Abstract
Background
Relatedness between group members is central to understanding the causes of animal dispersal. In many group-living mammals this can be complicated as extra-pair copulations result in offspring having varying levels of relatedness to the dominant animals, leading to a potential conflict between male and female dominants over offspring dispersal strategies. To avoid resource competition and inbreeding, dominant males might be expected to evict unrelated males and related females, whereas the reverse strategy would be expected for dominant females.
Methodology/Principal Findings
We used microsatellites and long-term data from an urban fox (Vulpes vulpes) population to compare dispersal strategies between offspring with intra- and extra-group fathers and mothers of differing social status in red foxes. Relatedness to the dominant male had no effect on dispersal in offspring of either sex, whereas there was a strong effect of relatedness to resident females on offspring dispersal independent of population density. Males with dominant mothers dispersed significantly more often than males with subordinate mothers, whereas dispersing females were significantly more likely to have subordinate mothers compared to philopatric females.
Conclusions/Significance
This is the first study to demonstrate that relatedness to resident females is important in juvenile dispersal in group-living mammals. Male dispersal may be driven by inbreeding avoidance, whereas female dispersal appears to be influenced by the fitness advantages associated with residing with the same-sex dominant parent. Selection pressure for paternal influence on offspring dispersal is low due to the limited costs associated with retaining unrelated males and the need for alternative inbreeding avoidance mechanisms between the dominant male and his female offspring. These findings have important implications for the evolution of dispersal and group living in social mammals, and our understanding of a key biological process.
Introduction
Dispersal is a fundamental biological process. Despite many studies into its underlying causes [1]–[3], little is known about genetic influences. At the population level, dispersal is thought to be a principal method of inbreeding avoidance [3]. Whilst an underlying assumption of this theory, hitherto no one has identified a role of intra-group relatedness on dispersal. Most canids are socially monogamous, but extra-pair copulations are common [4]–[6] and polygynandry creates social groups with juveniles of varying degrees of relatedness. Here, we tested whether relatedness to dominants determines offspring dispersal patterns in the red fox (Vulpes vulpes).
In group-living carnivores, dominant animals tolerate the presence of subordinates provided the reproductive and/or survival benefits outweigh the intra-specific costs [7]. Red fox social groups may contain additional subordinate adults, either offspring from previous years or individuals that have dispersed into the group [8]. Extra-pair copulations, and opportunistic subordinate matings, result in offspring with varied degrees of relatedness to the dominant pair [6]. Variation in relatedness is important in the evolution of dispersal, as kin competition and inbreeding avoidance are believed to be ultimate causes of dispersal [3].
In the absence of any fitness advantage for a dominant male, unrelated subordinate males might be expected to be forcefully evicted since they may compete with related males for resources, mates and future dominance, reducing the dominant male's indirect fitness. Forced dispersal of unrelated males would also reduce future conflicts between related and unrelated offspring e.g. over territory inheritance. Since dominant male foxes mate with resident subordinate females [6], the potential for inbreeding with related female offspring is high. If the costs of inbreeding outweigh the costs of dispersal, female offspring related to the dominant male should disperse whereas unrelated females, fathered through extra-group copulations, would be expected to be philopatric, providing future unrelated subordinate mating opportunities. However, a serious weakness in this argument is that juvenile dispersal in foxes is male biased [9].
If relatedness to dominant females is important, there is a potential conflict between dominant males and females if they parent the same offspring. Related males may disperse, avoiding inbreeding with dominant females, whereas unrelated males may disperse, avoiding resource competition with dominant males. Dominant females can potentially increase their fitness by allowing related females to remain and provide alloparental care to future offspring, but reduce intra-specific resource competition by evicting unrelated females.
Our aim was to determine whether juvenile dispersal is influenced by their relatedness to resident dominants and, if so, understand how the conflict between the dominant pair over offspring dispersal is resolved. We compared dispersal strategies of direct descendants of the dominants (related offspring) and cubs parented by subordinate females or extra-group males (unrelated offspring) using data from a long-term capture-mark-recapture study on red foxes to test three contrasting hypotheses: (i) paternal social status affects offspring dispersal; (ii) maternal social status affects offspring dispersal; and (iii) extra-group paternity affects offspring dispersal. We predicted that unrelated male offspring and related female offspring would disperse if relatedness to the dominant male was the major influence, whereas related male offspring and unrelated female offspring would disperse if relatedness to the dominant female had a stronger effect.
Methods
Study site and study animals
The study was undertaken in north-west Bristol, UK: this urban fox population has been studied continuously for over 30 years. Population density varied widely over the study period due to a sarcoptic mange (Sarcoptes scabiei) epizooty from 1994, which eliminated foxes on the study area in 1996 [10]; recovery thereafter has been slow [11]. Therefore we divided the samples into two time periods: (i) cohorts of cubs born in 2004–2009 when population density was 5.5–25.5 adults per km2, referred to as the high-density sample [12]. To include putative parents, we genotyped 410 foxes (212 males, 198 females) captured between 2002–2009; these comprised 92% of captures; and (ii) cohorts of cubs born in 1999–2003 when fox population density was 4.0–5.5 adults per km2, referred to as the low-density sample [12]. To include putative parents, we genotyped 146 foxes (76 males, 70 females) captured between 1998–2003; these comprised 95% of captures. Forty-four animals were included in both time periods, as cubs born during the low-density period were candidate parents during the high-density period.
