Abstract
Keloids are benign collagenous tumors that occur during dermal wound healing in genetically predisposed individuals. The lesions are characterized by over-proliferation of fibroblasts, some leukocyte infiltration, and prolonged high rates of collagen synthesis. To determine whether leukocyte chemoattractants or chemokines are participating in this disease process, immunohistochemical staining for the CXC chemokine, MGSA/GROα, and its receptor, CXCR2, was performed on tissue from keloids, hypertrophic scars and normal skin. Immunoreactive MGSA/GROα was not observed in hypertrophic scars or normal dermis, but was present in some myofibroblasts and lymphocytes in nodular areas of the keloid samples. This staining positively correlated with the degree of inflammatory infiltrate in the lesions. Keloids, but not hypertrophic scars or normal dermis, also exhibited intensive immunoreactivity for the CXCR2 receptor in endothelial cells and inflammatory infiltrates with occasional staining of myofibroblasts. In contrast, cultured fibroblasts from either keloids or normal skin did not express detectable amounts of mRNA for MGSA/GRO or CXCR2, although interleukin-1 strongly induced MGSA/GRO mRNA in both cell types. Interleukin-1 induction of MGSA/GRO was inhibited by glucocorticoid in normal and keloid fibroblasts, and the effect was more pronounced in keloid fibroblasts. This event was not correlated with inhibition of nuclear activation of NF-κB, AP-1 or Sp1, and might therefore be mediated by another mechanism such as decreased mRNA stability or transcriptional repression through the glucocorticoid response element in the MGSA/GRO promoter. Data from in vitro wounding experiments with cultured normal and keloid fibroblasts indicate that there were no significant differences in MGSA/GRO or CXCR2 receptor levels between normal and keloid fibroblasts. We also show that cultured keloid fibroblasts exhibit a delayed wound healing response. We postulate that the inflammatory component is important in development of keloid lesions and chemotactic cytokines may participate in this process.
Keloids are benign collagenous tumors that form in the reticular layer of the dermis during a prolonged wound healing process in persons with a genetic predisposition.1,2 Keloids occur predominantly in Black and Asian populations. The altered tissue repair mechanism appears to be restricted to dermal wound healing, because other growth or connective tissue abnormalities are not frequently reported in keloid patients. The disorder may be genetically heterogeneous, with both dominant and recessive modes of inheritance having been reported. Genetically susceptible individuals form keloids after wounding. Abnormalities in cell migration, proliferation, inflammation, synthesis and secretion of extracellular matrix proteins and cytokines, and remodeling of the wound matrix have all been described in keloids.3,4 Black patients with keloids often exhibit increased activity of fibrogenic cytokines5,6 as well as an altered cytokine profile.7 The exaggerated wound healing process in keloids appears to be due in part to loss of glucocorticoid suppression of collagen and elastin gene expression in cells derived from these lesions.8,9
Because glucocorticoids also suppress the activation of NF-κB, decreased glucocorticoid suppression in keloid lesions could potentially lead to enhanced NF-κB dependent cytokine gene transcription10 and thus significantly alter the wound healing profile within these lesions. The chemotactic cytokines melanoma growth stimulatory activity/growth-regulated protein (MGSA/GRO) and interleukin-8 (IL-8), are regulated in part by NF-κB, in cooperation with AP-1, Sp1 or other transcription factors.11–15 Glucocorticoids have been shown to suppress the expression of MGSA/GRO homologs in rat fibroblasts.16 Moreover, synovial fibroblasts cultured from patients with rheumatoid arthritis, another fibro-proliferative disease, express receptors for MGSA/GRO.17 We have shown that the expression of MGSA/GRO and its receptor is temporally increased during the wound healing process.18 Based upon these findings, we proposed that chemokine and chemokine receptor expression might be exaggerated in keloid lesions.
To test this hypothesis we examined the expression of the chemokine, MGSA/GRO, and its receptor, CXCR2, in keloid lesions as compared to hypertrophic scars, and normal skin, as well as the endogenous mRNA expression of MGSA/GRO and its receptor, CXCR2, in cultured fibroblasts from normal skin and keloid lesions. The effects of glucocorticoids on the IL-1 activation of nuclear NF-κB and AP-1 complexes in fibroblasts cultured from keloid lesions and normal skin using gel shift assay with probes for NF-κB and AP-1 as compared to the noninducible transactivator, Sp1, were determined. Lastly, the expression of CXCR2 and MGSA/GRO in cultured keloid and normal fibroblasts that have been subjected to in vitro wounding and the in vitro wound closure rates for these cultured keloid and normal fibroblasts was assessed.
MATERIALS AND METHODS
Actively growing keloid tissues (N = 10), hypertrophic scars from traumatic linear scars greater than 2 years of age (N = 10) and normal skin (N = 5) were collected from patients undergoing elective excision of keloids, scar revisions or abdominoplasty procedures. Tissue samples were obtained in accordance with procedures approved by the Institutional Review Board. None of the keloids had received corticosteroid injection within a one-year period and all were removed from the truncal region.
Tissue processing
Whenever possible, specimens were divided with a portion homogenized for RNA preparations and the other portion fixed in 4% paraformaldehyde. After 18 hours of fixation, the tissues were embedded in paraffin, sectioned, and sections used for immunohistochemical analyzes. Sections were deparaffinized, endogenous peroxidase activity was quenched for 20 minutes in a 3% H2O2/methanol solution, and preincubated in normal 10% porcine serum (Dako, Carpinteria, CA) for 20 minutes. Sections were incubated overnight in a humidified chamber at 4°C in rabbit antiserum (Dako, Carpinteria, CA) for MGSA/GROα at a concentration of 2 mg/ml as previously described.18 For CXCR2 immunostaining, samples underwent antigen retrieval at neutral pH (Biogenix, San Ramon, CA) by microwave irradiation before overnight incubation with antibody against CXCR2 at a concentration of 660 ng/ml. For identification of myofibroblasts, sections were incubated in goat anti-human α-smooth muscle actin antisera (C-11; Santa Cruz Technologies, Santa Cruz, CA). Negative controls included sections incubated in identical concentrations of nonspecific rabbit immunoglobulin (Dako, Carpinteria, CA) G or phosphate buffered saline solution (PBS) substituted for the primary antisera. All sections were further reacted with the reagents in a commercial avidin-biotin staining kit (Dako, Carpinteria, CA). Immunoreactive sites were visualized with 3,3-diaminobenzidine as the chromogen (Biogenex). Sections were rinsed in water, counterstained with hematoxylin, dehydrated, and cover-slipped. Images were captured with an Olympus AH Vanox light microscope (Melville, NY) interfaced to a Kontorn Eliktronik 3008 camera (Eching, Germany). Images were arranged through the use of Adobe Photoshop software.
