNA expression was analyzed as described by Ulrich-Lai, et al (2007). Briefly, data from each brain region were analyzed by two-way ANOVA. If there were group differences, Fisher’s least significant comparisons procedure was used to determine specific planned pairwise comparisons; no further adjustments were made to control for the experimentwise error rate. Outliers were removed only if they differed from the mean by more than 1.96 times the SD and they were outside the lower or upper quartiles by more than 1.5 times the interquartile range (McClave, 1994). Statistical significance is reported at p<0.05.
RESULTS
ENK mRNA Expression
There was extensive ENK mRNA labeling in regions of the hypothalamus, striatum, hippocampus, and amygdala as reported previously (Harlan et al., 1987) (see Figure 2a). Sucrose drink reduced ENK mRNA labeling in the anterior BST (Figure 3, BSTad (CVS x drink interaction F(2,48)=3.3, p=0.0479); BSTav (main effect of drink F(2,45)=3.4, p=0.0436, CVS x drink interaction F(2,47)=3.8, p=0.0310)) and the lateral shell of the NAc (Table 1, main effect of CVS F(1,45)=10.8, p=0.0022; main effect of drink F(2,45)=3.4, p=0.0436), and these effects were prevented by chronic stress (Figure 3, Table 1, p<0.05).
Figure 2.
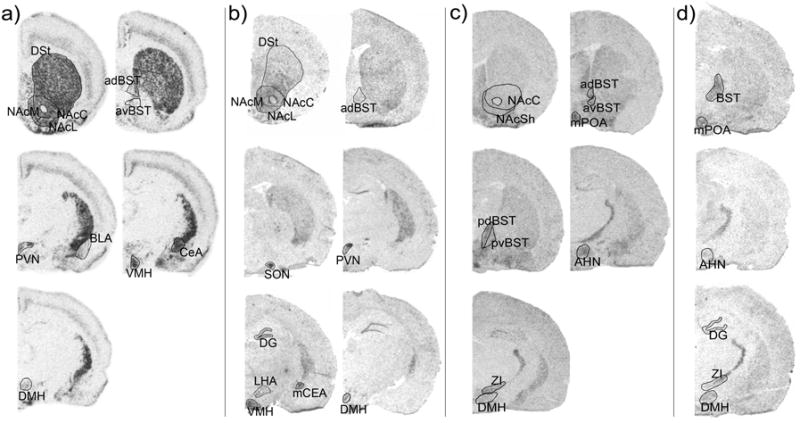
Representative images and regions analyzed for ENK (a), DYN (b), GAD65 (c), and GAD67 (d) mRNA expression by in situ hybridization. Abbreviations: anterior hypothalamic nucleus (AHN), basolateral amygdala (BLA), the bed nucleus of the stria terminalis (BST) (anterodorsal (adBST), anteroventral (avBST), posterior (posteriorBST), posterior dorsal (pdBST), posterior ventral (pvBST), central amygdala (CeA), dentate gyrus (DG), dorsal striatum (DSt), dorsomedial hypothalamic nucleus (DMH), lateral hypothalamic area (LHA), medial amygdala (MeA), medial preoptic area (mPOA), nucleus accumbens NAc (medial shell (NAcM), lateral shell (NAcL), and core (NAcC)), paraventricular hypothalamic nucleus (PVN), ventromedial hypothalamic nucleus (VMH), and zona inserta (ZI). Images for each probe are displayed rostral to caudal from left to right with the most rostral image at the top left.
Figure 3.
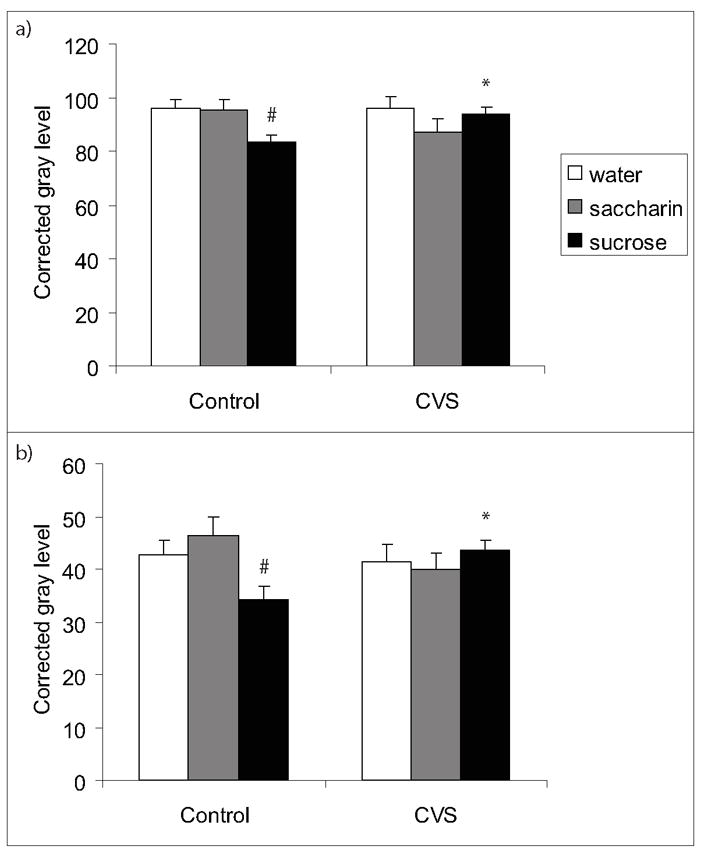
ENK mRNA in the anterior bed nucleus of the stria terminalis (BST). Sucrose drink reduced ENK mRNA expression in the anterior BST and this reduction was prevented by chronic stress in both the anterodorsal area (a) and the anteroventral area (b). An * indicates a significant difference from non-CVS and a # indicates a significant difference from water by post-hoc analysis. Data are shown as mean ± SEM with n=5-9 per group.
