Summary
Wound healing requires a complex series of reactions and interactions among cells and their mediators, resulting in an overlapping series of events including coagulation, inflammation, epithelialization, formation of granulation tissue, matrix and scar formation. Cytokines and chemokines promote inflammation, angiogenesis, facilitate the passage of leukocytes from circulation into the tissue, and contribute to the regulation of epithelialization. They integrate inflammatory events and reparative processes that are important for modulating wound healing. Thus both cytokines and chemokines are important targets for therapeutic intervention.
The chemokine-mediated regulation of angiogenesis is highly sophisticated, fine tuned, and involves pro-angiogenic chemokines, including CXCL1-3, 5–8 and their receptors, CXCR1 and CXCR2. CXCL1 and CXCR2 are expressed in normal human epidermis and are further induced during the wound healing process of human burn wounds, especially during the inflammatory, epithelialization and angiogenic processes. Human skin explant studies also show CXCR2 is expressed in wounded keratinocytes and Th/1/Th2 cytokine modulation of CXCR2 expression correlates with proliferation of epidermal keratinocytes. Murine excision wound healing, chemical burn wounds and skin organ culture systems are valuable models for examining the role of inflammatory cytokines and chemokines in wound healing.
Keywords: Chemokine, CXCR2, Epidermal wound healing, Cytokines
Introduction
Response to injury is an essential innate host immune response for restoration of tissue integrity. Tissue disruption results not only in tissue regeneration, but in a rapid repair process leading to formation of a fibrotic scar (Martin, 1997). Wound healing, whether initiated by trauma, microbes or foreign materials, proceeds via an overlapping pattern of events including coagulation, inflammation, epithelialization, formation of granulation tissue, matrix and tissue remodeling. Many of these processes are regulated by chemokines, a large superfamily of 8–15kD proteins that possess diverse biologic activities. Defined by a tetra-cysteine motif, these small proteins are subdivided into four distinct families according to the configurations of the cysteine residues at the amino terminus.
Chemokines are structurally related and are usually secreted upon cell stimulation. Most cell types, have the potential to actively participate in chemokine production. Chemokines selectively mediate the regionally specific recruitment of neutrophils, macrophages and lymphocytes. The role of individual chemokines or their receptors in wound healing has been studied mainly in rodent models. For example, transgenic mice over-expressing CXCL10 (an angiostatic chemokine that recruits T lymphocytes) exhibit impaired wound healing (Luster et al., 1998). Moreover, loss of CXCR2 (a receptor for angiogenic chemokines) results in delayed cutaneous wound healing, impaired angiogenesis, and repaired neutrophil recruitment into the wound bed (Devalaraja et al., 2000). It is not clear whether human and murine chemokine homologues exhibit similar functions in vivo, leaving open the question whether their physiological roles during inflammatory reactions are comparable (Engelhardt et al., 1998). However, topical application of CXCL-8 to human skin grafts on chimeric mice resulted in enhanced wound healing (Rennekampff et al., 2000).
The wound healing process modeled in the skin
Skin serves as a protective barrier against the environment. The initial injury triggers coagulation and an acute local inflammatory response followed by mesenchymal cell recruitment, proliferation and matrix synthesis. Failure to resolve the inflammation can lead to chronic non-healing wounds, whereas uncontrolled matrix accumulation, often involving aberrant cytokine pathways, leads to excess scarring and necrosis. Better understanding of the essential and complex role of cytokines in wound healing will provide opportunities to investigate pathways to inhibit/enhance cytokines to control or modulate pathologic healing (Efron and Moldawer, 2004).
Most types of cutaneous injury include damage of the blood vessels, and coagulation as a rapid response to initiate homeostasis and protect the host from excessive blood loss. With the adhesion, aggregation and degranulation of circulating platelets within the forming fibrin clot, several mediators and cytokines are released, including transforming growth factor beta (TGF-β), platelet derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) (Anitua et al., 2004). As the inflammatory mediators accumulate, the nearby blood vessels vasodilate and increase cellular trafficking.
Cytokines and their role in the wound-healing process
The response to injury is an essential host immune response for restoration of tissue integrity. The process of repair is mediated in large part by interacting molecular signals, primarily cytokines, which orchestrate cellular activities, and underscore inflammation and healing. The concept is that some cytokines function primarily to induce inflammation, while others suppress. Under pathologic conditions, anti-inflammatory mediators may either provide insufficient control over pro-inflammatory activities or may overcompensate and inhibit the immune response, rendering the host at risk from systemic infection. On the other side pro-inflammatory cytokines clearly promote inflammation and facilitate the passage of leukocytes from the circulation into the tissues. Depending on the cytokine and its role, it may be appropriate to either enhance (using some recombinant cytokines and gene transfer), or inhibit (cytokine receptor antibodies, soluble receptors, signal transduction inhibitors) the cytokine to achieve the desired outcome. Gene therapy is the future for wound-healing strategies.