Cell cultures
Tissue sources and methods of isolation, propagation and freezing of fibroblasts have been reported.19 Normal fibroblast cell strains 21, 116, 130, 131 and 170 and keloid fibroblast cell-lines 33, 50, 124, 125 and 261 were used in these studies. Cell-lines were routinely tested for Mycoplasma infection by the Hoechst staining method and were found to be negative. Normal and keloid fibroblast cell strains, at passage numbers under 6, were grown to about 80% confluence in Hams F-10 medium (GIBCO-BRL Life Technologies, Rockville, MD) supplemented with 10% fetal bovine serum (GIBCO-BRL Life Technologies, Rockville, MD). Wherever indicated, 24 hours prior to IL-1 treatment the medium was replaced with Hams F-10 medium containing 10% fetal bovine serum and 10 units/ml (50 ng/ml) hydrocortisone (Sigma Chemical Co., St. Louis, MO). For some experiments, 3 hours prior to harvesting, the medium was again replaced with Hams F-10 medium plus 10% fetal bovine serum and 5 units/ml IL-1 (1 ng/ml; R & D Laboratories, Minneapolis, MN).
Preparation of nuclear extracts
The procedures of Dignam et al.20 were followed for preparation of nuclear extracts with modification. Approximately 1 × 106 cells were rinsed twice in 1X PBS and scraped in 1X PBS + 2 mM ethylenediamine tetra-acetic acid (EDTA). Cells were suspended in Buffer A, comprised of 25 mM HEPES-NaOH [pH 7.9], 10 mM NaCl, 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), then were lyzed in Buffer A + 1% NP-40 with frequent vortexing on ice for 10 minutes. Nuclei were sedimented with a brief spin in a microfuge (12,000 r.p.m.) and suspended in Buffer A + 1 M sucrose. After centrifugation (12,000 r.p.m.), the nuclear pellet was rinsed once in Buffer C (Buffer A + 20% glycerol). Nuclear proteins were extracted in Buffer C containing 400 mM NaCl for 30 minutes on ice. The nuclear extract was cleared of debris by a 5-minute centrifugation (12,000 r.p.m.) at 4 °C. Nuclear extracts were dialyzed against 25 mM Hepes-NaOH [pH 7.9], 100 mM NaCl, 0.1 mM EDTA, 0.1 mM PMSF and 1 mM DTT. Protein was estimated by the BioRad dye binding method.
Preparation of cellular RNA
This protocol was essentially as described by Chomczynski and Sacchi.21 Approximately 1 × 106 cells were rinsed in 1 × PBS. Cells were scraped in 1 ml of a 1 : 1 mixture of guanidium isothiocyanate and phenol, collected in microcentrifuge tubes and extracted with 0.5 volumes of chloroform. The extracted RNA was precipitated in ethanol. RNA pellets were washed in 70% ethanol prior to drying and solubilizing in RNase free water. RNA was estimated by absorbance at 260 nm.
Electrophoretic mobility shift analysis
This protocol is a modification of the one described by Chodosh et al.22 About 10 ng of nuclear extracts from fibroblast cell lines was preincubated with 2 mg of a cold nonspecific competitor poly dI.dC: poly dI.dC in a buffer containing 20 mM Hepes-NaOH (pH:7.6), 50 mM NaCl, 0.2 mM EDTA (pH:8), 1 mM DTT and 2% glycerol for 15 minutes at room temperature. The reactions were incubated for an additional 20 minutes after the addition of 40,000 cpm of 32P end-labeled oligonucleotide probe corresponding to the consensus binding sites for NF-κB (5′ AGTTGAGGGGACTTTCCCAGGC 3′), AP1 (5′ CGCTTGATGAGTCAGCCGGAA 3′) or Sp1 (5′ ATTCFGATCGGGGCGGGGCGAG 3′) transcription factors. The combined nuclear extract and labeled probe was then electrophoresed through a 6% native polyacrylamide gel until the tracking dye reached the bottom of a 16-cm long gel. Gels were dried and exposed for autoradiography.
Northern blot analysis
Twenty to 25 micrograms of RNA/sample was mixed in loading dye containing 50% formaldehyde, 0.025% ethidium bromide, 0.01% xylene cyanol, 0.01% bromophenol blue, heated at 65 °C for 5 minutes and loaded on a 1.5% formaldehyde-agarose gel. Samples were electrophoresed until the lower tracking dye reached the bottom of a 10-cm gel. After electrophoresis, 28S and 18S RNA species were visualized by UV-trans-illumination to ensure equal loading. The gel was soaked in 10X Saline sodium chloride solution (SSC) for 10 minutes, according to Maniatis et al.23 RNA was transferred overnight to nitrocellulose by capillary transfer and efficiency of transfer was confirmed by viewing the blot on a transilluminator. The blot was allowed to dry and then baked for 2 hours in vacuo. The blot was prehybridized at 42 °C overnight in hybridization buffer containing 2X SSC, 1X Denhardt’s solution, 50% formamide, 200 mg/ml salmon sperm DNA and 0.1% SDS.23 Blots were hybridized overnight, to 108 cpm of 32P labeled probes generated by random-prime labeling of cDNA corresponding to either MGSA, IL-8 or interferon-8 inducible protein-10 (IP-10) and CXCR2, with cyclophilin as an internal control. Blots were washed twice with 2X SSC, twice with 0.2X SSC, and twice with 0.1X SSC. All washes were of 200 ml each, contained 0.1% SDS, and were for 20 minutes each at 50°C. Blots were dried and exposed to film for autoradiography. For graphical representations, autoradiographs were densitometrically scanned on ImageQuant software (Molecular Dynamics, Sunnyvale, CA) and values normalized against those of cyclophilin mRNA.