Table 1.
Forebrain expression of pro-enkephalin mRNA (corrected gray levels)
| Control
|
CVS
|
|||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| mPOA | 38.66± 3.0 | 39.3 ± 2.5 | 36.0 ± 2.2 | 40.6 ± 3.4 | 39.6 ± 2.4 | 41.8 ± 3.1 |
| AHN | 84.6 ± 5.8 | 90.5 ± 3.6 | 84.7 ± 2.5 | 91.9 ± 4.4 | 87.4 ± 4.1 | 92.8 ± 5.2 |
| Posterior BST | 31.0 ± 1.1 | 31.2 ± 2.8 | 27.8 ± 2.4 | 31.5 ± 3.0 | 29.7 ± 2.5 | 31.0 ± 2.9 |
| Dorsal striatum | 114.9± 2.5 | 123.5± 4.1 | 115.6 ± 3.3 | 118.1 ± 4.3 | 120.2 ± 3.1 | 118.8 ± 2.3 |
| Anterior MeA | 79.3 ± 3.2 | 80.2 ± 3.8 | 72.2 ± 2.3 | 78.0 ± 6.3 | 76.1 ± 3.1 | 82.5 ± 3.5 |
| Posterior MeA | 64.4 ± 3.4 | 61.45± 1.4 | 57.9 ± 4.0 | 61.7 ± 4.8 | 58.9 ± 2.2 | 64.2 ± 4.6 |
| BLA | 55.8 ± 2.4 | 53.9 ± 2.0 | 56.2 ± 3.1 | 60.0 ± 2.9 | 60.5 ± 3.8 | 58.5 ± 3.9 |
| NAc Core a | 102.4± 2.5 | 101.4± 2.1 | 97.8 ± 4.8 | 112.5 ± 3.0* | 116.4 ± 2.4* | 110.7 ± 2.8* |
| NAc Med Shell a | 95.1 ± 4.9 | 95.9 ± 3.2 | 95.8 ± 1.8 | 106.1 ± 3.6* | 109.6 ± 3.0* | 105.3 ± 2.8 |
| NAc Lat Shell ab | 118.1± 4.0 | 123.8± 5.0 | 108.2 ±3.2# | 126.1 ± 2.5 | 126.5 ± 1.0 | 124.7 ± 3.7* |
| CeA a | 125.6± 4.9 | 123.2± 5.5 | 117.4 ± 6.3 | 129.3 ± 3.3 | 130.3 ± 3.5 | 131.1 ± 2.7* |
| PVN a | 87.1 ± 2.2 | 83.2 ± 3.2 | 85.2 ± 4.7 | 91.0 ± 5.1 | 92.1 ± 4.2 | 96.7 ± 6.9 |
| DG a | 55.8 ± 2.4 | 53.9 ± 2 | 56.2 ± 3.1 | 60.0 ± 2.9 | 60.5 ± 3.8* | 58.5 ± 3.9 |
| VMH c | 118.8± 2.5 | 125.4± 3.7 | 115.2 ± 4.2 | 123.9 ± 2.6 | 119.2 ± 1.8 | 124.2 ± 1.6* |
Additional forebrain regions expressing pro-enkephalin mRNA. An a indicates regions with a main effect of stress, a b indicates regions with a main effect of drink, and a c indicates regions with stress/drink interaction p<0.05. An * indicates a significant difference from non-CVS control and a # indicates a significant difference from water control by post hoc analysis. Data are shown as mean ± SEM with n=5-9 per group. List of abbreviations: chronic variable stress (CVS), medial preoptic area (mPOA), anterior hypothalamic nucleus (AHN), posterior bed nucleus of the stria terminalis (posterior BST), anterior medial amygdala (anterior MeA), posterior medial amygdala (posterior MeA), basolateral nucleus of the amygdala (BLA), nucleus accumbens core (NAc core), nucleus accumbens medial shell (NAc medial shell), nucleus accumbens lateral shell (NAc lateral shell), central nucleus of the amygdale (CeA), paraventricular nucleus of the hypothalamus (PVN), dentate gyrus of hippocampus (DG), ventromedial hypothalamic nucleus (VMH).
In contrast, chronic stress induced an overall increase in ENK mRNA in numerous brain regions (Table 1, NAc core (main effect of CVS F(1,42)=24.2, p=0.0001), CeA (main effect of CVS F(1,48)=4.7, p=0.0356), PVN (main effect of CVS F(1,44)=4.5, p=0.0395), and DG (main effect of CVS F(1,47)=7.5, p=0.0088)). Post-hoc analysis revealed that for the NAc lateral shell, CeA, DG, and VMH, the stress-induced increase in labeled ENK mRNA occurred primarily in groups with sucrose or saccharin drink (p<0.05). Conversely, palatable drink (sucrose and saccharin) blunted the effects of chronic stress in the DMH (Figure 4, main effect of CVS F(1,45)=6.5, p=0.0144; main effect of drink F(2,45)=3.8, p=0.0305; CVS x drink interaction F(2,45)=3.7, p=0.0333) and NAc medial shell (Table 1, main effect of CVS F(1,42)=16.1, p=0.0003). ENK mRNA labeling was not affected by palatable snacking or chronic stress in the other examined brain regions (Table 1).