The CXC chemokine family of chemotactic cytokines has been implicated in the regulation of proliferation and differentiation of normal keratinocytes in inflammatory processes that involve the skin. CXC chemokines have been shown to be angiogenic, and the first angiogenic chemokine to be described was CXCL8 (Koch et al., 1992). Angiogenesis is stimulated concomitantly with neutrophil recruitment on post-wound day 1 and continues through post-wound day 4, resulting in granulation tissue formation (Romagnani et al., 2004). Chemokines play a critical role in the recruitment of leukocytes to sites of inflammation, in the migration of keratinocytes during epithelial repair, and in angiogenesis during wound repair (McKay and Leight, 1991; Gillitzer and Goebeler, 2001). The chemokine receptors, CXCR2 and CXCR1, are involved differentially in all of these events. The CXCR2 receptor is the main target receptor for the angiogenic chemokines. We have demonstrated that CXCL1 and CXCR2 are expressed in normal human epidermis and are induced during the wound healing process of human burn wounds, especially during the inflammatory, epithelialization and angiogenic processes (Nanney et al, 1995). When studying wound healing in CXCR2(−/−) mice, (Devalaraja et al., 2000) showed decreased recruitment of neutrophils, reduced keratinocyte migration and proliferation during epithelialization, and a significant delay in neovascularization (Fig. 1). The authors concluded that CXCR2(−/−) mice exhibited a delayed wound healing response, impaired neovascularization, compromised neutrophil recruitment, altered monocyte recruitment, and decreased secretion of IL-1β into the wound bed (Table 1A). In vitro wound healing experiments performed with primary mouse keratinocytes cultures from CXCR2−/− mice demonstrated a defective proliferative response in these cells. Mice with a targeted deletion of β-arrestin exhibited enhanced recruitment of neutrophils to wound beds after excisional wounding, faster re-epithelialization post wounding, and exhibited enhanced recruitment of neutrophils into an artifically made air pouch injected with CXCL1 (Su et al., 2005). These data suggested that β-arrestin binding to CXCR2 serves as a negative regulator of CXCR2 in vivo signaling.
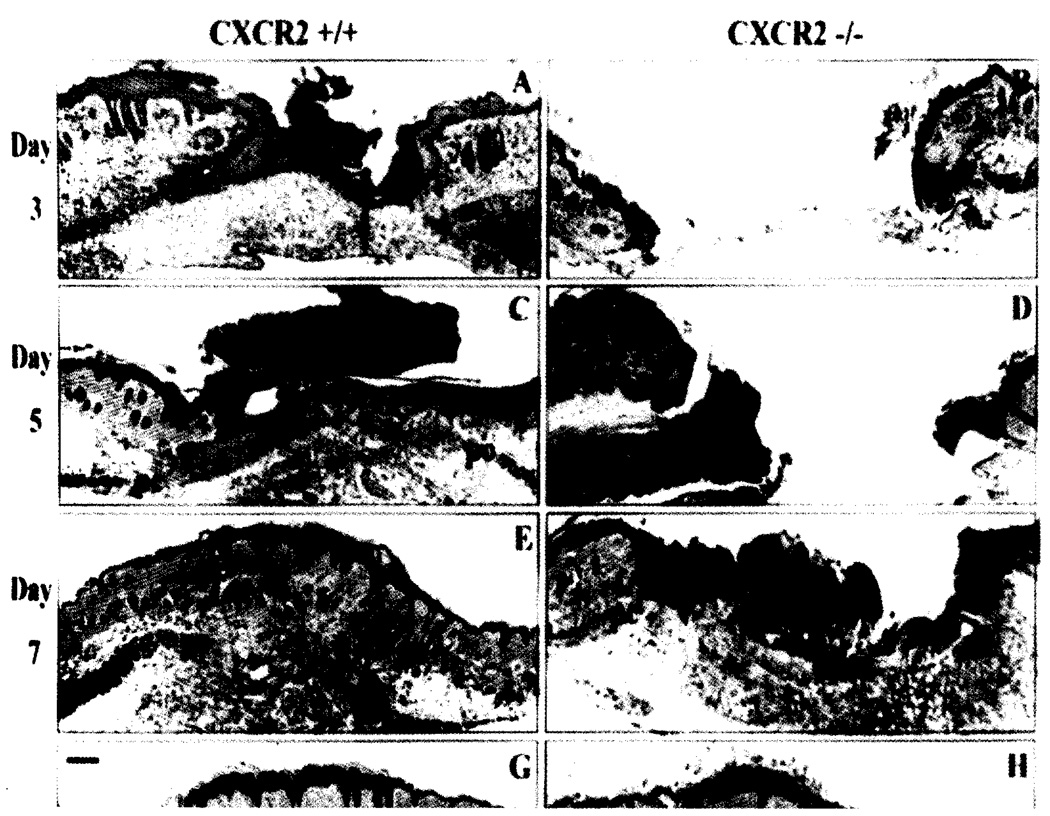
Fig. 1.
Delayed epithelial resurfacing and wound closure in CXCR2−/− mice. Representative micrographs from trichome-stained sections in parts A-H show responses to excisional injury on days 3, 5, 7 and 10 post-injury. Parts A, C, E and G depict the normal sequence of repair in +/+ (wild-type) mice. Epithelialization underneath the vivid red scab is nearly complete by day 3 with a hypertrophic epidermis by day 5. Robust cellularity in the wound bed, increasing collagen deposition, and wound contracture are evident at days 5, 7 and 10. By contrast, wounds from CXCR2 knockout mice shown in B, D, F, and H reveal impaired reparative responses: Resurfacing across the wound bed remains incomplete until days 7–8. Severely inhibited cellularity of the granulation tissue (gt) is noted at days 3–5 and delays in wound contracture are evident at days 3, 5 and 7. Immature granulation tissue is visible at day 7 followed by eventual wound closure and complete resurfacing by day 10. All wounds eventually show complete epidermal and dermal healing with no scarring. After resurfacing is achieved, sites of excisional wounding can only be detected by a discontinuity in the underlying panniculus-camosus layer (pc). Scale bars: 100 µm. (Reprinted with permission from Devalaraja et al., J. Invest. Dermatol. (2000) 115, 234–244.)
Table 1A.