Foxes were captured either by netting from den sites or in baited box traps set in residential gardens [13]. They were handled manually or anaesthetized with an intramuscular injection of ketamine hydrochloride (Vetalar 100 mg/ml solution; Pfizer Limited, Sandwich, Kent, UK), sexed and marked with plastic ear tags (Rototags, Dalton Supplies Ltd., Henley-on-Thames, Oxfordshire, UK); selected animals were also fitted with a radio collar. Three age classes were recognized based on body size and incisor wear [14]: cubs were <6 months old, subadults 6–12 months, and adults >12 months. All animals were assumed to have been born on April 1st each year [9]. Animal capture and handling procedures conformed to guidelines by the American Society of Mammalogists [15], were approved by the University of Bristol's ethics committee and were carried out under the Animals (Scientific Procedures) Act 1986 license number PPL3002434.
Foxes were assigned to social groups using capture-mark-recapture data and radio telemetry [16]. Any individual found on the same territory for two successive captures was assumed to have been resident on that territory, and thus a member of that social group, for the intervening period [6]. Dominant animals were those that elicited submissive behavior during interactions with all other same-sex group members and, for females, the individual most closely associated with the cubs when only a single litter was present [6]. Only animals first captured as cubs were classified as dispersers or philopatric and all cubs were assumed to have been captured on their natal territory. Dispersers were any subadult or adult recovered dead or recaptured one or more territory diameters away from the point of first capture. Philopatric animals were any adult recovered dead or recaptured less than one territory diameter away from the point of first capture: subadult recaptures were not classified as philopatric since this is the age-class when dispersal occurs [9]. Territory diameter was calculated separately for each year to take account of changes in fox population density.
Laboratory work
Genomic DNA was extracted from ear tissue ejected during marking, using an ammonium acetate precipitation method [17], [18]. Twenty-four markers (Table 1) were tested for establishing parentage in the high-density sample. All loci were unique based on a BLAST comparison of their sequences using a stand-alone BLAST and utilizing the GenBank nucleotide database (nr). The genome locations of the loci in the dog genome [19] were obtained based on sequence homology to check for potential physical linkage between loci or if any of the loci were located on the sex chromosomes. Duplex touchdown PCR (conditions below) were carried out on 24 unrelated individuals (12 males and 12 females) from the Bristol population to assess the loci. Fifteen markers, 11 from domestic dogs [20] and 4 from red foxes ([21]; Table 1) were selected for parentage analysis because they showed robust amplification, were autosomal (based on genotyping of known sexes and sequence homology to the assembled dog genome), had a low estimated frequency of null alleles (<0.1), had low levels of allelic drop out, lacked linkage disequilibrium and adhered to Hardy-Weinberg equilibrium (p>0.05). Observed and expected heterozygosity and estimated null allele frequencies were calculated using CERVUS version 3.0 [22]. Hardy-Weinberg equilibrium and linkage disequilibrium was calculated using GENEPOP version 4.0.10 [23]. PEDANT version 1.3 [24] was used to estimated allelic dropout rate per allele and the false allele rate per genotype.
Table 1. Details of microsatellite markers used to genotype Bristol's fox population and markers rejected for genotyping.
| Marker | Sequence accession number | Locus source species | Forward primer sequence | Reverse primer sequence |
| 1DGN14 | NW_876272 | Canis familiaris | TCACACAAAGTGGGTAAGATGG | GATTATGGTGCTATCCCTCTGG |
| 1DGN3 | NW_876269 | Canis familiaris | TTTTTTCTGTAAACCTAAAGCTGC | GGAAAGGTACAGGCATGTAGTTGG |
| 1FH2017b c d | NW_876259 | Canis familiaris | AGCCTCTATAATCACGTGAGCC | CCCAGTACCACCTTCAGGAA |
| 1FH2131a | NW_876308 | Canis familiaris | ATGAAGCCTCACGCCAAG | TGATCACACTCATCTCCCCA |