Figure 4.
ENK mRNA in the DMH. Chronic stress increased ENK and this increase was prevented by palatable drink in the DMH. An * indicates a significant difference from non-CVS control and a # indicates a significant difference from water by post-hoc analysis. Data are shown as mean ± SEM with n=5-9 per group.
DYN mRNA Expression
DYN mRNA was labeled in regions of the hypothalamus, striatum, hippocampus, and amygdala as reported previously (Morris et al., 1986, Sato et al., 1991) (Figure 2b). Chronic stress increased DYN mRNA labeling in the NAc core (main effect of CVS F(1,44)=8.2, p=0.0067), and this CVS-induced increase was prevented by sucrose or saccharin drink (Figure 5, p<0.05). In contrast, palatable drink and chronic stress did not affect DYN mRNA labeling in any of the other examined brain regions (Table 2).
Figure 5.
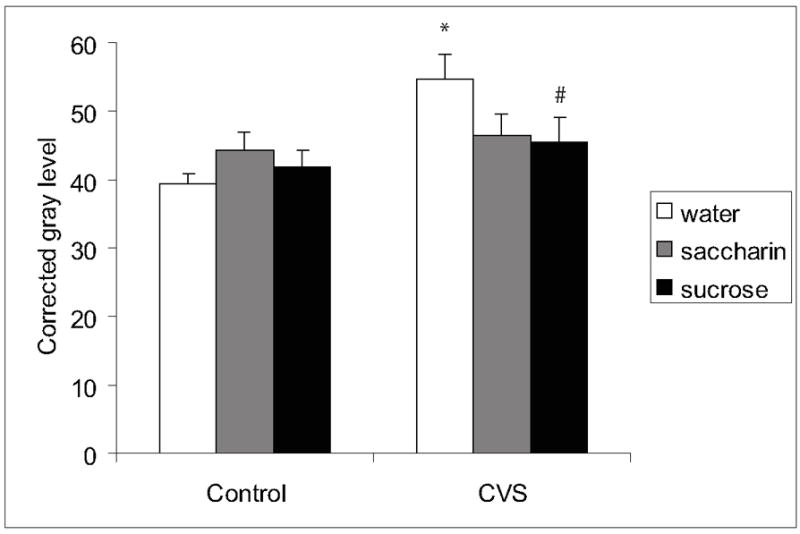
DYN mRNA in the NAc core. Chronic stress increased DYN mRNA expression in the NAc core and this increase was prevented by sucrose or saccharin drink. An * indicates a significant difference from non-CVS control and a # indicates a significant difference from water by post-hoc analysis. Data are shown as mean ± SEM with n=5-9 per group.
Table 2.
Forebrain expression of pro-dynorphin mRNA(corrected gray levels)
| Control
|
CVS
|
|||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| VMH | 54.1 ± 1.7 | 66.0 ± 4.4 | 61.8 ± 3.3 | 60.4 ± 4.5 | 62.2 ± 3.7 | 62.2 ± 4.5 |
| LHA | 39.9 ± 1.5 | 40.0 ± 3.7 | 36.0 ± 1.8 | 37.1 ± 2.5 | 33.6 ± 2.3 | 40.0 ± 2.5 |
| PVN | 54.7 ± 2.7 | 57.0 ± 4.2 | 54.4 ± 2.6 | 54.1 ± 4.6 | 49.6 ± 1.6 | 55.5 ± 4.2 |
| SON | 102.3 ± 6.5 | 87.0 ± 6.5 | 92.9 ± 5.5 | 98.3 ± 6.7 | 98.7 ± 5.2 | 98.6 ± 6.3 |
| NAc Med Shell | 38.8 ± 2.4 | 44.0 ± 3.1 | 43.1 ± 2.0 | 49.3 ± 2.0 | 44.8 ± 4.0 | 43.7 ± 3.0 |
| NAc Lat Shell | 34.8 ± 4.3 | 38.8 ± 2.9 | 39.9 ± 3.2 | 45.9 ± 3.6 | 41.7 ± 3.5 | 40.3 ± 3.5 |
| Dorsal Striatum | 24.5 ± 1.3 | 30.8 ± 2.1 | 26.3 ± 2.5 | 30.4 ± 2.4 | 28.0 ± 2.1 | 29.4 ± 2.8 |
| DG | 37.1 ± 5.0 | 41.5 ± 4.9 | 42.9 ± 2.2 | 43.8 ± 2.8 | 37.5 ± 2.8 | 42.9 ± 1.9 |
| DMH | 40.6 ± 5.9 | 53.2 ± 5.1 | 50.0 ± 4.4 | 53.3 ± 3.9 | 46.4 ± 3.0 | 50.6 ± 5.1 |
| mCeA | 51.0 ± 2.1 | 44.8 ± 3.9 | 43.2 ± 2.9 | 48.6 ± 2.6 | 47.5 ± 3.2 | 54.7 ± 3.3 |
| adBST | 23.0 ± 2.1 | 23.6 ± 3.2 | 26.2 ± 3.7 | 27.8 ± 2.0 | 26.9 ± 3.2 | 32.0 ± 4.1 |
Additional forebrain regions expressing pro-dynorphin showed no differences with stress or access to palatable drink. Data are shown as mean ± SEM with n=5-9 per group. List of abbreviations: chronic variable stress (CVS), ventromedial hypothalamic nucleus (VMH), lateral hypothalamic area (LHA), paraventricular nucleus of the hypothalamus (PVN), supraoptic nucleus (SON), nucleus accumbens medial shell (NAc medial shell), nucleus accumbens lateral shell (NAc lateral shell), dorsal striatum, dentate gyrus of hippocampus (DG), dorsomedial hypothalamus (DMH), medial central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis anterodorsal (BSTad).