Delayed neutrophil recruitment in CXCR2(−/−) mice after excision wounding.
| Day post–wounding | CXCR2 Genotype (Neutrophil Number) |
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| 3 | 56±2.80 | 32±1.60 | 6.0±0.47* |
| 5 | 48±2.33 | 42±2.05 | 2.8±0.99* |
| 7 | 17±1.70 | 60±3.48 | 12.5±1.37 |
| 10 | 11 ±1.28 | 37±2.64 | 16.5±0.75* |
Neutrophil recruitment to the wound bed at days 3, 5, 7 and 10 after cutaneous wounding. Total numbers of neutrophils in CXCR2 wild-type (+/+), CXCR2 heterozygous (−/+), and CXCR2 knockout mice (−/−) were manually counted within standardized areas of hematoxylin and eosin sections of the wound bed. Data are expressed as the mean ±SEM for the total number of neutrophils around the wounds based on six different mice at each time point after injury. The level of statistical significance of the comparison between wild type CXCR2(−/−) mice is indicated by the asterisk to denote p<0.05 based upon the Student’s two-tailed test and ANOVA.
In healing human skin excision wounds, CXCL8 is highly expressed along the denuded wound surface exactly where keratinocytes migrate from the wound edge to the closure point. The importance of CXCR2, and its interaction with ELR-motif CXC chemokines in the wound healing process is supported by the fact that CXCR2(−/−) mice or CXCR2(+/+) mice treated with the CXCR2 specific non-peptide antagonist SB 225002 (White et al., 1998) also showed delayed wound healing (Milatovic et al., 2003).
CXCL10 acting through activation of its receptor, CXCR3, plays a role in inhibiting wound repair. Transgenic mice developed to over-express CXCL10 in the skin exhibited an altered wound healing response characterized by enhancement of the inflammatory phase with lengthened and disorganized granulation phase that was characterized by impaired formation of the vasculature of the wound bed (Luster et al., 1998). Another chemokine that is reported to affect angiogenesis during wound repair is CXCL12. When human skin biopsies were analyzed for CXCL12 expression after excisional wounding, it was noted that CXCL12 accumulates at the margins of the wounds where it can potentially influence angiogenesis, where in response to TNFα and IFNγ, the ligand is down-regulated (Toksoy et al., 2007).
The re-epithelialization process can also be influenced by the influx of bone marow-derived keratinocyte stem cells. Another chemokine, CCL27 is reported to regulate this process through recruitment of these stem cells into wounded skin through interaction with its receptor, CCR10. The influx of these cells into the wound bed in response to CCL27 speeds up the wound healing process without affecting angiogenesis or the proliferation of keratinocytes (Inokuma et al., 2006).
Neutrophil recruitment
Neutrophils are the first type of immune cells found at the site if injury; they arrive first within a few minutes, followed by monocytes and lymphocytes. After acute injury, platelets and neutrophils are released passively from disrupted blood vessels. Their primary role is phagocytosis, and they are moved into the injured area by interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and transforming growth factor-beta (TGF-β). Some authors hypothesize that neutrophils may also contribute to early induction of angiogenesis in wound healing (Lingen, 2001). CXCL1, 5, and 8 are normally expressed in keratinocytes and can be released by wounding and further induced by proinflammatory cytokines such as IL-1 and TNF-α, bacterial products, and hypoxia. The simultaneous expression of several neutrophil-attractant chemokines in the acute phase of inflammation during wound healing may reflect the redundancy and robustness of the chemokine system (Mantovani, 1999). The spatially and timely differential expression of multiple neutrophil-specific chemokines, however, argues strongly in favor of a sophisticated multistep event, which enables neutrophils to leave occluded vessels and rapidly travel a rather long distance to enter the denuded wound surface, which is severely prone to infections with extrinsic pathogens.
Pro-inflammatory cytokines such as TNF-α not only induce CXCL8 and other chemokines, but may also suppress CXCR2 expression on neutrophils. In addition, the peak level of CXCL8 below the wound surface may stimulate migration and proliferation of keratinocytes, which express the CXCR2 at the wound edge. CXCL8 also stimulates the expression of CXCR1 on neutrophils (Murdoch and Finn, 2000), so they will be able to mount a second response and migrate to the superficial wound bed. It is interesting that the induction of the respiratory burst in neutrophils depends on interaction of CXCL8 with CXCR1 rather than CXCR2 (Jones et al., 1996) In addition to its neutrophil chemoattractant properties, CXCL8 also directly stimulates keratinocyte migration and proliferation. In our skin explant study we have shown that CXCL8 stimulates the proliferation of epidermal keratinocytes. CXCL 1, 5 and 8 may act as mediators of neutrophil inflammation in the early catabolic phase of wound repair as well as act as stimulators of re-epithelialization and neo-angiogenesis in the anabolic phase of wound repair. Thus, CXCL 1,5, 8 might be used as specific therapeutic agent in the regulation of wound healing process (Engelhardt et al., 1998).
Our previous studies (Devalaraja et al., 2000; Milatovic et al., 2003) showed that recruitment of neutrophils to the wound site was significantly reduced in CXCR2(−/−) mice through post-wound day 10 (Table 1A,B). Normally, recruitment of monocytes and lymphocytes sequentially follows neutrophilic recruitment, then rapidly subsides. Moreover, whereas monocyte recruitment diminished after post-wound day 4 in CXCR2(+/+) mice, the monocyte infiltrate was still elevated in CXCR2(−/−) mice on post-wound days 7 and day 10. CXCR2 is expressed to some extent on monocytes in addition to its robust expression on neutrophils. This data imply that other chemokine/ chemokine receptor interactions also modulate monocyte infiltration into the wound bed. Clearly loss of CXCR2 delays the timing of recruitment of neutrophils into the wound bed and significantly delays the wound healing process.