RESULTS
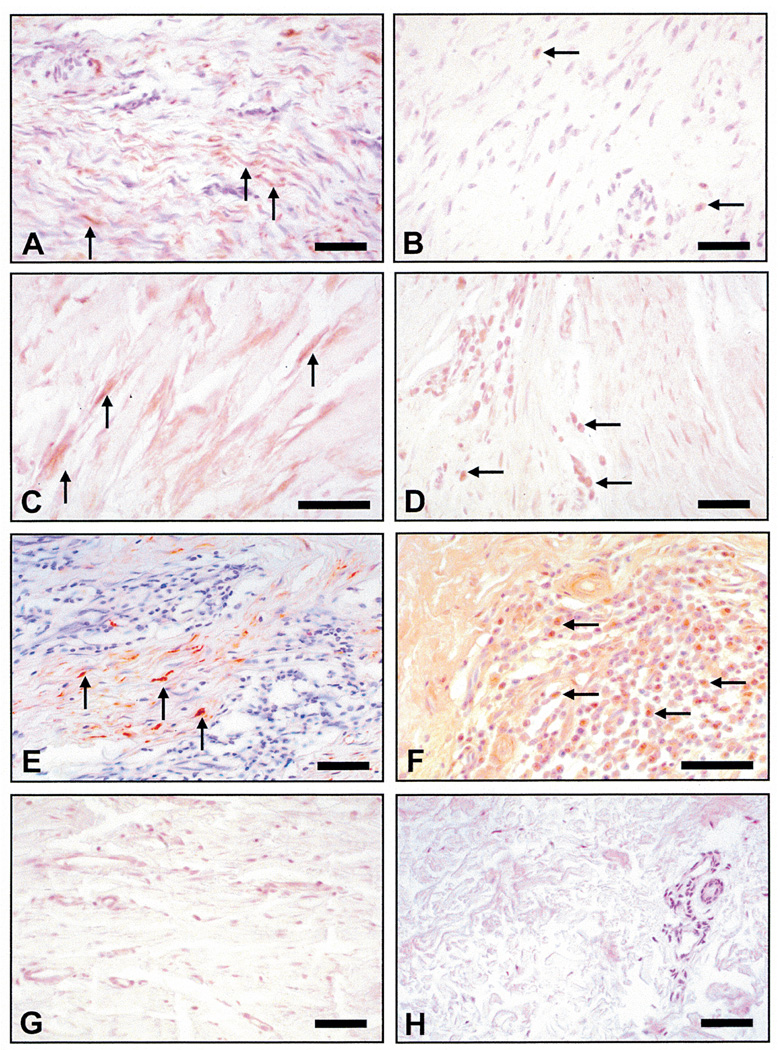
Keloid tissues displayed a wide spectrum of immunoreactive staining patterns for MGSA/GRO. In approximately half of the keloid samples examined in this study, the fibroblastic/myofibroblastic population showed MGSA/GRO reactive cells in 40–60% of these cells (Figures 1A, C and E). The remaining keloid lesions either showed a few MGSA/GRO positive cells with modest immunoreactivity (Figures 1B and D) or little or no staining (Figures 1B and D). While we initially hypothesized that expression of this chemokine would be highest in those cells at the periphery of these ever expanding lesions, this expected pattern was not observed in any of the lesions examined. Instead, the spatial localization for MGSA positive fibroblasts/myofibroblasts appeared to correlate best with the presence of inflammatory foci (Figures 1E and F). In addition, this chemokine was also detected in roughly 50% of the infiltrating inflammatory cells (mostly lymphocytes, judging by the cytoplasmic to nuclear size ratio)(Figure 1F).
Figure 1.
Immunohistochemical staining for MGSA/GROα. (A) Region within a keloid showing numerous immunoreactive (brown) fibroblasts/myofibroblasts (arrows); (B) Another keloid where immunoreactivity is present in very few cells (arrows). (C) This keloid shows intense staining for MGSA in cells with an elongated fibroblastic/myofibroblastic phenotype (arrows). (D) A keloid showing minimal staining for MGSA in fibroblasts but strong immunoreactivity for lymphocytes (arrows). (E) A keloid showing an intensive inflammatory focus with positive staining in the majority of myofibroblasts (arrows). (F) This keloid with an intensive inflammatory focus reveals that approximately 50% of the inflammatory cells show immunoreactivity for MGSA (arrows). (G) A representative hypertrophic scar from a 20-year-old Caucasian male shows minimal to no staining for the ligand). (H) Normal skin from a 20-year-old Caucasian female excised from an equivalent region of deeper reticular dermis shows no immunoreactivity for ligand in the dermal cell populations). All size bars indicate 50 µm, except Figure 1(H), which is 100 µm.
In the absence of a definitive marker for either the fibroblast or myofibroblast population, it was difficult to leukodetermine with certainty that the elongated MGSA/GRO positive cells were indeed myofibroblasts or simply fibroblasts. Our presumptive identification of fibroblasts/myofibroblasts is based on several studies that have established that these highly differentiated fibroblasts often contain an abundance of α-smooth muscle actin filaments.24–26 Within the keloids examined in the present study, many of these highly elongated cells with MGSA/GRO immunostaining also showed α-smooth muscle actin immunoreactivity, leading us to conclude that there is a great variability among keloid lesions but that some hyfibroblasts/myofibroblasts do contain this chemokine.
MGSA/GRO positive cells were not detected in the adjacent margins of normal dermis that were removed during the excisional procedure. MGSA/GRO immunoreactivity was not detected within the dermal cell populations present in either hypertrophic scars (Figure 1G) or cell populations within the papillary or reticular dermis of normal skin removed from nonkeloid forming individuals (Figure 1H).18
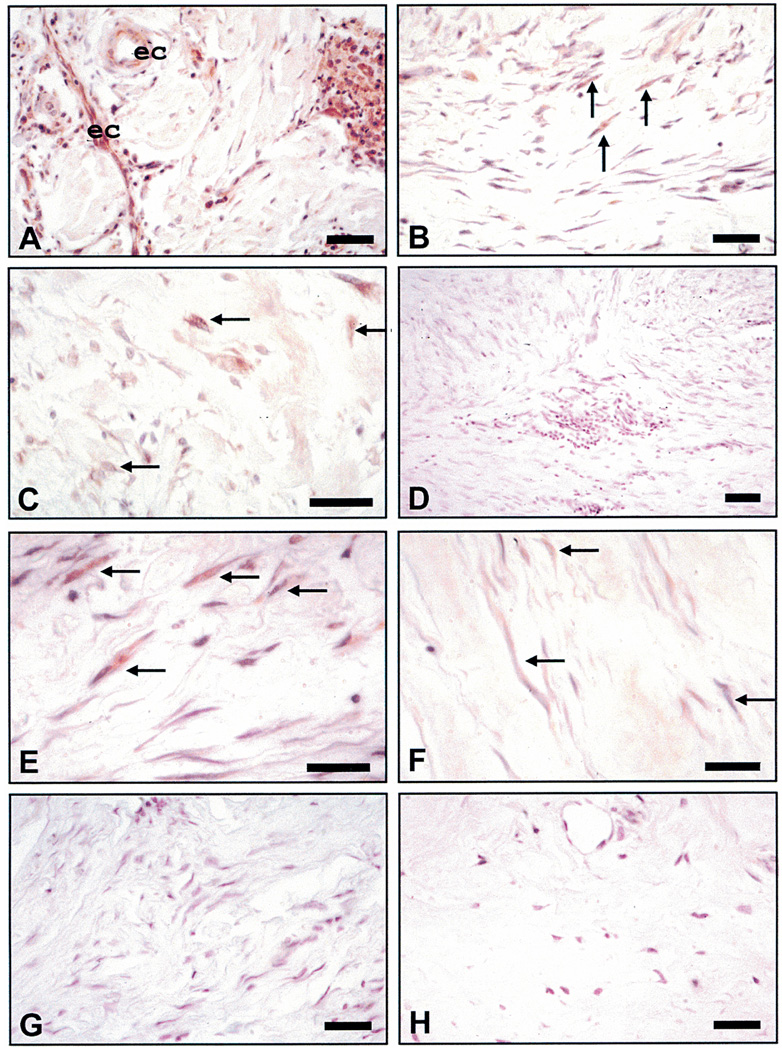
Immunostaining for CXCR2 in keloids, hypertrophic scars, and normal skin
Keloid tissues exhibited a somewhat different pattern of immunoreactive sites for the CXCR2 type of receptor. In several lesions, this receptor was present on endothelial cells lining capillaries and inflammatory infiltrates (Figure 2A). Myofibroblasts also occasionally exhibited CXCR2 immunoreactivity in some (Figures 2B and C) but not all keloid tissue samples (Figures 2D and F). In contrast, the keloid tissue shown in Figure 2E showed robust CXCR2 immunoreactivity in cells with a fibroblastic/myofibroblastic phenotype. Hypertrophic scars showed minimal to no staining for the CXCR2 receptor (Figure 2G). Normal skin from an equivalent area of deep dermis also showed no immunoreactivity for receptor within the dermal population (Figure 2H). Results from immunohistochemistry suggest that in some lesions, a small population of keloid fibroblasts express the MGSA/GRO ligand. Sizeable numbers of fibroblasts/myofibroblasts also express the CXCR2 receptor and may respond to chemokines produced by infiltrating leukocytes. Taken together these data suggest that this ligand and its receptor may play a role in the unwanted dermal proliferation/stimulation that is the hallmark of keloid formation.