GAD65 mRNA Expression
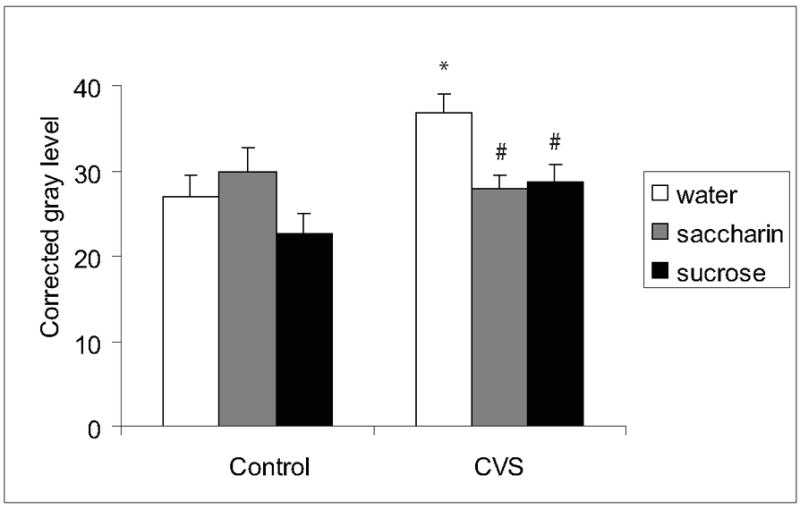
The pattern of GAD65 mRNA labeling observed in hypothalamic, hippocampal, and amygdalar subregions (Figure 2c) matched expression patterns reported previously (Bowers et al., 1998). Palatable drink caused a reduction in labeled GAD65 mRNA levels in the BSTpd that was reversed by chronic stress (Table 3, main effect of CVS F(1,44)=7.7, p=0.0086; main effect of drink F(2,44)=3.7, p=0.0326). In contrast, chronic stress differentially altered GAD65 mRNA levels in a region-specific manner. Chronic stress decreased GAD65 mRNA in the DMH and this effect was prevented by palatable drink (Figure 6, main effect of CVS F(1,46)=10.8, p=0.0020).
Table 3.
Forebrain expression of GAD65 mRNA(corrected gray levels)
| Control
|
CVS
|
|||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| mPOA | 38.6 ± 3.3 | 45.0 ± 2.9 | 40.2 ± 1.3 | 47.5 ± 3.7 | 40.9 ± 3.1 | 42.3 ± 3.4 |
| ZI | 36.3 ± 2.9 | 39.5 ± 1.0 | 33.2 ± 2.1 | 30.9 ± 2.1 | 35.7 ± 1.9 | 35.5 ± 2.6 |
| BSTpd ab | 43.1 ± 1.5 | 42.0 ± 2.0 | 36.9 ± 1.9# | 48.2 ± 2.2 | 43.9 ± 1.6 | 43.5 ± 2.9* |
| BSTpv a | 34.0 ± 1.8 | 34.6 ± 1.9 | 31.0 ± 1.7 | 36.8 ± 1.9 | 34.7 ± 1.6 | 38.4 ± 3.4* |
| BSTad | 26.0 ± 1.2 | 26.8 ± 1.6 | 26.8 ± 1.6 | 26.0 ± 1.6 | 24.3 ± 1.1 | 26.1 ± 1.2 |
| BSTav | 28.1 ± 1.5 | 28.3 ± 1.4 | 27.6 ± 2.3 | 29.3 ± 1.2 | 27.3 ± 1.3 | 31.0 ± 1.1 |
| NAc core | 21.6 ± 1.2 | 24.1 ± 1.1 | 22.1 ± 1.4 | 22.3 ± 1.6 | 22.7 ± 1.5 | 23.1 ± 1.8 |
| NAc shell | 27.2 ± 1.7 | 31.3 ± 1.4 | 29.8 ± 2.6 | 30.5 ± 2.5 | 30.0 ± 1.9 | 31.0 ± 2.5 |
| DG | 25.2 ± 1.7 | 20.5 ± 1.4 | 21.4 ± 2.6 | 23.5 ± 1.2 | 23.1 ± 1.9 | 23.2 ± 1.1 |
Additional regions expressing forebrain pro-dynorphin. An a indicates regions with a main effect of stress, a b indicates regions with a main effect of drink p<0.05. An * indicates a significant difference from non-CVS control and a # indicates a significant difference from water control by post hoc analysis. Data are shown as mean ± SEM with n=5-9 per group. List of abbreviations: chronic variable stress (CVS), medial preoptic area (mPOA), zona inserta (ZI), bed nucleus of the stria terminalis posteriordosal subregion (BSTpd), bed nucleus of the stria terminalis posterioventral subregion (BSTpv), bed nucleus of the stria terminalis anterodorsal subregion (BSTad), bed nucleus of the stria terminalis anteroventral subregion (BSTav), nucleus accumbens core (NAc core), nucleus accumbens shell (NAc shell), dentate gyrus of hippocampus (DG).
Figure 6.