Table 1B.
Delayed neutrophil recruitment in CXCR2 (+/+, +/−, −/−) mice after chemical wounding with nitrogen mustard.
| Day post–wounding | CXCR2 Genotype (Neutrophil number) |
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| 4 | 102.00±10.50 | 88.00±9.01 | 36.33±4.33* |
| 7 | 90.60±3.84 | 79.33±4.66 | 47.00±6.02* |
| 10 | 77.66±1.76 | 56.66±2.33 | 63.66±2.40 |
Neutrophil recruitment to the wound bed at days 4, 7, and 10 after cutaneous wounding. Total numbers of neutrophils in CXCR2 knockout, wild type, and heterozygous mice were manually counted within standardized areas of H&E stain sections of the wound bed at 100_. Data are expressed as the mean ± SEM for the total number of neutrophils in wounded skin sections based on wounds from three different mice at each time point after wounding, The level of statistical significance of the comparison between wild type and CXCR2(−/−) mice was based upon Student’s two-tailed t-test and is indicated by the * where p values were < 0.05.
Macrophage recruitment
Macrophages are essential for normal wound repair (DiPietro, 1995). Their primary role is to exhibit immunological functions as antigen-presenting cells and phagocytes. Macrophages are also an important source of growth factors (Riches, 1996). With exception to the direct presence of neutrophils through extravasation after vessel injury and the, immediate action of NAP-2, both leukocyte subtypes migrate equally fast into the injured tissue (Engelhardt et al., 1998). It is known that considerable amounts of additional monocyte-attractant chemokines are present in human skin wounds. In a murine skin-wound healing model, macrophage-inflammatory protein (MIP-lα), monocyte chemoattractan proein MCP-1 and RANTES play a critical role in macrophage recruitment (DiPietro, 1995; DiPietro et al., 1998; Frank et al., 2000; Jackman et al., 2000; Wetzler et al., 2000) in addition to MCP-1. Accumulating evidence indicates that MCP-1, a CC chemokine also named CCL2, also displays immunoregulatory functions and may be involved in Th subset differentiation. Monocyte chemotactic protein-1/CCL2, plays a role in the recruitment of monocytes to sites of injury and infection. Monocytes attracted to the wounded tissue 48 to 96 hours after injury, where they differentiate into macrophages. Activated macrophages are important for the transition into the proliferative phase of wound repair and they are the most important mediators in process of angiogenesis, fibroplasia and synthesis of nitric oxide (Rosenkilde and Schwartz, 2004). Some authors present macrophages as essential cells involved in wound healing process, because of their role in development of angiogenesis, which is the most critical phase of successful wound healing process (Broughton et al., 2006). The macrophage orchestrates tissue repair and seems to be the only inflammatory cell type absolutely required (Prockop et al., 1979).
Numerous enzymes and cytokines are secreted by the macrophages, including collagenases (Singer and Clark, 1999).Their role is to clean the wound and help to remove debris from the wound bed. In general, macrophages can induce angiogenesis via three different mechanisms. First, macrophages can secrete factors that can directly induce new blood vessel growth. There are many mechanisms by which macrophages become activated to secrete angiogenic factors. Macrophages can be activated to induce angiogenesis under conditions of hypoxia. In addition, cytokines produced by endothelial cells activate macrophages, which then secrete factors that degrade connective tissue matrix (Knighton et al., 1983). Finally, macrophages may secrete factors that stimulate secretion of angiogenic factors by other cell types, such as endothelial cells, fibroblasts, or keratinocytes.
Macrophages have long been known to participate in the induction of new blood vessel growth in a number of different settings, including pathologic and physiologic wound healing and tumors. They are essential for optimal wound healing, and the introduction of activated macrophages into healing wounds results in enhanced wound repair (Singer and Clark, 1999). Newly formed blood vessels not only allow leukocyte migration into the wound site, but also supply the oxygen and nutrients necessary to sustain the growth of granulation tissues (Suh et al., 2005), in the last phase of tissue remodeling, converting granulation tissue into a mature scar.
The role of CXCR2 in wound healing process
Our laboratory has previously characterized the role of the CXC chemokine receptor, CXCR2, in excision wound repair (Devalaraja et al., 2000) and in wound repair after chemical burn (Milatovic et al., 2003). CXCR2 expression is reported to be suppressed by LPS, TNF-α, and induced by IFN-γ (Khandaker et al., 1999). In our recent study, we have investigated the wound healing response of wounded human skin tissue explants to pro-inflammatory and anti-inflammatory cytokines. We also evaluated the proliferation of epidermal cells, as an important step in the healing process in response to wounding using a skin organ culture system. The skin organ culture system used in our study seems to be useful for studying the effects of interleukins in cutaneous wound healing.
Proinflammatory cytokines, including interleukins IL-lα and IL-1β, IL-6, and TNF-α, play an important role in wound repair. They likely influence various processes at the wound site, including stimulation of keratinocyte and fibroblast proliferation, synthesis and breakdown of extracellular matrix proteins, fibroblast chemotaxis, and regulation of the immune response (Glaser et al., 1999).