Figure 2.
Immunohistochemical staining for CXCR2. (A) Keloid showing intensive immunoreactivity for receptor in endothelial cells (ec) and inflammatory infiltrates (arrows). (B) A keloid showing immunoreactivity for receptor in multiple myofibroblasts. (C) A keloid showing moderate immunoreactivity for the receptor in only a few of the fibroblasts/myofibroblasts (arrows). (D) A keloid exhibiting little or no evidence that the receptor is expressed. (E) A keloid showing robust immunoreactivity in cells with a fibroblastic/myofibroblastic phenotype. (F) A keloid showing minimal immunoreactivity in the fibroblast population (arrows). (G) A representative hypertrophic scar showing minimal to no staining for the receptor. (H) Normal skin from an equivalent area of deep dermis showing no immunoreactivity for CXCR2 in dermal cell population. All size bars indicate 50 µm.
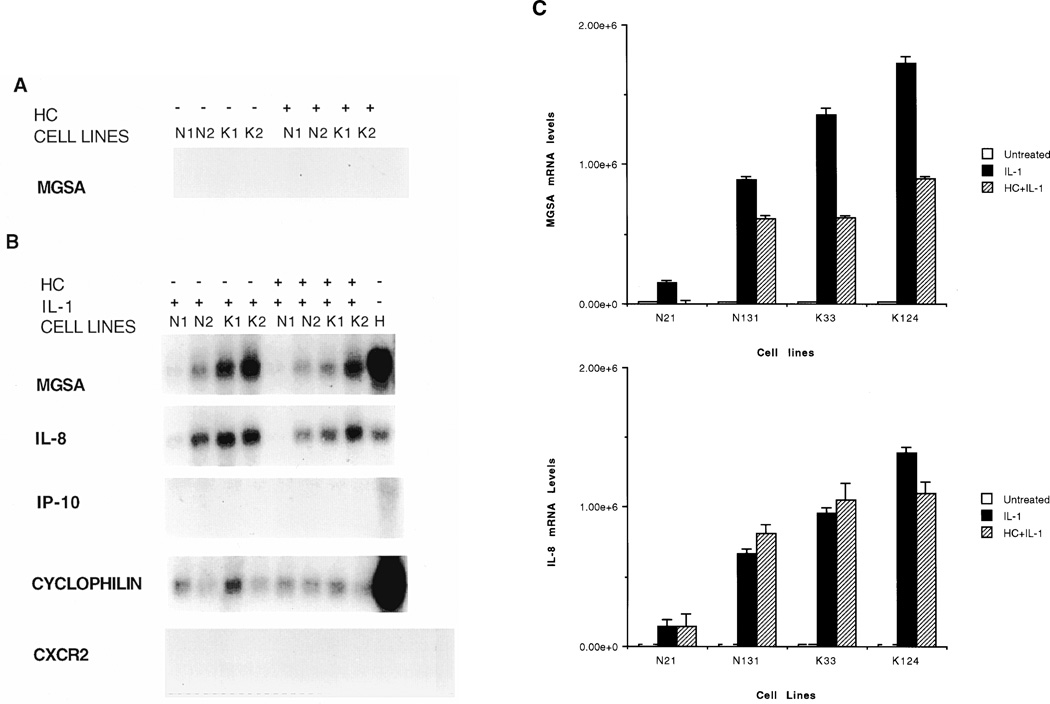
Northern blot analysis for chemokines and the CXCR2 receptor in fibroblasts
Expression of MGSA, IL-8, interferon inducible protein-10 (IP-10) and CXCR2 by 2 normal and 2 keloid fibroblasts cultures in response to IL-1 (1 ng/ml of IL-1α with a specific activity of 5000 units/mg) with or without hydrocortisone pretreatment (50 ng/ml of a hydrocortisone preparation with a specific activity of 200 units/mg)) was examined by Northern analysis. The concentrations of IL-1 and hydrocortisone were chosen based upon dose response experiments and the time course chosen for the experiments was based upon time course experiments where it was determined that maximal induction of MGSA/GRO mRNA occurred after 2–3 hours. Basal mRNA expression of all chemokines and CXCR2 was undetectable in both normal and keloid cells. IL-1 dramatically induced levels of MGSA and IL-8 but not IP-10 or CXCR2 (Figure 3A). The IL-1 induction of MGSA mRNA but not IL-8 mRNA was markedly inhibited by hydrocortisone pretreatment (Figure 3B). This inhibition was more pronounced in keloid fibroblasts. A representative Northern blot is shown and the bar graph shows the quantitation from three separate experiments. The glucocorticoid inhibition of IL-1 induced MGSA mRNA was significantly impaired in keloid fibroblast (p < 0.05) but not in normal fibroblasts (p > 0.7) (Student’s t-test).
Figure 3.
Northern blot analysis of chemokine gene expression. Total RNA from two normal fibroblast cell-lines, N1 & N2, and two keloid fibroblast cell-lines, K1 & K2, was resolved on formaldehyde-agarose gels, transferred to nitrocellulose membranes and probed with 32P labeled probes as indicated on the left. (A) Cells were treated with hydrocortisone (HC) or left untreated. (B) Cells, either pretreated with HC or left untreated, received a 3-h IL-1 treatment prior to harvesting. Total RNA from Hs294T malignant melanoma cells (H) was used as a positive control. (C) Autoradiograms of MGSA/GRO, cyclophilin B and IL-8 northern blots were densitometrically scanned using the ImageQuant software (Molecular Dynamics). Values obtained for MGSA/GRO and IL-8 mRNA were normalized against values of cyclophilin B mRNA and the results represented graphically for levels of MGSA/GRO mRNA (top) and IL-8 mRNA (bottom). The Y-axis represents a ratio of MGSA/GRO mRNA levels (top) or IL-8 mRNA levels (bottom) to cyclophilin B mRNA levels. Each graph is based on three experiments. Error bars represent standard deviations.