| 1FH2174 | NW_876323 | Canis familiaris | CACCTGTTCTCATAGAATGCAG | AAGTCTCGCCTCGGGGTC |
| 1FH2201a | NW_876323 | Canis familiaris | ATCAACAATGCATGCCACAT | GAGAACAAATAAATGCAAGCCC |
| 1FH2226b d | NW_876323 | Canis familiaris | GGACTACCCCATTGCATTTG | GAATCGAGTCCCATATCGGG |
| 1FH2281 | NW_876277 | Canis familiaris | TGCTGGCACGTATACCAAGA | AGTGTGATGCAGAGGTTCCC |
| 1FH2289a | NW_876284 | Canis familiaris | CATGGTCTCAGGATCCTAGGA | CTAAGCATTCTCTCTGATGGTCTT |
| 1FH2309 | NW_876270 | Canis familiaris | GACTGAGTTCTTTCAGCACAGTG | GGCAGCCTTATTATTCATGGA |
| 1FH2316 | NW_876307 | Canis familiaris | AAATGGCCTGACGAATATGC | GTGCCATGGCATATGGTAAA |
| 1FH2348b d | NW_876256 | Canis familiaris | GCATGCAAAGGTGTTAATTGG | ACACAAGGAAGCTTTGGGG |
| 1FH2541 | NW_876307 | Canis familiaris | CGTATGAGTTGGTATAATCTCAGG | TGCTTTTCACCTCCCTCTTG |
| 1FH2658 | NW_876258 | Canis familiaris | TCTTAGAAATTGCTGGTGGG | TAAGAAACTGCCAGTCTGTGG |
| 1REN161A12 | NW_876308 | Canis familiaris | GCCAAATGTCTCAGATGGGT | TGTCCACAGCTCATGAAAGG |
| 1REN162B09 | NW_876269 | Canis familiaris | CAAACTTGACAGTCTTTTCAGGA | GCATTCAAGATGCACCAATG |
| 1REN69B24 | NW_876323 | Canis familiaris | TGTAGGGCAGTGAATAAAAG | GCCTGGCTCAAGCTCACAAGT |
| 1V142 | DQ118707 | Vulpes vulpes | AAGCAGATCCTAGAGCAGCA | CCCCACAGTTTAGAAATATCTGC |
| 1V374 | DQ118709 | Vulpes vulpes | TACACACAGGAAGTAATGGGG | GACAGAAAGACAGAAGGCTTAG |
| 1V402c d | DQ118717 | Vulpes vulpes | GGGTAATTCATCCAGTGCCTT | TATGCAAACATGCAAACATGC |
| 1V468 | DQ118718 | Vulpes vulpes | TCTCCCACCCAAATCTCTTG | GCATTCAAGATGCACCAATG |
| 1V502b c d | DQ118722 | Vulpes vulpes | ACCCAAGTGTCCTCCATAGAT | TGGCCAAGTACTCTTCCACT |
| 1V602 | DQ118723 | Vulpes vulpes | CAGCCTGGACTACAATTCTCTTT | CCCCAAGTCTTTTGTCCAGA |
| 1V622a | DQ8730 | Vulpes vulpes | TTTTTTGAAAAGCACACCC | TGCTTTGTGTATCTTTTCTTTC |
| 2aht130 | NW_876266 | Canis familiaris | CCTCTCCTGGTAATTGCTGC | TGGAACACTGGTCCCCAG |
| 2c2001 | L78573 | Canis familiaris | TCCTCCTCTTCTTTCCATTGG | TGAACAGAGTTAAGGATAGACACG |
| 2c2006 | L78577 | Canis familiaris | TGGGGGCGTTAAGAGTAATG | CTAGGCCTAAACCCCTGAGC |
| 2c2010 | L78579 | Canis familiaris | AAATGGAACAGTTGAGCATGC | CCCCTTACAGCTTCATTTTCC |
| 2c2017 | L78583 | Canis familiaris | AGCCTCTATAATCACGTGAGCC | CCCAGTACCACCTTCAGGAA |
| 2c2054 | L78589 | Canis familiaris | GCCTTATTCATTGCAGTTAGGG | ATGCTGAGTTTTGAACTTTCCC |
| 2c2079 | L78596 | Canis familiaris | CAGCCGAGCACATGGTTT | ATTGATTCTGATATGCCCAGC |
| 2c2088 | L78599 | Canis familiaris | CCCTCTGCCTACATCTCTGC | TAGGGCATGCATATAACCAGC |
| 2ucb466 | L27191 | Canis familiaris | TCTGGATTGTGGTCACAACC | ACTGGACACTTCTTTTCAGACG |
| 2ucb646 | L29310 | Canis familiaris | TGGGATTCCAAAATGTTTTT | TCCCAGGATTAAGTCCCACA |
markers tested for establishing parentage in the high-density sample.
markers used for establishing parentage in the low-density sample.
marker not used in genotyping due to poor amplification.
marker not used in genotyping due to high frequency of nulls alleles.
marker not used in genotyping due to linkage disequilibrium.
marker not used in genotyping due to violations of Hardy Weinberg equilibrium.
The 15 selected loci were amplified in 4 multiplex sets (Table 2) using the Qiagen multiplex kit (Qiagen, Hilden, Germany) following Kenta et al. [25]. Each 2-µl multiplex reaction contained 50 ng genomic DNA, 1 µl Qiagen master mix and 1 µl primer mix (where all primers were at 0.2 µM). Due to differences in primer annealing temperatures, a touchdown program (65-55°C) was used for PCR amplification using a DNA Engine Tetrad thermocycler (MJ Research, Waltham, USA) with the following program: 3 minutes at 95°C followed by cycles of 30 seconds at 94°C, 90 seconds at 65-55°C (annealing temperature dropped by 1°C every cycle with 35 cycles at 55°C) and 60 seconds at 72°C, followed by a final extension stage of 30 minutes at 60°C. A negative PCR control (containing no DNA) was used to check for PCR contamination and individuals of known genotype were included on each plate to enable consistent allele size scoring. PCR products were separated using a 48-well capillary ABI 3730 automated DNA Sequencer (Applied Biosystems, Warrington, UK) with ABI ROX500 size standard (Applied Biosystems). Data were analyzed using GENEMAPPER version 3.7 (Applied Biosystems). All 410 foxes were examined to assess if any of the loci were sex-linked.