In support of a role for proinflammatory cytokines in wound repair, expression of IL-1α, IL-6, and TNF-α were shown to be strongly up-regulated during the inflammatory phase of healing (Hübner et al., 1996). Interferon-gamma (IFN-γ) is secreted predominantly by T-lymphocytes. The primary effect of IFN-γ is not limited to PMN leukocytes and macrophage activation and cytotoxicity (Rumalla and Borah, 2001) IFN-γ also induces tissue remodeling and directly reduces wound contraction (Tredget et al., 2000). Essential to wound healing PMN leukocytes and macrophages were shown to be the major source of these cytokines, but expression was also observed in some resident cell types (Feiken et al., 1995). Another early proinflammatory cytokine IL-1 which exists in two forms IL-1α and IL-1β, is released principally by monocytes, and shares many properties similar to TNF-α. Initial IL-1 expression is required and beneficial to the wound healing process by increasing collagen synthesis as well as keratinocyte and fibroblast growth. High levels of IL-1 after the first week of healing process appear to be deleterious and pathogenic (Broughton and Attinger, 2006). Thus, the coordinated expression of these cytokines is likely to be important for normal repair.
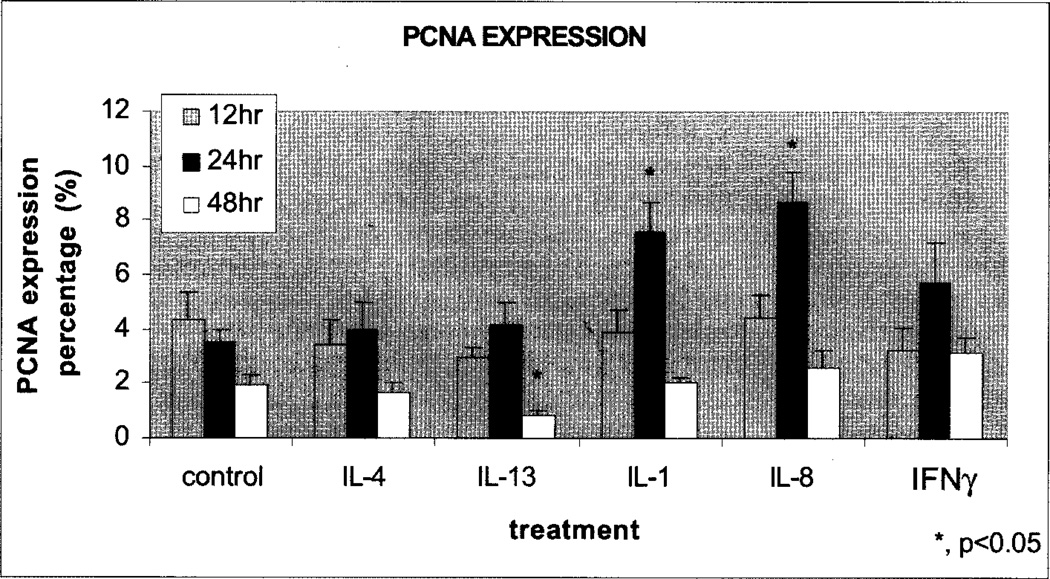
Epidermal injury represents a dramatic change and keratinocytes that normally differentiate to form rigid structures are required to become more flexible so that they are able to facilitate epidermal migration across the wound bed and rapidly close the wound. Though epidermal keratinocytes are the major source of cytokines in cutaneous wound healing, melanocytes, Langerhans cells, and leukocytes also produce several cytokines. Type 1 and Type 2 polarized T cells secrete cytokines that interact with various subsets of leukocytes. It appears that cytokines released by Thl and Th2 lymphocytes may exert a pro-inflammatory function on keratinocytes in terms of chemokine induction, but the role of soluble factors derived by the Thl (IFN-γ, IL-2, TNF-α), and Th2 (IL-4, IL-5, IL-6, IL-9, IL-10, IL-13) lymphocytes in promoting chemokine/chemokine receptor expression in keratinocytes is poorly defined. We have examined the role of cytokines produced by Thl, and Th2 lymphocytes in vitro in wound repair and expression of CXCR2 using an organ explant culture system. CXCR2 is thought to be predominantly responsible for neutrophil recruitment in response to its many ligands including CXCL8, and antagonists of CXCR2, open promise for treatment of inflammation. Using a skin explant model, we evaluated the proliferation of epidermal basal cells, an important process in wound healing, in the wound margin using human skin culture and immunohistochemical labeling with proliferating cell nuclear antigen (PCNA) as marker of cell proliferation (Fivenson et al., 1997). We postulated that cytokine treatment of wounded skin would induce proliferation of keratinocytes as monitored by PCNA staining. In the tissue sections examined, cells containing PCNA positive nuclei were present sparsely in migratory epidermis. PCNA positive cells were also found among epidermal basal cells in contact with the migratory epidermis and in one or two of the prickle cell layers immediately above the basal cells. We observed that keratinocytes from the skin explants treated for 24 hours with either IL-1 or CXCL8 showed increased proliferation, compared to untreated controls and compared to the 12 hours time point, as monitored by PCNA staining. In contrast IL-13 treatment inhibited proliferation after 48 hours treatment (Fig. 2).
Fig. 2.
Evaluation of the proliferative cellular nuclear antigen (PCNA) in human skin explant cultures treated with cytokines. Paraformaldehyde fixed, paraffin embedded human skin tissue sections were immunostained for proliferating cell nuclear antigen (PCNA). PCNA immunostaining showed a significant decrease (p<0.05) in the number of proliferating cells in the skin tissue explants treated with IL-13 after 48 hours of cytokine treatment. Treatments with IL-1 and IL-8 showed significant increase (p<0.05) in the number of proliferative cells (p<0.05) after 24 hours as compared to untreated control.