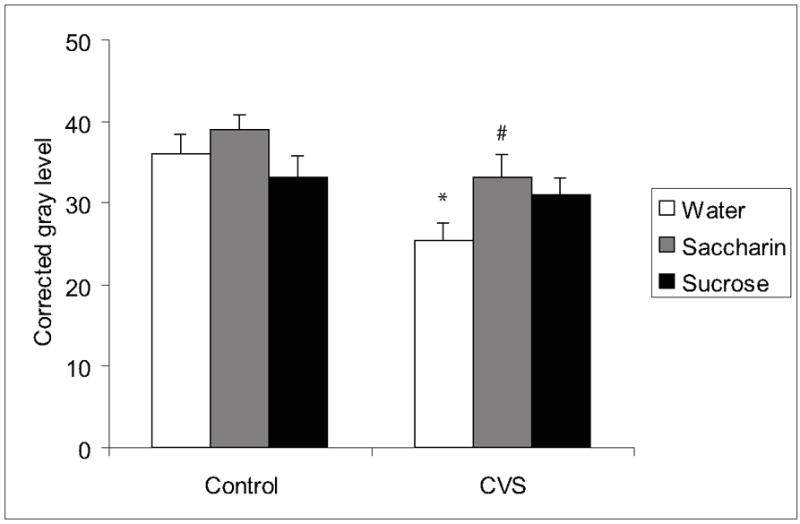
GAD65 mRNA in the DMH. Chronic stress decreased GAD65 mRNA in the DMH and this effect was prevented by palatable drink. An * indicates a significant difference from non-CVS control and a # indicates a significant difference from water by post-hoc analysis. Data are shown as mean ± SEM with n=5-9 per group.
Conversely, chronic stress increased labeled GAD65 mRNA in the AHA (CVS x drink interaction F(2,47)=4.2, p=0.0226), BSTpd (main effect of CVS F(1,44)=7.7, p=0.0086; main effect of drink F(2,44)=3.7, p=0.0326) and BSTpv (main effect of CVS F(1,47)=4.2, p=0.0468), with palatable drink preventing these effects in the AHA (Figure 7, Table 3, p<0.05). GAD65 mRNA labeling was not affected by palatable snacking or chronic stress in the other examined brain regions (Table 3).
Figure 7.
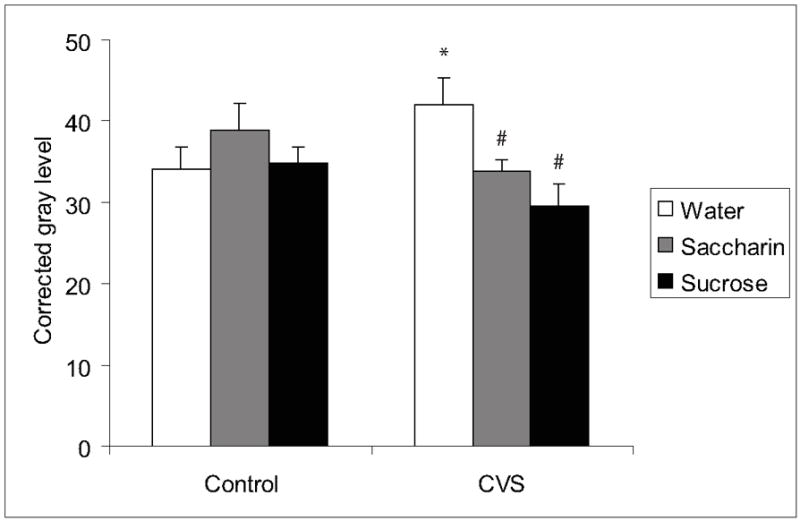
GAD65 mRNA in the AHA. Chronic stress increased GAD65 mRNA in the AHA and palatable drink prevented these effects. An * indicates a significant difference from non-CVS control and a # indicates a significant difference from water by post-hoc analysis. Data are shown as mean ± SEM with n=5-9 per group.
GAD67 mRNA Expression
GAD67 mRNA labeling occurred in limited hypothalamic, hippocampal, and amygdalar subregions (Figure 2d) which matched expression patterns reported previously (Bowers et al., 1998). Neither palatable drink nor CVS altered the labeling of GAD67 in any of the examined brain regions (Table 4).
Table 4.
Forebrain expression of GAD67 mRNA (corrected gray levels)
| Control
|
CVS
|
|||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| mPOA | 40.0 ± 5.9 | 38.3 ± 3.8 | 38.5 ± 7.7 | 36.8 ± 5.9 | 38.0 ± 5.4 | 42.2 ± 5.2 |
| AHN | 28.8 ± 1.5 | 32.9 ± 2.7 | 33.2 ± 1.8 | 29.6 ± 2.5 | 28.4 ± 3.4 | 25.2 ± 1.6 |
| DMH | 27.9 ± 2.8 | 35.6 ± 3.8 | 28.0 ± 1.8 | 30.5 ± 4.1 | 30.4 ± 1.3 | 27.9 ± 1.8 |
| DG | 23.9 ± 1..3 | 23.3 ± 2.2 | 23.3 ± 1.4 | 19.7 ± 2.0 | 29.4 ± 4.3 | 25.4 ± 2.3 |
| ZI | 42.7 ± 2.9 | 38.6 ± 3.5 | 40.3 ± 4.7 | 41.2 ± 4.1 | 35.6 ± 3.8 | 38.7 ± 2.2 |
| BST | 38.7 ± 4.5 | 35.5 ± 3.9 | 29.7 ± 3.8 | 21.3 ± 5.8 | 28.9 ± 2.1 | 39.6 ± 3.7 |
There were no differences in GAD67 mRNA expression after palatable drink access or stress. Data are shown as mean ± SEM with n=5-9 per group. List of abbreviations: chronic variable stress (CVS), medial preoptic area (mPOA), anterior hypothalamic nucleus (AHN), dorsomedial nucleus of the hypothalamus (DMH), dentate gyrus of hippocampus (DG), the zona inserta (ZI); bed nucleus of the stria terminalis (BST).