Table 2. Characterisation of 15 microsatellite markers in 24 unrelated foxes from Bristol's high-density population.
| Marker | Multiplex set | N | Florescent label | Alleles observed | Allele size range (bp) | HWE p-value | Non-exclusion probability parent pair | Estimated frequency of null alleles | HObs | HExp |
| FH2541 | 1 | 23 | HEX | 8 | 170–206 | 0.5691 | 0.2122 | +0.0530 | 0.783 | 0.814 |
| REN69B24 | 1 | 20 | 6-FAM | 7 | 228–280 | 0.7865 | 0.2293 | −0.0391 | 0.750 | 0.771 |
| REN161A12 | 1 | 22 | HEX | 5 | 295–303 | 0.8629 | 0.4392 | −0.0680 | 0.682 | 0.608 |
| V374 | 1 | 23 | HEX | 4 | 110–116 | 0.6962 | 0.3335 | −0.0647 | 0.739 | 0.738 |
| V468 | 1 | 21 | 6-FAM | 5 | 83–93 | 0.6550 | 0.2844 | −0.0609 | 0.762 | 0.736 |
| V602 | 1 | 23 | 6-FAM | 5 | 137–162 | 0.1400 | 0.3753 | +0.0281 | 0.609 | 0.663 |
| DGN3 | 2 | 22 | 6-FAM | 9 | 192–250 | 0.7768 | 0.1517 | −0.0177 | 0.864 | 0.854 |
| DGN14 | 2 | 18 | HEX | 7 | 224–250 | 0.7546 | 0.2102 | +0.0105 | 0.722 | 0.811 |
| REN162B09 | 2 | 24 | HEX | 2 | 190–194 | 1.0000 | 0.6793 | −0.0237 | 0.500 | 0.507 |
| V142 | 2 | 23 | HEX | 6 | 131–143 | 0.0906 | 0.2945 | +0.0634 | 0.652 | 0.727 |
| FH2174 | 3 | 20 | HEX | 9 | 232–276 | 0.6574 | 0.1690 | +0.0102 | 0.850 | 0.831 |
| FH2658 | 3 | 13 | 6-FAM | 14 | 352–449 | 0.2187 | 0.0677 | +0.0682 | 0.846 | 0.938 |
| FH2281 | 4 | 23 | 6-FAM | 9 | 429–465 | 0.8526 | 0.1571 | −0.0777 | 0.957 | 0.849 |
| FH2309 | 4 | 23 | 6-FAM | 5 | 350–370 | 0.2808 | 0.2980 | +0.0754 | 0.652 | 0.766 |
| FH2316 | 4 | 21 | HEX | 11 | 282–368 | 0.9346 | 0.1232 | −0.0582 | 1.000 | 0.878 |
The low-density sample was genotyped at 10 loci (Table 1) as part of a previous study [26]. Genotypes were assigned using a Megabase™ 1000 capillary sequencer (Amersham Pharmacia Biotech Ltd, Buckinghamshire, UK) with fragment sizing and allele calling completed with the associated software GENETIC PROFILER v.1.5. To minimize genotyping errors, allele scoring was undertaken independently by 2 people. The DNA extraction method, microsatellite loci identities, PCR reaction conditions, genotyping methods, heterozygosities and Hardy-Weinberg probabilities are described in Soulsbury et al. [26].
Parentage analysis
Since foxes breed during their first year [27], candidate mothers were females aged >9 months known or believed to be present on the cubs' territory from January to October i.e. from the onset of the mating season to the onset of dispersal by that cohort of cubs. Candidate fathers were all males present on the study area during the mating season i.e. the January and February prior to the cubs' birth. Analysis was carried out separately for each cohort of cubs, and the two sets of microsatellite genotyping data were run independently.
We adopted a likelihood method to assign parentage, using the software COLONY version 2.0 [28]. Mating systems were defined as polygynandrous without inbreeding for both sexes. No maximum number of siblings or known relatives were assumed. The proportion of candidate parents sampled was estimated at 70% using capture records [13]. However, since trapping rates could vary with population density, we used the 2009 cohort cubs to compare the effects of a variety of trapping rates (10%, 25%, 70%, 90%). PEDANT-estimated allelic dropout rate of each locus and mean genotyping error from regenotyping were incorporated into the analysis. For consistency, parentage analysis was rerun for the low-density sample using COLONY and the results compared with the previous study [12]. Three problematic loci from the low-density sample, c2006, c2088 and aht130, were not included due to poor scoring, high allelic dropout and/or significant departures from Hardy-Weinberg equilibrium [26]. Re-genotyping data were not available for this sample so the same error rate was assumed. Full likelihood analyses were run 3 times to ensure consistent parentage assignments.
Statistical analysis
The effect of relatedness to dominants on offspring dispersal was assessed separately for each sex using two-way Fisher's exact tests (SPSS version 16, IBM Corporation 2010, Chicago, USA) for all samples combined and for high-density samples separately. Low-density samples could not be analyzed separately due to sample size. Relatedness to dominant males was tested by comparing offspring dispersal strategies between dominant fathers and extra-group fathers. Relatedness to dominant females was tested by comparisons of offspring dispersal strategies between dominants and subordinates. Means are given ± SE.