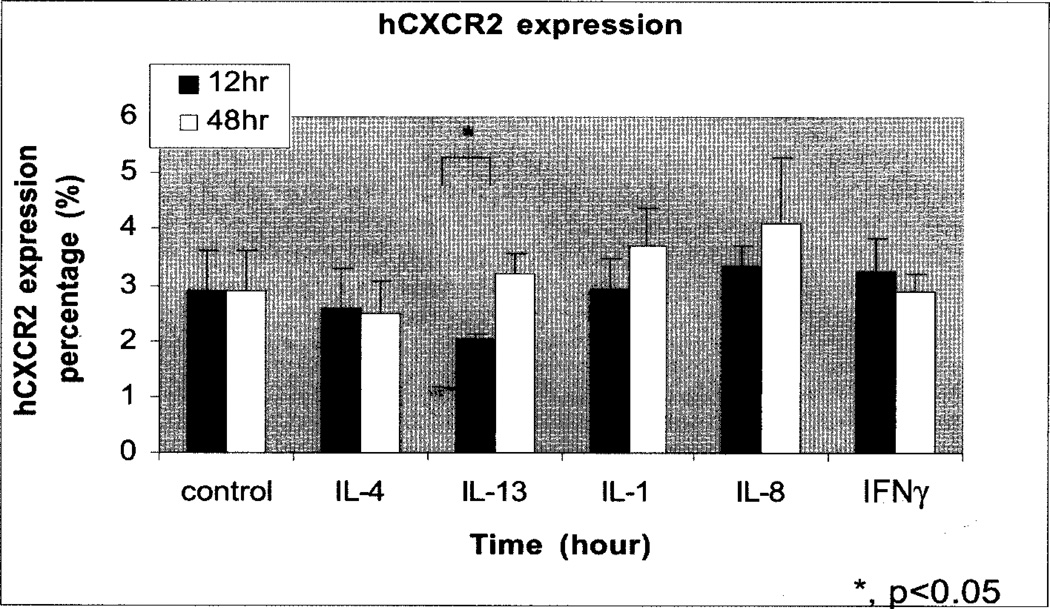
We also noticed that treatment of human tissue with IL-1, and CXCL8, was associated with increased CXCR2 expression. However, IL-13 produced a small but not statistically significant CXCR2 expression compared to untreated control after 12 hours of treatment (Fig. 3, Fig. 4B). This suppression in CXCR2 expression in response to IL-13 correlates with a reduction in PCNA positive cells at the same time point (Fig. 2). IL-13 and IL-4 strongly increased CXCR1 and CXCR2 chemokine receptor expression in human monocytes, macrophages, and dendritic cells (Bonecchi et al., 2000). Alternatively, macrophages can be activated by IL-4 and IL-13, two Th2 cytokines. The results presented by Bonecchi (Bonecchi et al., 2000) showed that IL-4 and IL-13 convert ELR+ CXC chemokines into potent monocyte attractants by up regulating receptor expression. Their study reported that two Th2 pathologies characterized by the presence of IL-4 and IL-13 are characterized by an infiltrate of IL-8 receptor positive mononuclear cells. Interestingly, they found that IL-4 and IL-13 down-regulate CXCR1 and CXCR2 expression in neutrophils and reduce their ability to migrate in response to IL-8.
Fig. 3.
Expression of hCXCR2 in the epidermis of human skin explant cultures treated with cytokines. Paraformaldehyde fixed, paraffin embedded human skin tissue sections were immunostained for hCXCR2. IL-13 showed a significant decrease (p<0.05) in the number of CXCR2 positive cells after 12 hours of treatment as compared to untreated control.
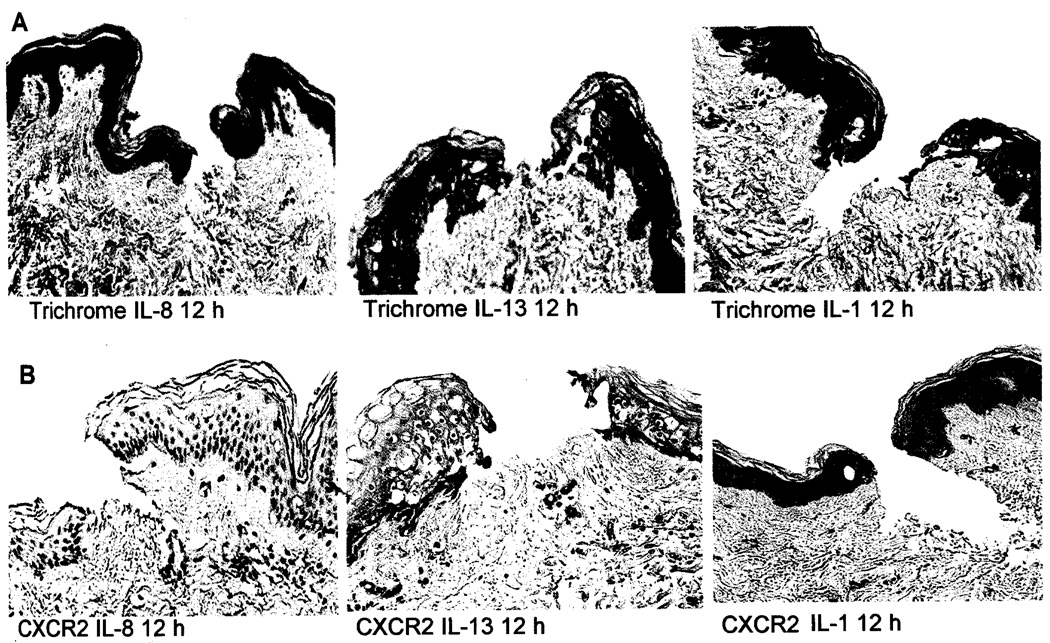
Fig. 4.