DISCUSSION
The primary aim of the current study was to assess changes in GABA and opioid gene transcription in specific reward and stress regulatory brain regions following comfort food intake in rats with or without a history of chronic stress. Importantly, these data enabled identification of potential neurochemical mechanisms for comfort food-mediated stress buffering.
Palatable Food
Palatable food intake, on its own, altered mRNA expression of inhibitory neurotransmitters in the NAc and the BST. Sucrose decreased ENK mRNA in the NAc lateral shell, in agreement with previous data (Kelley et al., 2003). Previous studies link increased ENK and DYN transcription with increased neuronal signaling (Giraud et al., 1991, Simpson and McGinty, 1995, Isola et al., 2008). The current data suggest decreased activity in enkephalinergic neurons in the NAc is commensurate with palatable food-induced neuroadaptation in reward brain circuitry (Kelley et al., 2003).
Sucrose decreased GAD65 mRNA in the dorsal portion of the posterior BST. GAD mRNA levels are regulated by GABA agonists in an inverse manner, in that high levels of GABA reduce GAD expression and activity (Rimvall and Martin, 1994, Sheikh and Martin, 1998, Raol et al., 2005, Martyniuk et al., 2007). Taken with the present results, this suggests that sucrose increases GABA release in the BSTpd. The BST is an important integrator of limbic stress input (Choi et al., 2007), and the BSTpd is thought to be stress inhibitory, particularly to acute stress responses (Choi et al., 2007, Choi et al., 2008). Collectively, the data suggest that sucrose-dampening of HPA responses to acute stress (Ulrich-Lai et al., 2007) may be mediated, at least in part, via altered GABAergic tone in the posterior BST.
In some cases, gene expression effects were more pronounced with sucrose than saccharin, suggesting that regulatory changes may be related to either metabolic and/or postingestional properties of sucrose or an enhanced hedonic value of the sucrose solution relative to saccharin (Sclafani and Abrams, 1986) (e.g., calories themselves can increase reward value (Lucas and Sclafani, 1989, Ackroff et al., 2010)).
Chronic Stress
Chronic stress altered expression of inhibitory neurotransmitters in numerous stress and reward brain regions. Specifically, chronic stress increased ENK mRNA in the DMH, NAc shell, CeA, PVN, and DG; increased DYN mRNA in the NAc core; increased GAD65 mRNA in the AHA and BST and decreased GAD65 in the DMH. Chronic stress increased ENK mRNA in the hippocampus and the PVN as described previously (Dumont et al., 2000, Chen et al., 2004). Glucocorticoids can drive ENK mRNA expression in the forebrain (Ahima et al., 1992) suggesting stress-induced signaling in the DMH, NAc shell, CeA, PVN, and DG. Chronic stress increased DYN mRNA expression in the NAc core, agreeing with previous results and suggesting increased signaling in dynorphinergic neurons (Isola et al., 2008). These data suggest that opioidergic neuropeptides may be important in the organization of stress responses (Dumont et al., 2000, Palkovits, 2000, Isola et al., 2008). Chronic stress induced increases in GAD65 mRNA expression in the BST and AHN agrees with previous studies (Bowers et al., 1998). However, unlike previous studies (Bowers et al., 1998), GAD65 mRNA was decreased in the DMH. This discrepancy may be due to differences in the strain of rats or duration of stress exposure. Overall, the changes in GAD65 mRNA suggest that chronic stress may have decreased GABAergic tone in the AHA and BST, and increased it in the DMH.
Importantly, gene expression changes induced by chronic stress were prevented by comfort food intake in the DMH (ENK and GAD65), AHN (GAD65), BSTpd (GAD65) and the NAc core (DYN). The DMH is a known stress regulatory region, and DMH efferents directly target the PVN (Thompson et al., 1996, DiMicco et al., 2002). The DMH mediates changes in HPA axis and autonomic responses to stress (DiMicco et al., 2002) and is held under tonic inhibition by GABAergic neurotransmission (Brandao et al., 1986, Milani and Graeff, 1987, Canteras, 2002, DiMicco et al., 2002). In the DMH, chronic stress decreased GAD65 mRNA expression, which may indicate a chronic stress-induced increase in GABAergic tone (Rimvall and Martin, 1994, Sheikh and Martin, 1998, Raol et al., 2005, Martyniuk et al., 2007). In contrast, ENK mRNA was increased in the DMH. Increases in ENK mRNA are generally thought to be a compensatory increase in synthesis that occurs after periods of high neuronal activity and high peptide release (Giraud et al., 1991, Simpson and McGinty, 1995, Isola et al., 2008), suggesting that chronic stress may increase neuronal activity of both GABA and ENK neurons in the DMH. Conversely, in the AHN, chronic stress increases GAD65 mRNA, consistent with a decrease in GABAergic tone in AHN neurons. Like the DMH, the AHN is also a central component of defensive response circuitry. However, neurons in the AHN respond similarly to appetitive and aversive stimuli, suggesting a role in attentional tuning to emotionally salient encounters (Ono and Nakamura, 1985). The ability of palatable drink to reverse the changes in gene expression in the DMH and AHN, brain regions critical for the HPA, behavioral, and autonomic responses to stress, implicate these regions as likely mediators for comfort food-induced stress buffering.
