Tumor cells adapt many ways to escape immune surveillance, resulting in immune tolerance to the tumor. Not uncommonly, tumor cells produce factors that direct the immune cells in the stroma to tolerate the tumor. A new study by Shields et al. introduces one major effector of tumor cell tolerance, CCL21, a CC chemokine that binds and activates the chemokine receptor, CCR7 (Shields et al.) CCL21 is normally expressed in the lymph node stroma to guide CCR7+ naïve T cells and mature dendritic cells, but it is also used to activate T regulatory (TReg) cells which are the major regulators of tolerance. Shields et al. show that tumors can use CCL21 to trigger a cascade of events that leads to tolerance. For example, B16-F10 melanoma cells constitutively express CCL21, which recruits CCR7+ TReg+ cells as well as antigen presenting APCs and other leukocytes, into the tumor microenvironment. Depending on the level of expression of CCL21 (high or low), a tumor tolerant or a tumor suppressive T cell phenotype develops. In the absence of CCR7 expression in the host – or CCR7 activation by CCL21 – tumors grow more slowly. CCL21 affects the host response to the tumor through paracrine interactions, rather than autocrine effects of CCL21 directly on the tumor itself.
CCL21Low tumors had a higher population of macrophages that differentiate into the anti-tumorigenic phenotype, as characterized by increased levels of secretion of IFN-γ, IL-2 and IL-4. Alternatively, CCL21High tumor cells created a microenvironment that was high in TGF-β1. TGFβ1 is known to regulate tumor tolerance by suppressing antigen-specific CD8+ T cell function, promoting TRegs, and shifting the macrophage from a tumor suppressing M1 phenotype to tumor promoting M2 phenotype. Not surprisingly, this was accompanied by increased CCR7 dependent recruitment of CD11b+CD11c F/4/80-Grhigh myeloid-derived suppressor cells (MDSCs). Thus, CCL21 High tumors grew larger than CCL21Low tumors. Surprisingly however, when athymic Foxn1nu/nu mice that are lacking T cells were the recipient of tumor xenografts, there was no growth disadvantage for the CCL21Low group. Since the CCL21Low tumors were defective in recruitment of MDSCs (CD11b+CDLLe+F4/80-Gr1high cells) the suggestion is that CCL21 overexpression prevents the tumor suppression by cytotoxic T cells that should normally take place.
To explore the type of T cells involved in the CCL21 mediated tumor growth, Shields et al., show that the peripheral margins of CCL21High tumors undergo lymphoid node-like stromal changes, including high expression of the tumor suppressor indoleamine 2,3-dioxygenase, complement receptor 1-related gene/protein-y that maintains self-tolerance and inhibits anti-tumor immunity. In fact, the authors were able to rescue non-syngeneic B16-CCL21High melanoma tumor allografts from rejection in BALB/C, 129/P2 and S2 mice. This finding may provide a very useful tool for translational studies using human tumors. Moreover, this finding of a CCL21-directed formation of a lymph node like stroma surrounding the tumor which promotes tolerance in the host immune response is truly exciting. The mechanism for this CCL21 directed shift in T cell populations likely involves recruitment of naïve T cells to peripheral sites in the tumor, a differentiation of the naïve T cells into TReg, and induction of effector T cell senescence, with the latter two events resulting in part from the creation of a peripheral stroma around the tumor as a consequence of CCL21/CCR7 mediated changes in gene expression.
Another recent paper by Kim et al. (Kim et al., 2009) demonstrates that expression of CXCL1 by MDA-231 breast cancer cells plays an important role in self-seeding of tumor cells through the recruitment of CD45+ cells into the stroma of seeded MDA231 tumors. In this elegant study, GFP-tagged circulating tumor cells (CTCs) derived from primary contra lateral lesions or from metastatic lesions were observed to ‘seed’ a primary contra lateral lesion. The properties of these ‘self-seeding’ tumor cells were examined by determining the gene expression profile of the self seeding cells and the effects of seeding by these CTCs on growth, angiogenesis, and leukocyte recruitment of contra-lateral lesions. Kim et al. observed that IL-6 and IL-8 produced by the tumor cells attracted the CTCs to infiltrate and ‘seed’ the tumor, while MMP1 and fascin-1 facilitated the infiltration of CTCs into the tumor. These self-seeded tumors grew faster, likely due to enhanced angiogenesis and enhanced recruitment of leukocytes of the neutrophil and macrophage lineage (CD45+CD11b+ and CD45+neut+).