A. Histological analysis of wounded human skin explants treated with IL-8, IL-13. or IL-1. Trichrome stained human skin tissue samples showed new collagen and immature epidermal cells at 12 hours after injury. Pictures were taken at 10× magnification. B. Immunohistochemical analysis of CXCR2 expression in IL-1, IL-13, and IL-8 treated human skin tissue explants. Analysis showed that IL-13 significantly decreased the number of CXCR2 positive cells after 12 hours of treatment as compared as IL-1 and IL-8.
Conclusions
Chemokines and their receptors are growing family of inflammatory molecules that are associated with many tissue specific inflammatory events. Chemokines regulate the migration and/or accumulation of leukocytes at a particular tissue site to function in infection clearance and tissue repair (Karpus and Fife, 2001). In our skin-explant study we evaluated the proliferation of epidermal cells, as an important step in the healing process, in response to wounding using a skin organ culture system. The results obtained in cell culture systems do not necessarily reflect the actual process in vivo (Akasaka et al., 2004). The methods used in our experiments are more closely aligned with the in vivo process as compared to cell culture. The skin organ culture system offers utility for studying the effects of some interleukins and chemokines, such as IL-1 and CXCL8 that have been reported to stimulate the proliferation of epidermal cells (Balkwill and Manotovani, 2001).
Some in vitro studies have shown an inhibitory effect of CXCL8 on keratinocyte proliferation, suggesting that an increased level of this chemokine may directly contribute to delayed wound healing (Iocono et al., 2000). In contrast to these studies, others found a stimulatory effect of CXCL8 on keratinocyte proliferation in vitro (Rennekampff et al., 2000). Based on our histology work we noticed that topically applied IL-1 and IFN-α caused a delay in the wound healing process based upon wound closure. It seems likely that potent activators of keratinocytes such as IL-1 will perhaps enhance keratinocyte proliferation and prolong wound closure (data not shown).
In contrast to the work of Bonecchi, we have shown that treatment of human tissue with IL-4 did not significantly affect CXCR2 expression. Expression of IL-4 was detected by immunohistochemistry in the lower epidermis below the wound as early as 24 hours after wounding. IL-4 is mostly expressed by T lymphocytes, basophils and mast cells (Brown and Hural, 1997) and is known to have pleiotropic biological effects (Peyron and Banchereau, 1994) on not only hematopoetic cells but also non-hematopoetic cells, especially fibroblasts, where it stimulates extracellular matrix synthesis (Gillery et al., 1992). The effects of IL-4 include suppressing the expression of proinflammatory cytokines, as well as promoting B cell proliferation, and mediating IgE production (Banchereau, 1995). Animal studies by Salmon-Her showed that application of 250ng of IL-4 once a day on mouse wounds induced a rapid and dramatic increase in formation of the granulation tissue (Salmon-Her et al., 2000). It has also been shown that IL-4 enhanced the production of collagen and other extracellular matrix macromolecules by human fibroblasts (Serpier et al., 1997). Taken together, these data suggest that IL-4 could be involved in connective tissue formation during normal and pathological wound healing.
Chemokines regulate the migration of resident and inflammatory cells during wound repair mechanisms. However, data obtained from animal models, particularly in mice with a different skin morphology, are not easily applicable to the human wound-healing situation. It is becoming increasingly clear that the host defense system involves many interactive processes. These interactive processes range from non-specific reaction to pattern recognition molecules to the exquisitely specific cell-mediated response to a single antigen (Coehlo et al., 2005). The regulated expression of chemokines and chemokine receptors enables appropriate modulation of leukocyte development and differentiation, lymphocyte trafficking, and immune surveillance.
The human skin organ culture system presented here should be useful for evaluation of topical wound healing treatments in humans. The ability to correlate molecular events with more obvious morphologic changes will assist clinicians in understanding the wound healing process, and allow dissection of molecular events during this process.
Acknowledgements
This work was supported by a grant from the Department of Defense and the VA Medical Center awards to Ann Richmond and Lillian B Nanney and by a grant from NIH (CA34590) to Ann Richmond and a VA Research Career Scientist Award to Ann Richmond. We are grateful to the Skin Disease Research Center Histology Core Laboratory and Lillian B Nanney for assistance with the immunostaining.