Sucrose decreased GAD65 mRNA expression in the BSTpd in the absence of chronic stress. However, during chronic stress GAD65 mRNA expression of rats receiving sucrose levels was not different from the water controls. The posterior BST is stress inhibitory, particularly in response acute stressors (Choi et al., 2007, Choi et al., 2008). This suggests that chronic stress overcomes the effects of the palatable drink on gene expression, possibly decreasing GABAergic tone and increasing GAD65 mRNA expression levels similar to water controls.
Chronic stress also increased DYN mRNA expression in the NAc core in water, but not sucrose or saccharin rats, suggesting an increase in NAc activity during chronic stress that is prevented by palatable drink. Stress activates the NAc (Perrotti et al., 2004) and dynorphin-dependent opioid receptor phosphorylation increases in the NAc after stress (Land et al., 2008). In addition, the dynorphin system generally promotes anxiety-like and dysphoric behaviors (Bruchas et al., 2010). Together, the data suggest that the NAc may be another key region in palatable comfort food-mediated stress reduction.
Although palatable drink did not prevent CVS-induced facilitation of the hormonal stress response in these rats (Ulrich-Lai et al., 2007), a history of sucrose decreased the hormonal response to acute stress regardless of chronic stress history, suggesting that altered inhibitory neurotransmission in striatal and hypothalamic brain regions may mediate acute stress-dampening by comfort food. In the chronically stressed rats, the diminished stress-buffering by the saccharin drink may be due, at least in part, to the decrease in saccharin intake after the onset of the CVS paradigm (Ulrich-Lai et al., 2007). However, two weeks of intake prior to CVS, together with a small intake during CVS, is sufficient to induce changes in opioid and GAD mRNA expression in many of the same stress and reward brain regions as sucrose.
Perspectives
The regulation of stress and reward pathways is extremely complex, therefore requiring thorough examination of stress and reward brain regions and, not surprisingly, revealing multiple brain regions affected by chronic stress or palatable snacking. Importantly, there is significant overlap in the stress and reward pathways and the current study demonstrates that palatable “snacks” can prevent neurochemical alterations induced by chronic stress (see summary diagram in Figure 8). These data suggest that engagement of forebrain reward circuits may play a role in inhibiting (buffering) responses of physiological or behavioral effector systems following chronic stress exposure.
Figure 8.
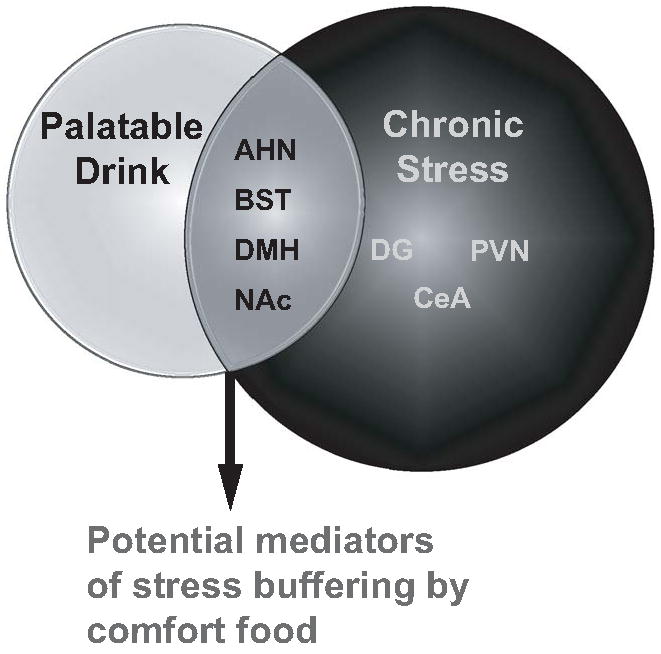
Summary diagram of neuroanatomical changes in response to palatable snacking and chronic stress. In a number of brain regions, shown in the overlapping circles, neurochemical changes induced by chronic variable stress were prevented by palatable snacking, thus identifying these regions as potential mediators of comfort food-induced stress buffering. Abbreviations: bed nucleus of the stria terminalis (BST), nucleus accumbens (NAc), dentate gyrus of hippocampus (DG), dorsomedial hypothalamus (DMH), anterior hypothalamic nucleus (AHN), paraventricular nucleus of the hypothalamus (PVN), central amygdale (CeA).
In summary, the data identify the BST, NAc, DMH, and AHA as brain reward and stress regions that may mediate HPA-dampening following chronic snacking, and suggest that there are clear interactions between brain reward and stress pathways that serve to determine the eventual response to physical or psychological adversity. These findings are consistent with the hypothesis that palatable food-induced alterations in stress-inhibitory neurocircuitry result in a decreased stress response. These observations are in agreement with the “comfort food hypothesis”, suggesting engagement of inhibitory signaling mechanisms in stress regulatory circuits. Understanding the endogenous neural mechanisms by which comfort food buffers the stress response could help improve strategies for the prevention and/or treatment of stress-related disorders and obesity.
Acknowledgments
The authors would like to thank Amanda Jones and Ben Packard for their technical assistance and Colin Kennard for his assistance in preparing the figures. This work was supported by DK059803 (AMC), MH069725 (JPH), MH049698 (JPH), DK067820 (YMU), and DK078906 (YMU).