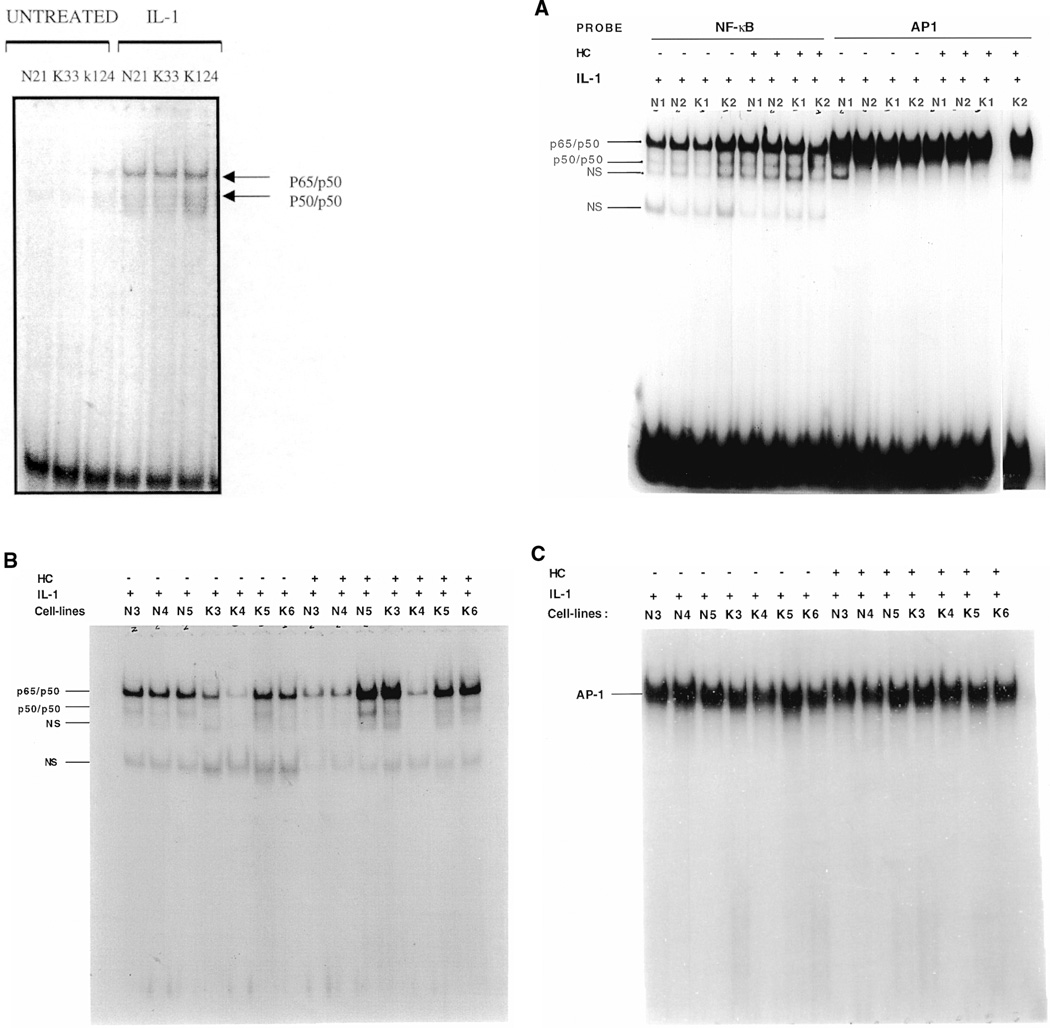
Nuclear levels of AP-1, NF-κB, AND Sp1 in normal and keloid fibroblasts
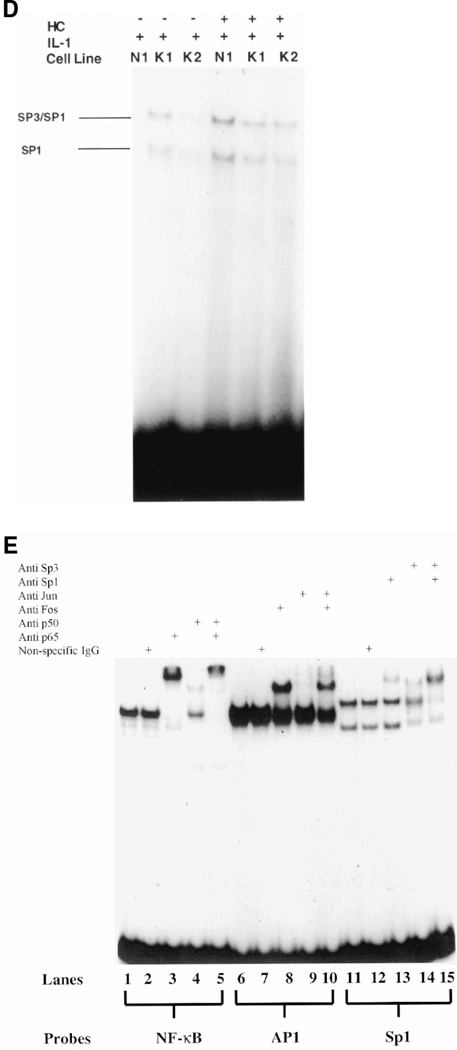
Nuclear levels of AP-1, NF-κB and Sp1 in response to IL-1 (1 ng/ml) with or without glucocorticoid pretreatment (50 ng/ml which was equivalent to 10 units/ml) were examined in normal and keloid fibroblasts by electrophoretic mobility shift analysis (EMSA). In these experiments, variability in keloid and control fibroblasts was noted in response to glucocorticoid suppression of NF-κB and AP-1. Results from EMSAs using nuclear extracts from 5 normal and 5 keloid fibroblast cultures indicated that IL-1 treatment increased binding of the p65 (Rel A) and p50 subunits of NF-κB (Figure 4A [left and right] and B). Hydrocortisone pretreatment of these cultures did not decrease nuclear levels of p65 (RelA) and p50 subunits of NF-κB. Based upon EMSA, hydrocortisone treatment appeared to have slightly increased the nuclear levels of these transactivators in K1, K3, K5 keloid fibroblasts and in N1, N2 and N5 normal fibroblasts. However, K2, N3 and N4 appeared to show a decrease in nuclear p65/p50 binding, and K4 exhibited no change in p65/p50 in response to glucocorticoid. Because the slight increase in NF-κB in response to glucocorticoid was variable, the conclusion that can be derived from these experiments is that hydrocortisone does not significantly inhibit nuclear localization or DNA binding of NF-κB p65/p50. Hydrocortisone pretreatment had no effect on AP1 or Sp1 nuclear levels (Figures 4C and D, respectively). Immunosupershift analysis (Figure 4E) indicates that complexes generated with the NF-κB element largely consist of p50/p65 heterodimers. Complexes obtained with the Sp1 probe contain Sp1 and Sp3. The AP1 complex appears to contain mostly c-Fos but not c-Jun. In general, the increases in NF-κB nuclear levels with hydrocortisone pretreatment appear to be more pronounced in keloid fibroblasts as compared to normal cells (Figure 4B). We conclude from these experiments that hydrocortisone probably inhibits IL-1 induction of MGSA expression in keloid fibroblasts and in normal fibroblasts by acting through a negative regulatory element such as the steroid response element or by effecting mRNA stability, rather than by inhibiting positive transactivators of MGSA/GRO transcription such as NF-κB, AP-1 or Sp1.
Figure 4.