Results
Microsatellite data
For the high-density population, re-genotyping of 23 individuals (5% of samples) provided a genotype error rate of 3.68%. All 15 microsatellite loci used to genotype the high-density samples were polymorphic and easily scorable. Locations of the loci on the dog genome were assigned using the ENSEMBL web interface (http://www.ensembl.org/Canis_familiaris/blastview) when the E-value was stronger than E-05. The following sequences were utilized to assign genome locations: (i) the amplified region; (ii) the EMBL sequence; and (iii) a 12,180 bp fragment of the sequence surrounding the amplified region. Fourteen loci were assigned to 8 different autosomes based on sequence homology. When multiple sequence sources were available and used for the same locus, all assigned locations were consistent. One fox sequence, V602 (EMBL accession number DQ118723), could not be assigned a location.
The 212 males and 198 females were all genotyped at each locus. No loci were X-linked based on genotyping, as all loci were heterozygous in a proportion of both males and females: X-linked loci would appear homozygous in all males (XY). Females (XX) amplified at all loci, so no loci were Y-linked. The average number of alleles per locus was 7.13, with a mean observed heterozygosity of 0.76±0.07 and mean expected heterozygosity of 0.77±0.05. Estimated mean allelic dropout rate per allele was 0.06±0.03. There was a mean false allele rate per genotype of 0.03±0.01. No pairs of loci were found to display linkage disequilibrium following a sequential Bonferroni correction [29] and no locus deviated from Hardy-Weinberg equilibrium (Table 2). However, FH21746 and REN69B24 were mapped closely on dog chromosome 7. To ensure any potential physical linkage between these loci did not affect parentage assignments, cubs from 2009 were analyzed three times: (i) with both markers; (ii) excluding FH2174; and (iii) excluding REN69B24. No differences in parentage assignments between the three analyses were found and the results adhered to what was expected from behavioral observations. Similarly, there were no differences in 2009 parentage assignments between simulations run with varying sampling proportions of candidate parents i.e. trapping rates of 10%, 25%, 70% and 90%. Thus, 70% was used for each cohort. The combined non-exclusion probability for the parent pair at all 15 loci, calculated using CERVUS, was 4.33e−10.
For the low-density sample, the average number of alleles per locus was 7.90, with a mean observed heterozygosity of 0.60±0.06 and mean expected heterozygosity of 0.72±0.04 [26]. Markers were tested for violations of Hardy-Weinberg equilibrium [12], [26] and linkage disequilibrium [12]. Parentage was assigned using CERVUS v. 2.0 and a decision matrix [12]. Mean polymorphic information content was 0.687; exclusionary power of the first parent was 0.989 and 0.999 for the second parent [12]. We also examined genotypes for sex linkage. All loci were heterozygous in a proportion of both males and females. Females amplified at all loci, so no loci were Y-linked. For the loci reanalyzed using COLONY, no inconsistencies were found between methods.
Effect of parentage on offspring dispersal
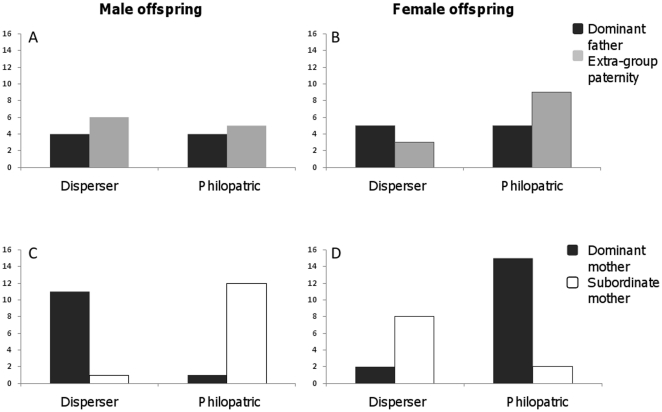
Only foxes with known dispersal status and assigned parents with established social status and/or extra-group paternity could be analyzed; thus 24% of all cubs captured between 1998 and 2009 were used (Table 3). Paternity did not affect male (Fig. 1A, p = 1, N = 19) or female (Fig. 1B, p = 0.378, N = 22) offspring dispersal, whereas maternity did. Males with dominant mothers dispersed significantly more often than males with subordinate mothers (Fig. 1C, p<0.001, N = 25). In contrast, dispersing females were significantly more likely to have subordinate mothers compared to philopatric females (Fig. 1D, p<0.001, N = 27). Analyzed separately, the high-density samples showed the same pattern. Paternity did not have an effect on offspring dispersal for males (p = 0.070, N = 13) or females (p = 0.999, N = 17), whereas maternity significantly affected male (p = 0.004, N = 18) and female (p<0.001, N = 22) dispersal. Low-density samples showed a similar trend but samples sizes were too low (N = 24) for statistical analysis. Only two cubs with known dispersal status were assigned subordinate fathers. They followed the expected dispersal strategies if maternal social status affects juvenile dispersal: a male with a dominant mother dispersed, a male with a subordinate mother was philopatric.