Footnotes
DECLARATION OF INTEREST The authors have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Garcia MM, Harlan RE. Glucocorticoid regulation of preproenkephalin gene expression in the rat forebrain. Brain Res Mol Brain Res. 1992;16:119–127. doi: 10.1016/0169-328x(92)90201-l. [DOI] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao ML, Di Scala G, Bouchet MJ, Schmitt P. Escape behavior produced by the blockade of glutamic acid decarboxylase (GAD) in mesencephalic central gray or medial hypothalamus. Pharmacol Biochem Behav. 1986;24:497–501. doi: 10.1016/0091-3057(86)90547-2. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Chen JX, Li W, Zhao X, Yang JX, Xu HY, Wang ZF, Yue GX. Changes of mRNA expression of enkephalin and prodynorphin in hippocampus of rats with chronic immobilization stress. World J Gastroenterol. 2004;10:2547–2549. doi: 10.3748/wjg.v10.i17.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33:659–669. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran EJ, Watson SJ., Jr Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. J Comp Neurol. 1995;361:57–76. doi: 10.1002/cne.903610106. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann N Y Acad Sci. 2009;1163:1–16. doi: 10.1111/j.1749-6632.2009.04433.x. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Dufour VL, Arnold MB. Taste of saccharin as sufficient reward for performance. Psychol Rep. 1966;19:1293–1294. doi: 10.2466/pr0.1966.19.3f.1293. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Kinkead R, Trottier JF, Gosselin I, Drolet G. Effect of chronic psychogenic stress exposure on enkephalin neuronal activity and expression in the rat hypothalamic paraventricular nucleus. J Neurochem. 2000;75:2200–2211. doi: 10.1046/j.1471-4159.2000.0752200.x. [DOI] [PubMed] [Google Scholar]
- Giraud P, Kowalski C, Barthel F, Becquet D, Renard M, Grino M, Boudouresque F, Loeffler JP. Striatal proenkephalin turnover and gene transcription are regulated by cyclic AMP and protein kinase C-related pathways. Neuroscience. 1991;43:67–79. doi: 10.1016/0306-4522(91)90418-n. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Shivers BD, Romano GJ, Howells RD, Pfaff DW. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987;258:159–184. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse. 2008;62:448–455. doi: 10.1002/syn.20515. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas F, Sclafani A. Flavor preferences conditioned by intragastric fat infusions in rats. Physiol Behav. 1989;46:403–412. doi: 10.1016/0031-9384(89)90011-5. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108–115. doi: 10.1016/j.brainres.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70:333–342. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Chang JP, Trudeau VL. The effects of GABA agonists on glutamic acid decarboxylase, GABA-transaminase, activin, salmon gonadotrophin-releasing hormone and tyrosine hydroxylase mRNA in the goldfish (Carassius auratus) neuroendocrine brain. J Neuroendocrinol. 2007;19:390–396. doi: 10.1111/j.1365-2826.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- McClave Ja, D F. Statistics. McMillian College Publishing Company, Inc; Edgewood Cliffs, NJ: 1994. [Google Scholar]
- Milani H, Graeff FG. GABA-benzodiazepine modulation of aversion in the medial hypothalamus of the rat. Pharmacol Biochem Behav. 1987;28:21–27. doi: 10.1016/0091-3057(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Haarmann I, Kempter B, Hollt V, Herz A. Localization of prodynorphin messenger rna in rat brain by in situ hybridization using a synthetic oligonucleotide probe. Neurosci Lett. 1986;69:104–108. doi: 10.1016/0304-3940(86)90423-4. [DOI] [PubMed] [Google Scholar]
- Ono T, Nakamura K. Learning and integration of rewarding and aversive stimuli in the rat lateral hypothalamus. Brain Res. 1985;346:368–373. doi: 10.1016/0006-8993(85)90872-8. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M. Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur J Pharmacol. 2000;405:161–166. doi: 10.1016/s0014-2999(00)00549-5. [DOI] [PubMed] [Google Scholar]
- Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000;80:142–152. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, W C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1986. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1356–1365. doi: 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60:1039–1042. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience. 2005;132:399–407. doi: 10.1016/j.neuroscience.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Morita Y, Saika T, Fujita M, Ohhata K, Tohyama M. Localization and ontogeny of cells expressing preprodynorphin mRNA in the rat cerebral cortex. Brain Res. 1991;541:41–49. doi: 10.1016/0006-8993(91)91071-8. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Abrams M. Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav. 1986;37:253–256. doi: 10.1016/0031-9384(86)90228-3. [DOI] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res. 2008;64:97–105. doi: 10.1016/j.jpsychores.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Sheikh SN, Martin DL. Elevation of brain GABA levels with vigabatrin (gamma-vinylGABA) differentially affects GAD65 and GAD67 expression in various regions of rat brain. J Neurosci Res. 1998;52:736–741. doi: 10.1002/(SICI)1097-4547(19980615)52:6<736::AID-JNR12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JN, McGinty JF. Forskolin induces preproenkephalin and preprodynorphin mRNA in rat striatum as demonstrated by in situ hybridization histochemistry. Synapse. 1995;19:151–159. doi: 10.1002/syn.890190302. [DOI] [PubMed] [Google Scholar]
- Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–848. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol. 1995;268:R183–191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1998. [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Wu SX, Liu XY, Wang W, Li YQ. Effects of c-fos antisense oligodeoxynucleotide on 5-HT-induced upregulation of preprodynorphin, preproenkephalin, and glutamic acid decarboxylase mRNA expression in cultured rat spinal dorsal horn neurons. Biochem Biophys Res Commun. 2003;309:631–636. doi: 10.1016/j.bbrc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