Electrophoretic mobility shift assays. (A, right) Two normal (N1,N2) and 2 keloid (K1, K2) fibroblast cell-lines were treated with IL-1 in the presence or absence of a 24-h hydrocortisone pretreatment. The nuclear extract from each sample was competed with polydI.dC: polydI.dC and probed for binding with 32P labeled double-stranded oligonucleotides corresponding to the consensus binding sites for either NF-κB or AP1. Binding reactions were then electrophoresed on 8% native polyacrylamide gels which were dried and subjected to autoradiography. Arrows on the left refer to the homo/heterodimeric composition of the p65 (RelA) and p50 subunits of the NF-κB complexes, while NS is a nonspecific complex. (A, left) Three samples, 1 normal and 2 keloid, were treated with IL-1 or without prior to EMSA showing the increase in NF-κB binding. (B) Nuclear extracts from 3 normal (N3–N5) and 4 keloid (K3–K6) fibroblast cell lines were treated with IL-1 with or without a 24-h hydrocortisone (HC) pretreatment and probed for binding with 32P labeled NF-κB consensus sequence oligonucleotide. Arrows indicate NF-κB subunit composition of complexes. NS refers to a nonspecific complex. (C) Nuclear extracts from 3 normal (N3–N5) and 4 Keloid (K3–K6) fibroblast cell-lines treated with IL-1 with or without a 24-h hydrocortisone pretreatment were probed with 32P labeled AP-1 consensus oligonucleotide. Arrow indicates position of the AP-1 complex. The free probe in this gel has been run out.
(D) One normal (N1) and 2 keloid fibroblast cell-lines (K1, K2) were treated with IL-1 in the presence or absence of a 24-h hydrocortisone pretreatment. Nuclear extracts were competed with polydI.dC:polydI.dC and probed for binding with labeled SP1 binding-site oligonucleotide. Arrows on the left represent SP1 monomeric or the SP1/SP3 heterodimeric complexes formed with the SP1 consensus site. (E) Nuclear extract from one keloid fibroblast cell line (K2) was probed for binding with either NF-κB, lanes 1 though 5, or AP1, lanes 6 through 10, or Sp1, lanes 11 through 15, consensus oligonulceotides. Reactions containing the NF-κB oligonucleotide were preincubated with antibodies against either NF-κB p65 (Rel A) (lane 3) or NF-κB p50 (lane 4) or both antibodies (lane 5). Reactions containing the AP1 oligonucleotide were preincubated with antibodies against either Fos (lane 8) or Jun (lane 9) or both antibodies (lane 10). Reactions containing the Sp1 oligonucleotide were preincubated with antibodies against either Sp1 (lane 13) or Sp3 (lane 14) or both antibodies (lane 15). A nonspecific antibody was used as a control (lanes 2, 7 and 12).
In vitro wound healing model
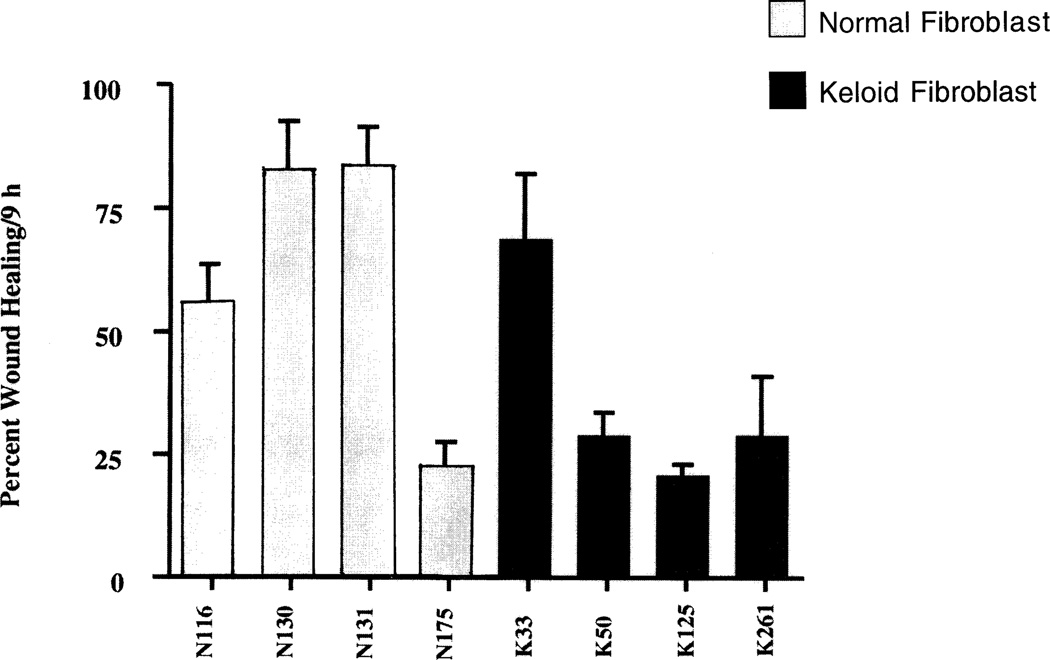
To determine whether the differences between normal and keloid tissue in the expression of the CXCR2 receptor were intrinsic properties of the fibroblasts or were induced by inflammatory components present in the in vivo setting, an in vitro series of wounding experiments were employed. Circular wounds of 400 microns were made as described in Methods on cultures of 4 normal and 4 keloid fibroblast strains grown in 24-well plates. The wound healing response was measured by the extent of wound closure. The wound area was measured at 0 and 9 hours postwounding and the percentage of wound closure was quantified. The averages and standard deviations were obtained from 4 wounds in 3 different experiments. Wound closure rates were slower in injured keloid fibroblasts than in control fibroblastic populations (Figure 5), a finding that suggested that the intrinsic migrational or proliferative properties of the keloid fibroblast were not inherently greater than normal fibroblasts. This is surprising in view of the previous work showing that keloid fibroblasts exhibit enhanced collagen expression, a metabolic event associated with enhanced wound repair.8 Simultaneous immunofluorescence staining of immunoreactive CXCR2 at corresponding time periods postwounding did not reveal an up-regulation of immunoreactivity for MGSA/GROα or CXCR2 after wounding (data not shown). These studies suggest that in the absence of inflammatory components (in vitro), little induction of MGSA/GROα or its CXCR2 receptor is evident in wounded keloid or normal fibroblasts in the culture dish. These data support the hypothesis that the inflammatory components are pivotal in the regulation of CXCR2 receptor expression and possibly MGSA/GROα expression in vivo.
Figure 5.
In vitro wound closure of keloid and normal fibroblasts. Cultures of keloid or normal fibroblasts were cultured in 24 well tissue culture plates. Wounds (400–500 µm) on monolayered keratinocytes were monitored over a period of 24 hours and the area of the wound defect determined using Bioquant software. Fibroblasts from keloids exhibited a wound closure which was slower than that of normal fibroblasts. Values represent mean ± SEM obtained from cultures from cultures of 4 normal and 4 keloid fibroblasts.