Table 3. Numbers of foxes genotyped.
| High density | Low density | |||
| Males | Females | Males | Females | |
| Total number of individuals genotyped | 212 | 198 | 76 | 70 |
| Total number of cubs genotyped | 85 | 83 | 46 | 36 |
| Cubs with determined paternity only | 4 | 5 | 2 | 3 |
| Cubs with determined maternity only | 36 | 36 | 7 | 7 |
| Cubs with determined paternity and maternity | 30 | 29 | 13 | 11 |
| Cubs with known dispersal and paternal group association only | 2 | 3 | 2 | 0 |
| Cubs with known dispersal and maternal social status only | 5 | 7 | 3 | 1 |
| Cubs with known dispersal and both paternal group association and maternal social status | 12 | 15 | 5 | 4 |
Figure 1. Frequency of dispersing and philopatric red fox offspring from parents of differing social status.
Paternal group association is shown for male offspring (A) and female offspring (B). Maternal social status is shown for male offspring (C) and female offspring (D). Maternal social status had a sex-dependent influence on offspring dispersal, whereas the father's social group had no effect.
Data quality
Difficulties with identifying dispersal strategy, parentage, parental social status and group association potentially created biased sampling. During 2002–2009, 52% of captured cubs were male, 48% female. It is unlikely therefore that there was a bias due to sex differences in births or trapping rates. Similarly, there was no difference between intra- (49%) and extra-group (51%) paternity assignments. This was particularly important as the trapping regime and identification of candidate fathers could have lead to an underestimate of extra-group paternity. Whilst only 16 (8%) of the cubs assigned paternity had subordinate fathers, dominant males monopolize intra-group breeding, so it is unlikely that the low frequency of subordinate fathers is a result of sampling bias. We identified four mixed paternity litters and subordinate mothers were present in each year at all densities. Thus, we believe that our sample is a true representation of the population.
Discussion
We found a strong effect of maternal social status on dispersal in both male and female offspring, which was not affected by population density. In contrast, relatedness to dominant males did not affect dispersal in offspring of either sex.
Dominant males and offspring dispersal
There are a number of reasons why relatedness to the dominant male may not influence dispersal in red foxes. Unrelated subordinate males and dominant males are not in direct reproductive competition because dominant males monopolize breeding with resident females, whereas subordinates seek matings on other territories [6]. It is unlikely that unrelated males compete for food, as this is available in excess [30]. So unrelated philopatric male offspring impose little cost on the dominant male, but equally the advantages of retaining male offspring are limited. Alloparental care by subordinates provides minimal fitness benefits to the dominant pair [16]. Moreover, at high population density few philopatric males gain dominance in their natal group [12], so there is a low chance of inheriting the territory. Therefore there is little selection pressure on dominant males to influence male dispersal.
Whilst we expected female offspring sired by dominant males to avoid inbreeding through dispersal, we found no such effect. This may be because dominant males tend to be unrelated to other adults in the social group due to extra-pair copulations and polygynous group reproductive output [12]. Moreover, interannual turnover of dominant males was high during periods of low population density [12], so there was limited risk of a dominant male mating with his philopatric female offspring. In addition, red fox dispersal is sex biased, with males leaving more frequently and travelling further than females, which generally move into adjacent social groups [9]. This creates high levels of inter-group relatedness across adjacent territories [12], and may explain why dominant males travel up to 2.7 territory diameters in search of extra-pair copulations [6]. Consequently, dispersal is an inadequate mechanism of inbreeding avoidance between dominant males and their female offspring, and so there is little selection pressure on dominant males to influence female offspring dispersal.
Dominant females and offspring dispersal
Numerous studies have suggested male-biased dispersal patterns evolved as an inbreeding avoidance mechanism [3]. The majority of within-group matings in red foxes are confined to the dominant male, with dominant females breeding at every opportunity, and subordinate females breeding at 56% of opportunities [6]. This creates a differential risk of inbreeding between mother and male offspring, dependent upon relatedness to the dominant female. We propose that males from dominant females have a higher probability of breeding with their mothers, and so disperse to avoid future inbreeding costs. In contrast, males with subordinate mothers adopt philopatry, as the costs of dispersal outweigh any potential risks of inbreeding.
This assumes that foxes disperse voluntarily. In some mammals the presence of the opposite sex parent or kin is sufficient stimulus to cause offspring dispersal [3], [31], although many species show increased aggressive behavior during the dispersal period, leading to the forced eviction of selected group members. If male red foxes are selectively evicted by their mothers, this implies the existence of kin recognition. Whilst widely reported in vertebrates [32], it is unknown whether foxes can recognize their own sub-adult offspring. However, if they can, the costs of dispersal could be avoided through alternative behavioral mechanisms of inbreeding avoidance, such as refusal to mate. Furthermore, in canid populations with limited dispersal, extra-pair copulations are highly efficient mechanisms of inbreeding avoidance [4]. Thus more data are needed on the role of agonistic interactions between male offspring and their mothers to conclude that inbreeding avoidance is the true explanation for the effect of relatedness to the dominant female on male dispersal.