Of interest, there was a 9-fold increase in the expression of the neutrophil and macrophage chemoattractant, CXCL1, in the seeder cell lines established from the seeded tumor. While knocking down CXCL1 expression in tagged-GFP-tagged MDA-231 seeder tumor cell populations did not alter the migration across the endothelium of tumor cells, or the efficiency of seeding established tumors, knockdown of CXCL1 did markedly reduce the infiltration of CD45+ leukocytes into the tumor mass. However, the effect of knock-down of CXCL1 in the CCTCs on tumor growth was not reported. However, it can be concluded that expression of chemokines by CTCs facilitates seeding and re-growth of tumor and thus may be a crucial factor in the local recurrence at the site of a previously excised tumor lesion.
We have recently come to understand that expression of chemokines by tumor cells plays a crucial role in the growth of the tumor. While certain chemokines recruit leukocytes into the tumor microenvironment that are pro-tumorigenic, others recruit leukocytes that antagonize tumor growth (Andreu et al., 2010, Denardo et al., 2009, 2008; Fridlender et al., 2009; Kim et al., 2009, Shields et al.). It will be important to understand better the properties of the recruited neutrophils and macrophages in the work described by Kim et al. Presumably the macrophages are the M2/arginase+ type and the neutrophils are the pro-tumorigenic N2 type of neutrophils, since the tumors that recruited these leukocytes grew faster than the primary tumor grew. Chemokines also play an important role in the recruitment of CTCs to distant target organs. For example expression of CXCR4 by breast cancer cells is a key factor in modulating metastasis to lung, lymph node, bone and liver (Li et al., 2004).
Clearly, learning how to appropriately antagonize the inflammatory chemokines will be important for the future of cancer chemotherapy. Moreover, future work needs to be directed to learning how to shift the make-up of the infiltrating leukocytes from one that is tumorigenic and tumor growth promoting to one that is cytotoxic for tumor cells. Is it possible to convert an M2 macrophage to an M1 macrophage by shifting the gene expression profile? Is it also possible to switch N2 neutrophils to N1 phenotype, and Th2 lymphocytes to the Th1 phenotype by altering their gene expression profile? Alternatively, reversion from a tumor promoting to a tumor fighting leukocytic infiltrate may turn out to be much more complex. Either way, these questions need to be answered with haste if we are to provide better therapies for cancer patients.
Figure 1.
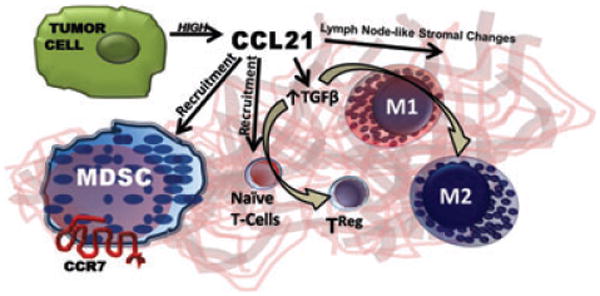
The paper by Shields et al. shows that tumor cells use CCL21 induce a cascade of events that leads to tolerance, including stromal expression of TGFβ and alterations in the peripheral tumor stroma to one that is lymph-node-like. This results in conversion of naïve T cells to TRegs, and recruitment of MDSCs. Both TRegs and MDSCs contribute to the maintenance of self tolerance, reduced tumor surveillance, and enhanced tumor growth.
Figure 2.
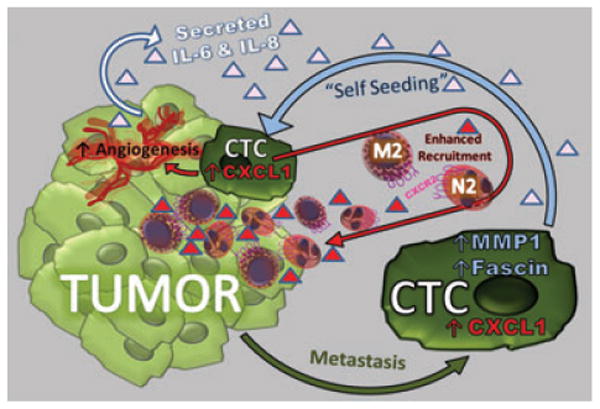
Kim et al. show that when circulating tumor cells (CTCs) re-seed the tumor, they express high levels of CXCL1, which recruits tumor promoting myeloid cells into the tumor and enhances angiogenesis. CTCs also exhibit enhanced expression of fascin and MMP1 which facilitate their invasive properties, resulting in greater infiltration into the tumor bed. Production of IL-6 and IL-8 by the tumor provides important attractants for recruitment of the CTCs into the tumor.
References
- Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denardo DG, Johansson M, Coussens LM. Inflaming gastrointestinal oncogenic programming. Cancer Cell. 2008;14:7–9. doi: 10.1016/j.ccr.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Denardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]