DISCUSSION
Keloids are benign collagenous tumors that form in the dermis as a result of an aberration in the process of wound healing in genetically predisposed individuals. In comparison to normal wound healing, keloid wound healing is characterized by an extended period of fibroblast proliferation and an elevated rate of collagen synthesis. This extended proliferation of keloid fibroblasts as compared to fibroblasts from normal scars may be in part due to diminished apoptosis due to down-regulation of apoptosis-related genes including defender of cell death-1(DAD-1), nucleoside diphosphate kinase B, glutathione S-transferase, glutathione S-transferase microsomal, glutathione peroxidase, tumor necrosis factor receptor 1-associated protein(TRADD), 19 kDa interacting protein 3 (NIP3), and cytoplasmic dynein light chain 1.27 The exaggerated wound healing process may be due in part to altered response to fibrogenic cytokines3,5,6,18 and to loss of glucocorticoid suppression of collagen and elastin gene expression in cells derived from these lesions.8,9 Moreover, an altered cytokine profile has been reported in black patients with keloids.7
Several reports link keloid formation to the immune system.28 Such studies have produced evidence that T lymphocytes are important modulators of wound healing29–31 and that an imbalance of their activity and of their products can result in wound failure or in excessive fibrosis of repairing tissue.29,32,33 In an immunocytochemical study of normal wounds, hypertrophic scars and keloids,28 T lymphocytes were observed in early stages of all three tissue types. However, they were markedly reduced in normal wounds by 14 weeks, whereas hypertrophic scars showed abundant T cells for about a year and keloid samples, which had the greatest lymphocyte presence, continued to have a diffuse lymphocyte infiltrate over a wide range of age of scar. In a more recent report Castagnolli et al.34 report T lymphocytes, mainly of the CD4 + type, in the epidermis and dermis of active hypertrophic scars.
Chemokines are the major mediators of leukocyte migration into the wound bed during wound healing.35 Sequential expression of IL-8, MGSA/GRO, monocyte chemotactic protein-1 (MCP-1), IP-10, and monokine induced by interferon-8 (mig) regulate the migration of first neutrophils, then monocytes/macrophages, and finally lymphocytes into the wound to facilitate wound repair. The cytokine milieu reportedly regulates the expression of chemokines and their receptors. IL-1 and tumor necrosis factor-α have been shown to induce the expression of all three MGSA/GRO genes.13 In contrast, interferon-g (IFN-γ) and hydrocortisone suppress the expression of these chemokines. IL-4 and IL-13 induce the expression of CXCR2 in monocytes.36 In T cells, IFN-γ and tumor necrosis factor-α induce CXCR2, while IL-4, 10 and 13 suppress CXCR2 expression.37 In contrast, in B cells IL-4 and IL-13 are reported to induce CXCR2 expression while IFN-γ and IL-2 suppress CXCR2 expression.38 We postulate that factors favoring a Th1 lymphocyte activation (secretion of IFN-γ) would be parallel with the induction of CXCR2 expression in T cells. In contrast factors favoring a Th2 lymphocyte activation (secretion of IL-4, IL-10, IL-13 and IL-1) would occur under conditions where B cells express CXCR2 and where IL-1 is secreted to activate the expression of the MGSA/GRO ligand. We did not detect significant levels of immunoreactive IL-4 in keloid tissues (data not shown). In fixed sections of keloid tissues we did observe the expression of both MGSA/GRO and its receptor, CXCR2. However, when the keloid fibroblasts were cultured in vitro, we did not observe expression of MGSA/GRO or its receptor, CXCR2. We were able to induce the expression of the chemokine with IL-1, pointing to the pivotal role for the inflammatory component in the regulation of chemokines and their receptors in keloid fibroblasts.
Our experiments show that hydrocortisone inhibits IL-1 mediated MGSA/GRO expression in keloid fibroblasts. It has been reported previously that glucocorticoid suppresses expression of the rat homolog of MGSA/GRO by impairing the activation of NF-κB.16 Expression of other CXC chemokines is also attenuated by glucocorticoids.39 Another mechanism for suppression of CC chemokine expression is through glucocorticoid mediated destabilization of chemokine mRNA.40 Based upon our gel shift analyzes, hydrocortisone inhibition of MGSA/GRO mRNA expression does not appear to be mediated by suppression of NF-κB, AP-1 or Sp1, but may be through effects on the putative Steroid Responsive Element [AGAACAT] located in the MGSA/GROα promoter at −601 bp to −596 bp relative to the transcription start-site. Effects of hydrocortisone on MGSA/GRO mRNA stability cannot be ruled out.
The ability of keloid fibroblasts to respond to hydrocortisone is an essential component of therapy, since surgery is seldom recommended as a treatment for keloids. Instead, hydrocortisone treatment is often successful for shrinking these benign lesions. The architecture and histological characteristics of keloids vary greatly among samples. Fibroblasts in the central core have more of a keloid phenotype as compared to those in the periphery which may be normal. The phenotype of primary cell cultures derived from keloid fibroblasts will therefore depend almost entirely on the source, phenotype and location of the fibroblast within the keloid tissue. The cytokine milieu in the keloid tissue will also contribute to the keloid nature of the fibroblast. All these factors may have contributed to the variability of the fibroblast cell lines used in this study. Inhibition of the induction of chemokines which attract the inflammatory cells is proposed to be a major contributor to the suppression of keloid growth. By altering the cytokine milieu of the keloid fibroblasts, the growth of these lesions is predicted to be reduced.
Acknowledgments
We thank Aimee Self, Jesse Britton, and Nancy Cardwell for their expert technical assistance. This work was supported by the Department of Veterans’ Affairs [VA/HBCU grant (AR and SR) and a VA Career Scientist Award (AR)], as well as by grants from the National Institutes of Health [GM40439 (LBN), P30 AR 41943].