Females with subordinate mothers dispersed more frequently than those with dominant mothers. Since dominants breed more frequently, there is a higher probability that the following year's cubs will be more closely related to the dominant's offspring from the current year than offspring from subordinates [6]. Furthermore, philopatric females reproduced significantly more than dispersers because dispersing females often missed their first breeding opportunity [27]. So philopatric female offspring related to the dominant female avoid the costs of dispersal while gaining indirect fitness benefits through alloparental care [33]. Moreover, by retaining her same-sex offspring, a dominant female has a higher probability of one of them inheriting the territory [16], increasing both her own and one of her female offspring's fitness. In contrast, retention of unrelated subordinate females is costly. Alloparental care is of limited benefit to the dominant pair [16] and unrelated females may compete with related females for future dominance and mating opportunities.
Since a lack of affiliative behavior is associated with female red fox dispersal [34], unrelated females may opt to disperse rather than be evicted. Breeding by subordinate females is opportunistic [6], so there is a low probability that females with subordinate mothers will be closely related to future resident offspring. Hence remaining to provide alloparental care will not increase their fitness. Moreover, since dominants have a much longer life span than subordinates at high densities [16], philopatric females have a relatively low chance of territory inheritance and dispersal provides a greater chance of attaining dominance [27]. Thus daughters of subordinate females appeared to disperse voluntarily because there was a low probability of territory inheritance and limited indirect fitness benefits of remaining as a subordinate on their natal territory at both high and low densities.
Whilst this is the first study to demonstrate an effect of direct relatedness to the dominant female on offspring dispersal, several recent studies have highlighted the importance of a range of maternal factors in influencing dispersal behavior in vertebrates. For example, prolonged prenatal exposure to maternal stress levels resulted in extended philopatry in the common lizard, Zootoca vivipara [35]; offspring dispersal behaviors in great tits (Parus major) vary in response to maternal parasitism by differential transfer of maternal yolk androgens [36]; mothers regulate offspring dispersal in western bluebirds (Sialia mexicana) through egg-laying order in response to environmental conditions [37]; and maternal social dominance in spotted hyenas (Crocuta crocuta) gives dispersing males a fitness advantage through faster growth and immigration into stronger clans [38].
Conclusions
Relatedness to resident females is important in juvenile dispersal in group-living mammals: female offspring unrelated to, and male offspring related to, the dominant female disperse. Paternity had no effect on dispersal of either sex. Male dispersal may be driven by inbreeding avoidance, whereas female dispersal appears to be influenced by the fitness advantages associated with residing with the same-sex dominant parent. Selection pressure on paternal control of offspring dispersal was low due to the limited costs associated with retaining unrelated males and the need for alternative inbreeding avoidance mechanisms between the dominant male and his female offspring. These findings have important implications for the evolution of dispersal and group living in social mammals, and our understanding of a key biological process.
Acknowledgments
We thank members of the Mammal Research Group for access to their data, Andy Krupa for genotyping advice, Gavin Horsburgh for assistance with sequence BLAST analysis, Anna Santure and Hannah Dugdale for advice on analysis and comments on the manuscript, and Terry Burke for project support. Genotyping was performed at the NERC Biomolecular Genetics Facility.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Long-term funding for this study was provided by the Dulverton Trust, and the molecular work was supported by the Natural Environment Research Council, United Kingdom. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dobson FS, Jones WT. Multiple causes of dispersal. Am Nat. 1985;126:855–858. [Google Scholar]
- 2.Lambin X, Aars J, Piertney SB. Dispersal, intraspecific competition, kin competition and kin facilitation; a review of empirical evidence. In: Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford: Oxford University Press; 2001. pp. 110–122. [Google Scholar]
- 3.Bowler DE, Benton TG. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. [DOI] [PubMed] [Google Scholar]
- 4.Sillero-Zubiri C, Gottelli D, Macdonald DW. Male philopatry, extra-pack copulations and inbreeding avoidance in Ethiopian wolves (Canis simensis). Behav Ecol Sociobiol. 1996;38:331–340. [Google Scholar]
- 5.Roemer GW, Smith DA, Garcelon DK, Wayne RK. The behavioural ecology of the island fox (Urocyon littoralis). J Zool. 2001;255:1–14. [Google Scholar]
- 6.Baker PJ, Funk SM, Bruford MW, Harris S. Polygynandry in a red fox population: implications for the evolution of group living in canids? Behav Ecol. 2004;15:766–778. [Google Scholar]