References
- Akasaka Y, Ono I, Yamashita T, Jimbow K, Ishii T. Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J. Pathol. 2004;203:710–720. doi: 10.1002/path.1574. [DOI] [PubMed] [Google Scholar]
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Manotovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Banchereau J. Converging and diverging properties of human interleukin-4 and interleukin-10. Boehring Inst. Mitt. 1995:58–77. [PubMed] [Google Scholar]
- Bonecchi R, Facchetti F, Dusi S, Luini W, Lissandrini D, Simmelink M, Locati M, Bernasconi S, Allavena P, Brandt E, Rossi F, Mantovani A, Sozzani S. Induction of functional IL-8 Receptors by IL-4 and IL-13 in Human Monocytes. J. Immunol. 2000;164:3862–3869. doi: 10.4049/jimmunol.164.7.3862. [DOI] [PubMed] [Google Scholar]
- Broughton G, Janis J, Attinger CE. Wound healing: an overview. Plast. Reconstruct. Surg. 2006;177 Suppl.:1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- Coelho AL, Hogaboam CM, Kunkel SL. Chemokines provide the sustained bridge between innate and acquired immunity. Cytokine Growth Factor Rev. 2005;16:553–560. doi: 10.1016/j.cytogfr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Devalaraja RM, Nanney LB, Qian Q, Du J, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J. Invest. Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP1 alpha as a critical macrophage chemoattractant in murine wound repair. J. Clin. Invest. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron PA, Moldawer LL. Cytokines and wound healing: the role of cytokine and anticytokine therapy in the repair response. J. Burn Care Rehabil. 2004;25:149–160. doi: 10.1097/01.bcr.0000111766.97335.34. [DOI] [PubMed] [Google Scholar]
- Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiken E, Romer J, Eriksen J, Lund LR. Neutrophils express tumor necrosis factor-alpha during mouse skin wound healing. J. Invest. Dermatol. 1995;105:120–123. doi: 10.1111/1523-1747.ep12313429. [DOI] [PubMed] [Google Scholar]
- Fivenson DP, Faria DT, Nickoloff BJ, Polverini PJ, Kunkel S, Burdick M, Strieter RM. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen. 1997;5:310–322. doi: 10.1046/j.1524-475X.1997.50405.x. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillery P, Fertin C, Nicolas JF, Chastang F, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures. Potential role in fibrosis. FEBS Lett. 1992;302:231–234. doi: 10.1016/0014-5793(92)80448-p. [DOI] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- Glaser R, Rabin B, Chesney M, Cohen S, Natelson B. Stress-Induced Immunomodulation. JAMA. 1999;81:2268–2270. doi: 10.1001/jama.281.24.2268. [DOI] [PubMed] [Google Scholar]
- Hübner G, Brauchle Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- Iocono JA, Colleran KR, Remick DG, Gillespie BW, Ehrlich HP, Garner WL. lnterleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Repair Regen. 2000;8:216–225. doi: 10.1046/j.1524-475x.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- Inokuma D, Abe R, Fujita Y, Sasaki M, Shibaki A, Makamura H, McMillan JR, Shimizu T, Shimizu H. CTAK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes. Stem Cells. 2006;24:2810–2816. doi: 10.1634/stemcells.2006-0264. [DOI] [PubMed] [Google Scholar]
- Jackman SH, Yoak MB, Keerthy S, Beaver BL. Differential expression of chemokines in a mouse model of wound healing. Ann. Clin. Lab. Sci. 2000;30:201–207. [PubMed] [Google Scholar]
- Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NAPDH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc. Natl. Acad. Sci. USA. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus WJ, Fife BT. Chemokines and chemokines receptors. Keystone Symposia. 2001;1:549–553. doi: 10.1517/14712598.1.3.549. [DOI] [PubMed] [Google Scholar]
- Khandaker MH, Mitchell G, Xu L, Andrews JD, Singh R, Leung H, Madrenas J, Ferguson SS, Feldman RD, Kelvin DJ. Metalloproteinases are involved in lipopolysaccharide and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptors. Blood. 1999;93:2173–2185. [PubMed] [Google Scholar]
- Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. lnterleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch. Pathol. Lab. Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc. Assoc. Am. Physicians. 1998;110:183–195. [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol. Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- McKay IA, Leigh IM. Epidermal cytokines and their role in cutaneous wound healing. Br. J. Dermatol. 1991;124:513–518. doi: 10.1111/j.1365-2133.1991.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Milatovic S, Nanney LB, Yu Y, White RD, Richmond A. Impared healing of nitrogen mustard wounds in CXCR2 null mice. Wound Repair. Regen. 2003;8:179–191. doi: 10.1046/j.1524-475x.2003.11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of melanoma growth stimulatory activity of growth-regulated gene the interleukin-8 receptor B in human Peyron E. and Banchereau J. (1994) lnterleukin-4: Structure, function, and clinical aspects. Eur. J. Dermatol. 1995;4:181–188. [Google Scholar]
- Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. The biosynthesis of collagen and its disorders. New Engl. J. Med. 1979;301:13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res. 2000;93:41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- Riches DWH. Macrophage involvement in wound repair, remodeling and fibrosis. In: Clark RAF, editor. The molecular and cellular biology of wound repair. 2nd ed. London: Plenum New York; 1996. pp. 95–141. [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends. Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. The chemokine system- a mayor regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- Rumalla VK, Borah GL. Cytokines, growth factors, and plastic surgery. Plast. Reconstr. Surg. 2001;108:719–733. doi: 10.1097/00006534-200109010-00019. [DOI] [PubMed] [Google Scholar]
- Salmon-Her V, Ramont L, Godeau G, Phillipe B, Guenounou M, Bernard P, Maquart FX. Implication of lnterleukin-4 in wound healing. Lab. Invest. 2000;80:1337–1343. doi: 10.1038/labinvest.3780141. [DOI] [PubMed] [Google Scholar]
- Serpier H, Gillery P, Salmon-Her V, Garnotel R, Georges N, Kalis B, Maquart FX. Antagonistic effects of interferon-gamma and interleukin-4 on fibroblast cultures. J. Invest. Dermatol. 1997;109:158–162. doi: 10.1111/1523-1747.ep12319207. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. New Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J. Immunol. 2005;175:5396–5402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/ macrophages and neovascularization. Stem Cells. 2005;23:1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- Toksoy A, Muller V, Gillitzer R, Goebeter M. Biphasic expression of stromal cell-derived factor-1 during human wound healing. Br. J. Dermatol. 2007;157:1148–1154. doi: 10.1111/j.1365-2133.2007.08240.x. [DOI] [PubMed] [Google Scholar]
- Tredget EE, Wang R, Shen Q, Scott PG, Ghahary A. Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-alpha and IFN-gamma in vitro and in vivo. J. Interferon Cytokine Res. 2000;20:143–151. doi: 10.1089/107999000312540. [DOI] [PubMed] [Google Scholar]
- Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Invest. Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- White JM, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits lnterleukin-8-induced neutrophil migration. J. Biol. Chem. 1988;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]