Glossary
- DTT
Dithiothreitol
- EDTA
Ethylenediamine tetraacetic acid
- EMSA
Electrophonetic mobility shift assay
- GROα
Growth-regulated protein
- IL
Interleukin
- IP-10
Interferon-γ inducible protein-10
- MGSA
Melanoma growth stimulatory activity
- PBS
Phosphate buffered saline solution
- SSC
Saline sodium chloride solution
REFERENCES
- 1.Murray JC, Pollack SV, Pinnell SR. Keloids: a review. J Am Acad Dermatol. 1981;4:461–470. doi: 10.1016/s0190-9622(81)70048-3. [DOI] [PubMed] [Google Scholar]
- 2.Niessen FV, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–1457. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Tredget EF, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures: the cellular and molecular basis for therapy. Surg Clin North Am. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 4.Machesney M, Tidman N, Waseem A, Kirby L, Leigh I. Activated keratinocytes in the epidermis of hypertrophic scars. Am J Pathol. 1998;152:1133–1141. [PMC free article] [PubMed] [Google Scholar]
- 5.Babu M, Diegelmann R, Oliver N. Keloid fibroblasts exhibit an altered response to TGF-beta. J Invest Dermatol. 1992;99:650–655. doi: 10.1111/1523-1747.ep12668146. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K, Garner W, Cohen L, Rodriguez J, Phan S. Increased types I and III collagen and transforming growth factor-beta 1 mRNA and protein in hypertrophic burn scar. J Invest Dermatol. 1995;104:750–754. doi: 10.1111/1523-1747.ep12606979. [DOI] [PubMed] [Google Scholar]
- 7.McCauley RL, Chopra V, Li YY, Herndon DN, Robson MC. Altered cytokine production in black patients with keloids. J Clin Immunol. 1992;12:300–308. doi: 10.1007/BF00918154. [DOI] [PubMed] [Google Scholar]
- 8.Russell SB, Trupin JS, Myers JC, Broquist AH, Smith JC, Myles ME, Russell JD. Differential glucocorticoid regulation of collagen mRNAs in human dermal fibroblasts. Keloid-derived and fetal fibroblasts are refractory to down-regulation. J Biol Chem. 1989;264:13730–13735. [PubMed] [Google Scholar]
- 9.Russell SB, Trupin JS, Kennedy RZ, Russell JD, Davidson JM. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down-regulation in fibroblasts from normal dermis but not from keloids. J Invest Derm. 1995;104:241–245. doi: 10.1111/1523-1747.ep12612788. [DOI] [PubMed] [Google Scholar]
- 10.Auphan N, Didonata JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I-kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 11.Anisowicz A, Messineo M, Lee SW, Sager R. An NF-κB-like transcription factor mediates IL-1/TNFα induction of gro in human fibroblasts. J Immunol. 1991;147:520–527. [PubMed] [Google Scholar]
- 12.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- 13.Shattuck RL, Wood LD, Jaffe GJ, Richmond A. High basal transcription through the NF-κB element results in accumulation of MGSA/GROα in human melanoma. Mol Cell Biol. 1994;14:791–802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood LD, Richmond A. HMGI (Y) and Sp1 in addition to NF-κB regulate transcription of the MGSA/GROa gene. Nucleic Acids Res. 1995;23:4210–4219. doi: 10.1093/nar/23.20.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood LD, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GROa gene requires both NF-κB and novel constitutive factors. J Biol Chem. 1995;270:30619–30626. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka T, Kubota A, Hirano T, Watanabe K, Yoshida H, Tsurufuji M, Iizuka Y, Konishi K, Tsurufuji S. Glucocorticoid-mediated gene suppression of rat cytokine-induced neutrophil chemoattractant CINC/gro, a member of the interleukin-8 family, through impairment of NF-κB activation. J Biol Chem. 1996;271:1651–1659. doi: 10.1074/jbc.271.3.1651. [DOI] [PubMed] [Google Scholar]
- 17.Unemori EN, Amento EP, Bauer EA, Horuk R. Melanoma growth-stimulatory activity/GRO decreases collagen expression by human fibroblasts. Regulation by C-X-C but not C-C cytokines. J Biol Chem. 1993;268:1338–1342. [PubMed] [Google Scholar]
- 18.Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of MGSA (GRO) and the IL-8 Receptor B in human wound repair. Am J Pathol. 1995;147:1248–1260. [PMC free article] [PubMed] [Google Scholar]
- 19.Russell SB, Trupin KM, Rodríguez-Eaton S, Russell JD, Trupin JS. Reduced growth-factor requirement of keloid-derived fibroblasts may account for tumor growth. Proc Natl Acad Sci USA. 1988;85:587–591. doi: 10.1073/pnas.85.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucl Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanididium thiocynate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Chodosh LA, Carthew RW, Sharp PA. A single polypeptide possesses the binding and transcription activities of the adenovirus late transcription factor. Mol Cell Biol. 1986;6:4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 24.Skalli O, Schurch W, Seemayeer TA, Lagace R, Montandon D, Pittet B, Gabbiani G. Myofibroblasts from diverse pathological Gilsettings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989;60:275–285. [PubMed] [Google Scholar]
- 25.Darby I, Skalli O, Gabbiani G. α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 26.Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as markers of phenotypic modulation. Lab Invest. 1994;63:144–161. [PubMed] [Google Scholar]
- 27.Sayah DN, Soo C, Shaw WW, Watson J, Messadi D, Longaker MT, Zhang X, Ting K. Downregulation of apoptosis-related genes in keloid tissues. J Surg Res. 1999;87:209–216. doi: 10.1006/jsre.1999.5761. [DOI] [PubMed] [Google Scholar]
- 28.Martin CW, Muir IFK. The role of lymphocytes in wound healing. Br J Plast Surg. 1990;43:655–662. doi: 10.1016/0007-1226(90)90185-3. [DOI] [PubMed] [Google Scholar]
- 29.Wahl SM, Allen JB. T-lymphocyte dependent mechanism of fibrosis. In: Barbul A, et al., editors. Growth factors and other aspects of wound healing: Biological and clinical implication. New York: Alan R. Liss; 1988. [Google Scholar]
- 30.Barbul A, Breslin RJ, Woodyard JP, Wasserkrug HL, Efron G. The effect of in vitro T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209:479–483. doi: 10.1097/00000658-198904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbul A. T cell-dependent immune system in wound healing. In: Barbul A, et al., editors. Growth factors and other aspects of wound healing: biological and clinical implications. New York: Alan R. Liss; 1988. pp. 161–175. [PubMed] [Google Scholar]
- 32.Linares HA. Proteoglycan–lymphocyte association in the development of hypertrophic scars. Burns. 1990;16:21–24. doi: 10.1016/0305-4179(90)90201-7. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs EJ. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991;12:17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- 34.Castagnoli C, Trombotto C, Ondei S, Steela M, Calcagni M, Magliacani G, Alasia ST. Characterization of T-cell subsets infiltrating post-burn hypertrophic scar tissues. Burns. 1997;23:565–572. doi: 10.1016/s0305-4179(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 35.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker E-B, Gillitzer R. Chemokines IL-8, GROα, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sozzani S, Bonecchi R, Facchetti F, Dusi S, Lissandrini D, Locati M, Allavena P, Rossi F, Mantovani A. Induction of functional IL-8 receptors by IL-4 and IL-13 in human monocytes. Cytokine. 1999;11:936, A98. doi: 10.4049/jimmunol.164.7.3862. [DOI] [PubMed] [Google Scholar]
- 37.Tani K, Su SB, Utsunomiya I, Oppenheim JJ, Wang JM. Interferon-gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998;28:502–507. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Jinquan T, Moller B, Storgaard M, Mukaida N, Bonde J, Grunnet N, Black FT, Larsen CG, Matsushima K, Threstrup-Pedersen K. Chemotaxis and IL-8 receptor expression in B cells from normal and HIV-infected subjects. J Immunol. 1997;158:475–484. [PubMed] [Google Scholar]
- 39.Smith JB, Herschman HR. Glucocorticoid-attenuated response genes encode intercellular mediators, including a new CXC chemokine. J Biol Chem. 1995;270:16756–16765. doi: 10.1074/jbc.270.28.16756. [DOI] [PubMed] [Google Scholar]
- 40.Poon M, Liu B, Taubman MB. Identification of novel dexamethasone-sensitive RNA-destabilizing region on rat monocyte chemoattractant protein 1 mRNA. Mol Cell Biol. 1999;19:6471–6478. doi: 10.1128/mcb.19.10.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]