- 7.West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17:661–672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Baker PJ, Harris S. The behavioural ecology of red foxes in urban Bristol. In: Macdonald DW, Sillero-Zubiri C, editors. Biology and conservation of wild canids. Oxford: Oxford University Press; 2004. pp. 207–216. [Google Scholar]
- 9.Harris S, Trewhella WJ. An analysis of some of the factors affecting dispersal in an urban fox (Vulpes vulpes) population. J Appl Ecol. 1988;25:409–422. [Google Scholar]
- 10.Newman TJ, Baker PJ, Harris S. Nutritional condition and survival of red foxes with sarcoptic mange. Can J Zool. 2002;80:154–161. [Google Scholar]
- 11.Soulsbury CD, Iossa G, Baker PJ, Cole NC, Funk SM, et al. The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mamm Rev. 2007;37:278–296. [Google Scholar]
- 12.Iossa G, Soulsbury CD, Baker PJ, Edwards KJ, Harris S. Behavioral changes associated with a population density decline in the facultatively social red fox. Behav Ecol. 2009;20:385–395. [Google Scholar]
- 13.Baker PJ, Harris S, Robertson CPJ, Saunders G, White PCL. Differences in the capture rate of cage-trapped red foxes Vulpes vulpes and an evaluation of rabies control measures in Britain. J Appl Ecol. 2001;38:823–835. [Google Scholar]
- 14.Harris S. Age determination in the red fox (Vulpes vulpes): an evaluation of technique efficiency as applied to a sample of suburban foxes. J Zool. 1978;184:91–117. [Google Scholar]
- 15.Gannon WL, Sikes RS Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mamm. 2007;88:809–823. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker PJ, Robertson CPJ, Funk SM, Harris S. Potential fitness benefits of group living in the red fox, Vulpes vulpes. Anim Behav. 1998;56:1411–1424. doi: 10.1006/anbe.1998.0950. [DOI] [PubMed] [Google Scholar]
- 17.Nicholls JA, Double MC, Rowell DM, Magrath RD. The evolution of cooperative and pair breeding in thornbills Acanthiza (Pardalotidae). J Avian Biol. 2000;31:165–176. [Google Scholar]
- 18.Richardson DS, Jury FL, Blaakmeer K, Komdeur J, Burke T. Parentage assignment and extra-pair paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol Ecol. 2001;10:2263–2273. doi: 10.1046/j.0962-1083.2001.01355.x. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 20.Kukekova AV, Trut LN, Oskina IN, Kharlamova AV, Shikhevich SG, et al. A marker set for construction of a genetic map of the silver fox (Vulpes vulpes). J Hered. 2004;95:185–194. doi: 10.1093/jhered/esh033. [DOI] [PubMed] [Google Scholar]
- 21.Wandeler P, Funk SM. Short microsatellite DNA markers for the red fox (Vulpes vulpes). Mol Ecol Notes. 2006;6:98–100. [Google Scholar]
- 22.Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- 23.Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. Heredity. 1995;86:248–249. [Google Scholar]
- 24.Johnson PCD, Haydon DT. Maximum-likelihood estimation of allelic dropout and false allele error rates from microsatellite genotypes in the absence of reference data. Genetics. 2007;175:827–842. doi: 10.1534/genetics.106.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenta T, Gratten J, Haigh NS, Hinten GN, Slate J, et al. Multiplex SNP-SCALE: a cost-effective medium-throughput single nucleotide polymorphism genotyping method. Mol Ecol Resour. 2008;8:1230–1238. doi: 10.1111/j.1755-0998.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 26.Soulsbury CD, Iossa G, Edwards KJ, Baker PJ, Harris S. Allelic dropout from a high-quality DNA source. Conserv Genet. 2007;8:733–738. [Google Scholar]
- 27.Soulsbury CD, Baker PJ, Iossa G, Harris S. Fitness costs of dispersal in red foxes (vulpes vulpes). Behav Ecol Sociobiol. 2008;62:1289–1298. [Google Scholar]
- 28.Wang J, Santure AW. Parentage and sibship inference from multilocus genotype data under polygamy. Genetics. 2009;181:1579–1594. doi: 10.1534/genetics.108.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 30.Ansell RJ. The spatial organisation of a red fox (Vulpes vulpes) population in relation to food resources. 2004. PhD Thesis, University of Bristol, Bristol, UK.
- 31.Wolff JO. What is the role of adults in mammalian juvenile dispersal? Oikos. 1993;68:173–176. [Google Scholar]
- 32.Halpin ZT. Kin recognition cues of vertebrates. In: Hepper PG, editor. Kin recognition. Cambridge: Cambridge University Press; 1991. pp. 220–258. [Google Scholar]
- 33.Griffin AS, West SA. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302:634–636. doi: 10.1126/science.1089402. [DOI] [PubMed] [Google Scholar]
- 34.Harris S, White PCL. Is reduced affiliative rather than increased agonistic behaviour associated with dispersal in red foxes? Anim Behav. 1992;44:1085–1089. [Google Scholar]
- 35.De Fraipont M, Clobert J, John-Alder H, Meylan S. Increased pre-natal maternal corticosterone promotes philopatry of offspring in common lizards Lacerta vivipara. J Anim Ecol. 2000;69:404–413. [Google Scholar]
- 36.Tschirren B, Fitze PS, Richner H. Maternal modulation of natal dispersal in a passerine bird: an adaptive strategy to cope with parasitism? Amer Nat. 2007;169:87–93. doi: 10.1086/509945. [DOI] [PubMed] [Google Scholar]
- 37.Duckworth RA. Maternal effects and range expansion: a key factor in a dynamic process? Phil Trans R Soc B. 2009;364:1075–1086. doi: 10.1098/rstb.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Höner OP, Wachter B, Hofer H, Wilhelm K, Thierer D, et al. The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nature Commun. 2010;1:60. doi: 10.1038/ncomms1059. doi: 10.1038/ncomms1059. [DOI] [PMC free article] [PubMed] [Google Scholar]